Abstract
Objective
To address the inconsistent findings on whether childhood misfortune increases adult cancer occurrence.
Methods
This study uses longitudinal data from the National Survey of Midlife Development in the United States (MIDUS) that first sampled 3,032 respondents aged 25 to 74 during 1995–1996. A series of logistic regressions were estimated separately for men and women to test whether the effect of childhood misfortune on adult cancer was largely cumulative or specific to the type or profile of misfortune.
Results
For men, additive childhood misfortune, physical abuse by father, and frequent abuse by either parent increased cancer risk. For women, physical abuse by mother and frequent abuse by either parent increased cancer risk.
Discussion
Analyses revealed the importance of examining alternative specifications of childhood misfortune for men and women. Additive childhood misfortune predicted cancer for men only, whereas child abuse by parent of the same sex predicted cancer for men and women.
Keywords: life course, stress process, childhood misfortune, cancer
Introduction
Research on the life course has proliferated in many fields, including sociology, psychology, epidemiology, and gerontology, where scholars are intrigued by whether there are enduring effects of early misfortune on health and well-being. Considerable research has revealed the “long arm” of early misfortune: Childhood events and exposures have enduring effects on a host of adult health indicators such as inflammation, heart attack, and obesity (respectively, Danese, Pariante, Caspi, Taylor, & Poulton, 2007; Hamil-Luker & O’Rand, 2007; Williamson, Thompson, Anda, Dietz, & Felitti, 2002). Although evidence is emerging that adverse environments during childhood have long-term effects on health, the mechanisms by which this occurs and the outcomes affected are matters of continuing debate.
Biological embedding is the term used by Hertzman and Boyce (2010, p. 330) to describe “the process by which human experience alters biological processes in stable and long-term ways that influence health over the life course.” Although any human experience has the potential for biological embedding, childhood experiences are distinctive because of how biological systems may be altered at early ages to deal with environmental insults. Episodic or short-term misfortune during childhood (e.g., death of a parent) may precipitate changes in biological systems, but they may also be associated with later-life stress proliferation and future exposure to health-disrupting stressors (Pearlin, 1989, 2010; Pearlin, Schieman, Fazio, & Meersman, 2005). More enduring forms of childhood misfortune (e.g., socioeconomic strain) may likewise alter early-life physiology, but their effects on adult health may also be transmitted through later environmental or behavioral pathways (Cohen, Janicki-Deverts, Chen, & Matthews, 2010). At this point, however, much of the research has focused on accumulated misfortune across different life domains because chronic exposure to misfortune, including multiple forms of misfortune, is seen as more likely to result in systemic biological changes and compromised health (Felitti et al., 1998; Taylor, 2010).
Among the many diseases examined in studies of the effects of childhood misfortune, cancer has received relatively little attention—and the results have been mixed. Some studies reveal that adverse childhood experiences are associated with greater cancer risk (e.g., Felitti et al., 1998; Fuller-Thomson & Brennenstuhl, 2009), and others report no such association (e.g., Draper et al., 2008; Korpimäki, Sumanen, Sillanmäki, & Mattila, 2010). Moreover, what is meant by “adversity” or “misfortune” varies considerably across the studies. Some studies operationalize misfortune during childhood as events (e.g., death of a parent: Scherg & Blohmke, 1988), and others examine conditions (e.g., low social class: de Kok et al., 2008) or experiences (e.g., child abuse: Fuller-Thomson & Brennenstuhl, 2009). The current study seeks to address the inconsistency in previous findings by examining whether multiple indicators of childhood misfortune, spanning events, conditions, and personal experiences, are related to adult cancer risk. Drawing from a 10-year longitudinal study, the analysis also addresses whether adult cancer is influenced by accumulated misfortune or selected childhood experiences.
Life Course Influences on Cancer
Life course epidemiology draws attention to the importance of childhood for diseases that may not emerge until decades later (Kuh & Ben-Shlomo, 2004). Since childhood is a period of rapid change in biological systems, including neurological development, scholars view biological embedding during childhood as highly sensitive to influences of the social environment (Hertzman & Boyce, 2010). Indeed, Taylor (2010) notes that harsh family environments during childhood affect stress responses, stress regulatory systems, and gene expression during childhood, thereby increasing the risk of mental and physical health problems. Even animal studies have shown that rats exposed to stressors at a young age are more likely to develop and grow tumors (Laconi et al., 2000). Thus, early misfortune may initiate the biological embedding of health risks, including cancer risk.
Besides its importance for physiological development, childhood is a crucial period for establishing the health behaviors, psychosocial tendencies, and social skills that will be consequential for later-life health, including cancer risk (Singh-Manoux & Marmot, 2005). To the extent that misfortune increases the probability of excessive drinking, smoking, or overeating, decreases agency and psychological well-being, and disrupts trust in others or thwarts social flourishing, early life events may set into motion a cascade of disadvantage with a wide and long reach (Ferraro & Shippee, 2009). Stress process theory is useful in this context because it posits that stressors tend to proliferate over the life course both temporally (Pearlin et al. 2005, p. 210) and across life contexts or domains (Pearlin et al., 2005, p. 211). A plausible inference from this insight is that adult environmental conditions and health behaviors may represent an important link between childhood misfortune and adult disease risk (Cohen et al., 2010).
Our review of recent contributions to cancer epidemiology suggests three important considerations in the relationship between childhood misfortune and adult cancer risk. First, there is growing evidence for the social and environmental origins of cancer. While cancer rates have been increasing in the United States and worldwide, explanations attributing the growth solely to medical advancements, which helped improve diagnostic tools and increase life expectancy, have been insufficient (Irigaray et al., 2007).1 Accordingly, more attention has shifted toward environmental factors and gene–environment interactions (Irigaray et al., 2007; Lichtenstein et al., 2000; Suisman, Burt, McGue, Iacono, & Klump, 2011). Indeed, one study that examined 15 common types of cancer found that environmental effects were much larger than genetic effects for all except thyroid cancer (Czene, Lichtenstein, & Hemminki, 2002). We view childhood misfortune as a type of environmental influence and ask whether it raises the risk of cancer occurrence in adulthood.
Second, beyond emphasizing how environmental factors influence cancer, recent research suggests that there are important sex differences in cancer etiology. Cook et al. (2009) highlight the important role that sex plays in the risk, onset, and etiology of cancer, supporting the notion that the role of sex in cancer research should extend beyond sex as a covariate. In addition to cancer disparities due to sex-specific cancer sites, incidence and mortality rates for non–sex-specific cancers vary substantially between men and women. Sex disparities in cancers that affect both sexes have been attributed not only to differences in environmental exposures but also to differences in physiological responses to the same exposures (Cook et al., 2009). A growing body of literature suggests that hormonal sex differences may modify the environment–cancer relationship, producing different rates and risks even for the cancers that affect both sexes. For example, whereas large concentrations of estrogen in women may be a protective factor against liver cancer, a common cancer among men (Naugler et al., 2007), it may also increase the risk of thyroid cancer for women (Chen, Vlantis, Zeng, & van Hasselt, 2008). On the physiological level, boys and girls may, therefore, be exposed and might react differently to childhood insults. Cancer researchers should, as Cook et al. suggest, “be encouraged to design, analyze, and report sex-specific associations to aid our understanding of sex differences in cancer incidence” (p. 1181). By performing separate analyses for each sex, research may identify which types of childhood misfortune biologically embed as carcinogenic for one sex or the other.
Third, there is growing awareness of varying latency periods in cancer etiology. Researchers recognize that cancer begins with damage to a cell’s DNA, which initiates a process of mutated cell division that may eventually evolve into a malignant neoplasm; this genetic process is often initiated by a carcinogenic agent (Merlo, Pepper, Reid, & Maley, 2006). Therefore, there can be a substantial time lag from carcinogenic exposure to onset of cancer, allowing time for a carcinogenic agent to biologically embed. A common example is the 30-year gap between smoking initiation and onset of lung cancer (Lynch & Smith, 2005). Thus, one wonders whether childhood misfortune may manifest a parallel latency period in the development of cancer.
Childhood Misfortune and Cancer
As noted earlier, results from previous studies are inconsistent as to whether childhood misfortune raises cancer risk (see Felitti et al., 1998; Korpimäki et al., 2010). The inconsistency may be due to many reasons such as the nation or region studied (examples include Canada, Germany, Finland, and United States) or cancer outcome studied (i.e., prevalence, incidence, or mortality), but we identify four issues that are endemic to accurately assessing the effects of childhood misfortune on adult cancer risk.
First, there is the basic question of what is meant by childhood misfortune.2 Several specific childhood risks have been linked to adult cancer: poor health, maternal death, and low socioeconomic status (SES) during childhood (Blackwell, Hayward, & Crimmins, 2001; Jacobs & Bovasso, 2000; Power, Hypponen, & Smith, 2005; Scherg & Blohmke, 1988). Childhood physical abuse has also been shown to increase cancer risk, net of other early misfortunes such as parental divorce, unemployment, and substance abuse (Fuller-Thomson & Brennenstuhl, 2009). Although some studies consider multiple events and/or experiences, others are limited to investigating a single event such as parental divorce (e.g., Hemminki & Chen, 2006). The single-event studies, though very helpful for detailed consideration of one event or condition, do not address the clustering of multiple forms of misfortune: Some types of misfortune may increase the risk of additional misfortune (i.e., chain of risk). Thus, it is possible that the presumed effect in studies of single events is actually due to related but unmeasured events.
Although all studies of childhood misfortune are limited by the inventory of misfortunes queried, the limitation is critical for interpreting the literature. For example, some studies claim to study childhood misfortune but exclude experiences that others point to as predictive of cancer risk. The study by Korpimäki et al. (2010) taps adversities such as family alcohol problems and serious conflicts but omits child abuse and parental death—and reports that childhood adversity is not related to cancer in Finland. By contrast, Felitti et al. (1998) include child abuse in their sum of adversities and report that the number of adverse childhood experiences raises cancer risk in the United States (see also Brown et al., 2010). These substantive differences in what is meant by childhood misfortune are important for both research and clinical application.
Second, various scholars approach the issue of heterogeneity in misfortune in different ways. Felitti and his colleagues reported a dose–response relationship between adversity and a host of health conditions, including cancer, asserting that it is the accumulation of multiple hazards that is critical to the development of disease and disability. Aggregating multiple types of adverse childhood experiences may be referred to as cumulative or additive misfortune (Ferraro, 2011), and other scholars have found this to be useful for studying the “joint or cumulative effects of multiple traumas” (Turner & Lloyd, 1995:268; Turner, Wheaton, & Lloyd, 1995). By contrast, some studies measure multiple forms of childhood misfortune but report them separately: unique types of misfortune (Fuller-Thomson & Brennenstuhl, 2009). A third approach is what may be called the clustering of misfortune by using analytic tools to find the underlying structure of various forms of misfortune (Hamil-Luker & O’Rand, 2007; Schafer, Ferraro, & Mustillo, 2011). Finally, some recent studies integrate indicators of severity or frequency to formulate profiles of misfortune (e.g., Greenfield & Marks, 2009a, 2009b; Schilling, Aseltine, & Gore, 2008). Each of these approaches may be appropriate, but if multiple measures are available, it seems incumbent on investigators to at least test alternative forms of aggregation when assessing the effects of childhood misfortune on health outcomes. Failure to do so may lead to generalizations that aggregated misfortunes do not lead to cancer risk, when selected forms of misfortune may actually be related but undetected. Indeed, one study cogently reveals that these methodological decisions can yield inconsistent and perhaps misleading results (Schilling et al., 2008).
Third, although some forms of childhood misfortune touch persons regardless of SES, there is a recurring theme in the literature that lower SES often exposes children to additional risks, from air pollution and environmental tobacco smoke to risky lifestyles, expressed through family networks and neighborhoods. Some of the early studies of life events and cancer risk did not adjust for SES, especially those done on clinical samples (e.g., Scherg & Blohmke, 1988), but most recent studies make some adjustment for indicators of SES. In doing so, findings have been reported that cancer is not directly influenced by childhood disease or misfortune but that the effects are indirect (Blackwell et al., 2001; Smith, Hart, Blane, & Hole, 1998). In short, because of the well-established gradient between SES and health, the SES measures used and the analytic strategies for assessing their influence merit systematic consideration.
Finally, adequate incorporation of adult status characteristics and behaviors are essential to understanding whether childhood misfortune influences cancer risk. Failure to adequately adjust for adult characteristics and behavior is a concern on two fronts: (a) overestimating the effects of childhood misfortune, and (b) failure to identify how childhood misfortune indirectly contributes to cancer risk. Indeed, publications from the Kaiser Permanente Adverse Childhood Experiences Study show that adversity increases the risk of some health-related behaviors such as smoking, which in turn partially mediate the relationship between childhood adversity and cancer (Brown et al., 2010; Felitti et al., 1998). Thus, one may be overestimating the direct effect of childhood misfortune without adequately addressing the behavioral consequences of the early misfortune.
Recognizing the contributions and limitations of previous research, the current study uses a longitudinal national sample with multiple measures of childhood misfortune and adult characteristics to address the following research questions for men and women.
Research Question 1: Does childhood misfortune increase cancer risk in adulthood?
We expect to find an association between childhood misfortune and adult cancer, even after adjusting for a fairly comprehensive inventory of adult status characteristics and behaviors.
Research Question 2: Is the effect of childhood misfortune on adult cancer risk largely cumulative or specific to the type or profile of misfortune?
We are unaware of any prior studies that contain multiple measures of childhood misfortune and systematically compare the effects of alternative specifications of misfortune (e.g., additive, unique, or frequent) on cancer risk. Thus, we address this gap and test alternative specifications in order to potentially resolve inconsistencies in previous research.
Research Question 3: What pathways link childhood misfortune to cancer occurrence during adulthood?
To better understand how childhood misfortune increases cancer risk in adulthood, we examine multiple pathways, including adult socioeconomic, lifestyle, and psychosocial factors.
Method
Sample
Data were drawn from two waves of the National Survey of Midlife Development in the United States (MIDUS), a national study of health and well-being sponsored by the John D. and Catherine T. MacArthur Foundation Network on Successful Midlife Development. Wave 1 data were collected between 1995 and 1996. Respondents were drawn from a nationally representative random-digit-dial sample of noninstitutionalized, English-speaking adults aged 25 to 74 residing in the 48 contiguous states. In addition, older adults (aged 65–74) and men were oversampled using disproportionate stratified sampling. Data collection was carried out in two parts. Initially, respondents were contacted by phone and participated in a computer-assisted telephone interview, which yielded a 70% response rate. Respondents who participated in the telephone interview were mailed a self-administered questionnaire. The response rate from the mailed questionnaire was 86.6%, yielding an overall response rate of 61% (.70 * .87 = .61) and an overall sample size of 3,302 (n) for the Wave 1 main sample.
Surviving respondents from Wave 1 were contacted for a follow-up at Wave 2 data collection during 2004–2006. Of the 3,032 respondents from Wave 1, 2,101 completed the Wave 2 telephone surveys (response rate of 69.5%). For this study, Wave 1 data were drawn from respondents who completed both the telephone and mail surveys, and Wave 2 data were drawn from respondents who completed the telephone interviews only.
Measures
The dependent variable, cancer occurrence, is drawn from both Wave 1 and Wave 2. During the telephone interviews at both time points, respondents were asked if they ever had any type of cancer. Although MIDUS did not distinguish between types of skin cancer, many skin cancers are minor, relative to the severity of other types of cancer (e.g., breast, lung). Whereas many other surveys, such as the Health and Retirement Study, exclude skin cancer when measuring the disease, we also exclude skin cancer cases from our measurement protocol (if skin cancer was the only cancer mentioned). Thus, responses to the survey question were coded into a dummy variable, where one equals has had (non-skin) cancer, and zero equals otherwise.
We also considered the possibility of examining site-specific cancer, but dividing cancer occurrence into subgroups seemed impractical considering the small number of respondents with cancer (only 5.9% of men and 10.4% of women reported a cancer diagnosis by Wave 2; see Table 1) and that a significant proportion of cancer sites (21.7%) were documented as “other.” We acknowledge the limitation of using the MIDUS for this project, but recent studies have used a general measure of all-site cancer as an outcome variable. Moreover, our aim is to address the inconsistency in recent studies (e.g., Fuller-Thomson & Brennenstuhl, 2009; Korpimäki et al., 2010) by examining how different types of misfortune affect men and women’s cancer risk.3
Table 1.
Descriptive Statistics by Sex From the Midlife Development in the U.S. Study (Wave 1, 1995)
| Variables | Range | Men
|
Women
|
||
|---|---|---|---|---|---|
| M (SD) | Cancer casesa | M (SD) | Cancer cases | ||
| Cancer occurrenceb | 0–1 | 0.059 | — | 0.104*** | — |
| Additive childhood misfortune | 0–5 | 1.944 (1.932) | — | 1.928 | 1.906 |
| Types of childhood misfortune | |||||
| Household socioeconomic status (SES) | |||||
| Welfare | 0–1 | 0.062 | 7 | 0.068 | 7 |
| Financially worse than others | 0–1 | 0.286 | 35 | 0.275 | 47 |
| Low education head of household | 0–1 | 0.387 | 46 | 0.407 | 76 |
| Household composition | |||||
| Parental death | 0–1 | 0.062 | 8 | 0.077 | 18 |
| Parental divorce | 0–1 | 0.117 | 7 | 0.141 | 21 |
| No male in household | 0–1 | 0.054 | 4 | 0.076* | 11 |
| Health at age 16 | |||||
| Poor physical health | 0–1 | 0.034 | 1 | 0.044 | 6 |
| Poor mental health | 0–1 | 0.066 | 5 | 0.086* | 15 |
| Abuse by perpetrator | |||||
| Physical abuse—mom | 0–1 | 0.174 | 15 | 0.197 | 46 |
| Physical abuse—dad | 0–1 | 0.219 | 26 | 0.139*** | 27 |
| Physical abuse—sibling | 0–1 | 0.334 | 19 | 0.319 | 50 |
| Physical abuse—other | 0–1 | 0.253 | 21 | 0.135*** | 23 |
| Emotional abuse—mom | 0–1 | 0.193 | 11 | 0.252*** | 50 |
| Emotional abuse—dad | 0–1 | 0.285 | 23 | 0.228*** | 49 |
| Emotional abuse—sibling | 0–1 | 0.406 | 28 | 0.408 | 65 |
| Emotional abuse—other | 0–1 | 0.306 | 18 | 0.230*** | 34 |
| Frequency of emotional and physical abuse by parents | |||||
| Never physical or emotional abuse | 0–1 | 0.224 | 17 | 0.309*** | 47 |
| Rarely one or both types | 0–1 | 0.356 | 29 | 0.276*** | 26 |
| Frequently only one type | 0–1 | 0.188 | 11 | 0.180 | 26 |
| Frequently both types | 0–1 | 0.229 | 22 | 0.226 | 52 |
| Demographic | |||||
| Age | 20–74 | 46.676 (13.075) | 47.427 (13.158) | ||
| Black | 0–1 | 0.053 | 0.083*** | ||
| Adult SES | |||||
| Years of education | 4–20 | 14.009 (2.777) | 13.565 (2.439)*** | ||
| Incomec | 0–300 | 75.533 (62.473) | 58.476 (54.987)*** | ||
| Lifestyle | |||||
| Total number of cigarettes smokedd | 0–148.701 | 15.106 (21.882) | 8.838 (15.375)*** | ||
| Obese | 0–1 | 0.241 | 0.251 | ||
| Psychosocial factors | |||||
| Widowed | 0–1 | 0.022 | 0.095*** | ||
| Divorced | 0–1 | 0.139 | 0.227*** | ||
| Family strain | 1–4 | 2.041 (0.591) | 2.207 (0.629)*** | ||
| Depressive symptoms | 0–7 | 0.618 (1.715) | 1.005 (2.166)*** | ||
| Agency | 1–4 | 2.808 (0.615) | 2.654 (0.687)*** | ||
| N | 1,471 | 1,561 | |||
Absolute number of cancer cases per each childhood misfortune.
Measure of cancer occurrence is taken from both Wave 1 (1995) and Wave 2 (2005) and indicates report of cancer by Wave 2.
Income in thousands of dollars.
Number of cigarettes in tens of thousands.
p < .05.
p < .01.
p < .001 (two-tailed tests; indicate significant differences between sexes).
Item measures for childhood misfortune are from Wave 1. Drawing from previous literature (see Felitti et al., 1998; Greenfield & Marks, 2009a; Turner et al., 1995) and available MIDUS questions, 16 different indicators were used to measure childhood misfortune: (1) family receipt of welfare or Aid to Dependent Children assistance for a period of 6 months or longer; (2) self-report of being financially worse off than other families; (3) less than a high school education for father (or mother if father was absent); (4) lack of male in household; (5) parental divorce; (6) parental death; (7–10) physical abuse by father, mother, sibling or other; (11–14) emotional abuse by father, mother, sibling, or other; and (15–16) self-report of poor mental or physical health at age 16. All 16 item measures were initially coded as dummy variables, and then different variables were created for 3 separate models to systematically investigate how childhood misfortune affects cancer in adulthood.
Physical and emotional abuse categories were modeled after the Conflict Tactics Scale (Straus, 1979) using 15 different item measures from the MIDUS self-administered questionnaire. Respondents were asked how frequently their mother, father, siblings, or anybody else insulted or swore at them; sulked or refused to talk to them; did or said something spiteful; threatened to hit them; smashed or kicked something in anger; pushed, grabbed, or shoved them; slapped them; threw something at them; kicked, bit, or hit them with a fist; hit or tried to hit them with something; beat them up; choked them; burned or scaled them. Since physical and emotional response categories ranged in frequency from never to often, respondents who reported experiencing abuse as sometimes or often were coded as 1 and those who reported never or rare were coded as 0. Dummy variables for poor mental and physical health at age 16 were created by coding those who reported poor or fair health as 1 and those who reported good, very good, or excellent as 0.
We tested three alternative specifications of childhood misfortune. For additive misfortune, we drew from Felitti et al. as follows. The 16 misfortune measures were divided into 5 categories of misfortune: household SES (receipt of welfare, financially worse than others, and less than a high school education for head of household), household composition (lack of male in household, parental divorce, and parental death), health at 16 (poor mental and physical health at age sixteen), physical abuse (physical abuse by father, mother, sibling, and other), and emotional abuse (emotional abuse by father, mother, sibling, and other). Responses indicating the experience of one or more of the misfortunes in each category were coded as 1. Then, these 5 categories were summed to create a count variable ranging from 0 to 5 (e.g., Felitti et al., 1998).
To test the effect of each unique misfortune, dummy variables for each of the 16 childhood misfortune measures were used.
Finally, to test the effect of profiles of misfortune, the measures for physical and emotional abuse by parents were recategorized into profiles to indicate frequency of both types of abuse. Although Greenfield and Marks’ (2009a) profiles used six configurations of abuse, we used a four-category classification due to the small number of cases for cancer occurrence. Parental emotional and physical abuse response categories that ranged from never to often were recoded into four different profiles: (1) never experienced physical or emotional abuse, (2) rarely experienced one or both types of abuse rarely, (3) frequently experienced one type of abuse, and (4) frequently experienced both types of abuse.
All models were adjusted for age and race; both variables were drawn from Wave 1. A continuous variable for age was used and a dummy variable was created for race (coded as 1 for Black). Drawing from Pearlin’s (2010) stress process model, we included adult covariates from Wave 1 to adjust for possible later life mechanisms of SES, lifestyle, and psychosocial factors that may influence cancer risk in adulthood. To examine the role of adult SES, measures of education and income were used. Values for education were coded to indicate years of education, ranging from 4 to 20 years. Income in thousands of dollars was created by dividing the respondent’s raw income by 1,000.
Obesity and lifetime number of cigarettes smoked were used to examine the role of adult lifestyles that affect health. Obesity was coded as a dummy variable with respondents who had a body mass index (BMI) of 30 or greater coded coded as 1 to indicate obesity, and those with a BMI of less than 30 were coded as 0. Lifetime number of cigarettes was estimated to examine the cumulative effect of smoking.4 First, we calculated the approximate number of years smoked (current age minus age began smoking regularly for current smokers; age when last smoked regularly minus age began smoking regularly for former smokers). Next, we estimated the yearly number of cigarettes smoked (average number of cigarettes smoked daily multiplied by 365). The product of years smoked and annual number of cigarettes yielded lifetime number of cigarettes. Since lifetime number of cigarettes ranged from 0 to 1.49 million for Wave 1 and from 0 to 1.72 million for Wave 2, the variable was then divided by 10,000.
To examine the role of psychosocial factors, social and psychological measures of marital status, family strain, depressive symptoms, and agency were included. Dummy variables for respondents who were divorced and widowed were created with those divorced coded as 1 for the variable divorced and those widowed coded as 1 for the variable widowed. Scales were constructed for family strain, depressive symptoms, and agency. Family strain was constructed by calculating the mean of four items; it ranges from 1 to 4, with higher values indicating higher levels of strain (alpha = .80). Respondents were asked how often family members made demands on and criticized them, let them down, and got on their nerves. The depressive symptoms variable was constructed using seven indicators of both depressed affect and anhedonia and ranges from 0 to7. Measures for depressive symptoms were designed to tap into clinical depressive episodes over the past the year and are based on criteria for clinical depression as defined in the American Psychiatric Association’s (APA) Diagnostic and Statistical Manual of Mental Disorders (DSM-III-R; APA, 1987). Respondents indicated whether they had experienced loss of interest or appetite, fatigue, trouble sleeping or concentrating, feeling worthless, or frequent thoughts on death for 2 weeks during the past 12 months when they had felt sad or depressed. Previous research has used the MIDUS depressive symptoms scale as a measure of depression (Kessler, Mickelson, Walters, Zhao, & Hamilton, 2004). Agency was constructed by calculating the mean of five item measures and ranges from 1 to 4, with higher values indicating higher levels of agency (alpha = .79). Respondents were asked how much the following five adjectives described them: self-confident, forceful, assertive, outspoken, and dominant.
Descriptive statistics by sex for the dependent and independent variables are presented in Table 1. Because of the previously mentioned sex differences in biological mechanisms and cancer rates, separate analyses were performed for men and women.
Analysis
To systematically examine the relationship between childhood misfortune and cancer occurrence in adulthood, two stages of analyses were performed. To determine whether the effect of childhood misfortune on adult cancer risk was largely cumulative or specific to the type or profile of misfortune, three sets of logistic regression models were estimated in the first stage of analyses. In each analysis, cancer occurrence was modeled separately for men and women. To test whether there was a dose–response relationship between childhood misfortune and cancer, the first set of models used a count variable for childhood misfortune (see Felitti et al., 1998). To investigate whether unique forms of misfortune are associated with cancer, Model 2 used 16 variables as separate predictors of cancer occurrence. A third set of models gave greater specificity to parental physical and emotional abuse profiles, differentiating the frequency of both types of abuse (Greenfield & Marks, 2009a). In Stage 1, each of the models was adjusted for all adult covariates (demographic, SES, lifestyle, and psychosocial factors). In this stage of analysis, we also performed supplementary analyses to explore the relationship between age and childhood misfortune, as cancer is a disease associated with age. To test the possibility that childhood misfortune has different associations with cancer in various stages of adulthood, we reestimated the models with interaction terms for each specification of misfortune (Misfortune × Age, Misfortune × Age-squared, Misfortune × Aged 50 and Older; yes/no).
The second stage builds on Stage 1 to systematically investigate potential pathways of childhood misfortune. Guided by the findings in Stage 1, Stage 2 of the analyses introduced blocks of variables to examine potential mechanisms of influence: adult SES, lifestyle, and psychosocial factors. In this phase of the analyses, cancer occurrence was modeled for each sex using a series of logistic regression models with specified blocks of variables in separate hierarchical steps. For the first set of models, cancer occurrence was modeled adjusting only for age and race. Model 2 included item measures of childhood misfortune using the abuse profiles as those were significant for both men and women. To test mechanisms of adult SES, Model 3 added education and income to the equation. To test potential lifestyle pathways in adulthood, Model 4 was estimated using age, race, childhood misfortune, obesity, and smoking. To test the effect of psychosocial mechanisms, Model 5 was estimated using age, race, childhood misfortune, divorce, widowed, family strain, depressive symptoms, and agency. Model 6 is the full model that included all independent variables—age, race, childhood misfortune, education, income, obesity, smoking, marital status, family strain, depressive symptoms, and agency.
For both stages of analysis, all models included the main MIDUS sample weight, which consists of weights for respondents who completed both the telephone and mail questionnaire at Wave 1. Listwise deletion was also used for all models. The variable with the highest proportion of missing cases was obesity—4.15% missing for men and 5.57% missing for women. Income had the second highest proportion of missing cases, with 3.33% missing for men and 3.65% missing for women.
Sex differences were also assessed across each of the model specifications by testing whether logit coefficients differed for men and women. We used a Wald χ2 test to make these comparisons, but adjusted for unequal residual variation between subsamples using the method recommended by Allison (1999) and implemented in Stata by Hoetker (2007). Initially, we ran a Heckman selection model to test for sample attrition due to cancer mortality between Wave 1 and Wave 2. However, results indicated that there was no significant sample bias due to mortality; therefore, this preliminary analysis was not included in the final models presented below.
Results
As shown in Table 1, the central tendencies of most variables vary little between men and women, but several differences are noteworthy. Specifically, cancer occurrence was higher for women than for men (p < .001). Several of the types of misfortune also differed by sex. Women were more likely to have experienced no male in household, been emotionally abused by mother, have poor mental health by age 16, and experience neither type of parental abuse during childhood. Men were more likely than women to experience rare parental abuse in addition to physical and emotional abuse by their father or another person. Differences between women and men were also observed for adult SES, lifestyle, and psychosocial factors.
Table 2 presents the results of a series of logistic regression equations for the first analytic stage. As outlined earlier, the strategy for our first stage of the analyses was to investigate the association of early misfortune with adult cancer occurrence across a number of childhood misfortune specifications. Model 1 used the summary measure of misfortune, Model 2 focused on unique misfortune, and Model 3 replaced the simple reporting of emotional and physical abuse by parents with more nuanced profiles that tap the frequency of parental abuse. Although the models adjust for all adult covariates, only the main predictors of childhood misfortune are presented in Table 2, as our analytic aim was to compare the main effects of different specifications of childhood misfortune on adult cancer risk.
Table 2.
Logistic Regression of Cancer Occurrence on Independent Variables by Sex, Midlife Development in the United States (Wave 1–1995 and Wave 2–2005)
| Independent variables | Model 1a
|
Model 2
|
Model 3
|
|||
|---|---|---|---|---|---|---|
| Men | Women | Men | Women | Men | Women | |
| Additive childhood misfortune (0–5) | 1.307*b | 1.124 | — | — | — | — |
| (1.009, 1.693)c | (0.948, 1.332) | |||||
| Types of childhood misfortune | ||||||
| Household socioeconomic status (SES) | ||||||
| Welfare | — | — | 0.581 | 1.006 | 0.519 | 0.992 |
| (0.149, 2.265) | (0.265, 3.826) | (0.132, 2.033) | (0.258, 3.816) | |||
| Financially worse than others | — | — | 1.876 | 0.813 | 1.641 | 0.803 |
| (0.972, 3.623) | (0.486, 1.360) | (0.827, 3.256) | (0.480, 1.342) | |||
| Low education—head of household | — | — | 0.928 | 1.092 | 0.892 | 1.117 |
| (0.506, 1.702) | (0.685, 1.740) | (0.502, 1.587) | (0.670, 1.784) | |||
| Household composition | ||||||
| Parental death | — | — | 1.669 | 1.681 | 1.792 | 1.670 |
| (0.504, 5.520) | (0.767, 3.684) | (0.588, 5.463) | (0.752, 3.706) | |||
| Parental divorce | — | — | 0.796 | 1.036 | 0.756 | 1.010 |
| (0.291, 2.179) | (0.538, 1.995) | (0.272, 2.101) | (0.522, 1.951) | |||
| No male in household | — | — | 0.265 | 1.106 | 0.276 | 1.063 |
| (0.049, 1.445) | (0.414, 2.956) | (0.050, 1.520) | (0.398, 2.837) | |||
| Health at age 16 | ||||||
| Poor physical health | — | — | 0.805 | 0.746 | 0.804 | 0.789 |
| (0.064, 10.177) | (0.239, 2.333) | (0.051, 12.726) | (0.262, 2.381) | |||
| Poor mental health | — | — | 1.469 | 1.441 | 1.551 | 1.359 |
| (0.378, 5.716) | (0.642, 3.235) | (0.383, 6.278) | (0.624, 2.960) | |||
| Abuse by perpetrator | ||||||
| Physical abuse—mom | — | — | 0.870d | 2.211* | — | — |
| (0.324, 2.339) | (1.114, 4.388) | |||||
| Physical abuse—dad | — | — | 2.559* | 1.009 | — | — |
| (1.173, 5.581) | (0.482, 2.116) | |||||
| Physical abuse–sibling | — | — | 0.526 | 0.708 | 0.549 | 0.711 |
| (0.222, 1.250) | (0.379, 1.326) | (0.219, 1.375) | (0.392, 1.290) | |||
| Physical abuse—other | — | — | 1.124 | 0.780 | 1.188 | 0.786 |
| (0.503, 2.510) | (0.337, 1.805) | (0.505, 2.791) | (0.349, 1.769) | |||
| Emotional abuse— mom | — | — | 1.037 | 0.767 | — | — |
| (0.319, 3.375) | (0.417, 1.412) | |||||
| Emotional abuse—dad | — | — | 1.274 | 1.637 | — | — |
| (0.532, 3.052) | (0.910, 2.944) | |||||
| Emotional abuse—sibling | — | — | 2.051 | 1.018 | 1.887 | 1.027 (0.591, 1.784) |
| (0.907, 4.639) | (0.579, 1.792) | (0.850, 4.188) | ||||
| Emotional abuse—other | — | — | 0.552 | 1.032 | 0.549 | 1.000 (0.501, 1.974) |
| (0.229, 1.331) | (0.520, 2.046) | (0.218, 1.385) | ||||
| Frequency of emotional and physical abuse by parentse | ||||||
| Rarely one or both types | — | — | — | — | 2.121 | 0.681 |
| (0.969, 4.646) | (0.365, 1.271) | |||||
| Frequently only one type | — | — | — | — | 1.059 | 1.490 |
| (0.355, 3.155) | (0.786, 2.826) | |||||
| Frequently both types | — | — | — | — | 3.558** | 2.184** |
| (1.467, 8.629) | (1.221, 3.905) | |||||
| −2 log likelihood | 219.458 | 482.702 | 201.864 | 445.206 | 201.144 | 444.566 |
| N | 1,312 | 1,374 | 1,268 | 1,313 | 1,273 | 1,318 |
All Models are adjusted for age, race, education, income, smoking, obesity, marital status, family strain, depressive symptoms, and agency.
Odds ratio.
95% confidence interval.
Italicized odds ratio and confidence interval denote sex differences (p < .05) while adjusting for test of unequal residual variance.
Reference group is never experienced either physical or emotional abuse by parent.
p < .05.
p < .01.
p < .001 (two-tailed tests).
As evidenced in Model 1, additive childhood misfortune was a significant predictor of adult cancer occurrence for men (odds ratio = 1.307, p < .05), but not for women. The Wald χ2 test adjusting for unequal residual variation, however, suggests that we cannot ascribe this difference to a greater effect among men.
Several sex differences appear in Model 2, which differentiates unique forms of misfortune. Among men, physical abuse by father was associated with approximately a 2.5-fold increase in the risk of adult cancer relative to male respondents who were not abused by their father (odds ratio = 2.559, p < .05). The relationship was nonsignificant among women, but the test adjusting for unequal residual variation reveals that the apparent sex difference cannot be attributed to a greater effect size for men. Rather, the greater variability in women’s cancer risk is confounded with the potential sex differences for the consequences of physical abuse by father. For women, physical abuse by mother was the only type of childhood misfortune that predicted cancer occurrence in adulthood (odds ratio = 2.211, p < .05), and this effect was stronger for women than for men (χ2, 1 df = 9.40).
A more fine-grained analysis of child abuse in Model 3 reveals the importance of differentiating rare mistreatment by parents from more frequent forms of abuse. Among men, those who reported experiencing frequent emotional and physical abuse by either parent had an increased cancer risk compared to those who reported never experiencing abuse (odds ratio = 3.558, p < .01). This finding was also consistent for women; experiencing frequent emotional and physical abuse by either parent increased cancer risk (odds ratio = 2.184, p < .01), though the effect was greater for men (χ2, 1 df = 6.68).
As mentioned earlier, supplementary analyses examined different functional forms of the age variable (age, age-squared, and aged 50 and older) and interactions for age and each specification of misfortune. Among the additive, unique, and profiles of misfortune, only the interactions between physical abuse by sibling(s) and age and physical abuse by sibling(s) and age2 were significant for men, both indicating that the effect of physical abuse by sibling(s) is stronger for older men (Physical Abuse by Sibling × Age: odds ratio = 1.148, p < .001; Physical Abuse by Sibling × Age2: odds ratio = 1.001, p < .001).
The second stage of the analysis is presented in Tables 3 and 4. Analyses from Table 2 reveal the importance of incorporating the abuse profiles. Therefore, the remaining models are patterned after Model 3 in Table 2, but we sequentially introduce blocks of variables that might act as pathways between childhood misfortune and adult cancer risk.
Table 3.
Logistic Regression of Cancer Occurrence on Independent Variables for Men, Midlife Development in the United States (Wave 1–1995 and Wave 2–2005)
| Independent variables | Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 |
|---|---|---|---|---|---|---|
| Demographic characteristics | ||||||
| Age | 1.091***a | 1.100***c | 1.100*** | 1.100*** | 1.098*** | 1.098*** |
| (1.064, 1.119)b | (1.067, 1.133) | (1.065, 1.134) | (1.065, 1.137) | (1.065, 1.132) | (1.061, 1.135) | |
| Black | 1.112 | 1.671 | 1.810 | 1.809 | 2.093 | 2.341 |
| (0.326, 3.794) | (0.530, 5.272) | (0.563, 5.820) | (0.512, 6.386) | (0.658, 6.661) | (0.655, 8.368) | |
| Types of childhood misfortune | ||||||
| Household socioeconomic status (SES) | ||||||
| Welfare | — | 0.865 | 0.894 | 0.529 | 0.817 | 0.519 |
| (0.335, 2.236) | (0.332, 2.405) | (0.168, 1.668) | (0.280, 2.382) | (0.132, 2.033) | ||
| Financially worse than others | — | 1.538 | 1.520 | 1.635 | 1.537 | 1.641 |
| (0.775, 3.053) | (0.779, 2.964) | (0.832, 3.211) | (0.761, 3.101) | (0.827, 3.256) | ||
| Low education—head of household | — | 1.019 | 1.005 | 0.960 | 0.975 | 0.892 |
| (0.559, 1.859) | (0.553, 1.827) | (0.531, 1.734) | (0.538, 1.768) | (0.502, 1.587) | ||
| Household composition | ||||||
| Parental death | — | 1.438 (0.528, 3.916) | 1.570 (0.567, 4.344) | 1.554 (0.548, 4.407) | 1.463 (0.516, 4.143) | 1.792 (0.588, 5.463) |
| Parental divorce | — | 0.506 | 0.539 | 0.681 | 0.537 | 0.756 |
| (0.174, 1.472) | (0.185, 1.567) | (0.264, 1.757) | (0.179, 1.609) | (0.272, 2.101) | ||
| No male in household | — | 0.879 | 0.843 | 0.288 | 0.893 | 0.276 |
| (0.212, 3.636) | (0.201, 3.546) | (0.055, 1.520) | (0.220, 3.625) | (0.050, 1.520) | ||
| Health at age 16 | ||||||
| Poor physical health | — | 0.588 | 0.788 | 0.628 | 0.551 | 0.804 |
| (0.041, 8.383) | (0.069, 8.940) | (0.043, 9.247) | (0.029, 10.368) | (0.051, 12.726) | ||
| Poor mental health | — | 1.457 | 1.517 | 1.397 | 1.536 | 1.551 |
| (0.396, 5.358) | (0.450, 5.118) | (0.348, 5.611) | (0.380, 6.217) | (0.383, 6.278) | ||
| Abuse by perpetrator | ||||||
| Physical abuse—sibling | — | 0.679 | 0.682 | 0.588 | 0.632 | 0.549 |
| (0.262, 1.763) | (0.259, 1.796) | (0.223, 1.554) | (0.265, 1.506) | (0.219, 1.375) | ||
| Physical abuse—other | — | 1.237 | 1.230 | 1.203 | 1.209 | 1.188 |
| (0.554, 2.763) | (0.547, 2.768) | (0.518, 2.790) | (0.537, 2.723) | (0.505, 2.791) | ||
| Emotional abuse—sibling | — | 1.530 | 1.546 | 1.660 | 1.723 | 1.887 |
| — | (0.678, 3.453) | (0.685, 3.489) | (0.732, 3.763) | (0.786, 3.776) | (0.850, 4.188) | |
| Emotional abuse—other | — | 0.595 | 0.595 | 0.546 | 0.598 | 0.549 |
| (0.246, 1.439) | (0.246, 1.436) | (0.215, 1.386) | (0.246, 1.457) | (0.218, 1.385) | ||
| Frequency of emotional and physical abuse by parentsd | ||||||
| Rarely one or both types | — | 2.384* | 2.347* | 2.266* | 2.252* | 2.121 |
| (1.092, 5.203) | (1.052, 5.235) | (1.044, 4.918) | (1.028, 4.933) | (0.969, 4.646) | ||
| Frequently only one type | — | 1.122 | 1.199 | 0.937 | 1.200 | 1.059 |
| (0.399, 3.157) | (0.423, 3.399) | (0.320, 2.740) | (0.436, 3.302) | (0.355, 3.155) | ||
| Frequently both types | — | 3.241* | 3.171* | 3.107* | 3.655** | 3.558** |
| (1.248, 8.413) | (1.224, 8.214) | (1.230, 8.313) | (1.515, 8.820) | (1.467, 8.629) | ||
| Adult SES | ||||||
| Years of education | — | — | 1.023 | — | — | 1.014 |
| (0.929, 1.126) | (0.920, 1.117) | |||||
| Incomee | — | — | 0.998 | — | — | 0.997 |
| (0.993, 1.003) | (0.991, 1.003) | |||||
| Lifestyle | ||||||
| Total number of cigarettes smokedf | — | — | — | 1.002 | — | 1.000 |
| (0.990, 1.014) | (0.988, 1.013) | |||||
| Obese | — | — | — | 0.915 | — | 0.939 |
| (0.500, 1.677) | (0.505, 1.746) | |||||
| Psychosocial factors | ||||||
| Widowed | — | — | — | — | 0.377 | 0.386 |
| (0.067, 2.130) | (0.063, 2.379) | |||||
| Divorced | — | — | — | — | 0.560 | 0.527 |
| (0.202, 1.553) | (0.175, 1.585) | |||||
| Family strain | — | — | — | — | 0.983 | 0.927 |
| (0.545, 1.771) | (0.479, 1.794) | |||||
| Depressive symptoms | — | — | — | — | 0.860 | 0.837 |
| (0.669, 1.105) | (0.619, 1.131) | |||||
| Agency | — | — | — | — | 0.711 | 0.806 |
| (0.448, 1.128) | (0.507, 1.283) | |||||
| −2 log likelihood | 246.657 | 222.959 | 220.440 | 209.854 | 215.985 | 201.144 |
| N | 1,422 | 1,375 | 1,346 | 1,313 | 1,360 | 1,273 |
Odds ratio 95%.
95% confidence interval.
Italicized odds ratio and confidence interval denote sex differences (p < .05) while adjusting for test of unequal residual variance (comparison with Table 4).
Reference group is never experienced either physical or emotional abuse by parent.
Income in thousands of dollars.
Number of cigarettes in tens of thousands.
p < .05.
p < .01.
p < .001 (two-tailed tests).
Table 4.
Logistic Regression of Cancer Occurrence on Independent Variables for Women, Midlife Development in the United States (Wave 1–1995 and Wave 2–2005)
| Independent variables | Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 |
|---|---|---|---|---|---|---|
| Demographic characteristics | ||||||
| Age | 1.020**a | 1.017c | 1.025 | 1.021* | 1.021* | 1.025* |
| (1.006, 1.035)b | (0.999, 1.050) | (1.000, 1.051) | (1.003, 1.038) | (1.003, 1.040) | (1.006, 1.046) | |
| Black | 0.453 | 0.547 | 0.636 | 0.668 | 0.573 | 0.668 |
| (0.147, 1.397) | (0.190, 1.576) | (0.157, 2.565) | (0.226, 1.971) | (0.197, 1.664) | (0.227, 1.971) | |
| Types of childhood misfortune | ||||||
| Household socioeconomic status (SES) | ||||||
| Welfare | — | 0.908 | 0.948 | 0.970 | 0.886 | 0.992 |
| (0.234, 3.530) | (0.188, 4.772) | (0.247, 3.807) | (0.228, 3.444) | (0.258, 3.816) | ||
| Financially worse than others | — | 0.966 | 0.988 | 0.899 | 0.899 | 0.803 |
| (0.595, 1.567) | (0.518, 1.886) | (0.541, 1.494) | (0.556, 1.453) | (0.480, 1.342) | ||
| Low education—head of household | — | 1.337 | 0.790 | 1.179 | 1.292 | 1.117 |
| (0.854, 2.094) | (0.441, 1.416) | (0.745, 1.868) | (0.825, 2.023) | (0.670, 1.784) | ||
| Household composition | ||||||
| Parental death | — | 1.485 | 0.652 | 1.494 | 1.597 | 1.670 |
| (0.712, 3.100) | (0.179, 2.374) | (0.689, 3.238) | (0.751, 3.397) | (0.752, 3.706) | ||
| Parental divorce | — | 1.006 | 1.491 | 0.944 | 1.062 | 1.010 |
| (0.531, 1.906) | (0.658, 3.378) | (0.493, 1.807) | (0.560, 2.014) | (0.522, 1.951) | ||
| No male in household | — | 1.091 | 0.901 | 1.098 | 1.065 | 1.063 |
| (0.434, 2.746) | (0.257, 3.163) | (0.419, 2.880) | (0.417, 2.721) | (0.398, 2.837) | ||
| Health at age 16 | ||||||
| Poor physical health | — | 0.616 | 0.661 | 0.660 | 0.700 | 0.789 |
| (0.196, 1.937) | (0.123, 3.549) | (0.210, 2.073) | (0.230, 2.129) | (0.262, 2.381) | ||
| Poor mental health | — | 1.269 | 0.537 | 1.409 | 1.165 | 1.359 |
| (0.594, 2.711) | (0.160, 1.809) | (0.656, 3.026) | (0.533, 2.546) | (0.624, 2.960) | ||
| Abuse by perpetrator | ||||||
| Physical abuse—sibling | — | 0.635 | 0.563 | 0.700 | 0.639 | 0.711 |
| (0.352, 1.145) | (0.217, 1.459) | (0.389, 1.259) | (0.349, 1.170) | (0.392, 1.290) | ||
| Physical abuse—other | — | 0.891 | 0.842 | 0.716 | 0.908 | 0.786 |
| (0.373, 1.762) | (0.253, 2.801) | (0.312, 1.643) | (0.421, 1.959) | (0.349, 1.769) | ||
| Emotional abuse— sibling | — | 1.135 | 1.316 | 1.050 | 1.082 | 1.027 |
| (0.679, 1.897) | (0.661, 2.619) | (0.612, 1,800) | (0.642, 1.822) | (0.591, 1.784) | ||
| Emotional abuse—other | — | 1.072 | 1.369 | 1.084 | 0.968 | 1.000 |
| (0.558, 2.054) | (0.606, 3.095) | (0.546, 2.149) | (0.504, 1.860) | (0.501, 1.974) | ||
| Frequency of emotional and physical abuse by parentsd | ||||||
| Rarely one or both types | — | 0.776 | 1.016 | 1.415 | 0.747 | 0.681 |
| (0.440, 1.369) | (0.469, 2.203) | (0.741, 2.702) | (0.415, 1.346) | (0.365, 1.271) | ||
| Frequently only one type | — | 1.457 | 1.044 | 1.415 | 1.478 | 1.490 |
| (0.779, 2.725) | (0.419, 2.601) | (0.741, 2.702) | (0.794, 2.751) | (0.786, 2.826) | ||
| Frequently both types | — | 1.962* | 2.001* | 2.031* | 2.101* | 2.184** |
| (1.108, 3.476) | (1.130, 3.544) | (1.129, 3.655) | (1.196, 3.691) | (1.221, 3.905) | ||
| Adult SES | ||||||
| Years of education | — | — | 1.025 | — | — | 0.985 |
| (1.000, 1.051) | (0.897, 1.081) | |||||
| Incomee | — | — | 0.999 | — | — | 0.999 |
| (0.995, 1.003) | (0.995, 1.003) | |||||
| Lifestyle | ||||||
| Total number of cigarettes smokedf | — | — | — | 1.009 | — | 1.008 |
| (0.998, 1.020) | (0.997, 1.020) | |||||
| Obese | — | — | — | 0.617 | — | 0.634 |
| (0.376, 1.012) | (0.386, 1.042) | |||||
| Psychosocial factors | ||||||
| Widowed | — | — | — | — | 0.687 | 0.543 |
| (0.323, 1.461) | (0.229, 1.287) | |||||
| Divorced | — | — | — | — | 1.333 | 1.309 |
| (0.803, 2.214) | (0.754, 2.274) | |||||
| Family strain | — | — | — | — | 0.902 | 0.884 |
| (0.608, 1.338) | (0.592, 1.320) | |||||
| Depressive symptoms | — | — | — | — | 1.066 | 1.041 |
| (0.972, 1.168) | (0.943, 1.150) | |||||
| Agency | — | — | — | — | 0.903 | 0.885 |
| (0.679, 1.203) | (0.652, 1.202) | |||||
| −2 log likelihood | 543.715 | 499.672 | 489.515 | 462.490 | 488.691 | 444.566 |
| N | 1,519 | 1,446 | 1,407 | 1,363 | 1,426 | 1,318 |
Odds ratio 95%.
95% confidence interval.
Italicized odds ratio and confidence interval denote sex differences (p < .05) while adjusting for test of unequal residual variance (comparison with Table 3).
Reference group is never experienced either physical or emotional abuse by parent.
Income in thousands of dollars.
Number of cigarettes in tens of thousands.
p < .05.
p < .01.
p < .001 (two-tailed tests).
Table 3 displays the results of the hierarchical models for men. In the first model, age was associated with cancer risk among men and remained significant in all subsequent Models (1–6). In Model 2, men who reported experiencing at least one type of parental abuse rarely had an increased cancer risk compared to those who reported never experiencing abuse (odds ratio = 2.384, p < .05); however, among men reporting frequent emotional and physical abuse by either parent, the risk of cancer occurrence more than tripled (odds ratio = 3.241, p < .05) relative to those who reported no abuse. In Models 3, 4, and 5, there were no significant effects for SES, lifestyle, or psychosocial factors, and the effects of rare and frequent abuse for men remained significant. In Models 4 and 5, the effect of rare physical abuse was stronger for men than it was for women (Model 4: χ2, 1 df = 6.21; Model 5: χ2, 1 df = 3.90; for women, see Table 4). When the model was fully adjusted for demographic, SES, lifestyle, and psychosocial factors in Model 6, the effect of at least one type of abuse rarely was attenuated and became nonsignificant. Frequent emotional and physical abuse remained significant (odds ratio = 3.558, p < .01) and was the only form of childhood misfortune associated with cancer occurrence among men.
The results of the hierarchical models for women are presented in Table 4. In Model 1, older age was associated with cancer risk for women; age was significant in every model except Models 2 and 3. In Model 2, experiencing both types of abuse frequently was associated with an increased cancer risk (odds ratio = 1.962, p < .05). Although frequent abuse is significant for men and women, the effect appears to be stronger for men (comparing Model 2 across Tables 3 and 4: χ2, 1 df = 5.78). When blocks of variables for SES, lifestyle, and psychosocial factors were introduced in Models 3, 4, and 5, there were no significant effects for any measures of SES, lifestyle, or psychosocial factors, and the effect of frequent emotional and physical abuse for women remained significant and was not attenuated. By comparing each model by sex, one finds that the effect of frequent abuse was stronger for men than for women (Model 3: χ2, 1 df = 5.89; Model 4: χ2, 1 df = 7.08; Model 5: χ2, 1 df = 5.57). Similar to the previous models, when Model 6 was fully adjusted for demographic, SES, lifestyle, and psychosocial factors, only age and experiencing frequent emotional and physical abuse from either parent was associated with increased cancer risk (odds ratio = 2.184, p < .01) for women. Consistent with the other model comparisons by sex, the effect of frequent emotional and physical abuse was stronger for men (χ2, 1 df = 6.80).
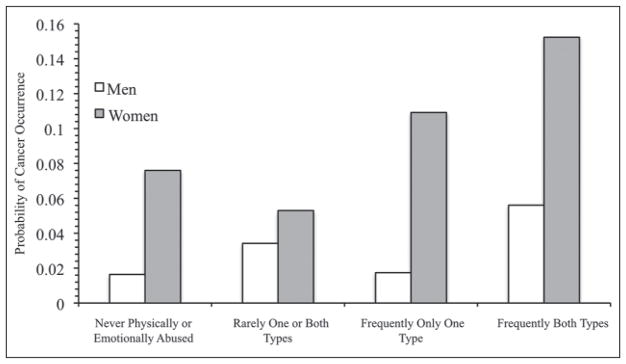
Figure 1 displays the predictive probabilities for cancer occurrence according to each abuse profile for men and women. Overall, women have a higher probability of cancer occurrence than men for each profile. For men and women, experiencing physical and emotional parental abuse frequently is associated with a noticeably higher probability of cancer occurrence compared to all other abuse profiles. The probability of cancer occurrence for women who experienced both types of parental abuse frequently is .1523, and for men, it is .0561.
Figure 1.
Predicted probabilities of cancer occurrence for men and women by parental abuse profile (National Survey of Midlife Development in the United States [MIDUS])
Note: Predicted probabilities are calculated from variables in Table 3, Model 6. All other variables in the table are held at their mean.
Discussion
Findings from the present investigation reveal a notable link between childhood misfortune and cancer occurrence during adulthood. Using a comprehensive list of possible childhood and adult risk factors, including childhood and adult SES, we found that childhood misfortune was associated with cancer occurrence for both men and women. However, the observed effects of additive and unique misfortune varied according to sex, suggesting that men and women may have different responses to childhood stressors. A large-scale study by Surtees and Wainwright (2007) found that between men and women who experienced similar types of misfortune, women not only reported the event as more upsetting but also took longer to recover from the experiences.
Sex differences were most apparent when testing an additive form of the 16 types of misfortune. The effect of additive childhood misfortune was significant for men only, not for women. Although the ACE (Adverse Child Experiences) study by Felitti et al. (1998) observed a cumulative effect on cancer for both men and women, the present study differs in analytic strategy and measures of misfortune. Unlike the Felitti et al. study, we estimated parameters for a sex-stratified analysis due to the nature of our outcome variable of cancer. Also, the present study examines an accumulation of different stressors than the ACE study: Data from the Kaiser Permanente ACE study include indicators of risky/dysfunctional families (e.g., substance abuse, mental illness), whereas the MIDUS data contain only indicators of household composition (e.g., lack of male in household, divorce). In addition to physical and emotional abuse, the Kaiser Permanente study has measures for sexual abuse. The MIDUS data do not include any measures of sexual abuse at Wave 1.5 Perhaps the accumulation of these more adverse childhood stress-ors may produce a dose–response relationship between childhood misfortune and cancer risk for women. Indeed, findings in the present study demonstrate that not all types of misfortune are equally influential on cancer risk.
Among the extensive vector of unique misfortunes examined, the long-term health consequences of child abuse became evident. When examining types of childhood misfortune, cancer risk was higher for men and women when child abuse was committed by parent of the same sex. For men, physical abuse by father increased cancer risk; for women, physical abuse by mother increased cancer risk. Whereas this unique effect of physical abuse for men and women varied by perpetrator, frequent abuse by either parent did not discriminate by sex; both men and women who experienced frequent emotional and physical abuse had a greater cancer risk compared to men and women who did not experience any type of parental abuse. When reduced models were analyzed, men who reported experiencing at least one type of parental abuse rarely had higher cancer risk relative to men who had never experienced either type of parental abuse. Although those who experienced at least one type of parental abuse frequently would be expected to have a higher risk for cancer, it is also reasonable that people who experienced minimal parental abuse would also have increased risk for health problems compared to those who never experienced abuse. Greenfield and Marks (2009a), who also found a similar pattern for functional limitations in adulthood, attributed this finding to the large number of respondents who reported rare parental abuse (as opposed to the relatively few who reported experiencing only one type of abuse frequently). In the male subsample, 35.6% of men reported experiencing at least one type of abuse rarely compared to 18.8% of men who reported experiencing only one type of abuse frequently. An alternative interpretation is that the relationship between child abuse and cancer risk varies in a nonlinear way.
When blocks of variables were sequentially introduced to help elucidate how such distal events in childhood can influence cancer occurrence in later life, there was little evidence for adult mechanisms of SES, lifestyle, and psychosocial factors as odds ratios and levels of significance remained fairly stable in each model. Although the effect of rare physical and emotional abuse was attenuated in the fully adjusted model, we cannot determine whether child abuse operates through a combination of SES, lifestyle, and psychosocial factors, but we note that the effect of frequent child abuse endured and that adult characteristics did not attenuate its effects for men or women.
Interpreting the results from this study requires consideration of several limitations. First, the retrospective nature of the childhood misfortune data creates the potential for recall bias; adults may have difficulty recollecting events from childhood accurately. Second, initial participant recruitment was via telephone; the 30-minute phone interview may have been a possible dis-incentive to participate for those with lung or mouth cancer, among other illnesses, who may have difficulty speaking for extended periods of time.
Third, grouping together all forms of cancer into one outcome variable does not allow us to identify types of childhood misfortune specific to certain types of cancer. However, our supplementary analyses of prostate cancer for men and breast cancer for women yielded similar results, suggesting that both abuse and the accumulation of childhood misfortune may place individuals at increased risk for cancer in adulthood. Regardless, this study advances recent contributions to the cancer literature that have also examined all-site cancer as an outcome variable (Fuller-Thomson & Brennenstuhl, 2009; Korpimäki et al., 2010). Fourth, MIDUS is not the ideal data set to explore the complex role of age in cancer risk. The relatively small number of people reporting cancer and the lack of information on age of cancer onset at Wave 1 limit our analytic ability to investigate the role of timing. Indeed, future research should investigate the relationship between childhood misfortune and cancer using age-stratified and event history analyses.
Fifth, those with the most adverse early life conditions are probably omitted due to higher risks of incarceration (e.g., prison), being institutionalized, or premature mortality. As with any longitudinal design, attrition, due to mortality or nonresponse, is another potential bias. Specifically, the lack of information provided by MIDUS on cause of death does not permit us to include cancer mortality in our measure of cancer occurrence. The likely result of this limitation is that the results presented here are an underestimate of the effect of childhood misfortune on cancer occurrence.
Despite these limitations, multiple indicators of child and adult risk factors enabled us to investigate the long arm of childhood misfortune over the life course and use a series of nested models to investigate later-life mechanisms. Childhood misfortune was related to cancer occurrence during adulthood, net of age, race, adult education, income, smoking, obesity, marital status, family strain, depressive symptoms, and agency. To our knowledge, this is the first study examining the effect of childhood misfortune on adult cancer occurrence while using three different specifications of misfortune: additive, unique, and abuse profiles. Had we used only the additive measure of misfortune, the story of parental abuse and cancer for women would have remained untold. Also, by analyzing profiles of abuse, the effect of the frequency of abuse for both men and women suggests another way to conceptualize accumulation. Therefore, we urge investigators to test alternative specifications of not only childhood misfortune but also different specifications of accumulated misfortune during childhood.
Identifying cancer risks that emerge early in the life course is imperative not only for aging research but also for public health prevention. In the United States, cancer is the second leading cause of death, and several status characteristics and risk factors are well known: age, sex, race, and SES (American Cancer Society, 2010). Although childhood misfortune is currently not a widely acknowledged risk factor for cancer, this study reveals that it should be: Some types of childhood misfortune, especially abuse, are implicated in the development of cancer in adulthood for both men and women.
Acknowledgments
We thank Margaret Favorite, Megan Gilligan, Emily Greenfield, and Linda Waite for helpful comments on an earlier version of this manuscript. Data were made available by the Inter-University Consortium for Political and Social Research, Ann Arbor, MI. Neither the collector of the original data nor the Consortium bears any responsibility for the analyses or interpretations presented here.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Support for this research was provided by a grant from the National Institute on Aging (R01 AG033541).
Footnotes
By diagnosing cancer in earlier stages and providing new cancer therapies, advanced medical technology significantly affects cancer mortality rates, not necessarily cancer incidence (Irigaray et al., 2007).
Various terms are used in the literature, including adversity (e.g., Brown et al., 2010; Felitti et al., 1998), misfortune (e.g., Schafer et al., 2011; Surtees & Wainwright, 2007), and trauma (e.g., Turner & Lloyd, 1995). One of the challenges in selecting among these terms is how the actor perceives the event, experience, or condition. For some experiences, such as physical abuse, the term adversity seems wholly appropriate. For others, such as parent’s educational level, it is more difficult to presume that the person viewed this as adverse or harmful. These assumptions are even more challenging when viewed in historical context or across cultures or subcultures because what some perceive as adverse may be considered a normal part of life for others (e.g., Edge & Rogers, 2005). Thus, we follow Surtees and Wainwright’s use of the term misfortune as the most general and encompassing. Our taxonomy is, therefore, hierarchical: Misfortune covers all of the measures described in our analysis (e.g., father did not complete high school), adversity describes those types of misfortune that are widely associated with distress or harm (e.g., abuse), and trauma describes the most vexing adverse conditions (e.g., frequent abuse).
Whereas most site-specific cancer analyses would have very few cases and insufficient statistical power, we did estimate supplementary models for the most common types of cancer for men (prostate cancer) and women (breast cancer). These results yielded similar findings to the main models we presented. For men, there were no differences between the all-site (non-skin) cancer model and the prostate cancer model regarding the main predictors of childhood misfortune; additive childhood misfortune, physical abuse by father, and frequent emotional and physical abuse were significant in the prostate cancer model. For women, the only difference from the all-site (non-skin) cancer model was that emotional abuse by father was a significant predictor of breast cancer occurrence. Significant predictors in the all-site (non-skin) cancer model—physical abuse by mother and frequent emotional and physical abuse—remained significant in the model for breast cancer.
Previous studies have also used available data to calculate cumulative exposure. For example, Asomaning et al. (2008) used daily number of packs smoked and years smoking to calculate number of packs per year to measure cumulative exposure.
Although National Survey of Midlife Development in the United States (MIDUS) includes a measure for sexual assault at Wave 2, there are substantial unit-missing and item-missing data (i.e., those who dropped out of the study and those who refused to answer the question at Wave 2, respectively). When we attempted to estimate the effect of Wave 2 sexual abuse on cancer occurrence, statistical diagnostics revealed model instability (i.e., extreme confidence intervals).
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Allison PD. Comparing logit and probit coefficients across groups. Sociological Methods and Research. 1999;28:186–208. [Google Scholar]
- American Cancer Society. Cancer facts & figures 2010. Atlanta, GA: Author; 2010. Retrieved from http://www.cancer.org/acs/groups/content/@nho/documents/document/acspc-024113.pdf. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3. Washington, DC: Author; 1987. [Google Scholar]
- Asomaning K, Miller DP, Liu G, Wain JC, Lynch TJ, Su L, Christiani DC. Second hand smoke, age of exposure and lung cancer risk. Lung Cancer. 2008;61:13–20. doi: 10.1016/j.lungcan.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell DL, Hayward MD, Crimmins EM. Does childhood health affect chronic morbidity in later life? Social Science & Medicine. 2001;52:1269–1284. doi: 10.1016/s0277-9536(00)00230-6. [DOI] [PubMed] [Google Scholar]
- Brown DW, Anda RF, Felitti VJ, Edwards VJ, Malarcher AM, Croft JB, Giles WH. Adverse childhood experiences are associated with the risk of lung cancer: A prospective cohort study. BMC Public Health. 2010;10:20. doi: 10.1186/1471-2458-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GG, Vlantis AC, Zeng Q, van Hasselt CA. Regulation of cell growth by estrogen signaling and potential targets in thyroid cancer. Current Cancer Drug Targets. 2008;8:367–377. doi: 10.2174/156800908785133150. [DOI] [PubMed] [Google Scholar]
- Cohen S, Janicki-Deverts D, Chen E, Matthews KA. Childhood socioeconomic status and adult health. Annals of the New York Academy of Sciences. 2010;1186:37–55. doi: 10.1111/j.1749-6632.2009.05334.x. [DOI] [PubMed] [Google Scholar]
- Cook MB, Dawsey SM, Freedman ND, Inskip PD, Wichner SM, Quraishi SM, … McGlynn KA. Sex disparities in cancer incidence by period and age. Cancer Epidemiology, Biomarkers, and Prevention. 2009;18:1174–1182. doi: 10.1158/1055-9965.EPI-08-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czene K, Lichtenstein P, Hemminki K. Environmental and heritable causes of cancer among 9.6 million individuals in the Swedish family-cancer database. International Journal of Cancer. 2002;99:260–266. doi: 10.1002/ijc.10332. [DOI] [PubMed] [Google Scholar]
- Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proceedings of the National Academy of Sciences. 2007;104(4):1319–1324. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kok IM, van Lenthe FJ, Avendano M, Louwman M, Coebergh JW, Mackenbach JP. Childhood social class and cancer incidence: Results of the GLOBE study. Social Science & Medicine. 2008;66:1131–1139. doi: 10.1016/j.socscimed.2007.11.035. [DOI] [PubMed] [Google Scholar]
- Draper B, Pfaff JJ, Pirkis J, Snowdon J, Lautenschlager NT, Wilson I, … Almeida OP Depression and Early Prevention of Suicide in General Practice Study Group. Long-term effects of childhood abuse on the quality of life and health of older people: Results from the Depression and Early Prevention of Suicide in General Practice Project. Journal of the American Geriatrics Society. 2008;56(2):262–271. doi: 10.1111/j.1532-5415.2007.01537.x. [DOI] [PubMed] [Google Scholar]
- Edge D, Rogers A. Dealing with it: Black Caribbean women’s response to adversity and psychological distress associated with pregnancy, childbirth, and early motherhood. Social Science and Medicine. 2005;61:15–25. doi: 10.1016/j.socscimed.2004.11.047. [DOI] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenburg D, Williamson DF, Spitz AM, Edwards V, … Marks JS. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. American Journal of Preventive Medicine. 1998;14(4):245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Ferraro KF. Health and aging: Early origins, persistent inequalities? In: Settersten RA, Angel JL, editors. Handbook of sociology of aging. New York, NY: Springer; 2011. [Google Scholar]
- Ferraro KF, Shippee TP. Aging and cumulative inequality: How does inequality get under the skin? The Gerontologist. 2009;49(3):333–343. doi: 10.1093/geront/gnp034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller-Thomson E, Brennenstuhl S. Making a link between childhood physical abuse and cancer. Cancer. 2009;115:3341–3350. doi: 10.1002/cncr.24372. [DOI] [PubMed] [Google Scholar]
- Greenfield EA, Marks NF. Profiles of physical and psychological violence in childhood as a risk factor for poorer adult health: Evidence from the 1995–2005 National Survey of Midlife in the United States. Journal of Aging and Health. 2009a;21:943–966. doi: 10.1177/0898264309343905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield EA, Marks NF. Violence from parents in childhood and obesity in adulthood: Using food in response to stress as a mediator of risk. Social Science & Medicine. 2009b;68:791–798. doi: 10.1016/j.socscimed.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamil-Luker J, O’Rand AM. Gender differences in the link between childhood socioeconomic conditions and heart attack risk in adulthood. Demography. 2007;44:137–158. doi: 10.1353/dem.2007.0004. [DOI] [PubMed] [Google Scholar]
- Hemminki K, Chen B. Lifestyle and cancer: Effect of parental divorce. European Journal of Cancer Prevention. 2006;15:524–530. doi: 10.1097/01.cej.0000220633.93104.64. [DOI] [PubMed] [Google Scholar]
- Hertzman C, Boyce T. How experience gets under the skin to create gradients in developmental health. Annual Review of Public Health. 2010;31:329–347. doi: 10.1146/annurev.publhealth.012809.103538. [DOI] [PubMed] [Google Scholar]
- Hoetker G. COMPLOGIT: Stata module to compare logit coefficients across groups. Boston, MA: Boston College Department of Economics; 2007. [Google Scholar]
- Irigaray P, Newby JA, Clapp R, Hardell L, Howard V, Montagnier L, … Belpomme D. Lifestyle-related factors and environmental agents causing cancer: An overview. Biomedicine & Pharmacotherapy. 2007;61:640–658. doi: 10.1016/j.biopha.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Jacobs JR, Bovasso GB. Early and chronic stress and their relation to breast cancer. Psychological Medicine. 2000;30:669–678. doi: 10.1017/s0033291799002020. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Mickelson KD, Walters EE, Zhao S, Hamilton L. Age and depression in the MIDUS survey. In: Brim OG, Ryff CD, Kessler RC, editors. How healthy are we? A national study of well-being at midlife. Chicago, IL: University of Chicago Press; 2004. pp. 227–251. [Google Scholar]
- Korpimäki SK, Sumanen MPT, Sillanmäki LH, Mattila KJ. Cancer in working-age is not associated with childhood adversities. Acta Oncologica. 2010;49:436–440. doi: 10.3109/02841860903521103. [DOI] [PubMed] [Google Scholar]
- Kuh D, Ben-Shlomo Y. A life course approach to chronic disease epidemiology. Oxford, UK: Oxford University Press; 2004. [PubMed] [Google Scholar]
- Laconi E, Tomasi C, Curreli F, Diana S, Laconi S, Serra G, … Pani P. Early exposure to restraint stress enhances chemical carcinogenesis in rat liver. Cancer Letters. 2000;161:215–220. doi: 10.1016/s0304-3835(00)00621-2. [DOI] [PubMed] [Google Scholar]
- Lichtenstein P, Holm NV, Verkasal PK, Iliadou A, Kaprio J, Koskenvuo M, … Hemminki K. Environmental and heritable factors in the causation of cancer: Analyses of cohorts of twins from Sweden, Denmark, and Finland. New England Journal of Medicine. 2000;343:78–85. doi: 10.1056/NEJM200007133430201. [DOI] [PubMed] [Google Scholar]
- Lynch J, Smith GD. A life course approach to chronic disease epidemiology. Annual Review of Public Health. 2005;26:1–35. doi: 10.1146/annurev.publhealth.26.021304.144505. [DOI] [PubMed] [Google Scholar]
- Merlo LMF, Pepper JW, Reid BJ, Maley CC. Cancer as an evolutionary and ecological process. Nature Reviews Cancer. 2006;6:924–935. doi: 10.1038/nrc2013. [DOI] [PubMed] [Google Scholar]
- Naugler WE, Sakurai T, Kim S, Maeda S, Kim KH, Elsharkawy AM, Karin M. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121–124. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- Pearlin LI. The sociological study of stress. Journal of Health and Social Behavior. 1989;30:241–256. [PubMed] [Google Scholar]
- Pearlin LI. The life course and the stress process: Some conceptual comparisons. Journal of Gerontology: Social Sciences. 2010;65B:207–215. doi: 10.1093/geronb/gbp106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlin LI, Schieman S, Fazio EM, Meersman SC. Stress, health, and the life course: Some conceptual perspectives. Journal of Health and Social Behavior. 2005;46:205–219. doi: 10.1177/002214650504600206. [DOI] [PubMed] [Google Scholar]
- Power C, Hypponen E, Smith GD. Socioeconomic position in childhood and early adult life and risk of mortality: A prospective study of the mothers of the 1958 British cohort. American Journal of Public Health. 2005;95(8):1396–1402. doi: 10.2105/AJPH.2004.047340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer MH, Ferraro KF, Mustillo SA. Children of misfortune: Early adversity and cumulative inequality in perceived life trajectories. American Journal of Sociology. 2011;116:1053–1091. doi: 10.1086/655760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherg H, Blohmke M. Associations between selected life events and cancer. Behavioral Medicine. 1988;14(3):119–124. doi: 10.1080/08964289.1988.9935133. [DOI] [PubMed] [Google Scholar]
- Schilling EA, Aseltine RH, Gore S. The impact of cumulative childhood adversity on young adult mental health: Measures, models, and interpretations. Social Science & Medicine. 2008;66:1140–1151. doi: 10.1016/j.socscimed.2007.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh-Manoux A, Marmot M. Role of socialization in explaining social inequalities in health. Social Science & Medicine. 2005;60(9):2129–2133. doi: 10.1016/j.socscimed.2004.08.070. [DOI] [PubMed] [Google Scholar]
- Smith GD, Hart C, Blane D, Hole D. Adverse socioeconomic conditions in childhood and cause specific adult mortality: Prospective observational study. British Medical Journal. 1998;316:1631–1635. doi: 10.1136/bmj.316.7145.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus MA. Measuring intrafamily conflict and violence: The Conflict Tactics (CT) scales. Journal of Marriage and Family. 1979;41:75–88. [Google Scholar]
- Suisman JL, Burt SA, McGue M, Iacono WG, Klump KL. Parental divorce and disordered eating: An investigation of a gene-environment interaction. International Journal of Eating Disorders. 2011;44:169–177. doi: 10.1002/eat.20866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surtees PG, Wainwright NWJ. The shackles of misfortune: Social adversity assessment and representation in a chronic-disease epidemiological setting. Social Science & Medicine. 2007;64:95–111. doi: 10.1016/j.socscimed.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Taylor SE. Mechanisms linking early life stress to adult health outcomes. Proceedings of the National Academy of Sciences. 2010;107:8507–8512. doi: 10.1073/pnas.1003890107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner RJ, Lloyd DA. Lifetime traumas and mental health: The significance of cumulative adversity. Journal of Health and Social Behavior. 1995;36:360–376. [PubMed] [Google Scholar]
- Turner RJ, Wheaton B, Lloyd DA. The epidemiology of social stress. American Sociological Review. 1995;60:104–125. [Google Scholar]
- Williamson DF, Thompson TJ, Anda RF, Dietz WH, Felitti V. Body weight and obesity in adults and self-reported abuse in childhood. International Journal of Obesity. 2002;26:1075–1082. doi: 10.1038/sj.ijo.0802038. [DOI] [PubMed] [Google Scholar]