Abstract
Therapy resistance represents a major clinical challenge in disseminated prostate cancer for which only palliative treatment is available. One phenotype of therapy-resistant tumors is the expression of somatic, gain-of-function mutations of the androgen receptor (AR). Such mutant receptors can use noncanonical endogenous ligands (e.g., estrogen) as agonists, thereby promoting recurrent tumor formation. Additionally, selected AR mutants are sensitized to the estrogenic endocrine-disrupting compound (EDC) bisphenol A, present in the environment. Herein, screening of additional EDCs revealed that multiple tumor-derived AR mutants (including T877A, H874Y, L701H, and V715M) are sensitized to activation by the pesticide 2,2-bis(4-chlorophenyl)-1,1-dichloroethylene (DDE), thus indicating that this agent may impinge on AR signaling in cancer cells. Further investigation showed that DDE induced mutant AR recruitment to the prostate-specific antigen regulatory region, concomitant with an enhancement of target gene expression, and androgen-independent proliferation. By contrast, neither AR activation nor altered cellular proliferation was observed in cells expressing wild-type AR. Activation of signal transduction pathways was also observed based on rapid phosphorylation of mitogen-activated protein kinase (MAPK) and vasodilator-stimulated phosphoprotein, although only MAPK activation was associated with DDE-induced cellular proliferation. Functional analyses showed that both mutant AR and MAPK pathways contribute to the proliferative action of DDE, as evidenced through selective abrogation of each pathway. Together, these data show that exposure to environmentally relevant doses of EDCs can promote androgen-independent cellular proliferation in tumor cells expressing mutant AR and that DDE uses both mutant AR and MAPK pathways to exert its mitogenic activity.
Introduction
Prostatic adenocarcinoma is the second leading cause of cancer death in the United States (1), and successful treatment of this disease is hindered by an inability to durably control androgen signaling. Disseminated prostate tumors are dependent on androgen for growth (2). Therefore, androgen deprivation therapy is the first line of intervention for such patients, as achieved via depletion of androgen synthesis and/or through the use of androgen receptor (AR) antagonists (3–5). These regimens are initially effective, as the majority of patients (>80%) incur remission (2). However, tumor remission is transient, as most patients relapse and develop therapy-resistant, androgen-independent cancer, in which the AR has been inappropriately reactivated (6). At present, there is no effective treatment for therapy-resistant prostate cancer; however, it is clear that AR reactivation is critical for the development of therapy-resistant tumors. As such, identifying the factors affecting androgen deprivation therapy efficacy is essential to improve the outcome of prostate cancer treatment and thereby increase patient survival.
AR is a member of the nuclear receptor superfamily of ligand-dependent transcription factors and has a major role in regulating function, growth, and differentiation of the prostate gland (7). Before ligand binding, AR is held inactive in the cytoplasm bound to heat shock proteins. In the prostate, testosterone is converted to dihydrotestosterone (DHT), which is a potent AR agonist (8). Binding of DHT to the receptor triggers dissociation of the inhibitory heat shock proteins and rapid translocation to the nucleus. Once in the nucleus, the complex binds to specific androgen-responsive elements (ARE) on target genes and, with the aid of coactivators, triggers target gene transcription (7, 9). The expression of a direct AR target gene, prostate-specific antigen (PSA), is a commonly used clinical marker for prostate cancer progression. Serum PSA levels directly correlate with AR activity, and monitoring PSA levels is thought to provide a measure of tumor cell proliferation and disease progression (10). Conversely, clinical depletion of androgen (e.g., via bilateral orchiectomy or gonadotropin-releasing hormone agonists; ref. 11) or the use of AR antagonists (e.g., Casodex; ref. 12, 13) results in a reduction of PSA expression (thus indicating efficient biochemical ablation of AR activity) and tumor cell cycle arrest or death. Resurgence of AR activity occurs through a myriad of pathways that restore AR signaling, PSA expression, and tumor cell proliferation, including (a) AR amplification, (b) overexpression of AR coactivators, (c) ligand-independent AR activation, (d) intratumoral androgen production, or (e) gain-of-function AR somatic mutations (6, 14–19). Studies indicate that gain-of-function mutations of AR are observed in 8% to 25% of recurrent tumors (14, 15) and are selected for during androgen ablation therapy. The ability of several such mutants to alter ligand specificity of the receptor promotes therapeutic resistance.
It was previously shown that low levels of a common environmental contaminant with endocrine-mimicking capabilities, bisphenol A (BPA), can negatively affect prostate cancer treatment in the presence of an AR mutant known to arise in tumor progression (AR-T877A; refs. 20, 21). BPA mediated androgen-independent activation of selected tumor-derived AR mutants (21), stimulated prostate cancer cell proliferation under conditions of androgen depletion (20), promoted xenograft tumor growth, and shortened the time to biochemical recurrence (PSA induction) in xenograft models of human disease (22). Because these findings showed that endocrine-disrupting compounds (EDC) can affect mutant AR activity, the present study endeavored to examine the effect of known EDCs on mutant AR activity and prostate cancer cell proliferation.
Here, we show that the known EDC 2,2-bis(4-chloro-phenyl)-1,1-dichloroethylene (DDE), a stable metabolite of the pesticide 1,1-bis(4-chlorophenyl)-2,2,2-trichloroethane (DDT), activates several tumor-derived, mutant ARs. In prostate cancer model systems expressing mutant AR, DDE induced AR recruitment to the regulatory region of the PSA target gene and increased expression of PSA mRNA. In the presence of mutant AR [but not wild-type AR (wtAR)], DDE induced androgen-independent cellular proliferation at low, environmentally relevant levels. These mitogenic effects of DDE proved to be mediated through both mutant AR and mitogen-activated protein kinase (MAPK) pathways. Combined, these data indicate that, for tumors harboring selected AR mutants, exposure to environmentally relevant levels of DDE could promote androgen-independent tumor cell proliferation. These data highlight the necessity to consider environmental agents for their ability to cause therapeutic bypass through inappropriate activation of AR while designing the second-generation therapeutics for prostate cancer.
Results
The Pesticide DDE Facilitates Transcriptional Activation of Selected Tumor-Derived AR Mutants
It has been shown that the EDC BPA is able to activate the tumor-derived AR mutant AR-T877A, resulting in ligand-independent receptor activation as measured by induction of AR target gene transcription, AR-T877A–dependent cellular proliferation, and reduced efficacy of androgen ablation therapy (20–22). Given the effect of this EDC on mutant AR activity, cellular proliferation, and resultant therapeutic outcome, a more comprehensive screen was used to identify whether mutant ARs are receptive to other EDCs. For these studies, a well-characterized yeast colorimetric screen was used for the detection of AR activity (23), wherein individual strains carrying an AR allele can be assessed for AR activity. Modulation of AR transcriptional potential on exposure to test compounds was assessed using the colorimetric score of yeast colonies (Table 1). Strains were assessed for each compound in parallel and controls included one inactive mutant originally isolated from a patient with complete androgen insensitivity syndrome (C784Y) and a constitutively active mutant (K580R) isolated from a prostate cancer specimen. Four additional mutants (AR-T877A, AR-H874Y, AR-L701H, and AR-V715M) carrying mutations thought to alter ligand specificity were also assessed (24–27). Moreover, these tumor-derived mutants were previously used and characterized for their responsiveness to noncanonical steroidal compounds such as 17-β-estradiol and progesterone (23, 28, 29). For each AR variant, ethanol was used as the negative (vehicle) control for AR activity, and DHT served as the positive control. No activation of AR-C784Y was observed on either treatment, as expected. By contrast, enhanced DHT-mediated activity was observed with wtAR as well as with the tumor-derived mutants AR-K580R, AR-T877A, AR-H874Y, AR-L701H, and AR-V715M. Thus, consistent with previous studies (23), this screen provides efficient assessment of mutant AR activity and was subsequently used to examine the effect of several EDCs on mutant AR function.
Table 1.
The Pesticide DDE Facilitates Transcriptional Activation of Select Tumor-Derived AR Mutants
| C784Y | K580R | wtAR | T877A | H874Y | L701H | V715M | |
|---|---|---|---|---|---|---|---|
| Ethanol | − | +++ | − | − | − | − | − |
| DHT (10−8 mol/L) | − | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ |
| Resveratrol (10−5 mol/L) | − | ++ | − | − | − | − | − |
| Coumestrol (10−5 mol/L) | − | ++ | − | − | − | − | − |
| Cadmium (10−5 mol/L) | − | ++ | − | − | − | − | − |
| DDE (10−5 mol/L) | − | ++++ | + | ++ | ++++ | +++ | ++++ |
| DDT (10−5 mol/L) | − | ++++ | ++ | ++ | ++++ | +++ | ++++ |
| Ethanol |
|
|
|
|
|
|
|
| DHT (10−8 mol/L) |
|
|
|
|
|
|
|
| DDE (10−5 mol/L) |
|
|
|
|
|
|
|
| DDT (10−5 mol/L) |
|
|
|
|
|
|
|
NOTE: Yeast strains containing the ARE-driven ADE2 reporter gene and expressing individual AR mutants were cultured on selective medium plates as described in Materials and Methods supplemented with either vehicle (0.1% ethanol), 10−8 mol/L DHT, 10−5 mol/L resveratrol, 10−5 mol/L coumestrol, 10−5 mol/L cadmium, 10−5 mol/L DDE, or 10−5 mol/L DDT. AR-mediated transactivational activity was scored based on the color of yeast colonies: white, complete activation of AR; pink, weak AR activation; red, no AR activation. Experiments were done in three biological replicates with representative images shown in bottom panel.
Based on epidemiologic studies, one explanation for the variation in the geographic incidence of prostate cancer has been attributed to differences in consumption of phytoestrogens (30). Specifically, men consuming diets rich in soy products (such as those from Asian countries) have a reduced prostate cancer–related death rate compared with men from Western countries (31, 32). Moreover, an effect of select phytoestrogens on prostate cancer development, cellular proliferation, and PSA secretion is also reported (33–35). Given the recent attention toward phytoestrogens as possible dietary supplements for chemoprevention and chemotherapeutic means of controlling prostate cancer (36, 37), the phytoestrogens, resveratrol and coumestrol, and known estrogenic EDCs were screened for their ability to activate AR variants. Given the dose-dependent response of prostate cells (21), cells were treated with increasing concentrations (10−10 to 10−5 mol/L). Although other phytoestrogens have been shown to bind wtAR (38), neither resveratrol nor coumestrol activated the negative control AR, showed altered K580R transactivation potential, or induced mutant AR function (Table 1; data not shown). Thus, these two phytoestrogens do not show enhanced agonist function with tumor-derived AR. AR mutants were also examined for their responsiveness to cadmium, as this metal has been implicated in increased risk for prostate cancer development and reported to activate AR in prostate cancer cells (39–41). Cadmium did not activate AR-C784Y, did not alter the activity of K580R, and, surprisingly, had no effects on wtAR or tumor-derived AR. These data indicate that resveratrol, coumestrol, and cadmium are not efficient for AR activation in yeast-based assays.
Lastly, the pesticide DDT and its stable metabolite DDE were examined for their effect on AR activity. There is strong epidemiologic data suggesting that long-term exposure to specific organochlorines (such as DDT/DDE) may contribute to increased risk of prostate cancer development (42–46). As expected, these agents had no effect on AR-C784Y activity; however, similar to DHT, they modestly enhanced AR-K580R activity. DDT and DDE also facilitated moderate activation of wtAR. This finding may be consistent with the previous observation that DDE can bind to wtAR with an IC50 of 1.53 × 10−5 mol/L (38, 47). The other tumor-derived mutant ARs also showed increased transactivational potential in the presence of DDT and DDE. Specifically, AR-L701H and AR-T877A were moderately activated by DDE and DDT, whereas AR-V715M and AR-H874Y were markedly sensitive to activation by DDT and DDE, as shown by white colony formation. These data reveal that, in yeast, the pesticide DDT and its stable metabolite DDE are able to enhance the transactivation function of wtAR and of several tumor-derived AR mutants.
Tumor-Derived AR Mutants Are More Susceptible to DDE-Mediated Activation in a Mammalian System
Although the yeast system supported DDE-mediated activation of mutant receptors, it was necessary to analyze the effects of DDE in a mammalian system. To avoid any discrepancy in data interpretation due to differing genetic backgrounds, reporter assays were carried out in CV1 cells (nontransformed, spontaneously immortalized epithelial cells that are refractory to androgen signaling). CV1 cells were transfected with either wtAR or AR mutants in the absence of androgen and receptor activity was monitored using the ARR2 (probasin) promoter after treatment with DHT, DDE, or vehicle control (ethanol). Two well-characterized AR mutants (AR-T877A and AR-H874Y) were chosen for this study, as these AR mutants are available for further analysis in prostate cancer models. Relative reporter activity was calculated for each AR construct after normalization to the internal transfection control (β-galactosidase; Fig. 1). Activity in the positive control (DHT) was set to 100 for each individual receptor variant representing maximal activation capacity under conditions of androgen induction. With wtAR, there was no significant induction in luciferase activity after DDE treatment. By contrast, DDE induced a modest but significant increase in AR-T877A and AR-H874Y activity over basal activity (9.9% and 36.5%, respectively). Similar to the results from yeast assays, these data indicate that DDE can activate mutant AR in a mammalian system; however, the extent of activation is mutation dependent.
FIGURE 1.
DDE induces mutant AR transcriptional activity in CV1 cells. CV1 cells were transfected in the absence of androgen with plasmids encoding wtAR or mutant AR (T877A or H874Y), ARR2-luciferase reporter, along with cytomegalovirus-β-galactosidase as an internal transfection control. After transfection, the cells were treated with either ethanol (EtOH), DHT, or DDE for 24 h. Cells were harvested using trypsin, lysed using reporter lysis buffer, and analyzed for luciferase activity. The luciferase/β-galactosidase ratio for endogenous ligand DHT was set to 100. Experiments were done with at least three independent replicates. *, P < 0.05.
DDE Effects on Mutant AR Activity in Prostate Cancer Cells Are Dose Dependent and Molecular Context Specific
Our previous studies indicate that inappropriate activation of mutant AR by the environmental BPA can affect the expression of AR target genes in prostate cancer cells (20–22). Additionally, it is well established that the cell-specific coregulatory milieu can dramatically alter the effect of ligand-mediated mutant AR activation (48, 49). Due to the paucity of prostate model cells that express mutant ARs, the effect of DDE in prostate cancer cell lines could be elucidated for only two mutant ARs (AR-T877A and AR-H874Y) along with the wtAR. To examine endogenous receptors, 22Rv1 cells (expressing AR-H874Y), LNCaP cells (expressing AR-T877A), and LAPC4 cells (harboring wtAR) were analyzed for endogenous AR activation, as monitored by quantitative reverse transcription-PCR analysis of endogenous PSA transcript levels after DDE exposure. Cells were cultured in androgen-depleted medium for 24 h and then treated for 24 h with ethanol (vehicle), DHT, or DDE. Considering the previous report indicating a biphasic response of prostate cancer cells to BPA (21), a range of DDE concentrations (10−11, 10−8, and 10−5 mol/L) was used (Fig. 2). These concentrations are comparable with DDT/DDE concentrations observed in human serum samples in several studies (50–54). Treatment with 10−8 and 10−5 mol/L DDE concentrations induced PSA transcription in a dose-dependent manner in 22Rv1 cells expressing endogenous AR-H874Y (Fig. 2A). Activation of AR reached a maximum (1.9-fold) in 22Rv1 cells at a DDE concentration of 10−5 mol/L compared with vehicle control. In parallel studies, DHT induced PSA mRNA by 1.7-fold (data not shown). To determine if this finding was recapitulated in prostate cancer cells that express an endogenous AR-T877A, PSA mRNA regulation by DDE was examined in the LNCaP model system (Fig. 2B). Therein, increased PSA expression was observed at a DDE concentration of 10−8 mol/L (1.7-fold), whereas higher doses (10−5 mol/L) induced cellular toxicity (data not shown). Although the response to these doses was not statistically significant, 10−7 mol/L DDE generated maximal induction of PSA (1.9-fold), whereas 10−6 mol/L DDE did not change PSA expression (data not shown). The positive control (DHT treatment) showed an 11-fold induction in PSA mRNA (data not shown). By contrast, no induction in PSA expression was observed for all concentrations of DDE in cells expressing wtAR (LAPC4; Fig. 2C), whereas DHT induced a 5.8-fold increase in PSA expression (data not shown). These data indicate that DDE induces PSA transcription in human prostate cancer cells expressing somatic AR mutants (55); however, the extent of induction in transcriptional activation varies based on the specific AR mutation and cell context.
FIGURE 2.

DDE effects on mutant AR activity in human prostate cancer cells are dose dependent and molecular context specific. 22Rv1 (A), LNCaP (B), or LAPC4 (C) cells were treated in steroid-free medium with either vehicle control (0.1% ethanol) or increasing doses of DDE ranging from 10−11 to 10−5 mol/L for 24 h. Total RNA was harvested using Trizol and used for cDNA synthesis followed by quantitative real-time PCR (Q-PCR). PSA/glyceraldehyde-3-phosphate dehydrogenase (GAPDH) for the ethanol treatments was set to 1 for each cell line, and the effect of DDE on PSA/GAPDH mRNA expression was set relative to the control. *, P < 0.05.
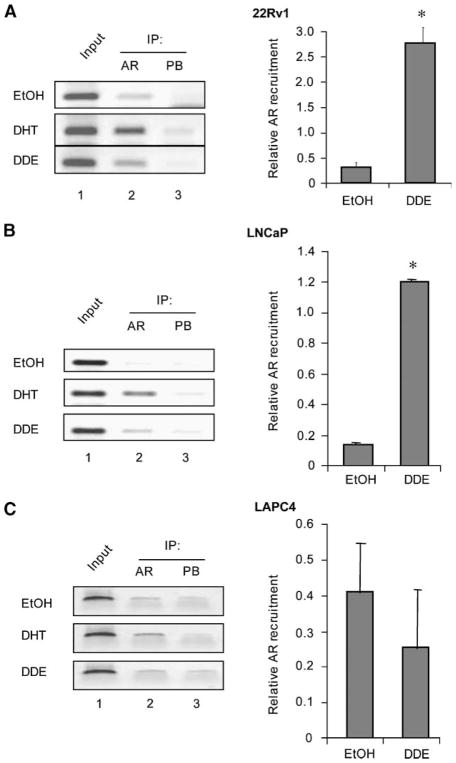
DDE Induces Mutant AR (AR-H874Y and AR-T877A) Recruitment to Target Gene Regulatory Regions
Previously published studies for another pesticide, 2,4-dichlorophenol, indicate that 2,4-dichlorophenol increases DHT-mediated AR activation by promoting nuclear translocation of the receptor (55). We investigated if DDE alone is able to exert similar effects in LNCaP cells. LNCaP cells were processed to collect nuclear and cytoplasmic fractions and AR expression analysis was carried out by immunoblotting. DDE increased the nuclear translocation of AR (data not shown). These results, along with the PSA mRNA expression studies (Fig. 2), suggest the possibility that DDE can modulate the transcriptional activity of AR by supporting AR recruitment to the enhancer region of target genes. To challenge this idea directly, AR recruitment to the PSA locus on DDE treatment was monitored by chromatin immunoprecipitation (ChIP) in cells expressing AR-H874Y, AR-T877A, and wtAR. As expected (given the established androgen independence of the 22Rv1 cell line; refs. 56, 57), AR-H874Y was detected in these cells at the PSA enhancer region in the absence of ligand (Fig. 3A, top left, lane 2), and DHT treatment further enhanced AR-H874Y recruitment on this regulatory region (middle, lane 2). Importantly, DDE (10−8 mol/L) treatment enhanced recruitment of AR-H874Y to PSA promoter regulatory regions (bottom, lane 2), indicating that DDE exposure directly affects AR-H874Y function (real-time quantitation of ChIP DNA is provided in right). More pronounced recruitment was observed using higher doses of DDE (10−6 mol/L; data not shown). In androgen-dependent LNCaP cells, endogenous AR-T877A was recruited to the PSA locus by both DHT and DDE (10−8 mol/L), whereas receptor detection was minimal in the absence of ligand (Fig. 3B). However, in LAPC4 cells (expressing wtAR), DDE (10−8 mol/L) was unable to promote AR recruitment compared with vehicle control (Fig. 3C), consistent with the failure of DDE to induce transcriptional activity of wtAR (Fig. 2C). Combined, these data indicate that DDE exposure induces recruitment of mutant AR but not wtAR to target gene regulatory regions in prostate cancer cells.
FIGURE 3.
DDE induces AR-H874Y and AR-T877A recruitment to target gene regulatory regions. 22Rv1 (A), LNCaP (B), or LAPC4 (C) cells cultured in the absence of androgen were treated with either vehicle control (0.1% ethanol) or 10−8 mol/L DDE before ChIP. Either anti-AR or the nonspecific IgG antibody control was used for the immunoprecipitation (IP) and primers specific to the regulatory regions within the PSA enhancer were used for the PCR. Shown here are the representative images (left) and their respective quantitation using real-time PCR (right). Each experiment was done thrice in duplicate. *, P < 0.05.
DDE Induces Proliferation in LNCaP (AR-T877A) Cells
Given the capacity of DDE to activate mutant AR, but not wtAR, the ability of DDE to induce cell cycle progression was analyzed in relevant model systems. First, cells were examined to analyze DDE-mediated effects on proliferation (Fig. 4). Interestingly, 22Rv1 cells require mutant AR but not androgen for cell proliferation, as these cells have acquired an androgen-independent phenotype (56, 57). Cell proliferation in the absence of steroids (ethanol) was set to 1. Due to the androgen-independent nature of this specific model system, DHT did not significantly increase the number of cells undergoing mitosis, as observed by bromodeoxyuridine (BrdUrd) incorporation assay (~1.3-fold over ethanol control). Similarly, DDE (10−11, 10−8, and 10−5 mol/L) failed to alter the number of cells undergoing mitosis (Fig. 4A). These data confirm the androgen independence of this model system, as cell proliferation is unchanged regardless of hormonal milieu. Furthermore, these data show that despite the ability of DDE to facilitate transactivation potential of AR-H874Y, DDE is unable to significantly enhance cell proliferation in 22Rv1 cells, similar to androgen.
FIGURE 4.
DDE induces proliferation in AR-T877A – expressing prostate cancer cells. 22Rv1 (A) or LNCaP (B) cells were cultured in the absence of steroid for 24 h. Then, either vehicle control (0.1% ethanol), 10−10 mol/L DHT, or increasing doses of DDE (10−11, 10−8, or 10−5 mol/L) were added for 24 h. Cells were labeled with BrdUrd for 16 h and BrdUrd incorporation was monitored via indirect immunofluorescence. Dotted line, the level of BrdUrd incorporation in the absence of ligand (vehicle control) for each cell type was set to 1. C. LNCaP cells were cultured in the absence of steroid for 24 h before the addition of ligand [ethanol control, 10−10 mol/L DHT, or DDE (10−11, 10−8, or 10−5 mol/L)] for the indicated times (24, 48, 72, 96, or 120 h) with fresh ligand added every 48 h. At each time point, cells were collected and cell number and viability were determined via trypan blue exclusion. Experiments were repeated with three biological replicates, each analyzed in triplicate. *, P < 0.05. D. LNCaP cells were steroid starved for 24 h before treating with either vehicle control (0.1% ethanol), 10−10 mol/L DHT, or 10−11 mol/L DDE. After 24 h, cell lysates were collected and subjected to SDS-PAGE followed by immunoblotting for total cyclin A, cyclin E, cyclin D1, or lamin B (loading control). E. LAPC4 cells were cultured in the absence of steroid, similar to LNCaP cells, for 24 h before addition of ligand [ethanol control, 10−10 mol/L DHT, or DDE (10−11, 10−8, or 10−5 mol/L)] for the indicated times (24, 48, 72, or 96 h) with fresh ligand added every 48 h. At each time point, cells were collected and cell number and viability were determined via trypan blue exclusion. Experiments were repeated with three biological replicates, each analyzed in triplicate. *, P < 0.05.
In contrast to 22Rv1 cells, LNCaP cells are dependent on ligand-induced AR-T877A activity for cellular proliferation (58, 59) and therefore represent therapy-sensitive prostate cancer. As expected, DHT elevated the number of proliferative LNCaP cells to ~3-fold over ethanol control (P < 0.01; Fig. 4B). Higher doses of DDE (10−5 mol/L) did not increase BrdUrd incorporation compared with ethanol control (0.6-fold); however, lower and environmentally more relevant doses of DDE (10−11 and 10−8 mol/L) facilitated a 2.1- and 1.8-fold increase in DNA synthesis, respectively (P < 0.05 for both). These observations are consistent with the established paradigm that hormone-dependent prostate cancer cells exhibit a biphasic response to androgens, wherein levels greater than 1 × 10−9 mol/L inhibit (rather than induce) cellular proliferation (20, 21, 59). To verify that low-dose DDE-mediated mitotic activity can actually affect cell number, proliferation assays were done. LNCaP cells were treated as indicated (ethanol, DHT, or increasing concentrations of DDE) and analyzed using trypan blue exclusion. As seen in Fig. 4C, LNCaP cells treated with ethanol failed to undergo cell doubling throughout the time course examined, whereas DHT triggered the expected increase in cell number. Congruent with the BrdUrd data, lower doses of DDE were more effective in inducing proliferation compared with higher doses. Combined, these data indicate that low, environmentally relevant levels of DDE increase cell cycle progression and proliferation in androgen-dependent LNCaP cells (AR-T877A) but not in androgen-independent 22Rv1 (AR-H874Y) cells.
To analyze the effect of DDE on key proteins responsible for cell proliferation, expression studies for cell cycle proteins were carried out. Considering that a lower (and environmentally relevant) concentration of DDE imparts maximal response on AR activity (as measured by PSA expression; Fig. 2B) and cell proliferation, 10−11 mol/L DDE was used for protein expression studies. Cell lysates from LNCaP cells treated with DDE for 24 h were used for protein expression analysis. Compared with control (ethanol)-treated cells, DDE samples showed increased expression of G1 cell cycle regulatory proteins, including cyclin A, cyclin E, and cyclin D1, in LNCaP cells, albeit to a lesser extent than DHT (Fig. 4D). Thus, DDE seems to induce a proliferative response similar to that induced by DHT in LNCaP cells. To investigate the effect of DDE on proliferation of cells containing wtAR, androgen-dependent LAPC4 (wtAR) cells were analyzed in parallel (Fig. 4E). LAPC4 cells were treated with control and test treatments (ethanol, DHT, and DDE) and counted at selected time points. LAPC4 cells are androgen dependent and therefore require androgen to show proliferative response. Accordingly, under the androgen-ablative condition, these cells showed no change in cell number, consistent with the literature (Fig. 4E; ref. 60). No apoptosis was noted, also consistent with the literature (data not shown). As shown, DHT treatments triggered the expected proliferative response in these cells, and control (ethanol) and DDE (10−11, 10−8, and 10−5 mol/L) treatments failed to induce cell doubling throughout the time course examined. These data suggest that DDE-mediated proliferation is predominantly observed in cells that have acquired specific mutations in the AR ligand-binding domain.
DDE Activates Cell Signaling Proteins MAPK and Protein Kinase A in Prostate Cancer Cells
Recent evidence has shown that in select model systems, including prostate cancer cells, organochlorines are able to activate the MAPK pathway (61). Additionally, AR activation through ligand-independent MAPK signaling cascades is an important mechanism in the development of hormone-independent prostate cancers (62). Therefore, the effect of DDE on the MAPK pathway under androgen-ablative conditions was examined. For these studies, LNCaP or LAPC4 cells were deprived of androgen for 24 h and subsequently treated with DHT (10−10 mol/L), DDE (10−11 mol/L), or vehicle control, and extracellular signal-regulated kinase (ERK) phosphorylation was monitored (Fig. 5). Total ERK levels were not altered by treatment (ethanol, DHT, or DDE). In vehicle-treated LNCaP cells, minimal phosphorylated ERK (p-ERK) was observed (Fig. 5A, top, lanes 1 and 4); however, DHT (10−10 mol/L) exposure increased ERK phosphory-lation after 40 min (lane 2), as reported previously (63, 64). DDE caused a similar induction of p-ERK at 40 and 120 min compared with vehicle (Fig. 5A). In cells expressing wtAR (LAPC4), little alteration in ERK phosphorylation levels was observed on DDE exposure (Fig. 5B, top, lanes 3 and 6) compared with vehicle (ethanol)-treated controls (lanes 1 and 4). These data show that MAPK activation can be induced by DDE exposure in cells expressing mutant AR.
FIGURE 5.
DDE activates cell signaling proteins MAPK and VASP in prostate cancer cells. LNCaP (A) and LAPC4 (B) cells were cultured in the absence of steroid for 24 h, and then either vehicle control (0.1% ethanol), 10−10 mol/L DHT, or 10−11 mol/L DDE was added. Following 40 or 120 min, cell lysates were collected and subjected to SDS-PAGE followed by immunoblotting for total ERK1/2, p-ERK1/2, or lamin B (loading control). C. LNCaP and LAPC4 cells were cultured in the absence of steroid for 24 h before treating with either 0.1% ethanol, 10−10 mol/L DHT, or 10−11 mol/L DDE. C. Following 10-min incubation, cells were harvested and the cell lysates were subjected to SDS-PAGE for expression analysis of phosphorylated VASP (p-VASP) or lamin B (loading control). Isoproterenol (ISO)-treated lysates were used as positive control for VASP phosphorylation.
Recent studies indicate that cell signaling molecules can play a crucial role in progression of prostate cancer cells from an androgen-dependent to androgen-independent state (65, 66). To verify the effect of DDE exposure on other cell signaling molecules, signal transduction downstream of G protein–coupled receptor pathways was analyzed. Vasodilator-stimulated phosphoprotein (VASP) is a well-characterized substrate of protein kinase A (PKA) that is required for the DHT-mediated activation of AR (65). In LNCaP cells that express mutated AR, treatment with DHT and DDE induced PKA activation, as measured by VASP phosphorylation (Fig. 5C). In LAPC4 cells, DDE but not DHT promoted PKA activation (Fig. 5C); however, these cells are not stimulated to proliferate by DDE. Thus, although rapid activation of signal transduction pathways can occur in response to DDE, induction of androgen-independent proliferation was associated with mutant AR expression and activation of the MAPK pathway. Combined, these data indicate that rapid activation of signal transduction pathways results from DDE exposure and that subsequent induction of androgen-independent proliferation is associated with mutant AR expression and activation of the MAPK pathway.
DDE Uses MAPK and Mutant AR Pathways for Induction of Cell Cycle Progression in LNCaP Cells
Because DDE exposure in AR-T877A–expressing cells resulted in both increased AR activity and MAPK activation, the relative effect of each pathway on the observed androgen-independent proliferation was assessed. For these experiments, LNCaP cells were seeded as described for Fig. 4B, and although not shown, analysis of BrdUrd incorporation rates showed that DHT induced proliferation by 3.4-fold compared with ethanol alone and DDE, 2.0-fold (similar to data in Fig. 4B; data not shown). To assess the effect of MAPK and AR pathways on the proliferative response, either Casodex or U0126 was added to the culture medium before ligand. BrdUrd incorporation rates for each treatment alone were set to 100% so as to delineate the effect of each inhibitor on cell cycle progression under each condition (Fig. 6). In the absence of androgen, Casodex did not alter cell cycle progression, as likely attributed to the already low BrdUrd incorporation in these cells under conditions of ligand depletion. However, inhibition of MAPK/ERK kinase (MEK) via U0126 abolished basal BrdUrd incorporation in the presence of ethanol (5% compared with androgen depletion alone), thus indicating that MEK activity may be important for residual proliferation under conditions of low androgen. Coadministration of Casodex and U0126 offered little additional benefit. These data indicate that under conditions of androgen ablation, MEK inhibition may prove effective in promoting a cytostatic response. Under conditions of DHT-mediated proliferation, Casodex reduced BrdUrd incorporation by ~60%. Interestingly, the MEK inhibitor also showed a similar level of BrdUrd decrease (70% compared with DHT alone, no statistical difference between singular treatments of Casodex and U0126; P > 0.05) and the combination of the two inhibitors (Casodex and U0126) resulted in BrdUrd incorporation in 9% of cells. Thus, DHT-mediated cellular proliferation seems to involve both AR and MAPK signaling.
FIGURE 6.

DDE uses MAPK and mutant AR pathways for induction of cell cycle progression in LNCaP cells. LNCaP cells were cultured in the absence of steroid (0.1% DMSO vehicle control; white columns) or in the presence of 10−6 mol/L Casodex (CSDX; black striped columns), 5 ×10−6 mol/L Casodex (black crisscrossed columns), 10−6 mol/L MEK inhibitor (U0126; gray columns), or combined 10−6 mol/L Casodex and 10−6 mol/L U0126 (black columns). Following 24 h of pretreatment, cells were stimulated with vehicle control (ethanol), 10−10 mol/L DHT, or 10−11 mol/L DDE for 24 h and labeled with BrdUrd during the last 16 h of treatment. BrdUrd incorporation for each ligand in the absence of inhibitory challenge was set to 100%. Experiments were done in triplicate. Columns, average; bars, SD. *, P < 0.05; **, P < 0.01.
In the presence of DDE, Casodex (1 μmol/L) modestly reduced DDE-mediated BrdUrd incorporation from 100% to 71% (Fig. 6). Increased concentrations of 5 μmol/L Casodex reduced BrdUrd incorporation even more efficiently (53.5%). These data show that AR-T877A activity is at least partially responsible for DDE-mediated cell cycle progression. Conversely, administration of the MEK inhibitor U0126 also suppressed DDE-mediated BrdUrd incorporation (5% BrdUrd incorporation). The combined effect of Casodex and U0126 was not statistically different from that of U0126 alone (P > 0.05). However, it is likely that MEK inhibition also impinges on AR, as MAPK has been shown to augment AR function, and the inhibitor U0126 has been shown to reduce AR transcriptional activity in a target gene–specific manner.5 Together, these data show that DDE acts, in part, through the MAPK pathway to promote cell cycle progression and that a cross-talk between the AR and MAPK pathways contributes to the proliferative action of DDE.
Discussion
Somatic mutations of the AR play a significant role in the development of recurrent disease and prostate cancer progression. Our studies identify for the first time that the ubiquitously present pesticide derivative DDE activates select mutant ARs frequently present in prostate cancer tumors (14). In prostate cancer cells expressing AR-H874Y or AR-T877A, DDE exposure induced AR recruitment to target gene regulatory regions and resulted in androgen-independent activation of target genes, including PSA, indicating that DDE can impinge on clinical markers of disease progression. Proliferation was induced in cells expressing AR-T877A under conditions of androgen ablation on DDE exposure, and this event was attributed to MAPK and mutant AR signaling in response to DDE. Previous studies from our lab for BPA indicate that the in vitro analysis does reflect on the implications on tumor progression in vivo (22). BPA exposure at physiologically relevant levels increased the growth of LNCaP xenografts under androgen-ablative status, which mimics the direct correlation between the EDC exposure and tumor progression in prostate cancer patients (22). In cells carrying the AR-H874Y mutation, although DDE-mediated AR activation was present, no additional mitogenic effect was observed, as these cells have already achieved androgen independence. Cells expressing wtAR were protected from DDE-mediated AR activation and proliferative response. Thus, these data show that environmentally relevant exposure to DDE can subvert androgen requirement and induce cellular proliferation in tumor cells with selected somatic mutations of AR.
Extensive use of the pesticide DDT in the 1960s and 1970s has rendered water sources and soil contaminated with DDT degradation product, DDE. DDT was banned in the United States in 1972, but it is still used for the control of malaria in many parts of the world. DDE, the stable breakdown product of DDT, is of particular interest due to its persistence in the environment and continued detection in human tissues (67–69). Moreover, lipid-soluble DDE penetrates the food chain and gets stored in the adipose tissues of poultry and farm animals, ultimately affecting the consumers. Additionally, there is a plethora of epidemiologic evidence linking DDT/DDE and other chlorinated pesticides with defects on male reproductive organs and fertility in both wildlife and human populations (68). Consistent with being characterized as a carcinogen, tissue levels of DDT and DDE are higher in cancer victims compared with patients suffering from other diseases (70). Global analysis shows that detectable amounts of DDE are present in 50% to 99% of human population (71). Therefore, exposure to DDE in prostate cancer patients is likely of concern, and our study is the first to address how DDE may adversely affect prostate cancer therapy and progression.
The effect of higher concentrations (5–50 μmol/L) of DDE on AR has been previously examined in the presence or absence of AR-ligand (R1881; ref. 47). In the present study, we found that the most pronounced effects of DDE on mutant AR activation occur at low nanomolar ranges. These concentrations fall within the known human exposure range for these compounds (between 6.6 and 19.36 μg/L in plasma samples; ref. 67). It is not without precedent for low doses of ligand to bind and activate steroid nuclear receptors (20, 21). Additionally, it is possible that low concentration of DDE is able to induce the proliferative effects in LNCaP cells through estrogen production. Specifically, DDE has been found to increase aromatase activity in endometrial stromal cells in culture, resulting in locally higher estrogen levels (72). As LNCaP cells express an estrogen-responsive AR (AR-T877A), this increase in local estrogen may drive AR activity and AR-dependent proliferation. Aromatase expression is regulated through several different promoter regions in a tissue-specific manner (73), so it is yet to be determined if and how DDE may regulate aromatase activity in LNCaP cells.
Given the results from disparate experiments comparing DDE-mediated AR activity in the yeast reporter system and effects on AR-dependent target gene transcription (in prostate cancer cells harboring AR-H874Y and AR-T877A), and DDE-facilitated proliferation especially in androgen-dependent cells expressing AR-T877A, it is important to further investigate the subtle factors responsible for facilitating DDE action. The underlying mechanisms responsible for DDE-mediated mutant AR activation and proliferation should be explored for coregulatory molecule usage to unravel target gene profile differences. The ability of DDE to bind to wtAR has been well documented (47). Most recently, it has been shown that DDE binds to recombinant rat wtAR with an IC50 of 1.53 × 10−5 mol/L (38). Interestingly, the chloride moieties present on DDE are proposed to be the key contributing factors responsible for binding to AR (38). DDE has also been shown to exert a dose-dependent competitive inhibition of DHT in competitive AR-binding assays (47). Therefore, our finding that DDE was able to activate wtAR in yeast is not surprising, but it has been reported that under specific conditions, DDE can serve as an antagonist with wtAR (47, 74). The role of coregulators in this process may be of importance, as different ligands impart different conformations to AR, invariably changing the accessibility and requirements of coregulators of AR (75, 76). Indeed, it is likely that mutant ARs require a specific set of cofactors to exert DDE-induced transcriptional response. Future studies will be directed at discerning how mutant AR becomes sensitized to DDE action.
The data herein also show that a component of DDE activity occurs through rapid induction of signal transduction pathways. DDE activated PKA in both LNCaP and LAPC4 cells, indicating that it may promote similar effects to those reported for DHT (76). It is imperative to delineate the consequence of DDE on G protein signaling, considering that G protein–coupled receptor signaling is influencial on prostate cancer development and progression (77). However, the G protein–coupled receptor signaling event tracked by VASP phosphorylation was not sufficient to induce androgen-independent cellular proliferation in response to DDE, thereby indicating that additional mechanisms must exist to override androgen dependence.
DDE-mediated proliferation in prostate cancer cells was attenuated by inhibition of AR function, thus validating the importance of mutant AR in DDE response. Interestingly, both DHT- and DDE-mediated cellular proliferation were abrogated by inhibition of MAPK signaling, thus indicating that the MAPK pathway contributes to the mitogenic action of AR agonists. However, it should be noted that there is increasing evidence of cross-talk between the AR and MAPK pathways, wherein MAPK can contribute under specific conditions to AR activity.6 These data pose an interesting paradigm, wherein DDE-mediated prostate cancer proliferation may occur through AR ligand binding and AR transactivation and/or through phosphorylation cascades involving MAPK signaling pathways that impinge on AR. It is reported that MAPK-mediated AR phosphorylation at either serine or threonine residues promotes activation and alters subsequent nuclear localization of AR (78, 79). In addition, activation of growth factor signaling pathways (e.g., HER2 kinase and interleukin-6) regulates AR function and hormone-independent growth (66, 80), although the mechanisms through which kinase signals modulate AR function in these model systems are still unknown. Ultimately, MAPK signaling pathways play important roles in prostate cancer disease progression (62). Because this study identified MAPK activation as an important component of DDE-induced proliferation, it is imperative to identify the means by which MAPK influences therapeutic response.
In summary, the present study shows that a prevalent environmental contaminant, DDE, may subvert the androgen dependence of specific prostate cancers through activation of mutant AR and MAPK pathways. The mitogenic actions of DDE were observed at low doses consistent with DDE exposure levels and raise concerns about the effect of this agent on prostate cancer management. As tumor-derived mutations of AR were shown to be sensitized to DDE action, the findings herein also underscore the importance of dissecting the differential responses of mutant AR to endocrine mimics during prostate cancer progression.
Materials and Methods
Reagents
DHT, DDT, DDE, resveratrol, coumestrol, and cadmium were obtained from Sigma-Aldrich. All reagents were solubilized in ethanol and stored at −20°C. Casodex (bicalutamide) was a generous gift from AstraZeneca Pharmaceuticals and was solubilized in DMSO to 10−2 mol/L and stored at −20°C. The MEK inhibitor U0126 from Promega was used as per the manufacturer’s protocol.
Cell Culture and Treatments
LNCaP cells were obtained from the American Type Culture Collection, used between passage 28 and 40, and maintained in IMEM (Cellgro, Mediatech) containing 5% heat-inactivated fetal bovine serum (Biofluids). The 22Rv1 cell line was the gift of Dr. J. Jacobberger (Case Western Reserve University, Cleveland, OH) and CV1 cells were obtained from the American Type Culture Collection. 22Rv1 and CV1 cells were maintained in DMEM containing 10% heat-inactivated fetal bovine serum. LAPC4 cells were a gift from Dr. C. Sawyers (Memorial Sloan-Kettering Cancer Center, New York, NY) and were maintained in Iscove’s modified Dulbecco’s medium (Cellgro, Mediatech) containing 10% heat-inactivated fetal bovine serum. Media for all cell types were supplemented with 100 units/mL penicillin-streptomycin and 2 mmol/L L-gluta-mine (Mediatech). Cells were cultured at 37°C in a 5% CO2 humidified incubator. For culture in steroid-free conditions, cells were seeded in phenol red–free IMEM (LNCaP), DMEM (22Rv1 and CV1), or Iscove’s modified Dulbecco’s medium (LAPC4) containing charcoal dextran–treated fetal bovine serum (CDT; Hyclone Laboratories).
Plasmids
Expression plasmid encoding wtAR (pSG5-AR) was a gift of Dr. C. Chang (University of Rochester, Rochester, NY). The AR-T877A–encoding plasmid was a gift of Dr. D. Feldman (Stanford University, Stanford, CA). The AR-H874Y construct was a gift from Dr. S. Balk (Harvard Medical School, Boston, MA). The probasin reporter plasmid ARR2-luciferase was previously described (81). The construct encoding the internal transfection control β-galactosidase was a gift of Dr. J.Y.J. Wang (University of California at San Diego, San Diego, CA). The pcDNA3.1 was purchased from Invitrogen.
Colorimetric Yeast Screen for AR Activity
yA(G)RE yeast strains (ade2 deficient) containing an integrated wt ADE2 sequence as a reporter gene under the control of an ARE-promoter (genotype MATade2-1 leu2-3, 112trp1-1his3-11, 15can1-100ura3-1 URA3 3xARE::pCY-C1::ADE2) were transformed to express distinct functional AR alleles and were the generous gift of Dr. Ralph W. de Vere White (Department of Urology, University of California at Davis School of Medicine, Sacramento, CA) and were maintained and grown as previously described (21). Single yeast colonies were grown for 3 d at 30°C in 3 mL minimal selective medium (Difco yeast nitrogen base without amino acids; Becton Dickinson) containing 0.5% adenine, 1% histidine, and 1% leucine and supplemented with arginine, valine, phenylalanine, aspartic acid, isoleucine, serine, methionine, threonine, and glutamic acid. Yeast culture in log phase of growth was diluted 1:2 into sterile PBS and 3 μL were inoculated onto selection plates containing the indicated concentrations of test compound (either 10−8 mol/L DHT, 10−5 mol/L resveratrol, 10−5 mol/L coumestrol, 10−5 mol/L cadmium, 10−5 mol/L DDT, or 10−5 mol/L DDE) and cultured at 35°C for 3 d. Failure of AR to transactivate ADE2 results in the formation of red colonies due to the accumulation of an intermediate red pigment in the adenine biosynthesis pathway. On AR activation by ligand and ADE2 activation, adenine biosynthesis is initiated and the red pigment is enzymatically reduced, resulting in the formation of white or pink yeast colonies, and the intensity of the color correlates directly to the extent of AR activation (23).
Reporter Assays
CV1 cells were seeded 24 h before transfection in 10% CDT containing phenol red–free DMEM medium. Transfections were carried out using N,N-bis(2-hydroxyethyl)-2-amino-ethanesulfonic acid buffered saline/calcium phosphate method. Cells were cotransfected with plasmids expressing the AR (wtAR or AR-T877A or AR-H874Y; 1.0 μg), the ARR2-luciferase reporter construct (1.0 μg), and cytomegalovirus-β-galactosidase (0.25 μg). Empty vector (pcDNA3.1) was used to bring the total amount of DNA per transfection to 4 μg/well in six-well plates. After transfection, cells were washed four times with PBS to remove the precipitate. The following treatments were then added in fresh medium: 10 nmol/L DHT, 10 μmol/L DDE, or 0.1% ethanol vehicle. After 24 h of treatment, cells were harvested using trypsin and stored at −20°C until further analysis. Reporter lysis buffer from Promega was used to lyse the cells and 20 μL of lysate were used for the analysis of luciferase activity (Promega), with 75 μL of luciferase reagent, as described in the manufacturer’s protocol. One microliter of the same lysates was also used to analyze β-galactosidase activity using the Galacto-Star System (Applied Biosystems).
Quantitative Reverse Transcription-PCR
Cells were seeded onto 6-cm plates in appropriate CDT medium at approximately 4 to 5 × 105 cells per plate. After 24 h, ethanol was added as the vehicle control, DHT (the canonical AR ligand) was added as a positive control, and DDE was added for 24 h. Trizol reagent (Invitrogen) was used to extract total RNA, of which 5 μg were used to generate cDNA with random hexamers using the ThermoScript reverse transcription-PCR system (Invitrogen). Quantitative PCR was done following Applied Biosystems methodologies. Briefly, cDNA was diluted 1:6 with PCR-grade water and mixed with Taqman Fast Universal PCR Master Mix (2×) No AmpErase UNG (Applied Biosystems) and primers from Applied Bio-systems: glyceraldehyde-3-phosphate dehydrogenase (Hs. 00266705_g1) and PSA (Hs. 00426859_g1). The following cycle variables were used: 95°C for 20 s followed by 40 cycles of 95°C for 3 s with a 60°C ramp for 30 s. For quantitation of genomic DNA obtained from ChIP, Taqman Fast Universal PCR Master Mix was used. The sequences of the primer used for this reactions are the following: AREIII, TTATACTGGGACAACTTCCAAACC (forward) and TCTGTTTTCAATCCAAGATCATGAA (reverse).
Chromatin Immunoprecipitation
LNCaP (4 × 106), 22Rv1 (3 × 106), and LAPC4 (3 × 106) cells were seeded into poly-L-lysine–coated 15-cm dishes in phenol red–free medium containing the appropriate concentration of CDT. After 72 h of incubation in serum-free condition, cells were treated with 0.1% ethanol, 10−8 mol/L DHT, or 10−8 mol/L DDE. After 3 h (DHT) or 8 h (DDE) of treatment, cells were fixed in 1% formaldehyde solution in PBS to allow for DNA-protein cross-linking. The cross-linking process was stopped using 125 mmol/L glycine. Cells were collected and treated with 750 μL cell lysis buffer [5 mmol/L PIPES (pH 8.0), 85 mmol/L KCl, 0.5% NP40, 12 ng benzamidine, 100 ng 1,10-phenanthroline, 100 ng aprotinin, 100 ng/mL leupeptine] for 5 min on ice. Nuclear lysis buffer [400 μL; 50 mmol/L Tris-Cl (pH 8.1), 10 mmol/L EDTA, 1% SDS] was added to the pellet for 10 min on ice and samples were sonicated to shear DNA to 300- to 500-bp fragments. Lysates were precleared with rotating incubation with 20 μL Sepharose beads for 2 h at 4°C. As a positive control for DNA fragmentation, 10 μL (10% volume of the immunoprecipitates) of input samples were collected at this stage. For immunoprecipitation, 100 μL of lysate were incubated with either 0.5 μg AR antibody (N-20; Santa Cruz Biotechnology) or equal amount of preimmune sera (prebleed control). The immunoprecipitation was carried out in 400 μL radioimmunoprecipitation assay buffer [150 mmol/L NaCl, 1.0% NP40, 0.5% deoxycholate, 0.1% SDS, 50 mmol/L Tris (pH 8.0)] for 2 h at 4°C. At this point, protein A-Sepharose (Amersham) was added for another 2 h at 4°C with rotation. Protein A beads were washed with Super-RIPA (radioimmunoprecipitation assay buffer plus 150 mmol/L NaCl) and then thrice with RIPA buffer and once with TE buffer [10 mmol/L Tris (pH 8.0), 1 mmol/L EDTA], with 5-min rotations between each wash at room temperature. To extract DNA, all samples (including inputs) were incubated with 150 μL of ChIP extraction buffer (1% SDS, 0.1 mol/L NaHCO3) along with 10 μL of 5 mol/L NaCl and 0.3 μL RNase A. This solution was incubated at 65°C overnight. DNA was purified using the QIAquick PCR Purification kit (Qiagen). PCR amplification was done on DNA recovered from the immunoprecipitated samples as well as input chromatin samples. For PCR amplification, the PSA promoter was amplified with previously published primers (9): primer G, ACAGACCTACTCTGGAG-GAAC; primer H, AAGACAGCAACACCTTTTT. The PCR conditions used were the following: 94°C for 5 min followed by 35 (LNCaP), 39 (22Rv1), or 32 (LAPC4) cycles of 94°C for 30 s, 50°C for 30 s, and 72°C for 30 s. The PCR product is ~200 bp and was visualized using ethidium bromide–stained 2% agarose gels. Quantification by real-time PCR was done as indicated in the previous section.
Immunoblotting
LNCaP cells (1 × 106) were seeded in 10-cm dishes in 5% CDT serum containing IMEM. After 24 h, cells were supplemented with either vehicle control (0.1% ethanol), 10−10 mol/L DHT, or 10−11 mol/L DDE. Following 40 or 120 min of treatment, cells were pelleted and whole-cell lysates were prepared. Lysates were subjected to brief sonication and clarified by centrifugation. Equal protein concentrations (20 μg) were subjected to SDS-PAGE. Proteins were transferred to Immobilon membranes (Millipore) and immunoblotted for p-ERK (E-4, mouse monoclonal, 1:250; Santa Cruz Biotechnology), total ERK [ERK 1 (K-23), rabbit polyclonal, 1:1,000; Santa Cruz Biotechnology], phosphorylated VASP (AB3846, rabbit polyclonal, 1:1,000; Chemicon International), and lamin B (M-20, goat polyclonal, 1:1,000; Santa Cruz Biotechnology). The secondary antibodies used were goat anti-rabbit (Alexa Fluor 680 A21076, 1:10,000; Molecular Probes; to detect total ERK), donkey anti-goat (Alexa Fluor 680 A21084, 1:10,000; Molecular Probes; to detect lamin B), and goat anti-mouse (1:10,000; Rockland; to detect p-ERK) to visualize the antibody-antigen complex. For cell cycle protein expression analysis, LNCaP cells were treated for 24 h and blotted for target proteins. Antibodies used for the analysis were the following: cyclin A (H4320; Santa Cruz Biotechnology), cyclin E (HE-12; Santa Cruz Biotechnology), and cyclin D1 (Ab-3; NeoMarkers). For VASP expression analysis, LNCaP and LAPC4 cells were exposed to DDE treatments for 10 min and subsequently processed for immunoblot using phosphorylated VASP antibody. Isoproterenol is a ligand agonist for the Gs protein–coupled β-adrenergic receptor and was used as a positive control to measure activated cyclic AMP–dependent PKA. VASP protein serves as a PKA substrate, and VASP phosphorylation status was monitored as an indicator for PKA activation.
BrdUrd Incorporation Assay
LNCaP and 22Rv1 cells were seeded in six-well dishes (24) on poly-L-lysine–coated coverslips at a density of 2.5 × 105 per well in appropriate CDT medium. Cells were then supplemented with vehicle control (0.1% ethanol), 10−10 mol/L DHT, or various doses of DDE. For experiments using Casodex (direct AR antagonist) or U0126 (MEK inhibitor), before the addition of ligand, either 10−6 mol/L Casodex or 10−6 mol/L U0126 or a combination of both was added to the culture medium. Following 72 h of treatment, cells were labeled with Cell Proliferation Labeling Reagent (Amersham) according to the manufacturer’s protocol. Labeling continued for 16 h and cells were then processed to detect BrdUrd via indirect immunofluorescence as previously described (20). Experiments were done with at least six independent biological replicates. At least 250 cells per experiment were counted for each condition. Averages and SDs are shown.
Cell Proliferation Assay
For growth curves, LNCaP (3 × 105) and LAPC4 (1 × 105) cells were seeded in six-well plates into appropriate medium containing CDT serum. Approximately 24 h later, the indicated concentrations of either ethanol (0.3%, vehicle control), DHT, or DDE were added. Cells were cultured in the designated conditions for the indicated time points and fresh reagents were added every 48 h. After treatment, viable cells were counted using a hemacytometer and trypan blue exclusion.
Statistical Analysis
Quantitative results are expressed as average ± SD. Statistical analyses were done using one-way ANOVA and Neuman-Keuls post-test. The criterion for statistical significance was P < 0.05.
Acknowledgments
Grant support: NIH grant R01-ES-16675 (K.E. Knudsen) and National Institute of Environmental Health Sciences Environmental Mutagenesis and Cancer training grant ES-07250-16 (J.K. Hess-Wilson). Y. Daaka is a Georgia Cancer Coalition Distinguished Cancer Scholar.
We thank Dr. Ralph W. de Vere White for providing the colorimetric yeast system and I. Agoulnik and N. Weigel (Department of Molecular and Cellular Biology, Baylor College of Medicine, Houston, TX) for sharing unpublished data.
Footnotes
I. Agoulnik and N. Weigel, submitted for publication.
I. Agoulnik and N. Weigel, personal communication.
Disclosure of Potential Conflicts of Interest
The authors declare that there is no conflict of interest that would prejudice the impartiality of this scientific work.
References
- 1.Jemal A, Murray T, Ward E, et al. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10– 30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 2.Klotz L. Hormone therapy for patients with prostate carcinoma. Cancer. 2000;88:3009– 14. doi: 10.1002/1097-0142(20000615)88:12+<3009::aid-cncr17>3.3.co;2-5. [DOI] [PubMed] [Google Scholar]
- 3.Sharifi N, Farrar WL. Androgen receptor as a therapeutic target for androgen independent prostate cancer. Am J Ther. 2006;13:166– 70. doi: 10.1097/00045391-200603000-00013. [DOI] [PubMed] [Google Scholar]
- 4.Damber JE, Khatami A. Surgical treatment of localized prostate cancer. Acta Oncol. 2005;44:599– 604. doi: 10.1080/02841860510029734. [DOI] [PubMed] [Google Scholar]
- 5.Kirkpatrick JP, Anscher MS. Radiotherapy for locally recurrent prostate cancer. Clin Adv Hematol Oncol. 2005;3:933– 42. [PubMed] [Google Scholar]
- 6.Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer. 2001;1:34– 45. doi: 10.1038/35094009. [DOI] [PubMed] [Google Scholar]
- 7.Trapman J, Brinkmann AO. The androgen receptor in prostate cancer. Pathol Res Pract. 1996;192:752– 60. doi: 10.1016/S0344-0338(96)80097-5. [DOI] [PubMed] [Google Scholar]
- 8.Russell DW, Wilson JD. Steroid 5α-reductase: two genes/two enzymes. Annu Rev Biochem. 1994;63:25– 61. doi: 10.1146/annurev.bi.63.070194.000325. [DOI] [PubMed] [Google Scholar]
- 9.Shang Y, Myers M, Brown M. Formation of the androgen receptor transcription complex. Mol Cell. 2002;9:601– 10. doi: 10.1016/s1097-2765(02)00471-9. [DOI] [PubMed] [Google Scholar]
- 10.Stephan C, Jung K, Diamandis EP, Rittenhouse HG, Lein M, Loening SA. Prostate-specific antigen, its molecular forms, and other kallikrein markers for detection of prostate cancer. Urology. 2002;59:2– 8. doi: 10.1016/s0090-4295(01)01449-2. [DOI] [PubMed] [Google Scholar]
- 11.Dehm SM, Tindall DJ. Molecular regulation of androgen action in prostate cancer. J Cell Biochem. 2006;99:333– 44. doi: 10.1002/jcb.20794. [DOI] [PubMed] [Google Scholar]
- 12.Mendelsohn LG. Prostate cancer and the androgen receptor: strategies for the development of novel therapeutics. Prog Drug Res. 2000;55:213– 33. doi: 10.1007/978-3-0348-8385-6_6. [DOI] [PubMed] [Google Scholar]
- 13.Hirawat S, Budman DR, Kreis W. The androgen receptor: structure, mutations, and antiandrogens. Cancer Invest. 2003;21:400– 17. doi: 10.1081/cnv-120018232. [DOI] [PubMed] [Google Scholar]
- 14.Taplin ME, Bubley GJ, Shuster TD, et al. Mutation of the androgen-receptor gene in metastatic androgen-independent prostate cancer. N Engl J Med. 1995;332:1393– 8. doi: 10.1056/NEJM199505253322101. [DOI] [PubMed] [Google Scholar]
- 15.Taplin ME, Bubley GJ, Ko YJ, et al. Selection for androgen receptor mutations in prostate cancers treated with androgen antagonist. Cancer Res. 1999;59:2511– 5. [PubMed] [Google Scholar]
- 16.Tilley WD, Buchanan G, Hickey TE, Bentel JM. Mutations in the androgen receptor gene are associated with progression of human prostate cancer to androgen independence. Clin Cancer Res. 1996;2:277– 85. [PubMed] [Google Scholar]
- 17.Visakorpi T, Hyytinen E, Koivisto P, et al. In vivo amplification of the androgen receptor gene and progression of human prostate cancer. Nat Genet. 1995;9:401– 6. doi: 10.1038/ng0495-401. [DOI] [PubMed] [Google Scholar]
- 18.Koivisto P, Kononen J, Palmberg C, et al. Androgen receptor gene amplification: a possible molecular mechanism for androgen deprivation therapy failure in prostate cancer. Cancer Res. 1997;57:314– 9. [PubMed] [Google Scholar]
- 19.Linja MJ, Savinainen KJ, Saramaki OR, Tammela TL, Vessella RL, Visakorpi T. Amplification and overexpression of androgen receptor gene in hormone-refractory prostate cancer. Cancer Res. 2001;61:3550– 5. [PubMed] [Google Scholar]
- 20.Wetherill YB, Petre CE, Monk KR, Puga A, Knudsen KE. The xenoestrogen bisphenol A induces inappropriate androgen receptor activation and mitogenesis in prostatic adenocarcinoma cells. Mol Cancer Ther. 2002;1:515– 24. [PubMed] [Google Scholar]
- 21.Wetherill YB, Fisher NL, Staubach A, Danielsen M, de Vere White RW, Knudsen KE. Xenoestrogen action in prostate cancer: pleiotropic effects dependent on androgen receptor status. Cancer Res. 2005;65:54– 65. [PubMed] [Google Scholar]
- 22.Wetherill YB, Hess-Wilson JK, Comstock CE, et al. Bisphenol A facilitates bypass of androgen ablation therapy in prostate cancer. Mol Cancer Ther. 2006;5:3181– 90. doi: 10.1158/1535-7163.MCT-06-0272. [DOI] [PubMed] [Google Scholar]
- 23.Shi XB, Ma AH, Xia L, Kung HJ, de Vere White RW. Functional analysis of 44 mutant androgen receptors from human prostate cancer. Cancer Res. 2002;62:1496– 502. [PubMed] [Google Scholar]
- 24.Thompson J, Saatcioglu F, Janne OA, Palvimo JJ. Disrupted amino- and carboxyl-terminal interactions of the androgen receptor are linked to androgen insensitivity. Mol Endocrinol. 2001;15:923– 35. doi: 10.1210/mend.15.6.0647. [DOI] [PubMed] [Google Scholar]
- 25.Zhao XY, Boyle B, Krishnan AV, Navone NM, Peehl DM, Feldman D. Two mutations identified in the androgen receptor of the new human prostate cancer cell line MDA PCa 2a. J Urol. 1999;162:2192– 9. doi: 10.1016/S0022-5347(05)68158-X. [DOI] [PubMed] [Google Scholar]
- 26.Steketee K, Timmerman L, Ziel-van der Made AC, Doesburg P, Brinkmann AO, Trapman J. Broadened ligand responsiveness of androgen receptor mutants obtained by random amino acid substitution of H874 and mutation hot spot T877 in prostate cancer. Int J Cancer. 2002;100:309– 17. doi: 10.1002/ijc.10495. [DOI] [PubMed] [Google Scholar]
- 27.Tan J, Sharief Y, Hamil KG, et al. Dehydroepiandrosterone activates mutant androgen receptors expressed in the androgen-dependent human prostate cancer xenograft CWR22 and LNCaP cells. Mol Endocrinol. 1997;11:450– 9. doi: 10.1210/mend.11.4.9906. [DOI] [PubMed] [Google Scholar]
- 28.Veldscholte J, Ris-Stalpers C, Kuiper GG, et al. A mutation in the ligand binding domain of the androgen receptor of human LNCaP cells affects steroid binding characteristics and response to anti-androgens. Biochem Biophys Res Commun. 1990;173:534– 40. doi: 10.1016/s0006-291x(05)80067-1. [DOI] [PubMed] [Google Scholar]
- 29.Veldscholte J, Berrevoets CA, Ris-Stalpers C, et al. The androgen receptor in LNCaP cells contains a mutation in the ligand binding domain which affects steroid binding characteristics and response to antiandrogens. J Steroid Biochem Mol Biol. 1992;41:665– 9. doi: 10.1016/0960-0760(92)90401-4. [DOI] [PubMed] [Google Scholar]
- 30.Adlercreutz H. Phyto-oestrogens and cancer. Lancet Oncol. 2002;3:364– 73. doi: 10.1016/s1470-2045(02)00777-5. [DOI] [PubMed] [Google Scholar]
- 31.Clinton SK, Giovannucci E. Diet, nutrition, and prostate cancer. Annu Rev Nutr. 1998;18:413– 40. doi: 10.1146/annurev.nutr.18.1.413. [DOI] [PubMed] [Google Scholar]
- 32.Cohen JH, Kristal AR, Stanford JL. Fruit and vegetable intakes and prostate cancer risk. J Natl Cancer Inst. 2000;92:61– 8. doi: 10.1093/jnci/92.1.61. [DOI] [PubMed] [Google Scholar]
- 33.Mentor-Marcel R, Lamartiniere CA, Eltoum IE, Greenberg NM, Elgavish A. Genistein in the diet reduces the incidence of poorly differentiated prostatic adenocarcinoma in transgenic mice (TRAMP) Cancer Res. 2001;61:6777– 82. [PubMed] [Google Scholar]
- 34.Landstrom M, Zhang JX, Hallmans G, et al. Inhibitory effects of soy and rye diets on the development of Dunning R3327 prostate adenocarcinoma in rats. Prostate. 1998;36:151– 61. doi: 10.1002/(sici)1097-0045(19980801)36:3<151::aid-pros2>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 35.Pollard M. Prevention of prostate-related cancers in Lobund-Wistar rats. Prostate. 1999;39:305– 9. doi: 10.1002/(sici)1097-0045(19990601)39:4<305::aid-pros12>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 36.Cos P, De Bruyne T, Apers S, Vanden Berghe D, Pieters L, Vlietinck AJ. Phytoestrogens: recent developments. Planta Med. 2003;69:589– 99. doi: 10.1055/s-2003-41122. [DOI] [PubMed] [Google Scholar]
- 37.Stephens FO. Phytoestrogens and prostate cancer: possible preventive role. Med J Aust. 1997;167:138– 40. doi: 10.5694/j.1326-5377.1997.tb138812.x. [DOI] [PubMed] [Google Scholar]
- 38.Fang H, Tong W, Branham WS, et al. Study of 202 natural, synthetic, and environmental chemicals for binding to the androgen receptor. Chem Res Toxicol. 2003;16:1338– 58. doi: 10.1021/tx030011g. [DOI] [PubMed] [Google Scholar]
- 39.Sahmoun AE, Case LD, Jackson SA, Schwartz GG. Cadmium and prostate cancer: a critical epidemiologic analysis. Cancer Invest. 2005;23:256– 63. doi: 10.1081/cnv-200055968. [DOI] [PubMed] [Google Scholar]
- 40.Achanzar WE, Diwan BA, Liu J, Quader ST, Webber MM, Waalkes MP. Cadmium-induced malignant transformation of human prostate epithelial cells. Cancer Res. 2001;61:455– 8. [PubMed] [Google Scholar]
- 41.Verougstraete V, Lison D, Hotz P. Cadmium, lung and prostate cancer: a systematic review of recent epidemiological data. J Toxicol Environ Health B Crit Rev. 2003;6:227– 55. doi: 10.1080/10937400306465. [DOI] [PubMed] [Google Scholar]
- 42.Blair A, Zahm SH. Agricultural exposures and cancer. Environ Health Perspect. 1995;103(Suppl 8):205– 8. doi: 10.1289/ehp.95103s8205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fleming LE, Bean JA, Rudolph M, Hamilton K. Cancer incidence in a cohort of licensed pesticide applicators in Florida. J Occup Environ Med. 1999;41:279– 88. doi: 10.1097/00043764-199904000-00010. [DOI] [PubMed] [Google Scholar]
- 44.Mills PK, Beeson WL, Phillips RL, Fraser GE. Cohort study of diet, lifestyle, and prostate cancer in Adventist men. Cancer. 1989;64:598– 604. doi: 10.1002/1097-0142(19890801)64:3<598::aid-cncr2820640306>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 45.Ritchie JM, Vial SL, Fuortes LJ, Guo H, Reedy VE, Smith EM. Organo-chlorines and risk of prostate cancer. J Occup Environ Med. 2003;45:692– 702. doi: 10.1097/01.jom.0000071510.96740.0b. [DOI] [PubMed] [Google Scholar]
- 46.Schreinemachers DM, Creason JP, Garry VF. Cancer mortality in agricultural regions of Minnesota. Environ Health Perspect. 1999;107:205– 11. doi: 10.1289/ehp.99107205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kelce WR, Stone CR, Laws SC, Gray LE, Kemppainen JA, Wilson EM. Persistent DDT metabolite p,p′-DDE is a potent androgen receptor antagonist. Nature. 1995;375:581– 5. doi: 10.1038/375581a0. [DOI] [PubMed] [Google Scholar]
- 48.Jenster G. The role of the androgen receptor in the development and progression of prostate cancer. Semin Oncol. 1999;26:407– 21. [PubMed] [Google Scholar]
- 49.Culig Z, Comuzzi B, Steiner H, Bartsch G, Hobisch A. Expression and function of androgen receptor coactivators in prostate cancer. J Steroid Biochem Mol Biol. 2004;92:265– 71. doi: 10.1016/j.jsbmb.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 50.Krauthacker B, Alebic-Kolbah T, Buntic A, Tkalcevic B, Reiner E. DDT residues in samples of human milk, and in mothers’ and cord blood serum, in a continental town in Croatia (Yugoslavia) Int Arch Occup Environ Health. 1980;46:267– 73. doi: 10.1007/BF00380016. [DOI] [PubMed] [Google Scholar]
- 51.Guardino X, Serra C, Obiols J, et al. Determination of DDT and related compounds in blood samples from agricultural workers. J Chromatogr A. 1996;719:141– 7. doi: 10.1016/0021-9673(95)00284-7. [DOI] [PubMed] [Google Scholar]
- 52.Rosell MG, Obiols J, Berenguer MJ, Guardino X, Lopez F, Brosa J. Determination of chlorinated insecticides in blood samples of agricultural workers. J Chromatogr. 1993;655:151– 4. doi: 10.1016/0021-9673(93)87023-f. [DOI] [PubMed] [Google Scholar]
- 53.Di Muccio A, Camoni I, Dommarco R, et al. Evaluation of p,p′-DDE, p,p′-DDT and polychlorobiphenyls (PCBs) levels in samples of human milk from Rome, Florence and the surrounding areas. Ann Ist Super Sanita. 1990;26:155– 60. [PubMed] [Google Scholar]
- 54.Li JY, Wu DS, Yang F, et al. Study on serum organochlorines pesticides (DDTs) level, CYP1A1 genetic polymorphism and risk of breast cancer: a case control study. Zhonghua Liu Xing Bing Xue Za Zhi. 2006;27:217– 22. [PubMed] [Google Scholar]
- 55.Kim HJ, Park YI, Dong MS. Effects of 2,4-D and DCP on the DHT-induced androgenic action in human prostate cancer cells. Toxicol Sci. 2005;88:52– 9. doi: 10.1093/toxsci/kfi287. [DOI] [PubMed] [Google Scholar]
- 56.Ting HJ, Bao BY, Reeder JE, Messing EM, Lee YF. Increased expression of corepressors in aggressive androgen-independent prostate cancer cells results in loss of 1α,25-dihydroxyvitamin D3 responsiveness. Mol Cancer Res. 2007;5:967– 80. doi: 10.1158/1541-7786.MCR-06-0318. [DOI] [PubMed] [Google Scholar]
- 57.Schalken J. Editorial comment on: Increased expression of tumor-associated trypsin inhibitor, TATI, in prostate cancer and in androgen-independent 22Rv1 cells. Eur Urol. 2007;52:1679. doi: 10.1016/j.eururo.2007.01.097. [DOI] [PubMed] [Google Scholar]
- 58.Kim IY, Kim JH, Zelner DJ, Ahn HJ, Sensibar JA, Lee C. Transforming growth factor-β1 is a mediator of androgen-regulated growth arrest in an androgen-responsive prostatic cancer cell line, LNCaP. Endocrinology. 1996;137:991– 9. doi: 10.1210/endo.137.3.8603613. [DOI] [PubMed] [Google Scholar]
- 59.Knudsen KE, Arden KC, Cavenee WK. Multiple G1 regulatory elements control the androgen-dependent proliferation of prostatic carcinoma cells. J Biol Chem. 1998;273:20213– 22. doi: 10.1074/jbc.273.32.20213. [DOI] [PubMed] [Google Scholar]
- 60.Sharma A, Comstock CE, Knudsen ES, et al. Retinoblastoma tumor suppressor status is a critical determinant of therapeutic response in prostate cancer cells. Cancer Res. 2007;67:6192– 203. doi: 10.1158/0008-5472.CAN-06-4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tessier DM, Matsumura F. Increased ErbB-2 tyrosine kinase activity, MAPK phosphorylation, and cell proliferation in the prostate cancer cell line LNCaP following treatment by select pesticides. Toxicol Sci. 2001;60:38– 43. doi: 10.1093/toxsci/60.1.38. [DOI] [PubMed] [Google Scholar]
- 62.Craft N, Shostak Y, Carey M, Sawyers CL. A mechanism for hormone-independent prostate cancer through modulation of androgen receptor signaling by the HER-2/neu tyrosine kinase. Nat Med. 1999;5:280– 5. doi: 10.1038/6495. [DOI] [PubMed] [Google Scholar]
- 63.Peterziel H, Mink S, Schonert A, Becker M, Klocker H, Cato AC. Rapid signalling by androgen receptor in prostate cancer cells. Oncogene. 1999;18:6322– 9. doi: 10.1038/sj.onc.1203032. [DOI] [PubMed] [Google Scholar]
- 64.Unni E, Sun S, Nan B, et al. Changes in androgen receptor nongenotropic signaling correlate with transition of LNCaP cells to androgen independence. Cancer Res. 2004;64:7156– 68. doi: 10.1158/0008-5472.CAN-04-1121. [DOI] [PubMed] [Google Scholar]
- 65.Kasbohm EA, Guo R, Yowell CW, et al. Androgen receptor activation by G(s) signaling in prostate cancer cells. J Biol Chem. 2005;280:11583– 9. doi: 10.1074/jbc.M414423200. [DOI] [PubMed] [Google Scholar]
- 66.Yeh S, Lin HK, Kang HY, Thin TH, Lin MF, Chang C. From HER2/Neu signal cascade to androgen receptor and its coactivators: a novel pathway by induction of androgen target genes through MAP kinase in prostate cancer cells. Proc Natl Acad Sci U S A. 1999;96:5458– 63. doi: 10.1073/pnas.96.10.5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pant N, Mathur N, Banerjee AK, Srivastava SP, Saxena DK. Correlation of chlorinated pesticides concentration in semen with seminal vesicle and prostatic markers. Reprod Toxicol. 2004;19:209– 14. doi: 10.1016/j.reprotox.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 68.Turusov V, Rakitsky V, Tomatis L. Dichlorodiphenyltrichloroethane (DDT): ubiquity, persistence, and risks. Environ Health Perspect. 2002;110:125– 8. doi: 10.1289/ehp.02110125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Younglai EV, Holloway AC, Lim GE, Foster WG. Synergistic effects between FSH and 1,1-dichloro-2,2-bis(P-chlorophenyl)ethylene (P,P′-DDE) on human granulosa cell aromatase activity. Hum Reprod. 2004;19:1089– 93. doi: 10.1093/humrep/deh252. [DOI] [PubMed] [Google Scholar]
- 70.U.S. Environmental Protection Agency. Integrated Risk Information System on p,p′-dichlorodiphenyldichloroethylene. Washington (DC): U.S. Environmental Protection Agency; 1988. [Google Scholar]
- 71.Charlier C, Desaive C, Plomteux G. Human exposure to endocrine disrupters: consequences of gastroplasty on plasma concentration of toxic pollutants. Int J Obes Relat Metab Disord. 2002;26:1465– 8. doi: 10.1038/sj.ijo.0802144. [DOI] [PubMed] [Google Scholar]
- 72.Holloway AC, Stys KA, Foster WG. DDE-induced changes in aromatase activity in endometrial stromal cells in culture. Endocrine. 2005;27:45– 50. doi: 10.1385/ENDO:27:1:045. [DOI] [PubMed] [Google Scholar]
- 73.Simpson ER, Zhao Y, Agarwal VR, et al. Aromatase expression in health and disease. Recent Prog Horm Res. 1997;52:185– 213. discussion -4. [PubMed] [Google Scholar]
- 74.Xu LC, Sun H, Chen JF, Bian Q, Song L, Wang XR. Androgen receptor activities of p,p′-DDE, fenvalerate and phoxim detected by androgen receptor reporter gene assay. Toxicol Lett. 2006;160:151– 7. doi: 10.1016/j.toxlet.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 75.Baek SH, Ohgi KA, Nelson CA, et al. Ligand-specific allosteric regulation of coactivator functions of androgen receptor in prostate cancer cells. Proc Natl Acad Sci U S A. 2006;103:3100– 5. doi: 10.1073/pnas.0510842103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bagchi G, Wu J, French J, Kim J, Moniri NH, Daaka Y. Androgens transduce the Gαs-mediated activation of protein kinase A in prostate cells. Cancer Res. 2008;68:3225– 31. doi: 10.1158/0008-5472.CAN-07-5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Daaka Y. G proteins in cancer: the prostate cancer paradigm. Sci STKE. 2004;2004:re2. doi: 10.1126/stke.2162004re2. [DOI] [PubMed] [Google Scholar]
- 78.Kraus S, Gioeli D, Vomastek T, Gordon V, Weber MJ. Receptor for activated C kinase 1 (RACK1) and Src regulate the tyrosine phosphorylation and function of the androgen receptor. Cancer Res. 2006;66:11047– 54. doi: 10.1158/0008-5472.CAN-06-0596. [DOI] [PubMed] [Google Scholar]
- 79.Gioeli D, Black BE, Gordon V, et al. Stress kinase signaling regulates androgen receptor phosphorylation, transcription, and localization. Mol Endocrinol. 2006;20:503– 15. doi: 10.1210/me.2005-0351. [DOI] [PubMed] [Google Scholar]
- 80.Culig Z, Steiner H, Bartsch G, Hobisch A. Interleukin-6 regulation of prostate cancer cell growth. J Cell Biochem. 2005;95:497– 505. doi: 10.1002/jcb.20477. [DOI] [PubMed] [Google Scholar]
- 81.Marshall TW, Link KA, Petre-Draviam CE, Knudsen KE. Differential requirement of SWI/SNF for androgen receptor activity. J Biol Chem. 2003;278:30605– 13. doi: 10.1074/jbc.M304582200. [DOI] [PubMed] [Google Scholar]