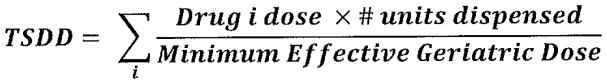Abstract
Study Objective
To determine the influence of anticholinergic medications on transitions in cognitive diagnosis of older adults in primary care.
Design
This observational cohort study was conducted over a mean follow-up of 3.2 years. Anticholinergic exposure was defined by pharmacy dispensing and claims records. Cognitive diagnosis was performed by an expert panel at baseline and annually up to 4 years.
Data Source
Medication exposure and other clinical data were extracted from the Indiana Network for Patient Care (INPC). The cognitive diagnosis was derived from a cognitive screening and diagnosis study.
Participants
A total of 350 adults 65 years and older without dementia and receiving primary care in a safety net health care system.
Measurement and Main Results
Cognitive diagnosis followed a two-phase screening and consensus-based neuropsychiatric examination to determine a baseline diagnosis as normal cognition, mild cognitive impairment (MCI), or dementia, with a follow-up neuropsychiatric examination and consensus-based diagnosis repeated annually. The Anticholinergic Cognitive Burden scale was used to identify anticholinergics dispensed up to 10 years before enrollment and annually throughout the study. A total standard daily dose of anticholinergics was calculated by using pharmacy dispensing data from the INPC. Among 350 participants, a total of 978 diagnostic assessments were completed over a mean follow-up of 3.2 years. Compared with stable cognition, increasing use of strong anticholinergics calculated by total standard daily dose increased the odds of transition from normal cognition to MCI (odds ratio [OR] 1.15, 95% confidence interval [CI] 1.01–1.31, p = 0.0342). Compared with stable MCI, strong anticholinergics did not influence the reversion of MCI to normal cognition (OR 0.95, 95% CI 0.86–1.05, p = 0.3266).
Conclusion
De-prescribing interventions in older adults with normal cognition should test anticholinergics as potentially modifiable risk factors for cognitive impairment.
Keywords: dementia, adverse drug reaction, primary care, anticholinergic, pharmacoepidemiology, modifiable risk factors, mild cognitive impairment
Medications with anticholinergic adverse effects are used by ~25% of older adults living in the community to manage symptoms such as incontinence, seasonal allergies, depression, and insomnia.1, 2 Governing bodies such as the American Geriatrics Society and the Center for Medicare and Medicaid Services recommend against using these medications in older adults because the risks of peripheral and central adverse events outweigh the therapeutic benefits.3, 4 Despite these recommendations and the availability of alternative treatments, population-based studies show no decrease in volume of prescription anticholinergic medications in older adults.5, 6
Cholinergic neurons are widely distributed throughout the basal forebrain with projections to the hippocampus, cortex, and medial temporal lobe, supporting learning, memory, organization, and attention.7, 8 Anticholinergic drugs characteristically block cholinergic receptors, and they were shown in mouse models to increase β-amyloid plaques and neurofibrillary tau proteins, hallmarks of Alzheimer’s dementia (AD).9–17 Interruption of cholinergic neurotransmission has been correlated with cell death and memory deficits that mimic AD.9, 15, 17 A 2016 study showed that anticholinergic users had smaller tissue volume in the cortex and temporal lobes, also correlating with worse performance in domains of memory, processing speed, and executive function compared with nonusers.18
Several epidemiological studies have identified the relationship between anticholinergic exposure and the diagnosis of cognitive impairment including international studies conducted in the United Kingdom, France, Australia, and the United States.2, 19–26 More recently, the quality of observational studies improved with the use of prescription records as a measure of medication exposure, as well as consensus-based diagnosis serving as the outcome. Two studies used pharmacy dispensing or claims data to show higher odds of cognitive impairment with increasing measures of anticholinergic exposure.23, 27
Prior work has predominantly shown relationships between anticholinergic exposure and dementia, and few studies have included mild cognitive impairment (MCI) as an outcome. MCI is a potentially reversible stage of cognitive decline between normal cognition and dementia.28 Two observational studies reported relationships between anticholinergics and MCI.21, 22 However, no prior studies have used dispensing data to evaluate the relationship between anticholinergic exposure and transitions between normal and impaired cognition.
Understanding the transition between cognitive states may improve our understanding of the reversibility of the adverse cognitive effects of anticholinergics. One randomized trial found no difference in a memory test in a nursing home population after reducing anticholinergic exposure by 50% over 8 weeks;29 however, the authors reported improvement in a small group who discontinued all anticholinergics. Epidemiological studies suggest that interventions to reduce anticholinergic use would have a more significant impact on populations with mild or no cognitive impairment as a preventive intervention.30
To address the question of reversibility, we conducted a secondary data analysis to model the reversibility of the adverse cognitive effects of anticholinergics by merging data from a longitudinal study in older adults in primary care containing multiple cognitive assessments with prescription dispensing records. Our hypothesis was that anticholinergic exposure would increase the transition from normal cognition to mild cognitive impairment and decrease the transition from MCI to normal cognition.
Methods
Study Population
This prospective observational study was conducted in older adults receiving primary care from Eskenazi Health (formerly known as Wishard Health) in Indianapolis, Indiana. Eskenazi Health, a large safety net health care system, is responsible for the care of the indigent, uninsured, or underinsured population in Indianapolis. Primary care was provided through nine primary care centers located throughout the city. Potentially eligible patients were screened if they were 65 years or older, had more than two primary care visits in the prior 12 months with a future visit scheduled, and no medical record diagnosis of dementia. Potentially eligible primary care patients were screened with the Mini Mental Status Examination (MMSE) or the Telephone Interview for Cognitive Status (TICS). Patients scoring 14 or greater on the MMSE or 22 or greater on the TICS, and who had an informant available, were eligible for enrollment in the diagnostic assessment.
This study was reviewed and approved by the institutional review board of the Indiana University-Purdue University of Indianapolis.
Cognitive Diagnosis
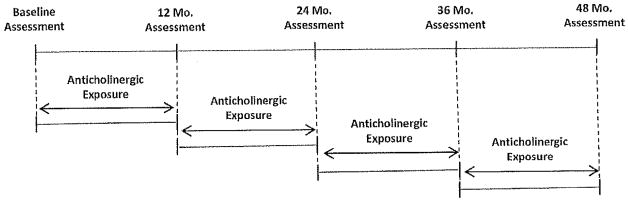
An expert-based consensus panel adjudicated cognitive diagnoses at each assessment that included the baseline and reassessment every 12 months up to 48 months (Figure 1). Participants completed a self-report of health history, depression by the Geriatric Depression Scale, and cognitive assessment with Wechsler Memory Scale-R Logical Memory, Wechsler Memory Scale-R Visual Reproduction, Consortium to Establish a Registry for Alzheimer’s Disease Word List Learning Task, Boston Naming, Animal Fluency, Wechsler Adult Intelligence Scale-Revised Block Design, and Trail Making Tests A & B. Informants were asked about the range, onset, and progression of cognitive symptoms, functional status with the Functional Assessment Questionnaire, and psychiatric status with the Neuropsychiatric Inventory. A neurologic and physical examination and a medication review completed the medical assessment. The consensus diagnosis conference included geriatric, psychiatric, neurologic, and neuropsychiatric experts along with standard criteria to attribute diagnoses of normal cognitive, MCI, and dementia for each participant at each wave. Participants with a diagnosis of normal cognition or MCI were included in the study; those with a diagnosis of dementia were excluded.
Figure 1.
Timeline for collection of cognitive outcome and exposure variables.
Measure of Medication Exposure
Medication data were retrieved from the Indiana Network for Patient Care that captures pharmacy dispensing data from outpatient pharmacies located within the Eskenazi Health network as well as dispensed prescriptions from retail pharmacies through Surescripts claims data.31 The Pharmacy module within the INPC contains dispensed drug name, strength, quantity dispensed, number of days supplied, and date dispensed for each prescription record. Prescription records were extracted from the 10 years before the initial cognitive assessment through to the final cognitive assessment completed by each participant to create two anticholinergic exposure variables used in the analysis. The time-varying exposure variable included anticholinergic prescription records between 12-month cognitive assessments (Figure 1); a historical exposure variable was defined using records for the 10 years of exposure before the baseline assessment.
Anticholinergics were defined according to the Anticholinergic Cognitive Burden (ACB) scale that was previously validated in similar populations.2, 22, 27, 32, 33 According to the ACB scale, medications with mild anticholinergic effects were attributed a score of 1 and defined as those with serum anticholinergic activity or in vitro affinity to muscarinic receptors but with no known clinically relevant adverse cognitive effects. Medications with clinically relevant anticholinergic properties were attributed a score of 2 or 3 based on the presence of peripheral anticholinergic side effects only (score of 2) or the presence of both peripheral and central anticholinergic side effects (score of 3).
We defined the total standard daily dose (TSDD) as a cumulative measure of anticholinergic exposure for all anticholinergics identified in the ACB scale (scores 1, 2, and 3) as well as for strong anticholinergics only. When calculating the TSDD, a standard daily dose (SDD) was derived by multiplying the number of medication units (tablets or capsules) dispensed by the strength (in milligrams) and then dividing by the minimum effective geriatric dose (MEGD). MEGD was defined by a geriatric medication reference34 that was previously used for similar adjustments.23, 35 The SDD for all anticholinergics also included a multiplier to weight each drug as defined in the ACB scale (score of 1, 2, or 3). For analyses using the SDD from strong anticholinergics only (score 2 and 3), no weighting was applied. Figure 2 shows that the time-varying anticholinergic exposure variable was defined as a cumulative total SDD (TSDD) by summing each of the SDDs for anticholinergic prescriptions for each subject. TSDD for the time-varying anticholinergic exposure variable was further adjusted to accommodate clinical interpretation by dividing TSDD for all ACB medications by 2000 and TSDD for strong anticholinergics by 365.
Figure 2.
Calculation of total standard daily dose (TSDD) for strong anticholinergic exposure. Anticholinergic exposure using all anticholinergics (mild, possible, and strong with scores 1, 2, and 3, respectively) included a multiplier corresponding to the score for each medication in the numerator.
Demographics and Other Variables
Demographics and comorbidities were collected through a combination of participant self-report and medical record review of diagnoses. Hypertension was defined as systolic blood pressure greater than 140 mm Hg or diastolic blood pressure greater than 90 mm Hg. Diabetes was defined as glucose greater than 126 mg/dl fasting or 160 mg/dl random, or glycosylated hemoglobin A1c greater than 10. The definition of coronary artery disease included myocardial infarction, angioplasty, coronary artery bypass graft, and stent placement. Congestive heart failure (CHF) included a diagnosis of CHF, cardiomyopathy, or left ventricular hypertrophy. Cancer included history of breast, prostate, lymphoma, colon, or lung cancers.
Statistical Analysis
The t test and Fisher exact test were used to compare the baseline characteristics between participants with normal cognition and MCI as well as comparisons between those whose diagnosis transitioned and remained stable. We used logistic regression models with generalized estimating equations (GEEs) with the outcomes defined as transition in cognitive diagnosis versus stable diagnosis at 12-month intervals. Transition from MCI to normal was compared with stable MCI; transition from normal to MCI was compared with stable normal cognition. Each participant could contribute up to four repeated transitional outcomes for each 12-month segment depending on the duration of participation in the study, and the repeated outcomes from the same individuals were adjusted using the GEE approach. Anticholinergic exposure variables were independent variables in each model and were collected during the 12-month period between diagnoses as time-dependent variables. A historical anticholinergic exposure variable was also defined using prescription records from the 10 years before the baseline cognitive assessment. Because prior studies suggest that only highest exposure over time adversely affects cognition,23 we defined historical anticholinergic exposure as a categorical variable of the highest tertile of anticholinergic exposure. Adjusted models included age, race, sex, education, stroke, CHF, and historical anticholinergic medication exposure. Covariates not included in the models were not significantly related to the model outcomes.
Missing dispensed amount fields occurred in 33% of all anticholinergic records; we addressed missing dispensed amount fields by substituting number of days supplied if available, or 60 days as the most common number of days supplied among all prescription records. Missing prescription strength fields occurred in 21% of all anticholinergic records and was addressed by substituting MEGDs. A sensitivity analysis excluding missing records was also conducted.
Results
Among 350 participants, the mean age at baseline was 71.2 (±5.1) years, 79.1% were female and 62.0% were African American (Table 1). Those diagnosed with MCI at baseline were more likely to be non–African American, have lower education, have more comorbidity, and have used any anticholinergic than those diagnosed as cognitively normal. Mean follow-up was 3.2 years (standard deviation [SD] 0.8 years, range 0.9–4.3 years).
Table 1.
Baseline Demographic Characteristics of Study Population
| All participants, N = 350 | Normal cognition, N = 206 | MCI, N = 144 | p valuea | |
|---|---|---|---|---|
| Age, mean (SD) | 71.2 (5.1) | 71.3 (5.0) | 71.2 (5.2) | 0.8304 |
| Race, n (%) | ||||
| African American | 217 (62.0) | 149 (72.3) | 68 (47.2) | <0.0001 |
| Non–African Americanb | 133 (38.0) | 57 (27.7) | 76 (52.8) | |
| Female, n (%) | 277 (79.1) | 168 (81.6) | 109 (75.7) | 0.2288 |
| Years of education, mean (SD) | 11.6 (2.2) | 11.9 (2.0) | 11.2 (2.4) | 0.0023 |
| APOE e4 carrier, n (%)c | 101 (29.7) | 64 (32.2) | 37 (26.2) | 0.2786 |
| Stroke/TIA, n (%) | 58 (16.6) | 25 (12.1) | 33 (22.9) | 0.0087 |
| CAD, n (%) | 55 (15.7) | 26 (12.6) | 29 (20.1) | 0.0728 |
| CHF, n (%) | 69 (19.7) | 27 (13.1) | 42 (29.2) | 0.0003 |
| Hypertension, n (%) | 308 (88.0) | 177 (85.9) | 131 (91.0) | 0.1821 |
| Diabetes, n (%) | 166 (47.6) | 92 (44.7) | 74 (51.7) | 0.2306 |
| Cancer, n (%) | 64 (18.3) | 38 (18.4) | 26 (18.1) | 1.0000 |
| Depression, n (%) | 112 (32.0) | 53 (25.7) | 59 (41.0) | 0.0035 |
| No. of non-ACB meds, mean (SD) | 5.2 (3.6) | 5.0 (3.5) | 5.5 (3.7) | 0.1928 |
| Any ACB 1 yr before baseline, n (%) | 247 (70.6) | 135 (65.5) | 112 (77.8) | 0.0169 |
| TSDD Any ACB 10 yrs before, mean (SD) | 6466 (9103) | 5388 (7969) | 8007 (10,350) | 0.0113 |
| Strong ACB 1 yr before baseline, n (%) | 107 (30.6) | 51 (24.8) | 56 (38.9) | 0.0066 |
| TSDD strong ACB 10 yrs before, mean (SD) | 908 (2601) | 647 (2165) | 1279 (3091) | 0.0355 |
ACB = anticholinergic cognitive burden; APOE = apolipoprotein E; CAD = coronary artery disease (defined as history of myocardial infarction, angioplasty, coronary artery bypass grafting, stent); CHF = congestive heart failure (defined as history of CHF, cardiomyopathy, left ventricular hypertrophy); MCI = mild cognitive impairment, not dementia; SD = standard deviation; TIA = transient ischemic attack; TSDD = total standard daily dose.
Cancer (nonskin): breast, prostate, lymphoma, colon, or lung.
Disease states identified by International Classification of Disease, Ninth Revision, codes, chart review, or patient report of diagnosis.
The p value for difference between MCI and normal cognition groups.
Non–African Americans included 129 whites, 2 biracial, 1 American Indian, and 1 participant indicating “other” as their race.
Due to missing data, sample size for APOE e4 is 340 for total population, 199 in normal cognition, and 141 for MCI.
A total of 978 consecutive diagnostic assessments were completed among 350 participants: 229 (22.7%) with initial MCI diagnoses remained as MCI at the subsequent evaluations; 135 (13.8%) with initial MCI diagnoses reverted to normal cognition; 7 (0.7%) with MCI who transitioned to dementia; 511 (52.2%) with initial normal cognition who remained normal at the subsequent evaluation; and 103 (10.5%) with normal cognition who transitioned to MCI. For the purpose of this analysis, the seven transitions from MCI to dementia (0.7% of all transitions) were included in the stable MCI group. Table 2 presents demographic and clinical characteristics between those who transitioned in cognitive diagnosis and those who remained stable stratified by initial diagnosis.
Table 2.
Participant Characteristics by Outcomes of Successive Transitions
| Initial diagnosis | Normal | p value | MCI | p value | ||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Follow-up diagnosis | Normal, n = 511b | MCI, n = 103b | Normal, n = 135b | MCI, n = 229b | ||
| Age, mean (SD) | 70.8 (4.8) | 72.5 (4.9) | 0.0015 | 70.7 (4.3) | 72.4 (5.7) | 0.0044 |
| Race, n (%) | ||||||
| African American, | 355 (69.5) | 64 (62.1) | 0.9392 | 67 (49.6) | 126 (55.0) | 0.5486 |
| Non-African American | 156 (30.5) | 39 (37.9) | 68 (50.4) | 103 (45.0) | ||
| Sex, n (%) | ||||||
| Female | 421 (82.4) | 84 (81.6) | 0.8498 | 107 (79.3) | 166 (72.5) | 0.2493 |
| Education, yrs, mean (SD) | 12.2 (1.9) | 11.1 (2.1) | <0.0001 | 11.3 (2.4) | 10.9 (2.3) | 0.1057 |
| APOE e4 carrier, n (%) | 153 (31.0) | 33 (32.7) | 0.6991 | 38 (28.6) | 73 (32.3) | 0.5124 |
| Stroke/TIA, n (%) | 56 (11.0) | 15 (14.6) | 0.3203 | 18 (13.3) | 56 (24.5) | 0.0100 |
| CAD, n(%) | 60 (11.7) | 16 (15.5) | 0.2715 | 26 (19.3) | 45 (19.7) | 0.8826 |
| CHF, n (%) | 71 (13.9) | 25 (24.3) | 0.0195 | 39 (28.9) | 68 (29.7) | 0.7615 |
| Hypertension, n (%) | 459 (89.8) | 92 (89.3) | 0.8170 | 121 (89.6) | 197 (86.0) | 0.2046 |
| Diabetes, n (%) | 251 (49.1) | 45 (43.7) | 0.3062 | 67 (49.6) | 97 (42.4) | 0.2229 |
| Cancer, n (%) | 110 (21.5) | 15 (14.6) | 0.1602 | 22 (16.3) | 30 (13.1) | 0.4685 |
| Depression, n (%) | 151 (29.5) | 42 (40.8) | 0.3951 | 54 (40.0) | 108 (47.2) | 0.4583 |
| Number of non-AC meds during transition, mean (SD) | 6.3 (3.6) | 7.1 (3.6) | 0.0430 | 6.7 (3.9) | 6.9 (3.8) | 0.6989 |
| N (%) exposed to any ACB in transition | 387 (75.7) | 84 (81.6) | 0.2965 | 106 (78.5) | 187 (81.7) | 0.3710 |
| N (%) exposed to strong ACB in transition | 150 (29.4) | 42 (40.8) | 0.0166 | 51 (37.8) | 96 (41.9) | 0.3211 |
AC = anticholinergic; ACB = anticholinergic cognitive burden; APOE = apolipoprotein E; CAD = coronary artery disease(defined as history of myocardial infarction, angioplasty, coronary artery bypass grafting, stent); CHF = congestive heart failure (defined as history of CHF, cardiomyopathy, left ventricular hypertrophy); MCI = mild cognitive impairment, not dementia; TIA = transient ischemic attack; SD = standard deviation.
Cancer (nonskin) defined as breast, prostate, lymphoma, colon, or lung.
Disease states identified by International Classification of Disease, Ninth Revision, codes, chart review, or patient report of diagnosis.
n=number of transitions in each group.
Sample size defined by number of transitions rather than number of participants.
Among the total study population, 70.6% were exposed to any ACB medication at baseline, and 30.6% were exposed to at least one strong ACB, with higher exposure in those diagnosed with MCI (Table 1). Table 3 presents the anticholinergics used in any period after the baseline assessment.
Table 3.
Report of Anticholinergic Exposure at Any Wave Following Baseline
| ACB score 1 | Proportion exposed, %a | ACB score 2 | Proportion exposed, %a | ACB score 3 | Proportion exposed, % a |
|---|---|---|---|---|---|
| Alprazolam | 2.0 | Carbamazepine | 1.4 | Amitriptyline | 7.4 |
| Aripiprazole | 0.3 | Cyclobenzaprine | 12.0 | Atropine | 0.6 |
| Atenolol | 10.9 | Oxcarbazepine | 0.3 | Chlorcyclizine | 0.9 |
| Bupropion | 6.6 | Chlorpheniramine | 0.3 | ||
| Captopril | 0.3 | Clemastine | 0.3 | ||
| Cetirizine | 2.6 | Dicyclomine | 2.0 | ||
| Chlorthalidone | 0.6 | Diphenhydramine | 1.1 | ||
| Codeine | 10.6 | Diphenoxylate-Atropine | 0.6 | ||
| Colchicine | 4.3 | Doxepin | 1.1 | ||
| Diazepam | 4.0 | Hydroxyzine | 12.9 | ||
| Digoxin | 4.3 | Meclizine | 8.0 | ||
| Fentanyl | 2.6 | Nortriptyline | 1.7 | ||
| Furosemide | 24.6 | Olanzapine | 0.9 | ||
| Haloperidol | 0.3 | Oxybutynin | 10.0 | ||
| Hydralazine | 11.1 | Paroxetine | 3.1 | ||
| Isosorbide | 10.6 | Promethazine | 7.7 | ||
| Levocetirizine | 0.3 | Propantheline | 0.3 | ||
| Loperamide | 1.1 | Quetiapine | 1.7 | ||
| Loratadine | 1.4 | Scopolamine | 0.3 | ||
| Metoprolol | 42.0 | Solifenacin | 0.3 | ||
| Morphine | 3.1 | Tolterodine | 2.6 | ||
| Nifedipine | 0.6 | Trifluoperazine | 0.3 | ||
| Prednisone | 18.3 | Trihexyphenidyl | 0.3 | ||
| Ranitidine | 17.4 | ||||
| Risperidone | 1.7 | ||||
| Theophylline | 1.1 | ||||
| Trazodone | 17.1 | ||||
| Triamterene-Hydrochlorothiazide | 10.6 | ||||
| Venlafaxine | 1.4 | ||||
| Warfarin | 13.7 |
ACB = anticholinergic cognitive burden.
Medication use reported as proportion of all participants who used the medication at any point after baseline. Denominator for the proportion was 350.
Among participants with an initial diagnosis of normal cognition, use of strong anticholinergics as measured by the TSDD significantly increased the likelihood of transition from normal cognition to MCI compared with stable normal cognition after adjusting for age, sex, race, education, stroke, CHF, and high prior exposure to strong anticholinergics (odds ratio [OR] 1.15, 95% confidence interval [CI] 1.01–1.31, p = 0.0342) (Table 4). Age also significantly increased the transition from normal cognition to MCI (OR 1.07, 95% CI 1.01–1.12, p = 0.0117); whereas education reduced the likelihood of transition from normal cognition to MCI (OR 0.78, 95% CI 0.69–0.88, p < 0.001). All other covariates, including stroke, sex, race, CHF, and high 10-year exposure to strong anticholinergics, did not influence the transition from normal cognition to MCI. Apolipoprotein E e4 did not significantly influence the results; however, when included in the model with TSDD of strong anticholinergics as the independent variable and transition from normal cognition to MCI as the dependent variable, the OR was 1.13 with a 95% CI of 1.00–1.28 (p = 0.0573). Notably, the TSDD of all anticholinergics was not significantly associated with the transition from normal cognition to MCI. A sensitivity analysis excluding missing data for days’ supply and strength from pharmacy fields also showed a significant relationship between increasing exposure to strong anticholinergics and transition from normal cognition to MCI (OR 1.17, 95% CI 1.01–1.34, p = 0.0304).
Table 4.
Results of Logistic Regression Models of the Transition in Cognitive Diagnosis Compared With Stable Diagnosis
| Outcomes | Unadjusted OR | Adjusted OR | Adjusted p valuea |
|---|---|---|---|
| Normal to MCI vs normal to normal | |||
| TSDD: All ACB | 1.18 (0.99–1.42) | 1.12 (0.93–1.36) | 0.2290 |
| TSDD: Strong ACB | 1.16 (1.04–1.29) | 1.15 (1.01–1.31) | 0.0342 |
| MCI to Normal vs MCI to MCI | |||
| TSDD: All ACB | 0.93 (0.82–1.05) | 0.92 (0.81–1.05) | 0.2121 |
| TSDD: Strong ACB | 0.96 (0.87–1.06) | 0.95 (0.86–1.05) | 0.3266 |
ACB = anticholinergic cognitive burden; MCI = mild cognitive impairment; OR = odds ratio; TSDD = total standard daily dose.
Adjusted p value includes age, sex, race, education, history of stroke, history of congestive heart failure, and highest tertile of 10-year TSDD for strong anticholinergics.
Among participants with a diagnosis of MCI, the OR estimates for the reversion to normal cognition were less than one for both measures of anticholinergic exposure suggesting a decreased likelihood of reversion from MCI to normal among anticholinergic users. However, the relationship was not statistically significant. A history of stroke and being male significantly reduced the likelihood of reversion from MCI to normal cognition (OR for stroke 0.43, 95% CI 0.23–0.81, p = 0.0088; OR for male 0.51, 95% CI 0.28–0.94, p = 0.0310).
Discussion
This analysis provides further evidence of the adverse cognitive effects of anticholinergics by showing higher odds of cognitive decline in community-dwelling older adults without dementia. Specifically, we demonstrate that cumulative exposure to strong anticholinergics, as defined by the TSDD, increased the odds of transitioning from normal cognition to MCI, whereas cumulative exposure to strong anticholinergics did not significantly influence the reversion from MCI to normal cognition. Our results show a 15% higher odds of transitioning from normal cognition to MCI among those using a minimally effective dose of a strong anticholinergic every day for 1 year, such as paroxetine 10 mg once/day. Increasing the dose or adding low-dose anticholinergics multiplied the odds: a 20-mg dose of paroxetine once/day for 1 year resulted in 30% higher odds of transitioning to MCI.
One study described a higher odds of dementia among the highest users of strong anticholinergics over 10 years when using the TSDD to quantify cumulative anticholinergic exposure.23 We also recently reported an increasing odds of dementia and MCI with cumulative anticholinergic exposure based on ACB score and duration of use.27 Other studies using anticholinergic drug scales to calculate exposure have used categorical variables (exposed vs unexposed) and sum of ACB scores (0, 1, 2, or 3). Our results support the biological gradient (cumulative exposure) condition of causality in epidemiological studies36 for the relationship between strong anticholinergics and MCI.
The hypothesis that anticholinergics increase β-amyloid and tau proteins to worsen cognition is supported by the findings of higher odds of transition from normal cognition to MCI. This hypothesis would also suggest it is unlikely that de-prescribing (or discontinuing) anticholinergics among current users would be expected to improve cognition immediately. Our results failed to show a statistically significant reduction in the odds of reversion from MCI to normal cognition with increasing exposure to anticholinergics, with one explanation being that the cholinergic pathway has little influence on cognition among older adults in primary care with a diagnosis of MCI. Another explanation from this analysis suggests other factors such as stroke and sex are more likely to influence this transition. To date, no study has demonstrated a reversible effect on cognition by de-prescribing anticholinergics; however, this area of research has not been widely pursued. Only one prospective intervention reducing anticholinergic burden has been published, and it showed that despite a 50% reduction in anticholinergic drug score, no improvement in memory or global cognition was reported after 8 weeks.29 Nearly 70% of participants in the study had at least mild dementia at baseline, and the target for anticholinergic reduction was not restricted to strong anticholinergics. Despite this, the authors reported an improvement in memory scores among a subgroup of five subjects who discontinued all anticholinergics. Beyond this study, no de-prescribing interventions have been conducted in older adults with MCI or in a primary care population at risk of cognitive impairment. De-prescribing interventions are needed to determine whether anticholinergic exposure is permanent or reversible.
Opportunities to prevent or delay dementia can have a significant impact on medical, social, and financial outcomes.37 Delaying the onset of dementia by 1 year is projected to prevent 9.2 million cases of dementia by 2050.38 Therefore, interventions focused on improving cognition or preventing decline are of great interest in the National Alzheimer’s Project Act.39
Strengths of this analysis are the objective measures of medication data and consensus-based diagnosis as the outcome. However, some limitations to the study should be noted. First, use of pharmacy dispensing and claims data does not include use of nonprescription (over-the-counter [OTC]) anticholinergics, such as diphenhydramine and chlorpheniramine. Participants in this study were incentivized to use Eskenazi pharmacies that recorded use of OTC medications in the dispensing database and improved but not assured the capture of OTC medications. Second, use of pharmacy dispensing and claims data as a measure of exposure assumes medications are consumed by participants. Third, unmeasured variables or severity of disease may have influenced our results, a potential source of bias in any observational study. We were, however, able to merge self-report with medical records to capture the presence or absence of diseases that are important in cognitive evaluation including depression, heart disease, and stroke. However, we note the lack of specific diagnoses such as incontinence and insomnia that have been associated with dementia in this analysis and therefore cannot rule out indication bias. Lastly, the limited sample size prevented other approaches to modeling reversibility of anticholinergic adverse effects, such as comparing diagnostic transitions among stoppers and starters of strong anticholinergics. Thus replication of our analysis in larger and more diverse populations would be of interest.
Conclusions
Our results demonstrate that cumulative exposure to strong anticholinergics increased the transition from normal cognition to MCI in community-dwelling older adults without dementia. Strong anticholinergics did not have a statistically significant influence on the reversion from MCI to normal cognition. Interventions preventing or de-prescribing anticholinergic use in community-dwelling older adults without dementia should be tested for potential impact on safely, as well as the ability to prevent or delay the onset of cognitive impairment.
Acknowledgments
Funding: This work was supported by grants from the National Institute of Health: K23AG044440; R01AG026096, R01AG09956, and P30AG10133. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Study concept, acquisition of data, conduct of the collection, management, and analysis of the study data, and interpretation of the results were conducted by all authors. Preparation of the manuscript was conducted by Dr. Campbell and Ms. Lane, with revisions and approval performed by Drs. Campbell, Gao, Unverzagt, and Ms. Lane. Dr. Campbell and Ms. Lane had full access to the data in the study and take responsibility for the integrity and the accuracy of the data analysis.
Footnotes
Conflict of interest: The authors have declared no conflicts of interest for this article.
Meeting presentation: Presented at the Alzheimer’s Association International Conference, London, UK, July 16–20, 2017.
References
- 1.Castelino RL, Hilmer SN, Bajorek BV, Nishtala P, Chen TF. Drug Burden Index and potentially inappropriate medications in community-dwelling older people: the impact of Home Medicines Review. Drugs Aging. 2010;27:135–48. doi: 10.2165/11531560-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 2.Campbell NL, Boustani MA, Lane KA, et al. Use of anticholinergics and the risk of cognitive impairment in an African American population. Neurology. 2010;75:152–9. doi: 10.1212/WNL.0b013e3181e7f2ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Society AG. Updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63:2227–46. doi: 10.1111/jgs.13702. [DOI] [PubMed] [Google Scholar]
- 4.CMS. [Accessed February 26, 2018];CMS Manual System [online] Available at http://www.cms.gov/Regulations-and-Guidance/Guidance/Transmittals/downloads/rl9soma.pdf.
- 5.Feiton M, Hanlon JT, Perera S, Thorpe JM, Marcum ZA. Racial differences in anticholinergic use among community-dwelling elders. Consult Pharm. 2015;30:240–5. doi: 10.4140/TCP.n.2015.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sumukadas D, McMurdo ME, Mangoni AA, Guthrie B. Temporal trends in anticholinergic medication prescription in older people: repeated cross-sectional analysis of population prescribing data. Age Ageing. 2014;43:515–21. doi: 10.1093/ageing/aft199. [DOI] [PubMed] [Google Scholar]
- 7.Mesulam M-M, Guillozet A, Shaw P, Levey A, Duysen E, Lockridge O. Acetylcholinesterase knockouts establish central cholinergic pathways and can use butyrylcholinesterase to hydrolyze acetylcholine. Neuroscience. 2002;110:627–39. doi: 10.1016/s0306-4522(01)00613-3. [DOI] [PubMed] [Google Scholar]
- 8.Everitt BJ, Robbins TW. Central cholinergic systems and cognition. Annu Rev Psychol. 1997;48:649–84. doi: 10.1146/annurev.psych.48.1.649. [DOI] [PubMed] [Google Scholar]
- 9.Scheiderer CL, McCutchen E, Thacker EE, et al. Sympathetic sprouting drives hippocampal cholinergic reinnervation that prevents loss of a muscarinic receptor-dependent long-term depression at CA3–CA1 synapses. J Neurosci. 2006;26:3745–56. doi: 10.1523/JNEUROSCI.5507-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perry EK, Kilford L, Lees AJ, Burn DJ, Perry RH. Increased Alzheimer pathology in Parkinson’s disease related to antimuscarinic drugs. Ann Neurol. 2003;54:235–8. doi: 10.1002/ana.10639. [DOI] [PubMed] [Google Scholar]
- 11.del Pino J, Zeballos G, Anadón MJ, et al. Cadmium-induced cell death of basal forebrain cholinergic neurons mediated by muscarinic M1 receptor blockade, increase in GSK-3β enzyme, β-amyloid and tau protein levels. Arch Toxicol. 2016;90:1081–92. doi: 10.1007/s00204-015-1540-7. [DOI] [PubMed] [Google Scholar]
- 12.Billings LM, Oddo S, Green KN, McGaugh JL, LaFerla FM. Intraneuronal Aβ causes the onset of early Alzheimer’s disease-related cognitive deficits in transgenic mice. Neuron. 2005;45:675–88. doi: 10.1016/j.neuron.2005.01.040. [DOI] [PubMed] [Google Scholar]
- 13.Caccamo A, Oddo S, Billings LM, et al. M1 receptors play a central role in modulating AD-like pathology in transgenic mice. Neuron. 2006;49:671–82. doi: 10.1016/j.neuron.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 14.Oddo S, Caccamo A, Shepherd JD, et al. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Aβ and synaptic dysfunction. Neuron. 2003;39:409–21. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 15.Geula C. Abnormalities of neural circuitry in Alzheimer’s disease Hippocampus and cortical cholinergic innervation. Neurology. 1998;51:S18–29. doi: 10.1212/wnl.51.1_suppl_1.s18. [DOI] [PubMed] [Google Scholar]
- 16.Sivaprakasam K. Towards a unifying hypothesis of Alzheimer’s disease: cholinergic system linked to plaques, tangles and neuroinflammation. Curr Med Chem. 2006;13:2179–88. doi: 10.2174/092986706777935203. [DOI] [PubMed] [Google Scholar]
- 17.Bierer LM, Haroutunian V, Gabriel S, et al. Neurochemical correlates of dementia severity in Alzheimer’s disease: relative importance of the cholinergic deficits. J Neurochem. 1995;64:749–60. doi: 10.1046/j.1471-4159.1995.64020749.x. [DOI] [PubMed] [Google Scholar]
- 18.Risacher SL, McDonald BC, Tallman EF, et al. Association between anticholinergic medication use and cognition, brain metabolism, and brain atrophy in cognitively normal older adults. JAMA Neurol. 2016;73:721–32. doi: 10.1001/jamaneurol.2016.0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fox C, Smith T, Maidment I, et al. Effect of medications with anti-cholinergic properties on cognitive function, delirium, physical function and mortality: a systematic review. Age Ageing. 2014;43:604–15. doi: 10.1093/ageing/afu096. [DOI] [PubMed] [Google Scholar]
- 20.Fox C, Richardson K, Maidment ID, et al. Anticholinergic medication use and cognitive impairment in the older population: the medical research council cognitive function and ageing study. J Am Geriatr Soc. 2011;59:1477–83. doi: 10.1111/j.1532-5415.2011.03491.x. [DOI] [PubMed] [Google Scholar]
- 21.Ancelin ML, Artero S, Portet F, Dupuy AM, Touchon J, Ritchie K. Non-degenerative mild cognitive impairment in elderly people and use of anticholinergic drugs: longitudinal cohort study. BMJ. 2006;332:455–9. doi: 10.1136/bmj.38740.439664.DE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cai X, Campbell N, Khan B, Callahan C, Boustani M. Long-term anticholinergic use and the aging brain. Alzheimers Dement. 2013;9:377–85. doi: 10.1016/j.jalz.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gray SL, Anderson ML, Dublin S, et al. Cumulative use of strong anticholinergics and incident dementia: a prospective cohort study. JAMA Intern Med. 2015;175:401–7. doi: 10.1001/jamainternmed.2014.7663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sittironnarit G, Ames D, Bush AI, et al. Effects of anticholinergic drugs on cognitive function in older Australians: results from the AIBL study. Dement Geriatr Cogn Disord. 2011;31:173–8. doi: 10.1159/000325171. [DOI] [PubMed] [Google Scholar]
- 25.Nishtala PS, Narayan SW, Wang T, Hilmer SN. Associations of drug burden index with falls, general practitioner visits, and mortality in older people. Pharmacoepidemiol Drug Saf. 2014;23:753–8. doi: 10.1002/pds.3624. [DOI] [PubMed] [Google Scholar]
- 26.Bottiggi KA, Salazar JC, Yu L, et al. Long-term cognitive impact of anticholinergic medications in older adults. Am J Geriatr Psychiatry. 2006;14:980–4. doi: 10.1097/01.JGP.0000224619.87681.71. [DOI] [PubMed] [Google Scholar]
- 27.Campbell NL, Perkins AJ, Bradt P, et al. Association of anticholinergic burden with cognitive impairment and health care utilization among a diverse ambulatory older adult population. Pharmacotherapy. 2016;36:1123–31. doi: 10.1002/phar.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao S, Unverzagt FW, Hall KS, et al. Cognitive impairment, incidence, progression, and reversion: findings from a community-based cohort of elderly African Americans. Am J Geriatr Psychiatry. 2014;22:670–81. doi: 10.1016/j.jagp.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kersten H, Molden E, Tolo IK, Skovlund E, Engedal K, Wyller TB. Cognitive effects of reducing anticholinergic drug burden in a frail elderly population: a randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2013;68:271–8. doi: 10.1093/gerona/gls176. [DOI] [PubMed] [Google Scholar]
- 30.Fox C, Livingston G, Maidment ID, et al. The impact of anticholinergic burden in Alzheimer’s dementia—the LASER-AD study. Age Ageing. 2011;40:730–5. doi: 10.1093/ageing/afr102. [DOI] [PubMed] [Google Scholar]
- 31.McDonald CJ, Overhage JM, Tierney WM, et al. The Regenstrief Medical Record System: a quarter century experience. Int J Med Inform. 1999;54:225–53. doi: 10.1016/s1386-5056(99)00009-x. [DOI] [PubMed] [Google Scholar]
- 32.Boustani MCN, Munger S, Maidment I, Fox C. The impact of anticholinergics on the aging brain: a review and practical application. Aging Health. 2008;4:311–20. [Google Scholar]
- 33.Campbell NL, Maidment I, Fox C, Khan BA, Boustani MA. The 2012 Update to the Anticholinergic Cognitive Burden Scale. J Am Geriatr Soc. 2013;61:S142–3. [Google Scholar]
- 34.Semla TP, Beizer JL, Higbee MD. Geriatric Dosage Handbook. 21. Hudson, OH: Lexicomp; 2016. [Google Scholar]
- 35.Gray SL, Dublin S, Yu O, et al. Benzodiazepine use and risk of incident dementia or cognitive decline: prospective population based study. BMJ. 2016;352:i90. doi: 10.1136/bmj.i90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hill AB. The environment and disease: association or causation? Proc R Soc Med. 1965;58:295–300. doi: 10.1177/003591576505800503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alzheimer’s Association. 2016 Alzheimer’s disease facts and figures. Alzheimer Dement. 2016;12:459–509. doi: 10.1016/j.jalz.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 38.Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. Forecasting the global burden of Alzheimer’s disease. Alzheimer Dement. 2007;3:186–91. doi: 10.1016/j.jalz.2007.04.381. [DOI] [PubMed] [Google Scholar]
- 39.National Plan to Address Alzheimer’s Disease: 2016 Update. US Department of Health and Human Services; 2016. [Google Scholar]