SUMMARY
Barrett's esophagus is a well-recognized risk factor for esophageal adenocarcinoma. The natural history of Barrett's esophagus classified as ‘indefinite for dysplasia’ (IND) is poorly characterized. The aim of this study is to characterize the natural history of IND by determining the rate of neoplastic progression and identifying risk factors for progression. Patients from the University of Pennsylvania Health System pathology database and Barrett's esophagus registry with a diagnosis of IND between 2000 and 2014 were identified. Exclusion criteria included: (1) prior diagnosis of low-grade dysplasia (LGD), high-grade dysplasia (HGD), or esophageal adenocarcinoma (EAC); (2) presence of LGD, HGD, or EAC at the time of diagnosis of IND; and (3) lack of follow-up endoscopy after diagnosis. Patients with neoplastic progression were classified as having either prevalent disease (LGD, HGD, or EAC on surveillance biopsy within 12 months of IND diagnosis) or incident disease (LGD, HGD, or EAC on surveillance biopsy >12 months after IND diagnosis). One hundred six patients were eligible for analysis. Of 87 patients with follow-up endoscopy and biopsies within 1 year of IND diagnosis, 7 (8%) had prevalent disease (2 LGD, 4 HGD, 1 EAC). The prevalence of LGD was 2.3%, HGD was 4.6%, and EAC was 1.1%. Importantly, four of the seven prevalent (2 LGD, 2 HGD) cases were found to have dysplasia within 6 months of IND diagnosis. No demographic or endoscopic characteristics studied were associated with prevalent disease. Of the 106 IND patients, there were 66 patients without prevalent dysplasia with >1-year follow-up. Three (4.5%) progressed (1 to LGD after 12 months, 2 to HGD after 16.5 and 28 months), yielding an incidence rate for any dysplasia of 1.4 cases/100 person-years and HGD/EAC of 0.9/100 person-years. Risk factors for incident disease were smoking (p = 0.02) and Barrett's esophagus segment length (p = 0.03). IND is associated with considerable risk of prevalent dysplasia, especially within the first 6 months after diagnosis. However, the incidence of HGD/EAC is low and similar to previous studies of IND. These data suggest that IND patients should have repeat endoscopy within 6 months with careful surveillance protocols. Longer BE length and smoking history may help predict which patients are more likely to develop dysplasia, and therefore identify patients who may warrant even closer monitoring.
Keywords: Barrett's esophagus, esophageal adenocarcinoma, indefinite for dysplasia
INTRODUCTION
Esophageal cancer accounts for 1.1% of all new cancer cases in the United States, and it is estimated that in 2015, there will have been 16,890 new cases and 15,590 deaths from esophageal cancer.1 Adenocarcinoma is the predominant histologic type of esophageal cancer in the United States, and Barrett's esophagus is the only known precursor lesion. Furthermore, incidence and mortality rates for esophageal adenocarcinoma continue to increase.2
Degree of dysplasia remains the best clinically available marker of cancer risk in patients with Barrett's esophagus and therefore currently determines both surveillance intervals and treatment strategies. Dysplasia in Barrett's esophagus has been classified in a five-tier system: non-dysplastic Barrett's esophagus (NDBE), indefinite for dysplasia (IND), low-grade dysplasia (LGD), high-grade dysplasia (HGD), and intramucosal carcinoma. ‘Indefinite for dysplasia’ is defined as ‘epithelial abnormalities insufficient to diagnose dysplasia or epithelial abnormalities that are unclear due to inflammation or sampling.’3
While there is substantial literature on progression of NDBE, LGD, and HGD, there is a paucity of data to guide the management of Barrett's esophagus IND. The aim of this study is to further characterize the natural history of IND by determining the rate of neoplastic progression and identifying risk factors for progression.
MATERIALS AND METHODS
Patient population
Patients from the University of Pennsylvania Health System pathology database and Barrett's esophagus registry with a histopathologic diagnosis of IND between 2000 and 2014 were identified.
Both patients who were diagnosed with IND in the University of Pennsylvania Health System as well as patients who were referred from other centers were included in the study. All referral cases to the University of Pennsylvania were reviewed by at least two gastrointestinal pathologists, although slides were not re-reviewed for the purpose of this study.
Patients were excluded if they had a prior diagnosis of any dysplasia or esophageal adenocarcinoma, if they had biopsy-proven dysplasia or esophageal adenocarcinoma concurrent to the IND diagnosis, or if they did not have follow-up endoscopy with biopsy after IND diagnosis. We obtained appropriate approval for this study from the Institutional Review Board of the University of Pennsylvania.
Demographic and clinical variables
Electronic medical record data were collected on demographic variables and endoscopic characteristics, including age, gender, body mass index (BMI), smoking history, proton pump inhibitor (PPI) use, nonsteroidal anti-inflammatory drug (NSAID) use, family history of Barrett's esophagus or esophageal adenocarcinoma, presence of hiatal hernia, length of BE segment, presence of mucosal nodularity, and presence of multifocal IND. Multifocal IND was defined as presence of IND in biopsies from different levels in the esophagus.
Outcome measures
Patients with neoplastic progression were classified as having either prevalent neoplasia (LGD, HGD, or EAC on surveillance biopsies within 12 months of IND diagnosis) or incident neoplasia (LGD, HGD, or EAC on surveillance biopsy more than 12 months following IND diagnosis).
Statistical analysis
Patients were divided into two groups: those who underwent follow-up endoscopy and biopsies within 12 months of IND diagnosis (prevalent dysplasia) and those who did not have prevalent dysplasia and had follow-up endoscopy and biopsies more than 12 months after IND diagnosis (incident dysplasia).
In the first group, the association of prevalent dysplasia with patient characteristics was assessed with Fisher's exact and Wilcoxon rank sum tests. In the second group, the univariate association of patient characteristics and progression rate to incident dysplasia (LGD, HGD, or EAC) and advanced neoplasia (HGD or EAC) was determined using the log-rank test for categorical data and the Cox proportional hazards likelihood ratio test for interval data. Statistical analysis was performed using the SAS 9.4.
RESULTS
A total of 425 patients with IND were identified within the study period, of whom 319 patients were excluded due to prior or concurrent diagnosis of dysplasia, lack of follow up endoscopy, or insufficient records leaving 106 patients eligible for analysis (Fig. 1). Patient demographics are shown in Table 1. Of the 106 patients analyzed, 40 patients were on twice daily PPI at the time of IND diagnosis, 64 patients were on once daily PPI at the time of IND diagnosis, and 2 patients did not have information on PPI dose in the electronic medical record. Of note, 19 of the 106 patients analyzed had findings of mucosal irregularity on endoscopy.
Fig. 1.
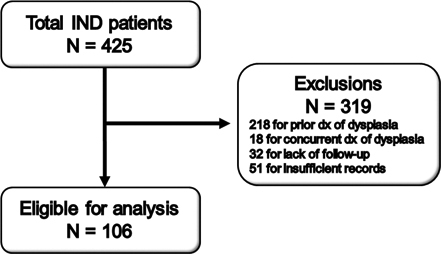
Flowchart showing identification of patients with indefinite for dysplasia (IND) for study inclusion and exclusions.
Table 1.
Study population characteristics
| Parameter | Total n = 106 |
|---|---|
| Gender | |
| Female | 37 (34.9%) |
| Male | 69 (65.1%) |
| Age (median/IQR) | 62 (54–69) |
| Smoker | |
| Current | 8 (7.5%) |
| Former | 37 (34.9%) |
| Never | 60 (56.6%) |
| PPI Use | |
| Yes | 93 (87.7%) |
| No | 12 (11.3%) |
| Aspirin/NSAID use | |
| Yes | 49 (46.2%) |
| No | 55 (51.9%) |
| BMI (median/IQR) | 27 (25–33) |
| Family history | |
| Yes | 11 (10.4%) |
| No | 83 (78.3%) |
| BE length | |
| ≥3 cm | 41 (38.7%) |
| <3 cm | 61 (57.5%) |
| Hiatal hernia present | |
| Yes | 70 (66.0%) |
| No | 27 (25.5%) |
| Hiatal hernia length (median/IQR) | 1 (0–3) |
| Mucosal irregularity | |
| Yes | 19 (17.9%) |
| No | 78 (73.6%) |
| Multifocal IND | |
| Yes | 17 (16.0%) |
| No | 39 (36.8%) |
BMI, body mass index; IND, indefinite for dysplasia; IQR, interquartile range; NSAID, nonsteroidal anti-inflammatory drug.
Prevalent disease
Of the 87 patients who had a follow-up endoscopy with biopsy within 1 year of IND diagnosis, seven (8.0%) had prevalent dysplasia (Fig. 2). Five (5.7%) were diagnosed with HGD/EAC within the first year. Of these five patients, one patient was found to have LGD at six months and subsequently found to have HGD at 12 months, and a second patient was diagnosed with HGD at six months, underwent endoscopic mucosal resection, and then was subsequently diagnosed with EAC at 12 months. The remaining two patients with prevalent disease (2.3%) were found to have LGD, one at six months following IND diagnosis and one at nine months. The time line of disease progression is shown in Figure 3. Importantly, four out of the seven cases of prevalent dysplasia were found within six months of diagnosis of IND. There were no demographic or endoscopic characteristics associated with prevalent disease.
Fig. 2.
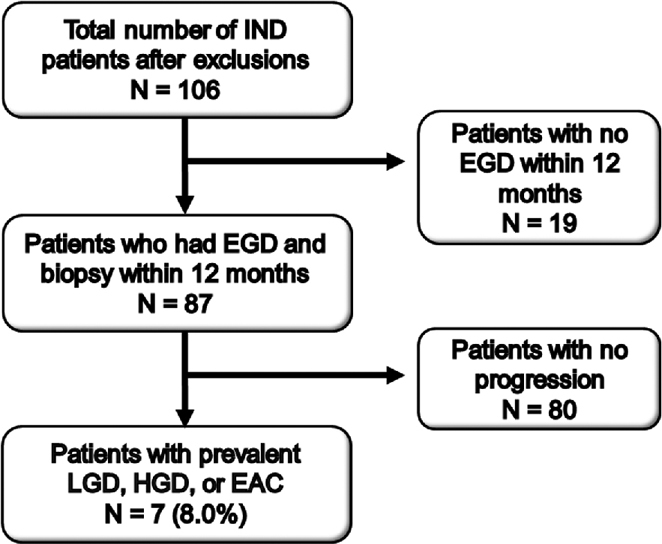
Flowchart showing identification of indefinite for dysplasia (IND) patients who underwent repeat endoscopy within 12 months of IND diagnosis and were subsequently found to have prevalent low-grade dysplasia (LGD), high-grade dysplasia (HGD), or esophageal adenocarcinoma (EAC).
Fig. 3.
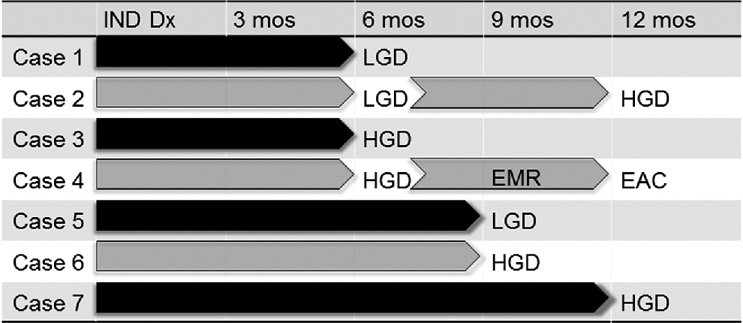
Timeline of progression from time of indefinite for dysplasia (IND) diagnosis to low-grade dysplasia (LGD), high-grade dysplasia (HGD), and esophageal adenocarcinoma (EAC) for the seven cases of prevalent dysplasia. Note: Case 2 was found to have HGD at six months after IND diagnosis, and then was found to have HGD on repeat endoscopy at 12 months after IND diagnosis. Case 4 was found to have HGD at six months, underwent endoscopic mucosal resection, and then at 12 months was found to have EAC.
Incident disease
Of the total 106 IND patients, there were 66 patients without prevalent dysplasia who had a follow-up endoscopy more than 1 year after IND diagnosis. These 66 patients were followed for a median of 32 months (range 12 to 160 months). Three (4.5%) progressed (one to LGD after 12 months, two to HGD after 16.5 and 28 months), yielding an incidence rate for any dysplasia of 1.4 cases per 100 person-years and 0.9 cases per 100 person-years for the combined endpoint of HGD/EAC, although there were no cases of incident EAC during the study period (Figs 4,5). Of note, two out of the three patients with incident dysplasia did not have a repeat endoscopy with biopsy within 1 year of IND diagnosis, therefore could have had prevalent dysplasia that was missed.
Fig. 4.
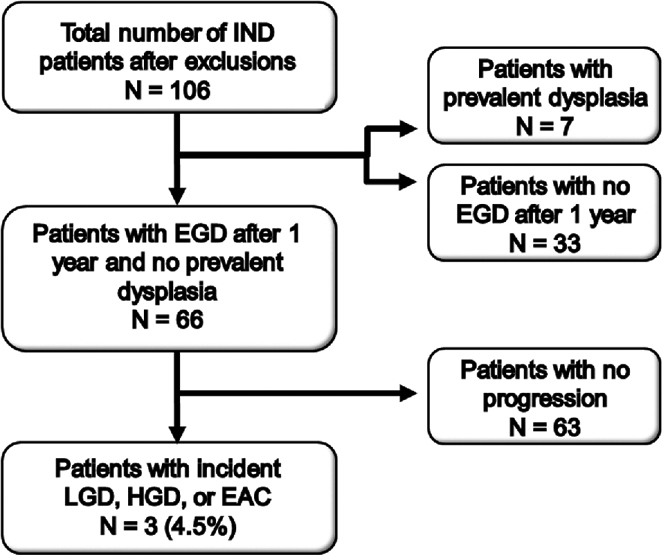
Flowchart showing identification of indefinite for dysplasia (IND) patients who did not have prevalent dysplasia and who underwent repeat endoscopy after greater than 12 months of IND diagnosis and were subsequently found to have incident low-grade dysplasia (LGD), high-grade dysplasia (HGD), or esophageal adenocarcinoma (EAC).
Fig. 5.
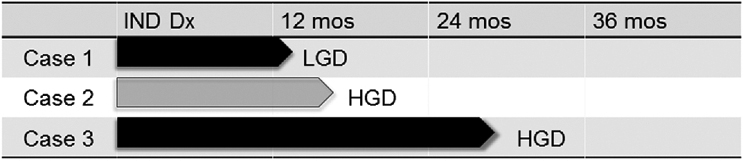
Timeline of progression from time of indefinite for dysplasia (IND) diagnosis to low-grade dysplasia (LGD), high-grade dysplasia (HGD), and esophageal adenocarcinoma (EAC) for the three cases of incident dysplasia. Case 1 progressed to LGD at 12 months, Case 2 progressed to HGD at 16.5 months, and Case 3 progressed to HGD at 28 months.
Patients who developed incident dysplasia had a longer BE segment length (p = 0.025) and were more likely to report a smoking history (p = 0.021) than patients who did not progress. No other clinical, pathologic, or endoscopic abnormalities were associated with incident disease.
DISCUSSION
In our study of Barrett's esophagus patients with IND, 8% were found to have dysplasia and 5.7% were found to have HGD or EAC within 1 year of IND diagnosis. Furthermore, over half of patients with prevalent dysplasia were identified within only six months of IND diagnosis. After excluding cases of prevalent disease, the risk of progression was 1.4 cases per 100 person-years for any dysplasia or EAC and 0.9 cases per 100 person-years for HGD/EAC.
In contrast to the extensive published literature on low-grade and high-grade dysplasia, there are surprisingly little data on the natural history of Barrett's esophagus IND. Early studies reported progression rates of IND to adenocarcinoma as high as 18%.4–6 However, several recent studies including our own suggest that progression risk is lower than previously thought.
Horvath et al. reported a prevalence of HGD/EAC of 4.7% and an incidence rate for HGD/EAC of 1.2 cases per 100 person-years, which is in line with our results indicating a higher risk of progression during the first year following IND diagnosis.7 A recently published multicenter cohort study by Sinh et al. reported a progression rate of 0.86 cases per 100 person-years for HGD/EAC, but did not separate prevalent from incident cases.8 Of note, while the population examined by Sinh et al. included the subset of patients in our study from the University of Pennsylvania Health System Barrett's esophagus registry, the current study also included patients from the larger health system's pathology database that identified additional patients not included in the registry or in the Sinh study. The largest study of IND progression to date is a nationwide cohort study from the Netherlands, which reviewed 842 patients with IND and reported an incidence rate for HGD/EAC of 1.4 cases per 100 person-years.9 These studies have produced results similar to our incidence rate for HGD/EAC of 0.9 cases per 100 person-years.
We also studied possible risk factors for progression of disease in hopes of identifying patients who may warrant closer endoscopic monitoring. Our data indicate that smoking history and the length of BE segment are associated with higher risk of incident disease, whereas age, gender, BMI, PPI use, NSAID use, family history of BE or EAC, presence of hiatal hernia, presence of mucosal nodularity, and presence of multifocal IND were not associated with progression. Prior studies have also found an association between progression and the BE segment length.7,10 While our study did not find an association between the BE segment length and prevalent disease, the small number of patients who were found to have prevalent disease limits our analysis of risk association. Other groups identified age8,9 and multifocal IND4,7,10 as risk factors for progression, which was not the case in our study.
This uncertainty in the natural history of IND is reflected in the variability of current practice guidelines. Current guidelines from the American Gastroenterological Association do not address IND.11 The American Society for Gastrointestinal Endoscopy12 and the British Society of Gastroenterology guidelines13 include recommendations for optimization of antisecretory therapy followed by repeat endoscopy, although with only low-quality supporting evidence. More recently, the international BOBCAT (Benign Barrett's and Cancer Taskforce) group released consensus guidelines using a Delphi process with an 80% agreement threshold, in which they define IND as an interim diagnosis and recommended repeat endoscopy with biopsy within 12 months, although with very low-quality evidence.14 New guidelines from the American College of Gastroenterology now recommend optimization of acid suppression for 3–6 months followed by repeat endoscopy, then a surveillance interval of 12 months if IND is confirmed, albeit also based on low quality evidence.15
Based on the finding of recent studies including our own of a substantial risk of prevalent dysplasia, we would recommend the following management strategy for IND (Fig. 6): if IND is found, it should first be reviewed by two gastrointestinal pathologists. It would then be reasonable to optimize acid suppression with twice daily PPI therapy and repeat endoscopy within 6 months. If the diagnosis of IND is confirmed, we would recommend a surveillance interval of 12 months.
Fig. 6.
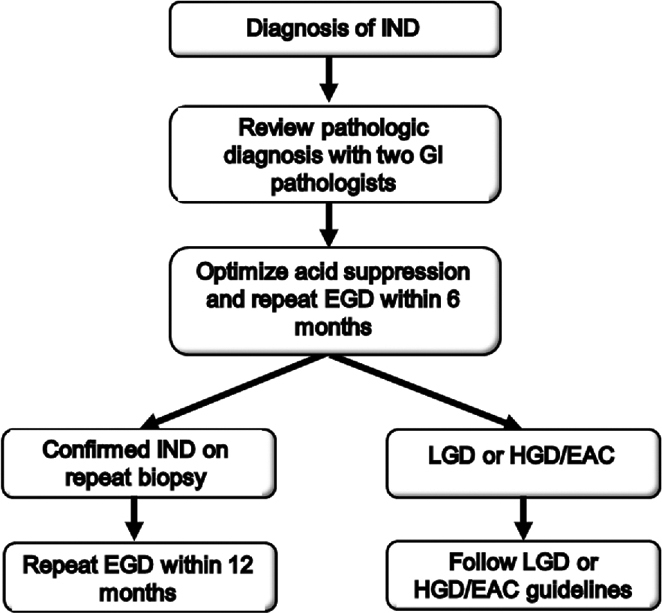
Suggested management strategy for indefinite for dysplasia (IND).
Our study includes one of the largest cohorts of IND patients in the United States to date, and by dividing the study population into two groups, we were able to distinguish between prevalent and incident dysplasia. We readily acknowledge limitations of our study. The study population was taken from a single tertiary referral center; therefore, results may not be generalizable to all IND patients. Furthermore, not all patients included in the study had all of the relevant demographic and endoscopic data in the electronic medical record, which somewhat limited analysis of possible risk factors. The small number of patients who progressed to incident dysplasia also limits our analysis of progression risk, although our incidence rate for HGD/EAC is similar to prior studies. Additionally, this study was limited by a median follow-up duration of 32 months, although our results are similar to other recent studies with longer follow-up durations. Finally, these slides were not re-reviewed to develop a consensus diagnosis by a single reader, although all IND specimens in our institution are routinely examined by two gastrointestinal pathologists. Of note, 19 of the 106 patients analyzed had findings of mucosal irregularity on endoscopy, and it is possible that pathologic diagnosis may have been affected by active inflammation. Fourteen were found to have non-dysplastic BE on follow-up biopsy, two had persistent IND on follow-up biopsy, and three had HGD/EAC on follow-up biopsy.
In conclusion, our study suggests that IND is an important diagnostic category that carries significant risk of prevalent dysplasia, particularly within the first six months of diagnosis. Risk of progression to advanced neoplasia after the first year following IND diagnosis is lower than previously thought and similar to other recent studies. Longer BE segment and smoking history may help predict which IND patients are more likely to progress to HGD/EAC and therefore identify patients who may warrant closer monitoring. Better objective markers for disease progression are needed for accurate risk stratification for IND to optimize patient management in the future.
Acknowledgments
This work was supported in part by the NIH/NCI U54-CA163004, NIH/NIDDK P30-DK050306 and its core facilities (Molecular Pathology and Imaging Core, Molecular Biology/Gene Expression Core, Transgenic and Chimeric Mouse Core, and Cell Culture Core), NIH/NCI P01-CA098101 and institutional funds.
References
- 1. Howlader N, Noone A M, Krapcho M et al. SEER Cancer Statistics Review, 1975–2012. Bethesda, MD: National Cancer Institute, 2015. [Google Scholar]
- 2. Hur C, Miller M, Kong C Y et al. Trends in esophageal adenocarcinoma incidence and mortality. Cancer 2013; 119.6: 1149–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schlemper R J, Riddell R H, Kato Y E et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut 2000; 47: 251–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Montgomery E, Goldblum J R, Greenson J K et al. Dysplasia as a predictive marker for invasive carcinoma in Barrett esophagus: a follow-up study based on 138 cases from a diagnostic variability study. Hum Pathol 2001; 32: 379–8. [DOI] [PubMed] [Google Scholar]
- 5. Younes M, Lauwers G Y, Ertan A et al. The significance of “indefinite for dysplasia” grading in Barrett metaplasia. Arch Pathol Lab Med 2011; 135: 430. [DOI] [PubMed] [Google Scholar]
- 6. Sonwalkar S A, Rotimi O, Scott N et al. A study of indefinite for dysplasia in Barrett's oesophagus: reproducibility of diagnosis, clinical outcomes and predicting progression with AMACR (α methylacyl CoA racemase). Histopathology 2010; 56: 900–7. [DOI] [PubMed] [Google Scholar]
- 7. Horvath B, Singh P, Xie H et al. Risk for esophageal neoplasia in Barrett's esophagus patients with mucosal changes indefinite for dysplasia. J Gastroenterol Hepatol 2015; 30: 262–7. [DOI] [PubMed] [Google Scholar]
- 8. Sinh P, Anaparthy R, Young P E et al. Clinical outcomes in patients with a diagnosis of" indefinite for dysplasia" in Barrett's esophagus: a multicenter cohort study. Endoscopy 2015; 47.8: 669–4. [DOI] [PubMed] [Google Scholar]
- 9. Kestens C, Leenders M, Offerhaus G J, van Baal J W, Siersema P D. Risk of neoplastic progression in Barrett's esophagus diagnosed as indefinite for dysplasia: a nationwide cohort study. Endoscopy 2015; 47: 409–14. [DOI] [PubMed] [Google Scholar]
- 10. Thota P N, Lee H J, Goldblum J R et al. Risk stratification of patients with Barrett's esophagus and low-grade dysplasia or indefinite for dysplasia. Clin Gastroenterol Hepatol 2015; 13: 45965. [DOI] [PubMed] [Google Scholar]
- 11. American Gastroenterological Association American Gastroenterological Association medical position statement on the management of Barrett's esophagus. Gastroenterology 2011; 140: 1084–91. [DOI] [PubMed] [Google Scholar]
- 12. Evans J A, Early D S, Fukami N et al. The role of endoscopy in Barrett's esophagus and other premalignant conditions of the esophagus. Gastrointest Endosc 2012; 76: 1087–94. [DOI] [PubMed] [Google Scholar]
- 13. Fitzgerald R C, di Pietro M, Ragunath K et al. British society of gastroenterology guidelines on the diagnosis and management of Barrett's oesophagus. Gut 2014; 63: 7–42. [DOI] [PubMed] [Google Scholar]
- 14. Bennett C, Moayyedi P, Corley D A et al. BOB CAT: a large-scale review and Delphi consensus for management of Barrett's esophagus with no dysplasia, indefinite for, or low-grade dysplasia. Am J Gastroenterol 2015; 110: 662–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shaheen N J, Falk G W, Iyer P G, Gerson L. ACG clinical guideline: diagnosis and management of Barrett's esophagus. Am J Gastroenterol 2016; 111: 30–50. [DOI] [PMC free article] [PubMed] [Google Scholar]


