Abstract
Aim: The aim of this study was to compare vascularity observed using contrast-enhanced 3D ultrasonography and pathological changes in human hepatocellular carcinoma (HCC) and surrounding non-tumorous areas after sorafenib treatment.
Materials and methods: Twelve patients with HCC were enrolled in this clinical study. The maximum tumor diameter as measured using sonography ranged from 15 to 33 mm (mean, 24.0 mm; SD, 5.7 mm). Assessments using contrast-enhanced (0.2 mL of Sonazoid suspension; Daiichi Sankyo, Tokyo, Japan) 3D ultrasonography (LOGIQ 7; GE Healthcare, Milwaukee) were performed in all the patients before and 1 week after sorafenib treatment. The microvessel density (MVD) of the HCC and surrounding non-tumorous area was evaluated based on the immunohistochemical staining of microvessels using an antigen for CD34.
Results: Blood flow in the tumor was decreased in all 12 cases after sorafenib treatment. The MVD of the tumorous area at 1 week after sorafenib administration (38.8 ± 5.2) was significantly lower than that observed before sorafenib administration (72.4 ± 13.0) (P < 0.01). Blood flow in the non-tumorous area had decreased in 6 cases at 1 week after sorafenib treatment and had not changed in the 6 other cases. In the reduced blood flow group, the MVD of the non-tumorous area at 1 week after sorafenib administration had decreased significantly, compared with the MVD of the non-tumorous area before sorafenib administration. However, in the group with no change in blood flow, the MVD of the non-tumorous area at 1 week after sorafenib treatment had not changed, compared with the MVD of the non-tumorous area before sorafenib treatment.
Conclusion: Contrast-enhanced 3D ultrasonography studies showed a correlation between vascularity and pathological changes in human HCC and the surrounding non-tumorous area after sorafenib treatment.
Keywords: sorafenib, hepatocellular carcinoma, contrast-enhanced 3D ultrasonography
Introduction
Nutrients are transported to tumor cells through tumor capillaries, so angiogenesis is an important step in tumorigenesis 1, and the development of new tumor capillaries is necessary for rapid tumor growth 2. Sorafenib inhibits two major angiogenesis pathways involving vascular endothelial growth factor receptor 2 (VEGFR2) and platelet-derived growth factor receptor (PDGFR) 3,4,5. Sorafenib has also been shown to confer a survival benefit in patients with advanced HCC 6.
Chen et al. evaluated blood flow in HCC using CT perfusion imaging 7. Dynamic contrast-enhanced MRI 8 and diffusion MRI imaging 9 have also been used to evaluate blood flow in HCC. Ultrasonography can depict tumor morphology, and the Doppler method can be used to analyze vascularity. Recently, enhanced image resolution using an increased probe frequency (13 MHz) has enabled the in vivo visualization of neovascularization in abundantly vascularized tumors, such as melanomas in animals 10 or humans 11. Rathmell et al. 12 studied antiangiogenic effects on renal cell carcinoma using ultrasound molecular imaging. Contrast-enhanced ultrasonography using microbubbles can be used to analyze changes in vascularity 13-15. Yoshida et al. 16 evaluated the relationship between arterial-phase contrast-enhanced ultrasonography findings for tumor parenchymal tissues and the results of pathological examinations after anti-angiogenesis treatment; they reported a reduction in tumor vessel density in some tumor areas in a rabbit model. Mertense et al. 17 also evaluated the effects of sorafenib when combined with radiofrequency ablation treatment in rat liver tissue after radiofrequency ablation (RFA) or a sham puncture with concomitant sorafenib administration; using Ki67 and CD31 staining, they showed a reduction in cell proliferation and microvessel density on days 1 and 3 after sorafenib treatment and concluded that sorafenib transiently promotes necrosis after radiofrequency ablation in rat liver. In a previous study, we compared the results of ablation for human HCC after RFA alone and after RFA plus sorafenib and reported that the area of ablation was significantly larger in the RFA plus sorafenib group 18. However, the relationship between vascularity findings obtained using contrast-enhanced ultrasonography and the results of pathological examinations of human tumors has not been previously reported.
The aim of this study was to compare vascularity observed using contrast-enhanced 3D ultrasonography and pathological changes in human HCC after 1 week of sorafenib treatment.
Materials and Methods
Patients
Between September 2010 and December 2011, 12 patients with HCC were enrolled in this clinical study. These 8 men and 4 women ranged in age from 65 to 82 years old (mean, 74.8 years). The maximum tumor diameter measured using sonography ranged from 15 to 33 mm (mean, 24.0 mm; SD, 5.7 mm). All the patients had Child-Pugh classification A liver cirrhosis. In all the patients, the diagnosis of hepatocellular carcinoma was confirmed using a percutaneous needle biopsy. Hepatitis B virus surface (HBs) antigen alone was positive in 2 (16.7%) patients, hepatitis C virus (HCV) antibody alone was positive in 8 (66.6%) patients, and both were negative in 2 (16.7%) patients. The histological features of the tumor were classified into well-differentiated, moderately differentiated, and poorly differentiated types in 4, 6 and 2 of the 12 cases, respectively. Sorafenib was started at a dose of 400 mg orally twice in 7 cases and at a dose of 800 mg orally twice in 5 cases. Our hospital ethics committee approved this study, and each patient signed an informed consent form at the time of enrollment.
Contrast-enhanced 3D ultrasonography
Contrast-enhanced (0.2 mL of Sonazoid suspension; Daiichi Sankyo, Tokyo, Japan) 3D ultrasonography (LOGIQ 7; GE Healthcare, Milwaukee) was performed in all the patients using a convex volume 4D3C-L probe (GE Healthcare). Sonazoid (0.2 mL) was injected intravenously through a 24-gauge cannula in all the patients. A coded harmonic angio (CHA) mode with a high mechanical index (0.5-0.9) at 8-13 frames per second was used for the contrast-enhanced, three-dimensional US procedure. The focus point was set under the tumor 19.
3D image reconstruction was performed using the functionalities of the LOGIQ 7 ultrasound system. The three orthogonal planes were represented as plane A (which could migrate from front to rear through the volume of interest [VOI]), plane B (left to right), and plane C (up to down). Tomographic ultrasound images (TUI) of several parallel slices in three orthogonal planes were reconstructed in three phases using Autosweep 3D 20. The distance between any two TUI slices could be adjusted to obtain the desired images.
The images were reviewed independently by two readers (K. Numata and K. Tanaka) with 25 years of experience in performing liver US and 5 years of experience performing CE 3D US imaging. The readers were asked to classify the 3D enhancement changes observed during the early phase as an increase, no change, or a decrease. The evaluations of the two readers were in concordance for all 12 lesions.
Histological Analysis
Specimens from hepatic masses suspected of being HCC and non-tumorous parenchyma were obtained using a modified 21-gauge Menghini needle under sonographic guidance (Sonopsy C1; Hakko, Tokyo, Japan). The specimens were fixed in formalin for 4 h, embedded in paraffin, sectioned serially in a longitudinal orientation, and stained with hematoxylin and eosin.
Microvessels were stained immunohistochemically using labeled streptavidin-biotin after antigen retrieval for CD34. The sections were incubated with an anti-CD34 monoclonal antibody (dilution 1:200). The stain was visualized using diaminobenzidine tetrahydroxychloride solution (DAB; DAKO Corp., Carpinteria, USA) as a substrate. The microvessel density (MVD) of the HCC and the surrounding non-tumorous area was evaluated according to the method described by Weidner et al. 21. Any positively stained endothelial cell or endothelial cell cluster that was clearly separated from adjacent microvessels and connective elements was counted as one microvessel, irrespective of the presence of a vessel lumen. At low power (×100), the tissue sections were screened and five areas with the most intense neovascularization were selected. The microvessel counts in these areas were performed at a low power (×200). The mean microvessel count of the five most vascular areas was taken as the MVD. The counts were measured as the number of microvessels per 0.2 mm2. The microvessel counts were analyzed by the same pathologist (with 5 years of experience in pathology) who had no knowledge of the contrast-enhanced ultrasonography findings.
Statistical Analysis
All the data were reported as the mean ± standard deviation. The difference in the MVD before and after sorafenib treatment was evaluated using the Wilcoxon signed rank test. Statistical significance was defined as a P value of less than 0.05.
Results
We evaluated blood flow in both tumorous and non-tumorous areas before and 1 week after sorafenib administration. Blood flow decreased in the tumorous areas in all 12 cases after sorafenib treatment. In addition, the MVD in the tumorous area was evaluated before and after sorafenib treatment, and the MVD of the tumorous area at 1 week after sorafenib treatment (38.8 ± 13.7) was significantly lower than that observed before sorafenib treatment (72.4 ± 13.1) (P < 0.01) (Table 2). No differences in the blood flow changes in either the tumorous or non-tumorous areas were seen between patients treated at a dose of 400 mg of sorafenib and those treated at a dose of 800 mg.
Table 2.
Change in MVD of tumors at 1 week after sorafenib use
| MVD | P value | ||
|---|---|---|---|
| Before sorafenib use | One week after sorafenib use | ||
| Tumor area (n = 12) | 72.4 ± 13.1 | 38.8 ± 13.7 | P < 0.01 |
MVD: microvascular density
On the other hand, blood flow in the non-tumorous area decreased after sorafenib treatment in 6 cases but did not change in the other 6 cases. In the reduced blood flow group, the MVD of the non-tumorous area at 1 week after sorafenib administration (23.0 ± 3.4) was significantly lower than the MVD of the non-tumorous area before sorafenib administration (43.0 ± 1.0) (P < 0.05) (Table 3). However, in the group without a change in blood flow, the MVD of the non-tumorous area after 1 week of sorafenib administration (44.3 ± 5.9) was not different from the MVD of the non-tumorous area before sorafenib administration (44.0 ± 5.2) (Table 3).
Table 3.
Change in MVD of non-tumorous area at 1 week after sorafenib use
| Change of blood flow in non-tumorous area | MVD | P value | |
|---|---|---|---|
| Before sorafenib use | One week after sorafenib use | ||
| Decrease (n = 6) | 43.0 ± 1.0 | 23.0 ± 3.4 | P < 0.05 |
| No change (n = 6) | 44.0 ± 5.2 | 44.3 ± 5.9 | NS |
MVD: microvascular density
Before sorafenib therapy, the blood flow in the tumor was higher than that in the non-tumorous area, and the MVD of the tumor (72.4 ± 13.1) was higher than that of the non-tumorous area (43.5 ± 3.4) (P < 0.05).
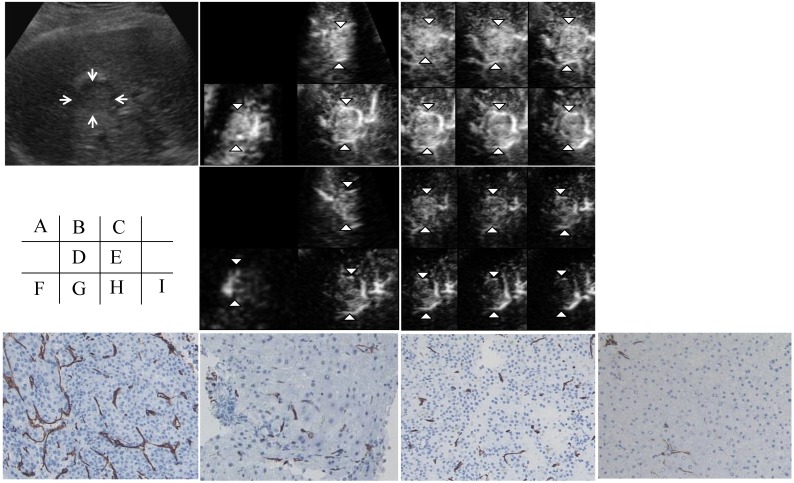
Figure 1 shows assessments made using contrast-enhanced 3D ultrasonography before and at 1 week after sorafenib treatment in patient No. 1 (a 57-year-old man). An HCC with a diameter of 25 mm was located in the anterior inferior segment of the right lobe. A hypoechoic nodule was depicted on a 2D sonogram. In the early phase, the nodule showed diffuse enhancement of the intratumoral vessels in all three orthogonal planes on a multiplanar rendering (MPR) display. A tomographic ultrasound image with a slice distance of 1.5 mm showed diffuse enhancement of the intratumoral vessels (arrowheads) during the early phase. The assessments of the three orthogonal planes on the MPR display and the tomographic ultrasound findings after sorafenib treatment showed decreases in vascular flow in both the tumor and the non-tumorous area during the early phase. The histology of the tumor before and after sorafenib treatment was moderate HCC. The MVD as assessed using CD34 staining of the tumor had decreased at 1 week after sorafenib treatment. The MVD of the non-tumorous area had also decreased after sorafenib treatment.
Figure 1.
Contrast-enhanced (CE) three-dimensional ultrasonography (3D US) images of the liver in a 57-year-old man with hepatocellular carcinoma (HCC) in the anterior inferior segment of the right lobe. A) A hypoechoic nodule with a diameter of 25 mm is visible on a 2D sonogram (arrows). B) The same nodule observed during the early phase shows diffuse enhancement of the intratumoral vessels in three orthogonal planes on a multiplanar rendering (MPR) display (arrowheads). C) A tomographic ultrasound image with a slice distance of 1.5 mm shows diffuse enhancement of the intratumoral vessels (arrowheads) during the early phase. D) Assessments of the three orthogonal planes on an MPR display show decreases in vascular flow in both the tumor (arrowheads) and the non-tumorous area during the early phase when examined after sorafenib treatment. E) Tomographic ultrasound images with a slice distance of 1.5 mm also show the decreased enhancement of intratumoral vessels (arrowheads) and vessels in the non-tumorous area during the early phase when examined after sorafenib treatment. The microvessel density (MVD) of the tumor (F) as assessed using CD34 staining decreased after sorafenib treatment (original magnification, x200) (H). The MVD of the non-tumorous area (G) also decreased after sorafenib treatment (original magnification, x200) (I).
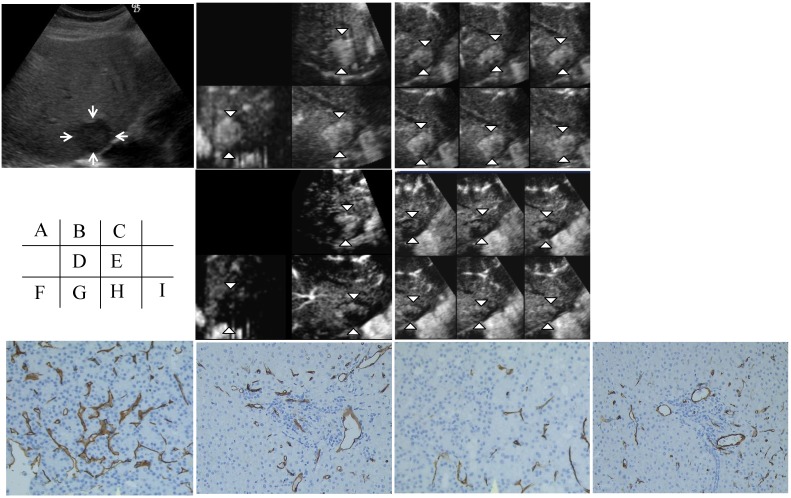
Figure 2 shows another case (an 88-year-old man). A hypoechoic nodule with a diameter of 28 mm was depicted on a 2D sonogram. The three orthogonal planes on the MPR display and the tomographic ultrasound findings showed a decreased vascular flow in the tumor; however, no change in blood flow in the non-tumorous area was seen. CD34 staining in the tumor showed a decrease in the MVD, but CD34 staining in the non-tumorous area showed no change in the MVD after sorafenib treatment.
Figure 2.
Contrast-enhanced (CE) three-dimensional ultrasonography (3D US) images of the liver in an 88-year-old man with hepatocellular carcinoma (HCC) in the anterior inferior segment of the right lobe. A) A hypoechoic nodule with a diameter of 28 mm is visible on a 2D sonogram (arrows). B) The same nodule observed during the early phase shows diffuse enhancement of the intratumoral vessels in three orthogonal planes on an MPR display (arrowheads). C) A tomographic ultrasound image with a slice distance of 1.5 mm shows diffuse enhancement of the intratumoral vessels (arrowheads) during the early phase. D) Assessments of the three orthogonal planes on an MPR display show decreases in vascular flow in the tumor during the early phase (arrowheads) when examined after sorafenib treatment; however, the blood flow in the non-tumorous area did not change. E) Tomographic ultrasound images with a slice distance of 1.5 mm also show the decreased enhancement of intratumoral vessels (arrowheads) during the early phase when examined after sorafenib treatment; however, the blood flow in the non-tumorous area did not change. The microvessel density (MVD) in the tumor (F) as assessed using CD34 staining decreased after sorafenib treatment (original magnification, x200) (H). On the other hand, the MVD of the non-tumorous area (G) as assessed using CD34 staining did not change, even after sorafenib treatment (original magnification, x200) (I).
Discussion
Angiogenesis is a phenomenon in which tumors induce the formation of new vessels to provide oxygen and nutrition for their growth, resulting in an increase in the total blood flow in the tumor. The anti-angiogenesis agent sorafenib significantly inhibits neovascularization in xenograft models of human cancers 22. Wilhelm et al. 23 evaluated the effect of sorafenib on the inhibition of angiogenesis and microvessel density, and they showed that analyses of microvessel density and microvessel area in the same tumor sections using antimurine CD31 antibodies demonstrated a significant inhibition of neovascularization in xenograft models. Yang et al. 24 evaluated the effect of sorafenib in an animal HCC model using iron oxide-enhanced susceptibility weighted imaging and found that CD31 was reduced pathologically.
These data demonstrated that sorafenib is a dual-action RAF kinase and VEGFR inhibitor that targets tumor cell proliferation and tumor angiogenesis. The efficacy of new antiangiogenic treatments can now be evaluated using imaging research, since the study of tumor neovascularization is now a major theme 25-27.
Contrast-enhanced Doppler ultrasound is a new noninvasive imaging technique that can be an effective tool for evaluating antiangiogenic drugs 22. The advantage of using contrast-enhanced ultrasonography to evaluate anti-tumor treatment is that the examinations are inexpensive, rapid, feasible, and repeatable without adverse effects 16. Lavisse et al. 28 evaluated early tumor vasculature-disrupting effects after anti-angiogenesis treatment and the feasibility of using contrast-enhanced ultrasonography for quantitative assessments of these effects. They concluded that contrast-enhanced US allowed quantitative in vivo evaluations of the functional effects of anti-angiogenesis treatment using the peak intensity (PI) and the time to PI (TPI), with the tumoral microvasculature exhibiting the greatest changes. There are also several methods for evaluating blood flow during tumor neovascularization, and Meresca et al. 29 used the color pixel density to measure the relative density of colored pixels compared with that of the entire series of pixels for the tumor. They used Photoshop software to calculate differences in luminosity in areas before and after the injection of contrast medium. On the other hand, contrast-enhanced three-dimensional (3D) US has recently been successfully used to accurately depict and provide detailed visualization of the vascular characteristics of various focal liver tumors 19,20. Contrast-enhanced 3D fusion sonography clearly visualized both the tumor vascularity and the tissue structure when used in combination with Sonazoid 30, 31. In the present study, we used 3D contrast-enhanced US to evaluate vascular flow before and after the administration of a new antiangiogenic treatment, and a strong correlation was found between the vascularity observed using contrast-enhanced 3D ultrasonography and pathological changes after sorafenib treatment. Although the TPI depicts only one slice of the whole tumor, 3D US has the advantage of being able to depict and evaluate vascular changes in multiple sections of both the tumor and non-tumorous areas simultaneously.
Yoshida et al. 16 reported that the absence of vascularization detected by contrast-enhanced ultrasonography correspond to the absence of intratumoral vascularization, as established histologically. A new computerized method has also been used to study tumor vascularization by quantifying the distribution of functional microvessels in vivo, resulting in a report that the tumor vessel density was reduced after sorafenib treatment 32. Lassau et al. 33 evaluated histological slides and ultrasonographic slices after contrast injection and concluded that the regions in which no vessels were detected using contrast-enhanced ultrasonography corresponded to regions in which vascularization was absent when examined histologically. In the present study, the microvascular density in the tumor, as revealed using anti-CD34 antibody staining, decreased in all 12 cases in which the vascular flow in the tumor area had decreased, and the microvascular density also decreased in the non-tumorous areas in 6 cases in which a decrease in vascular flow was noted. On the other hand, no changes in microvascular density, as revealed using anti-CD34 staining, were observed in 6 other cases in which vascular flow had not changed, so a strong correlation appears to exist between vascularity observed using 3D enhanced ultrasonography and histological findings. However, the reason why the vascularity of the tumors decreased in all the cases but the vascularity of the non-tumorous areas decreased in only half of the cases remains unclear. Since specimens from tumor and non-tumorous parenchyma were obtained before and after sorafenib treatment, this study was limited in that only a small number of cases were examined for a relatively short observation period. Thus, conclusive statements cannot be made because of the small number of cases and relatively short observation period, and further studies with a longer observation period are needed to determine the correlation between contrast-enhanced 3D ultrasonography and pathological changes in human HCC and surrounding non-tumorous areas according to the dose of sorafenib.
In conclusion, the use of contrast agents increases the sensitivity of ultrasound imaging for the detection of angiogenesis. A correlation exists between contrast-enhanced 3D ultrasonography findings and pathological changes in human HCC. However, these findings should be clarified in further studies investigating the change in blood flow after sorafenib treatment in a large number of cases.
Table 1.
Baseline clinical characteristics of patients with hepatocellular carcinoma (n = 12)
| Characteristics | |
|---|---|
| Number of patients | 12 |
| Sex (male/female) | 8/4 |
| Age (years) | 74.8 ± 5.4 (65-82) |
| Alcohol/HCV/HBsAg | 2/8/2 |
| Child A/B/C | 12/0/0 |
| Diameter (mm) | 24.0 ± 5.7 (10-100) |
| Dose (800 mg/400 mg) | 5/7 |
References
- 1.Folkman J. Tumor angiogenesis: Therapeutic implications. N Engl J Med. 1971;1:1182–6. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 2.Denekamp J. Angiogenesis, neovascular proliferation and vascular pathophysiology as targets for cancer therapy. Br J Radiol. 1993;66:181–96. doi: 10.1259/0007-1285-66-783-181. [DOI] [PubMed] [Google Scholar]
- 3.Folkman J. New perspectives in clinical oncology from angiogenesis research. Eur J Cancer. 1996;2A:2534–9. doi: 10.1016/s0959-8049(96)00423-6. [DOI] [PubMed] [Google Scholar]
- 4.Llovet JM, Bruix J. Molecular targeted therapies in hepatocellular carcinoma. Hepatology. 2008;48:1312–27. doi: 10.1002/hep.22506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Llovet JM, Bruix J. Novel advancements in the management of hepatocellular carcinoma in 2008. J Hepatol. 2008;48(Suppl. 1):S20–37. doi: 10.1016/j.jhep.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 6.Llovet JM, Ricci S, Mazzaferro V, SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med; 2008. p. 24. 359:378-90. [DOI] [PubMed] [Google Scholar]
- 7.Chen YW, Pan HB, Tseng HH. et al. Assessment of blood flow in hepatocellular carcinoma: correlations of computed tomography perfusion imaging and circulating angiogenic factors. Int J Mol Sci. 2013;14:17536–52. doi: 10.3390/ijms140917536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang JF, Zhao ZH, Zhang Y. et al. Dual-input two-compartment pharmacokinetic model of dynamic contrast-enhanced magnetic resonance imaging in hepatocellular carcinoma. World J Gastroenterol. 2016;22:3652–3662. doi: 10.3748/wjg.v22.i13.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chapiro J1, Wood LD, Lin M. et al. Radiologic-pathologic analysis of contrast-enhanced and diffusion-weighted MR Imaging in patients with HCC after TACE: diagnostic accuracy of 3D quantitative image analysis. Radiology. 2014;273:746–758. doi: 10.1148/radiol.14140033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lassau N, Paturel-Asselin C, Guinebretière JM. et al. New hemodynamic approach to angiogenesis: Color and pulsed Doppler ultrasonography. Invest Radiol. 1999;34:194–8. doi: 10.1097/00004424-199903000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Lassau N, Mercier S, Koscielny S. et al. Prognostic value of highfrequency sonography and color Doppler US for the pre-operative assessment of melanomas. AJR. 1999;172:457–61. doi: 10.2214/ajr.172.2.9930803. [DOI] [PubMed] [Google Scholar]
- 12.Rojas JD, Lin F, Chiang YC. et al. Ultrasound molecular imaging of VEGFR-2 in clear-cell renal cell carcinoma tracks disease response to antiangiogenic and notch-inhibition therapy. Theranostics. 2018;8:141–155. doi: 10.7150/thno.19658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiao JD, Zhu WH, Shen SR. Evaluation of hepatocellular carcinoma using contrast-enhanced ultrasonography: correlation with microvessel morphology. Hepatobiliary Pancreat Dis Int. 2010;9:605–10. [PubMed] [Google Scholar]
- 14.Roccarina D, Garcovich M, Ainora ME. et al. Usefulness of contrast enhanced ultrasound in monitoring therapeutic response after hepatocellular carcinoma treatment. World J Hepatol. 2015;7:1866–1874. doi: 10.4254/wjh.v7.i14.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reshu Saini, Kenneth Hoyt. Recent developments in dynamic contrast-enhanced ultrasound imaging of tumor angiogenesis. Imaging Med. 2014;6:41–52. doi: 10.2217/iim.13.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoshida K, Hirokawa T, Moriyasu F. et al. Arterial-phase contrast-enhanced ultrasonography for evaluating anti-angiogenesis treatment: a pilot study. World J Gastroenterol. 2011;17:1045–50. doi: 10.3748/wjg.v17.i8.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mertens JC, Martin IV, Schmitt J. et al. Multikinase inhibitor sorafenib transiently promotes necrosis after radiofrequency ablation in rat liver but activates growth signals. Eur J Radiol. 2012;81:1601–6. doi: 10.1016/j.ejrad.2011.04.042. [DOI] [PubMed] [Google Scholar]
- 18.Fukuda H, Numata K, Moriya S, et al.Hepatocellular carcinoma. concomitant sorafenib promotes necrosis after radiofrequency ablation-propensity score matching analysis. Radiology. 2014;272:598–604. doi: 10.1148/radiol.14131640. [DOI] [PubMed] [Google Scholar]
- 19.Numata K, Fukuda H, Ohto M. et al. Evaluation of the therapeutic efficacy of high-intensity focused ultrasound ablation of hepatocellular carcinoma by three-dimensional sonography with a perflubutane-based contrast agent. Eur J Radiol. 2010;75:e67–75. doi: 10.1016/j.ejrad.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 20.Luo W, Numata K, Morimoto M, et al.Clinical utility of contrast-enhanced three-dimensional ultrasound imaging with Sonazoid. findings on hepatocellular carcinoma lesions. Eur J Radiol. 2009;72:425–31. doi: 10.1016/j.ejrad.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 21.Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis-correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- 22.Lamuraglia M, Escudier B, Chami L, To predict progression-free survival and overall survival in metastatic renal cancer treated with sorafenib:Pilot study using dynamic contrast-enhanced Doppler ultrasound. Eur J Cancer; 2006. p. 42. 4272-9. [DOI] [PubMed] [Google Scholar]
- 23.Wilhelm SM, Carter C, Tang L. et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 24.Yang S, Lin J, Lu F, Han Z, Fu C, Gu H. Use of Ultrasmall Superparamagnetic Iron Oxide Enhanced Susceptibility Weighted Imaging and Mean Vessel Density Imaging to Monitor Antiangiogenic Effects of Sorafenib on Experimental Hepatocellular Carcinoma. Contrast Media Mol Imaging. 2017: 9265098. Published online; 2017. Jun 21. doi: 10.1155/2017/9265098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eggermont AM. Evolving imaging technology:contrast-enhanced Doppler ultrasound is early and rapid predictor of tumour response. Ann Oncol. 2005;16:995–6. doi: 10.1093/annonc/mdi230. [DOI] [PubMed] [Google Scholar]
- 26.Hochedez P, Lassau N, Bonvalot S. et al. Treatment of local recurrent melanomas by isolated limb perfusion: value of Doppler ultrasonography. J Radiol. 2003;84:597–603. [PubMed] [Google Scholar]
- 27.Lassau N, Chawi I, Rouffiac V. et al. Interest of color Doppler ultrasonography to evaluate a new anti-angiogenic treatment with thalidomide in metastatic renal cell carcinoma. Bull Cancer. 2004;91:629–35. [PubMed] [Google Scholar]
- 28.Lavisse S, Lejeune P, Rouffiac V. et al. Early quantitative evaluation of a tumor vasculature disruptive agent AVE8062 using dynamic contrastenhanced ultrasonography. Invest Radiol. 2008;43:100–11. doi: 10.1097/RLI.0b013e3181577cfc. [DOI] [PubMed] [Google Scholar]
- 29.Maresca G, Summaria V, Colagrande C. et al. New prospects for ultrasound contrast agents. Eur J Radiol. 1998;27:171–8. doi: 10.1016/s0720-048x(98)00059-x. [DOI] [PubMed] [Google Scholar]
- 30.Yukisawa S, Ohto M, Masuya Y. et al. Contrast-enhanced three-dimensional fusion sonography of small liver metastases with pathologic correlation. J Clinical Ultrasound. 2007;35:1–8. doi: 10.1002/jcu.20299. [DOI] [PubMed] [Google Scholar]
- 31.Ohto M, Ito R, Soma N, Contrast-enhanced 3D ultrasonography in hepatocellular carcinoma. J Med Ultrasonics; 2011. p. 38. 3-12. [DOI] [PubMed] [Google Scholar]
- 32.Righi M, Giacomini A, Lavazza C, Sia D, Carlo-Stella C, Gianni AM. A computational approach to compare microvessel distributions in tumors following antiangiogenic treatments. Lab Invest. 2009;89:1063–1070. doi: 10.1038/labinvest.2009.76. [DOI] [PubMed] [Google Scholar]
- 33.Lassau N, Koscielny S, Opolon P. et al. Evaluation of contrast-enhanced color Doppler ultrasound for the quantification of angiogenesis in vivo. Invest Radiol. 2001;36:50–5. doi: 10.1097/00004424-200101000-00007. [DOI] [PubMed] [Google Scholar]