Abstract
Background:
The aim of this study was to evaluate the effect of dydrogesterone in the outcome of idiopathic intrauterine growth restriction (IUGR).
Materials and Methods:
It is a double-blind randomized control clinical trial study that was done in Shahid Beheshti hospital of Isfahan during 2015–2016. In this study, 89 pregnant women with idiopathic IUGR fetus were selected and randomly divided into two intervention and control groups. Intervention group was treated with dydrogesterone 10 mg every 12 h for 2 weeks, while the control group received conventional management and treatment of IUGR, which also has been performed in the intervention group.
Results:
After 2 weeks of intervention, fetal weight was significantly increased in dydrogesterone group as compared to control group (2053.15 vs. 1736.36 g, P = 0.001); furthermore, we observed significant differences in the term of fetal abdominal circumference between the groups (27.25 vs. 25.92 cm, P = 0.006). Middle cerebral artery resistance index (0.67 vs. 0.83, P < 0.001) and uterine artery (UA) resistance index (0.68 vs. 0.81, P < 0.001) were significantly decreased in dydrogesterone group as compared to control group.
Conclusions:
Our results showed that dydrogesterone reduces resistance index of uterine artery and middle cerebral and increased fetal weight, while no sign of toxicity was observed. Dydrogesterone supplementation would have the potentiality to become a simple and economic means to prevent IUGR.
Keywords: Dydrogesterone, idiopathic, intrauterine growth restriction
Introduction
Intrauterine growth restriction (IUGR) is one of the most common adverse outcomes of pregnancy which affects up to 7% of pregnancies. IUGR increases the risk of perinatal morbidity and mortality.[1,2,3] Fetuses with IUGR are traditionally defined as a birth weight below the 10th percentile for the gestational age.[4,5] It is associated with the risk of difficult handling of stress in vaginal delivery, decreased oxygen levels, hypoglycemia, low resistance to infection, low Apgar scores, meconium aspiration, trouble maintaining body temperature, abnormally high red blood cell count, moreover the risk for stroke, type 2 diabetes mellitus, metabolic syndrome, dyslipidemia, cardiovascular and heart disease, reduction in growth as adults and changes in the pattern of puberty significantly increase in fetus with IUGR.[6] IUGR etiology may include primary deficits in placental function such as pregnancy-induced hypertension that interferes with placental blood flow. They may also include secondary placental dysfunction due to adverse maternal behaviors such as cigarette smoking or environmental influences including maternal malnutrition and stress.[7]
In total, the most common cause of IUGR is impaired nutrition supply to the fetus. This may result from impaired maternal nutrition, decreased blood flow to the uterus or placenta. This may require maternal nutritional supplementation, maternal hyperoxygenation, or pharmacological manipulations aimed at increasing blood flow.[8]
On the other hand, recently, some studies have established the association between IUGR and low progesterone levels.[9,10] Progesterone supplementation in IUGR may be associated with improved uteroplacental circulation. It acts on the myometrium and suppresses the estrogen action by inhibiting the replacement of cytosolic estrogen receptors and it has a direct effect on uterus biosynthetic processes through its own cellular receptor.[11] The immune modulatory effect of progesterone through progesterone-induced blocking factor (PIBF) may play a role in improving the outcome in pregnancy with fetal growth restriction.[12,13] However, there has been no previous investigation about the effect of dydrogesterone and blood flow to the uterus or placenta. In contrast, there are several studies on the effect of dydrogesterone on the other outcomes in IUGR cases such as miscarriage. Moreover, dydrogesterone does not have androgenic effects such as progesterone. Therefore, this study was designed to evaluate the effectiveness of dydrogesterone on the outcome of idiopathic IUGR with more focus on blood flow to the uterus or placenta.
Materials and Methods
This double-blind clinical trial was conducted in Obstetrics and Gynecology Department of Isfahan Alzahra and Shahid Beheshti educational hospitals of Isfahan during 2014–2015. In this study, 90 fetuses were selected and blocked randomly divided into two groups. The fetus outcome and resistance index of uterus or placenta of pregnant women receiving dydrogesterone (intervention group) were compared to women receiving conventional treatment in control group.
Therefore, after the approval of Ethical committee of Isfahan University of medical sciences the aim of the project was fully described for the patients and they have filled the consent form.
Inclusion criteria consisted of pregnant women referred to obstetrics and gynecology department of Alzahra and Shahid Beheshti hospitals with a diagnosis of idiopathic IUGR fetus (weight below the 10th percentile for the gestational age), signed a consent form to participate in the study, and having gestational age between 28–35 weeks. The women with fetal congenital or chromosomal anomaly and some complications such as hypertension, diabetes, renal disease, cardiovascular disease and connective tissue disease, dissatisfaction to continue the study participation, improper use of dydrogesterone, and stopping the follow-up for any reason were excluded from the study.
The sample size required for this study was calculated by using sample size formula for comparison of two ratio and taking into account the 95% confidence level, 80% power, and the prevalence of improvement which was estimated 50%, and 0.3 difference between the two groups was estimated 45 patients per groups.
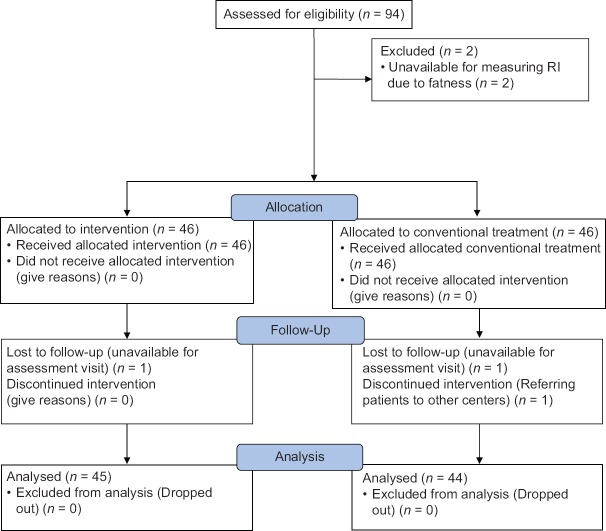
The study flowchart is shown in Figure 1. Ninety-four pregnant women with a diagnosis of idiopathic IUGR fetus who had been diagnosed by obstetricians and based on inclusion and exclusion criteria were included.
Figure 1.
Study flowchart (CONSORT format)
The fetuses were regarded as growth retarded when compared to the standard “Hadlock's Charts of gestational ageing.” If the fetal parameter was within ± 2 standard deviation (SD), then it was regarded as normal. However, if it was more than 2 SD < the mean gestational age for a particular week of pregnancy, then it was regarded as being abnormal. The biparietal diameter, head circumference, femur length, and the abdominal circumference (AC) were all tabulated in this manner and compared with the standard charts.
Ultrasonography was performed with an abdominal 3.5–5-MHz curvilinear transducer. Freeze-frame ultrasound capabilities and electronic on-screen calipers were used for the measurements. Each measurement was repeated three times in each fetus, and the mean value was determined. To measure middle cerebral artery (MCA) flow velocity, the transducer was moved toward the base of the skull to identify the circle of Willis. At this plane, MCA is easily distinguishable from the internal carotid and the anterior cerebral arteries. After localization of MCA, Doppler flow velocity was measured at the middle third of the MCA, away from the internal carotid and anterior cerebral arteries. Multiple waveform recordings were obtained using pulsed-wave Doppler. Resistance index was calculated according to the following formula: (S_D)/S.
The uterine artery (UA) was studied by the first placental site identification. If it was unilateral, then UA was studied on that site. In case of central placenta, both uterine arteries were evaluated. The main branch of UA is located at cervicocorporal junction, and Doppler velocimetry measurement was performed near to this location. As well as, resistance index was calculated. Umbilical artery Doppler velocimetry waveforms were obtained from midpoint between the fetal abdominal wall and the placental insertion site of the umbilical cord.
All eligible patients were randomly divided into two groups using a blocked randomization procedure with matched individuals in each block based on age. The first patient was arranged in one of the legs, and the subsequent patients were randomly distributed into two groups to obtain sufficient sample size in each group.
In order to make the study blind, patients were unaware of the received drug and prescribing was done by the researcher and the work of collecting information was done by another person who did not know about the allocation of patients to the groups.
Eighty-nine patients completed the study; 45 from intervention group and 44 from control group. The study received ethics approval from the Ethics Committee of Isfahan University of Medical Sciences, (394,763) and all participants gave written informed consent. Intervention group received oral dydrogesterone 10 mg every 12 h for 2 weeks with conventional management and treatment of IUGR, while control group only received conventional management and treatment of IUGR (included sonographic follow-up, improvement of nutrition, and rest). At the end of the study, weight and AC were measured and intrauterine ultrasound was performed for evaluating MCA and UA RI with same operator and in same previous center 2 weeks later. Other data including demographic and clinical characteristics were collected from patients' profile and entered to collected data form. All measuring data were collected by one observer.
Data were analyzed and reported only for patients who completed the trial. Statistical analysis of data was performed using SPSS version 22 software (Chicago, Illinois: SPSS Inc.). Chi-square test was performed to compare qualitative variables between the groups and Kolmogorov–Smirnov test was performed to check the normal distribution of all studied parameters. Student's t-test and paired t-test were used for variables which were distributed in a normal way. Furthermore, changes of quantitative variables between the two groups were tested by repeated measures ANOVA test. Two-tailed P < 0.05 was considered statistically significant. P < 0.05 was considered statistically significant.
Results
According to the results of the study, the mean of mothers' age in intervention and control groups were 26.55 ± 3.06 and 27.52 ± 3.44 years, respectively, and no statistically different was seen (P = 0.165). Furthermore, the mean of gestational age of the intervention and control groups were 30.86 ± 2.32 and 31.22 ± 2.45 weeks, respectively, and there is no statistical difference between the two groups (P = 0.479).
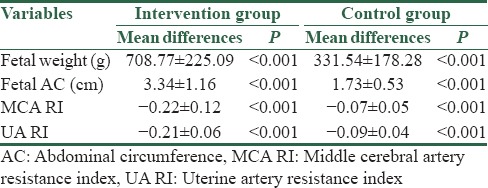
The mean of fetal weight, AC, MCA RI, and UA RI are shown in Table 1. According to Table 1, there was no statistical difference between the two groups in the beginning of the study, but at 2 weeks after, the difference between the two groups was statistically significant. Furthermore, the mean difference of the variables was statistically different between the two groups [Table 2]. In addition, no patients experienced any adverse effects.
Table 1.
Mean and standard deviation of demographic data in the two groups
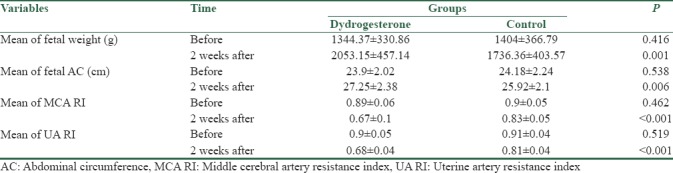
Table 2.
Mean differences in studied variables in the two groups
Discussion
Our results showed that after 2 weeks of intervention, fetal weight (2053.15 vs. 1736.36) was significantly increased in dydrogesterone group; moreover, MCA RI (0.67 vs. 0.83) and UA RI (0.68 vs. 0.81) were significantly decreased in dydrogesterone group.
Progesterone is a vasodilator and muscle relaxant which increases during pregnancy. Moreover, progesterone induces a rapid vasorelaxant effect on the fetoplacental vasculature, so it can be directly influenced on prostaglandin-stimulated smooth muscle contraction.[14] In total, the mechanism of progesterone vasodilator effect may act by various actions.[15,16] A study showed that inhibition of nitric oxide reduced the progesterone vasodilatation effect in rabbit's pulmonary artery.[15] Our results confirmed the findings of the two previous studies.[17,18] Vasodilator effects of progesterone have been reported in Paonessa et al.'s,[14] Li et al.'s,[15] and Hermenegildo et al.'s[18] studies.
Study performed by Hermenegildo et al.[18] showed that progesterone and medroxyprogesterone acetate induced prostacyclin (a potent vasodilator) synthesis in human umbilical venous endothelial cells by enhancing the expression and activity of cyclooxygenase-1 and 2. In IUGR pregnancies, prostacyclin production by placental cells was diminished, as compared with normal placental cells which can be due to the loss of synthesis, not due to the increase of metabolism.[19]
The basis for using dydrogesterone was related to reduction in levels of progesterone in IUGR pregnancies.[20,21] Furthermore, dydrogesterone may improve the myometrial perfusion in IUGR gestation by promoting myometrial small arterial vasodilatation, decreasing peripheral resistance, and increasing the flow in utero placental bed. On the other hand, T-cell during normal pregnancy predominantly secretes anti-inflammatory cytokines (such as secreting IL-3, IL-4, IL-10, and placental growth factor (PGF)) compared to proinflammatory cytokines (such as secreting IL-2 and IL-12, tumor necrosis factor-α).[4] About the inability of mother to switch from proinflammatory cytokines to anti-inflammatory cytokines it has been proposed cytokine profiles at the fetal-neonatal interface as one of the primary causes of miscarriage, IUGR, and preeclampsia.[22]
Dydrogesterone manifests an immunomodulatory effect by inducing PIBF production. PIBF inhibits natural killer cell activity, causes TH2 bias (promotes secreting anti-inflammatory cytokines), and increases asymmetric antibody which acts as a blocking antibody and protects the antigen from potentially harmful cell-mediated response. All these eventually lead to an improvement in IUGR fetuses.[8] In total, dydrogesterone administration is useful, safe, and effective way to treat IUGR. However, notice to limitation of our study such as lock of sample and follow-up time, more study is recommended.
Conclusions
Our results showed that dydrogesterone reduces resistance index of UA and MCA and increased fetal weight, while no sign of toxicity was observed. Dydrogesterone supplementation would have the potentiality to become a simple and economic means to prevent IUGR. However, future studies with larger sample sizes, multiple measurements with specific intervals, umbilical or placental sampling, and assessment of perinatal outcome are warranted to confirm the results of the present study.
Financial support and sponsorship
This study was financially supported by Isfahan University of Medical Sciences, Isfahan, center of Iran.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We gratefully acknowledge the dedicated efforts of the investigators, the coordinators, the volunteer patients who participated in this study, and the clinical research development units of Isfahan Alzahra hospital.
References
- 1.Garite TJ, Clark R, Thorp JA. Intrauterine growth restriction increases morbidity and mortality among premature neonates. Am J Obstet Gynecol. 2004;191:481–7. doi: 10.1016/j.ajog.2004.01.036. [DOI] [PubMed] [Google Scholar]
- 2.Koukoura O, Sifakis S, Spandidos DA. DNA methylation in the human placenta and fetal growth (review) Mol Med Rep. 2012;5:883–9. doi: 10.3892/mmr.2012.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Romo A, Carceller R, Tobajas J. Intrauterine growth retardation (IUGR): Epidemiology and etiology. Pediatr Endocrinol Rev. 2009;6(Suppl 3):332–6. [PubMed] [Google Scholar]
- 4.Dollberg S, Haklai Z, Mimouni FB, Gorfein I, Gordon ES. Birth weight standards in the live-born population in Israel. Isr Med Assoc J. 2005;7:311–4. [PubMed] [Google Scholar]
- 5.Figueras F, Gratacós E. Update on the diagnosis and classification of fetal growth restriction and proposal of a stage-based management protocol. Fetal Diagn Ther. 2014;36:86–98. doi: 10.1159/000357592. [DOI] [PubMed] [Google Scholar]
- 6.Chernausek SD. Update: Consequences of abnormal fetal growth. J Clin Endocrinol Metab. 2012;97:689–95. doi: 10.1210/jc.2011-2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Regev RH, Reichman B. Prematurity and intrauterine growth retardation – Double jeopardy? Clin Perinatol. 2004;31:453–73. doi: 10.1016/j.clp.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 8.Wadhwa L, Batra S, Tempe A. Role of dydrogesterone in the treatment of idiopathic IUGR. Int J Reprod Contracept Obstet Gynecol. 2013;2:157–60. [Google Scholar]
- 9.Martin CR. Michigan: BC Decker; 2009. Prenatal diagnosis and treatment. Manual of Neonatal Surgical Intensive Care; p. 238. [Google Scholar]
- 10.Bujold E, Roberge S, Lacasse Y, Bureau M, Audibert F, Marcoux S, et al. Prevention of preeclampsia and intrauterine growth restriction with aspirin started in early pregnancy: A meta-analysis. Obstet Gynecol. 2010;116:402–14. doi: 10.1097/AOG.0b013e3181e9322a. [DOI] [PubMed] [Google Scholar]
- 11.Barclay L. Vaginal progesterone reduces risk of preterm birth. Am J Obstet Gynecol. 2003;188:419–24. doi: 10.1067/mob.2003.41. [DOI] [PubMed] [Google Scholar]
- 12.Dosiou C, Hamilton AE, Pang Y, Overgaard MT, Tulac S, Dong J, et al. Expression of membrane progesterone receptors on human T lymphocytes and Jurkat cells and activation of G-proteins by progesterone. J Endocrinol. 2008;196:67–77. doi: 10.1677/JOE-07-0317. [DOI] [PubMed] [Google Scholar]
- 13.Miller L, Hunt JS. Regulation of TNF-alpha production in activated mouse macrophages by progesterone. J Immunol. 1998;160:5098–104. [PubMed] [Google Scholar]
- 14.Paonessa DJ, Shields AD, Howard BC, Gotkin JL, Deering SH, Hoeldtke NJ, et al. 17-hydroxyprogesterone caproate reverses induced vasoconstriction of the fetoplacental arteries by the thromboxane mimetic U46619. Am J Obstet Gynecol. 2006;195:1011–4. doi: 10.1016/j.ajog.2006.06.041. [DOI] [PubMed] [Google Scholar]
- 15.Li HF, Zheng TZ, Li W, Qu SY, Zhang CL. Effect of progesterone on the contractile response of isolated pulmonary artery in rabbits. Can J Physiol Pharmacol. 2001;79:545–50. [PubMed] [Google Scholar]
- 16.Cetingoz E, Cam C, Sakallı M, Karateke A, Celik C, Sancak A, et al. Progesterone effects on preterm birth in high-risk pregnancies: A randomized placebo-controlled trial. Arch Gynecol Obstet. 2011;283:423–9. doi: 10.1007/s00404-009-1351-2. [DOI] [PubMed] [Google Scholar]
- 17.Cianfarani S, Geremia C, Scott CD, Germani D. Growth, IGF system, and cortisol in children with intrauterine growth retardation: Is catch-up growth affected by reprogramming of the hypothalamic-pituitary-adrenal axis? Pediatr Res. 2002;51:94–9. doi: 10.1203/00006450-200201000-00017. [DOI] [PubMed] [Google Scholar]
- 18.Hermenegildo C, Oviedo PJ, García-Martínez MC, García-Pérez MA, Tarín JJ, Cano A, et al. Progestogens stimulate prostacyclin production by human endothelial cells. Hum Reprod. 2005;20:1554–61. doi: 10.1093/humrep/deh803. [DOI] [PubMed] [Google Scholar]
- 19.Jogee M, Myatt L, Elder MG. Decreased prostacyclin production by placental cells in culture from pregnancies complicated by fetal growth retardation. Br J Obstet Gynaecol. 1983;90:247–50. doi: 10.1111/j.1471-0528.1983.tb08618.x. [DOI] [PubMed] [Google Scholar]
- 20.Kaneoka T, Shimizu H, Matsuoka I, Taguchi S, Shirakawa K. Prenatal diagnosis and treatments of intrauterine growth retardation (author's transl) Nihon Sanka Fujinka Gakkai Zasshi. 1982;34:233–42. [PubMed] [Google Scholar]
- 21.Kaneoka T, Aso M, Nobori M, Aonuma M, Shimizu H, Shirakawa K, et al. Ultrasonic and biochemical detection and prenatal treatments of intra-uterine fetal growth retardation (author's transl) Nihon Sanka Fujinka Gakkai Zasshi. 1980;32:103–12. [PubMed] [Google Scholar]
- 22.Banerjee S, Smallwood A, Moorhead J, Chambers AE, Papageorghiou A, Campbell S, et al. Placental expression of interferon-gamma (IFN-gamma) and its receptor IFN-gamma R2 fail to switch from early hypoxic to late normotensive development in preeclampsia. J Clin Endocrinol Metab. 2005;90:944–52. doi: 10.1210/jc.2004-1113. [DOI] [PubMed] [Google Scholar]