Abstract
Background:
This study was aimed to evaluate the influence of oral N-acetylcysteine (NAC) application as an adjuvant to letrozole on induced ovulation outcomes in patients with polycystic ovary syndrome (PCOS).
Materials and Methods:
This was a placebo-controlled double-blind randomized clinical trial with 130 PCOS patients who were infertile. First, patients were randomly divided into two groups. Patients in Group 1 were administered letrozole 5 mg/d plus NAC 1.2 g/d and patients in Group 2 were administered letrozole plus placebo for 5 days starting at the 3rd day of the menstruation period. On the 12th day of the cycle, ultrasound evaluation was performed, and in whom at least one follicle with an 18–20 mm diameter was found, 10,000 unit human chorionic gonadotropin (hCG) was prescribed, and 36 h after hCG injection, timed intercourse was advised. On the 16th day, after hCG injection, serum β-hCG level was evaluated.
Results:
The number of follicles >18 mm was significantly higher in the letrozole + NAC group (P = 0.007). The ovulation and pregnancy rates were also significantly higher in the letrozole + NAC group (P = 0.045). No adverse side effects and no cases of ovarian hyperstimulation syndrome were observed in NAC group.
Conclusion:
NAC is demonstrated to be a safe and well-tolerated adjuvant to letrozole and can increase the pregnancy rates in PCOS patients.
Keywords: Letrozole, N-acetylcysteine, ovulation, polycystic ovary syndrome, pregnancy rate
Introduction
Polycystic ovary syndrome (PCOS) is a common and endocrine disorder in young women with a prevalence of up to 10%.[1] It manifests itself in a variety of clinical ways and 55%–75% of patients with PCOS are infertile due to chronic anovulation.[1,2,3] The diagnosis of PCOS is determined by the presence of two of the following conditions: oligoovulation or anovulation, hyperandrogenism and polycystic ovaries detected by ultrasonography. The three most bothersome symptoms commonly reported by affected women are hirsutism, regulate periods, and infertility.[4,5] A significant proportion of patients with PCOS have been found to suffer from defective insulin secretion and insulin resistance,[6] and hyperinsulinemia is present in >50% of patients with PCOS.[7] On the other hand, some authors believe that the hyperinsulinemia, secondary to insulin resistance, stimulates the secretion of both urine and adrenal androgen and suppresses the synthesis of sex hormone-binding globulins by the liver, causing a consequent increase of biologically active androgens in the circulation. Increase of ovarian androgen production would result in early follicular atresia and anovulation, including other typical clinical manifestations of hyperandrogens, such as hirsutism and acne.[8]
Letrozole is the most commonly used aromatase inhibitor (AI) for ovulation induction in women with PCOS.[9] Letrozole appears to result in higher live birth rates than clomiphene citrate (CC) and possibly lower multiple gestation rates.[10] AIs induce ovulation by inhibiting estrogen production and the consequent hypo estrogenic state increases gonadotropin-releasing hormone and pituitary follicle-stimulating hormone synthesis.[11]
N-acetylcysteine (NAC) is commonly used as a safe mucolytic drug with antioxidant and insulin regulatory effects.[12] NAC may also improve the circulating level of insulin and insulin sensitivity in hyperinsulinemic women with PCOS and ameliorate the homocysteine and lipid profile in PCOS.[13] NAC has been used effectively as an adjuvant to letrozole for ovulation induction in women with PCOS, and oral NAC administration has been proposed for the prevention of endothelial damage due to antioxidant agent.[14] In recent years, a limited number of studies have shown the possible benefits of NAC administration in improving insulin sensitivity and better induction of ovulation outcomes in patients with PCOS.[14,15]
This study was conducted to evaluate the effect of oral NAC administration as an adjuvant to letrozole in induction of ovulation outcomes in PCOS patients. The combination of these two drugs was evaluated for the first time in this study.
Materials and Methods
This placebo-controlled double-blind randomized clinical trial was conducted in Isfahan University infertility center, Shahid Beheshti Hospital, between 2015 and 2016. All infertile women who were referred to our center with PCOS (based on Rotterdam criteria, ESHRE/ASRM 2004),[4] aged 20–35 years, body mass index (BMI) <35 kg/m2, both patient tubes confirmed by hysterosalpingography, and with partner's normal semen analysis results (total volume >2 ml, concentration >20 million/ml, total motility >50%, and normal morphology >14%) were included in the study. Patients with thyroid dysfunction, hyperprolactinemia, history of large ovarian cyst formation (>6 cm), and asthma and/or allergy to medication were excluded from the study. Patients who received any hormonal medication (except for progesterone for withdrawal bleeding) and metformin were also excluded from the study. Furthermore, none of the patients or their male partners had any sexual dysfunction interfering with successful intercourse.
The total sample size of 120 patients was estimated to provide 80% power to detect the difference of 20% at the 0.05 level of significance. Taking into consideration a 10% dropout rate, the required minimum number of enrolled patients was determined to be 130 (65 in Group 1 and 65 in Group 2). Written informed consent was obtained from all the participants before entering into the study.
After obtaining an authorization from the Medical Ethics Committee of the Isfahan University, this article coded IR. MUI. REC.1394.3.716.
On the 3rd day of menstrual cycle (induced by 200–300 mg progesterone-in-oil injection in patients with amenorrhea), a baseline vaginal ultrasound examination and serum thyroid-stimulating hormone and thyrotropin and prolactin level assessment (all measured by immune reactive multianalysis) were performed for all patients who were candidates for induction of ovulation. Then, patients were randomly divided into two groups [Figure 1]. In the first group, patients received 5 mg letrozole (manufactured by Iran Hormone Company) plus 1200 mg NAC (produced by OSVE Company). From the 3rd until the 7th day of the menstrual cycle, NAC was given to the patients in the form of powder inserted in small pockets to be diluted into one standard glass of water and taken orally in two daily divided doses. In the second group in addition to 5 mg daily letrozole, patients received a placebo (oral rehydration solution [ORS] powder manufactured by Shafa company) from day 3 until day 7. ORS powder was given to the patients in the same pockets as NAC for two daily divided doses.
Figure 1.
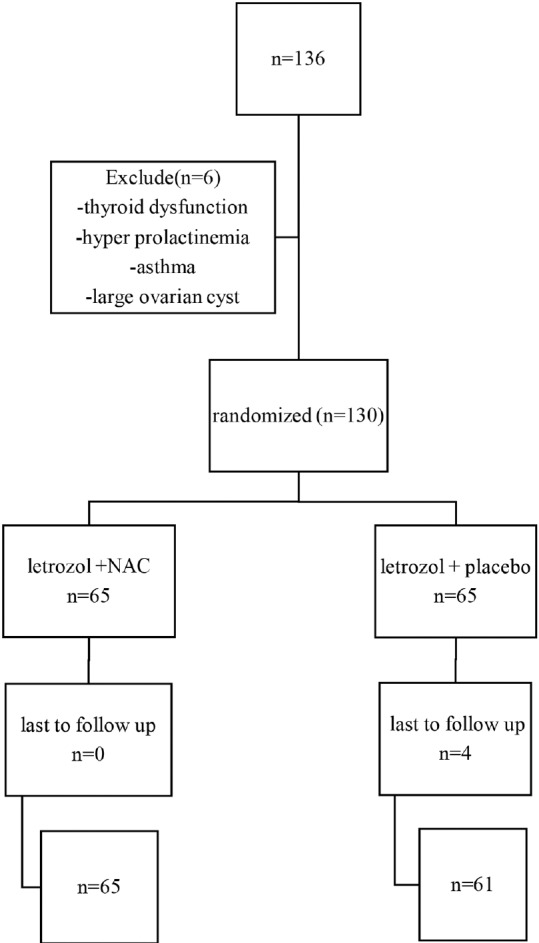
Study flowchart
On the 12th day of menstrual cycle, patients were monitored by transvaginal ultrasound examination to evaluate the mean follicular diameter. In the presence of at least one follicle with 18–20 mm in size, 10,000 u human chorionic gonadotropin (hCG) was injected intramuscularly and time of intercourse was advised 36 h after hCG injection. Serum β-hCG level was measured on the 16th day after hCG injection. With two serial positive β-hCG levels (at least 2 days apart), another transvaginal ultrasound examination was performed on the 6th week of gestation to determine clinical pregnancy.
Data were analyzed using SPSS 16 (Statistical Package for the Social Science) (Chicago, Illinois: SPSS Inc.). The significance of the difference between experimental and control groups for continuous data was assessed using the student's t-test. Fisher's exact test was used to compare the categorical data. P < 0.05 was considered statistically significant.
Results
In this study, a total of 130 infertile women had been evaluated, while 4 women were excluded due to discontinued cycle. There was no significant difference in age distribution, abdominal circumference, and BMI in two groups. The average age, abdominal circumference, and BMI in groups treated with letrozole + NAC and letrozole + placebo are shown in Table 1.
Table 1.
Mean and standard deviation of age, waist circumference, and body mass index in two groups

The average size of follicle in groups being treated by letrozole + NAC and letrozole + placebo was 15.8 ± 3.5 and 13.9 ± 3.7, respectively, and the size of follicle was considerably larger in letrozole + NAC group (P = 0.007). Based on the results, 19 patients of letrozole + NAC group and 9 of letrozole + placebo group had appropriate follicle size (31.7% vs. 16.1%), and the difference between the two groups was significant. Follicle size distribution is shown in Figure 2.
Figure 2.
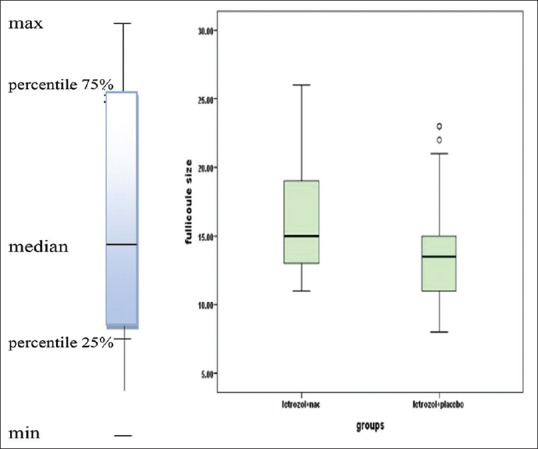
Median and percentile of 25% and 75% of follicle size in two groups
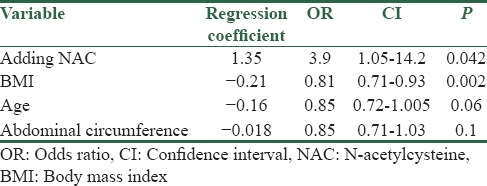
Based on the results obtained, induction of ovulation was achieved in twenty patients in letrozole + NAC group and nine in letrozole + placebo group (33.3% vs. 16.1%), and induction of ovulation in letrozole + NAC group was considerably higher (P = 0.032). Furthermore, pregnancy rate was considerably higher in letrozole + NAC group (P = 0.045). Results are shown in Table 2. Logistic regression analysis with backward conditional method on results obtained showed increased chance of pregnancy by 3.9 times using letrozole + NAC. In addition, patient's BMI had considerable impact on the chance of pregnancy in a way that increased BMI decreases the chance of pregnancy by 0.81 times, and according to the test, age and abdominal circumference had no significant impact on the chance of pregnancy [Table 3].
Table 2.
Frequency distribution of ovulation induction and succeed in pregnancy in two groups
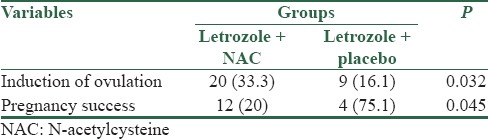
Table 3.
Odds ratio, confidence interval, and a significant level variables on the chances of becoming pregnant
Discussion
Infertility in women with polycystic ovary is a serious problem and several ways have been processed to succeed in their pregnancy. Letrozole has considerably increased the chance of pregnancy in these women, but success rate in this method is not certain and reaches to 20% in the best condition. On the other hand, there has been no research on the impact of adding NAC to letrozole, so this study uniquely explores the impact of letrozole alone and letrozole with NAC on pregnancy rate in infertile women with PCOS.
A total of 130 infertile women with polycystic ovary were evaluated in two groups of 65 patients receiving letrozole + placebo and letrozole + NAC, in which there was no significant difference in the age distribution, BMI, and abdominal circumference.
Based on the results, patients receiving letrozole + NAC had bigger size follicle and better chance in pregnancy compared to those receiving letrozole alone. In a study by Holzer et al., it was suggested that letrozole is successful in ovulation and pregnancy induction with the rate of 70%–84% and 20%–27%, respectively[15] that is similar to our study with 20% pregnancy rate. In addition, other studies demonstrated that adding NAC to clomiphene increases the chance of pregnancy and induction of ovulation in infertile women with PCOS. For example, in a study conducted by Riyahinezhad et al. in 2015, adding NAC to clomiphene has significantly increased the size of follicle (about 0.64 mm) and improved the pregnancy rate in PCOS women with infertility[16] comparing to our study, in which increasing the size of follicle was about 2 mm [Figure 1]. Rizk et al. also found increased pregnancy rate in CC-resistant PCOS patients with CC plus NAC administration.[17] Our data support the results of their study, but based on our findings, NAC may be beneficial as an adjuvant to CC for the induction of ovulation in a more expanded range of PCOS patients and not just limited to CC-resistant PCOS women, though further investigation is required to demonstrate certain impact of NAC on pregnancy rate. It is noteworthy that there have been studies on NAC impact on metabolism and hormonal variation in women with polycystic ovary and positive effects have been observed. In a study by Fulghesu et al., NAC administration has reduced the insulin area under the curve after OGTT) Oral Glucose Tolerance Test) and increased the peripheral insulin sensitivity.[12] A significant drop in testosterone level and free androgen index was also demonstrated with NAC treatment in PCOS patients in their study.[12] Kilicokman et al. had described NAC as an effective medication for reducing the serum insulin and testosterone levels and improving the homocysteine status as well as lipid profiles among PCOS patients.[13] In our previous study, we also observed a significant decrease in weight, BMI, waist/hip ratio, fasting blood sugar, serum insulin, total cholesterol, low-density lipoprotein levels, and homeostasis model assessment insulin resistance index after a 6-week treatment with NAC in PCOS patients.[18] High-density lipoprotein levels were also elevated significantly and NAC improved the lipid profile, hormonal levels, and ovulation status in women with PCOS in that study.[18] It has also been shown that treatment with NAC plus L-arginine for a long time may restore gonadal function in PCOS with improvement of insulin sensitivity.[19] Further studies are required to evaluate the beneficial effects of NAC on hormonal and metabolic profiles of PCOS patients in comparison with other insulin-sensitizing agents, such as metformin.
In addition to the NAC's effects on insulin-sensitizing and androgen-reducing, some other biological effects of NAC, such as inhibition of phospholipid metabolism, anti-apoptotic and antioxidant effects, pro-inflammatory cytokine release and protease activity, cause to Improve folliculogenesis in PCOS patients.[20,21]
However, there are also some unknown points about its effects on the ovulation and pregnancy.
Conclusion
Based on the results, NAC increases letrozole success rate in induction of ovulation in infertile women with PCOS and is recommended with letrozole considering the desired effect of NAC on estrogen, androgen, and lipid profile level in woman with PCOS. Further studies are required to approve decisive influence of NAC on drugs such as letrozole and clomiphene.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Goodarzi MO, Dumesic DA, Chazenbalk G, Azziz R. Polycystic ovary syndrome: Etiology, pathogenesis and diagnosis. Nat Rev Endocrinol. 2011;7:219–31. doi: 10.1038/nrendo.2010.217. [DOI] [PubMed] [Google Scholar]
- 2.Kovacs G, Wood C. The current status of polycystic ovary syndrome. Aust N Z J Obstet Gynaecol. 2001;41:65–8. doi: 10.1111/j.1479-828x.2001.tb01296.x. [DOI] [PubMed] [Google Scholar]
- 3.Guzick D. Polycystic ovary syndrome: Symptomatology, pathophysiology, and epidemiology. Am J Obstet Gynecol. 1998;179:S89–93. doi: 10.1016/s0002-9378(98)70238-8. [DOI] [PubMed] [Google Scholar]
- 4.Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004;19:41–7. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 5.Kitzinger C, Willmott J. 'The thief of womanhood': Women's experience of polycystic ovarian syndrome. Soc Sci Med. 2002;54:349–61. doi: 10.1016/s0277-9536(01)00034-x. [DOI] [PubMed] [Google Scholar]
- 6.Venkatesan AM, Dunaif A, Corbould A. Insulin resistance in polycystic ovary syndrome: Progress and paradoxes. Recent Prog Horm Res. 2001;56:295–308. doi: 10.1210/rp.56.1.295. [DOI] [PubMed] [Google Scholar]
- 7.Lanzone A, Fulghesu AM, Andreani CL, Apa R, Fortini A, Caruso A, et al. Insulin secretion in polycystic ovarian disease: Effect of ovarian suppression by GnRH agonist. Hum Reprod. 1990;5:143–9. doi: 10.1093/oxfordjournals.humrep.a137058. [DOI] [PubMed] [Google Scholar]
- 8.Utiger RD. Insulin and the polycystic ovary syndrome. N Engl J Med. 1996;335:657–8. doi: 10.1056/NEJM199608293350909. [DOI] [PubMed] [Google Scholar]
- 9.Badawy A, Mosbah A, Shady M. Anastrozole or letrozole for ovulation induction in clomiphene-resistant women with polycystic ovarian syndrome: A prospective randomized trial. Fertil Steril. 2008;89:1209–12. doi: 10.1016/j.fertnstert.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 10.Imani B, Eijkemans MJ, te Velde ER, Habbema JD, Fauser BC. A nomogram to predict the probability of live birth after clomiphene citrate induction of ovulation in normogonadotropic oligoamenorrheic infertility. Fertil Steril. 2002;77:91–7. doi: 10.1016/s0015-0282(01)02929-6. [DOI] [PubMed] [Google Scholar]
- 11.Requena A, Herrero J, Landeras J, Navarro E, Neyro JL, Salvador C, et al. Use of letrozole in assisted reproduction: A systematic review and meta-analysis. Hum Reprod Update. 2008;14:571–82. doi: 10.1093/humupd/dmn033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fulghesu AM, Ciampelli M, Muzj G, Belosi C, Selvaggi L, Ayala GF, et al. N-acetyl-cysteine treatment improves insulin sensitivity in women with polycystic ovary syndrome. Fertil Steril. 2002;77:1128–35. doi: 10.1016/s0015-0282(02)03133-3. [DOI] [PubMed] [Google Scholar]
- 13.Kilicokman T, Kucukm N-acetyl cysteine treatment for polycystic ovary syndrome. IntjGynaecolobstet. 2004;85:296–7. doi: 10.1016/j.ijgo.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Pieper GM, Siebeneich W. Oral administration of the antioxidant, N-acetylcysteine, abrogates diabetes-induced endothelial dysfunction. J Cardiovasc Pharmacol. 1998;32:101–5. doi: 10.1097/00005344-199807000-00016. [DOI] [PubMed] [Google Scholar]
- 15.Holzer H, Casper R, Tulandi T. A new era in ovulation induction. Fertil Steril. 2006;85:277–84. doi: 10.1016/j.fertnstert.2005.05.078. [DOI] [PubMed] [Google Scholar]
- 16.Riyahinezhad S, Tehrani HG, Movahedi M, Soltani N, Haghighat F. The effectiveness of using of N acetyl cysteine together with clomiphene citrate on ovulation indication in the patients with polycystic ovary syndrome referred to Isfahan reproduction and infertility center, 2013. J Sci Res Dev. 2015;2:210–4. [Google Scholar]
- 17.Rizk AY, Bedaiwy MA, Al-Inany HG. N-acetyl-cysteine is a novel adjuvant to clomiphene citrate in clomiphene citrate-resistant patients with polycystic ovary syndrome. Fertil Steril. 2005;83:367–70. doi: 10.1016/j.fertnstert.2004.07.960. [DOI] [PubMed] [Google Scholar]
- 18.Salehpour S, Tohidi M, Akhound MR, Amirzargar N. Nacetyl cysteine, a novel remedy for polycystic ovarian syndrome. Int J Fuzzy Syst. 2009;3:66–7. [Google Scholar]
- 19.Masha A, Manieri C, Dinatale S, Bruno GA, Ghigo E, Martina V, et al. Prolonged treatment with N-acetylcysteine and L-arginine restores gonadal function in patients with polycystic ovary syndrome. J Endocrinol Invest. 2009;32:870–2. doi: 10.1007/BF03345763. [DOI] [PubMed] [Google Scholar]
- 20.De Mattia G, Bravi MC, Laurenti O, Cassone-Faldetta M, Proietti A, De Luca O, et al. Reduction of oxidative stress by oral N-acetyl-L-cysteine treatment decreases plasma soluble vascular cell adhesion molecule-1 concentrations in non-obese, non-dyslipidaemic, normotensive, patients with non-insulin-dependent diabetes. Diabetologia. 1998;41:1392–6. doi: 10.1007/s001250051082. [DOI] [PubMed] [Google Scholar]
- 21.Odetti P, Pesce C, Traverso N, Menini S, Maineri EP, Cosso L, et al. Comparative trial of N-acetyl-cysteine, taurine, and oxerutin on skin and kidney damage in long-term experimental diabetes. Diabetes. 2003;52:499–505. doi: 10.2337/diabetes.52.2.499. [DOI] [PubMed] [Google Scholar]


