Abstract
Objective:
The objective of this study was to evaluate the extent of self-medication of antibiotics and dispensing practices in Kenya.
Methods:
A cross-sectional study was carried out at three selected pharmacies in Nairobi (Kenya), between January and March 2017, targeting principally antibiotic prescriptions for systemic use issued and dispensed as well as antibiotics sold over-the-counter without a prescription. The quality of antibiotics prescribed and dispensed was assessed against key WHO and other criteria. Benchmarking was used to assess the quality of antibiotics prescribed as there are no predetermined levels, just guidance and the rationale. Key indicators included: utilization of penicillins, percentage utilization of third-and fourth-generation of cephalosporins versus first and second generation, utilization of macrolides including lincosamides and utilization of quinolones as a percent of total systemic antibiotic use.
Findings:
There was a low level of dispensing of antibiotics without a prescription with over 90% (94.1%) of antibiotics dispensed with a valid prescription. The most common antibiotics dispensed were the penicillins at just over 50% of all antibiotics, the cephalosporins at over 12% (12.6%) and the fluoroquinolones at just under 12% (11.7%). There were concerns with high use of third and fourth generation cephalosporins versus first- and second-generation as well as co-amoxiclav versus other penicillins.
Conclusion:
Low levels of self-medication of antibiotics and high adherence to quality standards for dispensing are encouraging and provide direction to other countries. Educational initiatives are needed though to address high levels of co-amoxiclav prescribing.
KEYWORDS: Antibiotics, dispensing, Kenya, self-medication
INTRODUCTION
The inappropriate use of antibiotics appreciably increases antimicrobial resistance (AMR), increasing morbidity, mortality, and costs.[1,2,3,4] AMR is exacerbated by inappropriate prescribing of antibiotics for predominantly viral infections, as well as inappropriate dispensing and self-medication with antibiotics,[5,6] which is common in Africa with their co-payment levels, limited availability of physicians, and easy access to pharmacists. In addition, the perceived effectiveness of antibiotics including for minor complaints, and often patients cannot afford to see both a physician and purchase prescribed medicines, mean patients often just seek help from community pharmacies.[6,7,8] This recognizes the importance of pharmacists within the healthcare arena, enhanced by their training.[9,10,11] However, previous research in Kenya ascertained that more than 70% of pharmacists did not ask patients for a prescription before dispensing antibiotics, which is a concern as these are considered prescription-only medicines.[12]
There is also lack of knowledge surrounding the dispensing process in Kenya and what questions are asked if patients purchase antibiotics without a prescription. Both are important as dispensing is an integral service provided by pharmacists as part of the Medication Management Cycle, and separation of prescribing and dispensing provides a safety mechanism, ensuring an independent review of prescriptions before starting treatment.[13] In addition, there are issues surrounding the appropriateness, compliance, and costs of medicines that need to be discussed with patients to enhance their appropriate use.[14] The choice of antibiotic is also important to reduce AMR, with the WHO and others producing quality indicators for benchmarking including narrow versus broad-spectrum antibiotics.[15,16]
Consequently, there is a need to address this knowledge gap in Kenya as the country refines and implements its national antibiotic strategy. These include (i) determining the types of antibiotics commonly prescribed and dispensed in community pharmacies including the rate of self-medication and the extent of prescribing and dispensing of antibiotics for predominantly viral infections; (ii) identifying the nature of health professionals who prescribe and dispense antibiotics; (iii) documenting the current dispensing process; (iv) investigating current antibiotic documentation policies and practices against agreed quality criteria.
METHODS
A cross-sectional study was carried out at three selected pharmacies in Nairobi, among 19 pharmacies approved by the School of Pharmacy, University of Nairobi, between January and March 2017, targeting principally antibiotic prescriptions for systemic use (ATC J01),[17] issued and dispensed as well as antibiotics sold over-the-counter without a prescription.
Three sites were purposively selected on the basis of the socioeconomic status of their clientele. Most of the prescriptions of antibiotics are generated in the central business district of Nairobi (low socioeconomic area), and two sites (1 and 2) were selected form this area. In addition, pharmacies in low socioeconomic areas are more likely to dispense antibiotics without a prescription. The third site is located near the School of Pharmacy, and it serves wealthy clientele (high socioeconomic area). Random sampling was undertaken to avoid selection bias, with the 19 eligible pharmacies divided into two groups based on their location. Each pharmacy is a rotation site for University of Nairobi students for experiential learning. The final three pharmacies were selected by choosing every fourth pharmacy on the list. The aim of the study was subsequently discussed with the owner and staff to gain their agreement, and they were assured that the findings would not be used against them.
Within each site, prescriptions were selected if they contained an antibiotic and were received on the day the researcher (MMM) was present. The researcher (MMM) participated in the dispensing activities including wearing a laboratory coat to blend in and observed any dispensing of antimicrobials including any self-medication sale. The three pharmacies were visited randomly during each month (one pharmacy was selected each month) to help reduce bias.
The prescriptions were subsequently recorded to collect information on the type of antibiotic dispensed, the age of the patient (child or adult) and prescriber information. The researcher (MMM) also made a detailed observation of the dispensing process using a data collection tool. For instance, the labels of the dispensed items were examined to identify possible labeling errors, and the researcher observed if patients were counseled before leaving the pharmacy. The researcher also observed if any patients were dispensed antibiotics for influenza or a common cold. These two were selected as upper respiratory tract infections are common in ambulatory care, and patients should not be given antibiotics initially for these conditions as they are predominantly viral in origin.[3,4,5,6,7] The key information collected with the aid of a specifically designed data collection form included reviewing the dispensing process against Australian guidance.[13] This involved key areas including (i) accepting and checking the prescription; (ii) reviewing and process of the prescription; (iii) selecting the medicine, preparing the prescription and checking it; (iv) labeling and assembling the prescription, and (v) counselling the patient/their care provider.
The quality of antibiotics prescribed and dispensed was assessed against key WHO and other criteria.[4,15,16,18,19,20] Benchmarking was used to assess the quality of antibiotics prescribed as there are no predetermined levels, just guidance, and the rationale. Key indicators included.
Utilization of penicillins (J01C) as a percentage of total antibiotic systemic use as well as penicillins versus combinations (co-amoxiclav) as the high use of co-amoxiclav increases side effects for patients as well as resistance; consequently, it is recommended as second line after amoxicillin. Amoxicillin is also suggested especially in children versus broad-spectrum antibiotics such as cephalosporins and macrolides
Percentage utilization of third- and fourth-generation of cephalosporins (J01DD and DE) versus first and second generation (J01DB and DC), which enhances AMR rates and increases costs, as well as concerns with Clostridium difficile
Utilization of macrolides including lincosamides (J01F) as a percentage of total systemic antibiotic use
Utilization of quinolones (J01M) as a percentage of total systemic antibiotic use as concerns with their costs and growing resistance rates.
Overall, the WHO identified the fluoroquinolones, third-and fourth-generation cephalosporins, macrolides, and glycopeptides as being of the highest priority for risk management to improve future use.[4,15]
The quality of antibiotics prescribed and dispensed will also be assessed against current antibiotic prescribing and dispensing patterns among former Soviet Union Republics and Turkey as they typically have high copayment levels, variable antibiotic use by class, and variable enforcement of laws restricting self-medication with antibiotics.[4,15]
To determine the number of prescriptions that would be selected, Fischer's formula was used, since this was a cross-sectional study:[21]
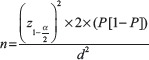
Where;
n = sample size (estimated)
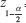 = the standard normal variate (at 5% type 1 error [P < 0.05] is 1.96 and at 1% type 1 error [P < 0.01] is 2.58). P values are considered statistically significant below 0.05, hence 1.96 was used in the formula
= the standard normal variate (at 5% type 1 error [P < 0.05] is 1.96 and at 1% type 1 error [P < 0.01] is 2.58). P values are considered statistically significant below 0.05, hence 1.96 was used in the formulaP = the estimated number of patients obtaining their antibiotics from community pharmacies, estimated at a 17.35% based on a previous study in Jordan where high rates of self-medication were observed.[22] Jordan was chosen as this is equivalent to the Nairobi regarding socioeconomics as well as perceived antibiotic prescribing and dispensing practices
d = the desired degree of accuracy for the study (5%).
The estimated sample size was 220 patients.
Data were entered on Epi info software (Centers for Disease Control and Prevention, Atlanta, GA) and analyzed using STATA (StataCorp. LLC, Texas, USA). All variables were subjected to descriptive analysis. The numerical values were subjected to tests for normal variation such as the Shapiro–Wilk test. Variables that were not normally distributed were potentially summarized as a median and interquartile range. Normally distributed variables were summarized as means and standard deviations where appropriate.
Inferential data analysis was conducted using one way ANOVA, the Kruskal–Wallis test and the Pearson's Chi-square test to compare the distribution of continuous and categorical variables across the three study sites. Bivariate analysis was undertaken to assess whether there was any statistically significant association between the antibiotics prescribed, patients and practice variables.
Ethical approval was given by Kenyatta National Hospital-University of Nairobi Ethics Research Committee (KNH-UoN ERC) and all pharmacies gave consent to participate in the study.
RESULTS
Details of antibiotics dispensed
A total of 222 prescriptions were analyzed during the study, with 61.6% being for females. There was no breakdown of patients' ages apart from recording adults (86.3%) and children (13.7%) as this information was not recorded on the prescription. Antibiotics were only dispensed on patient request for seven patients (3.15%) [Table 1].
Table 1.
Dispensing process undertaken (n=222)
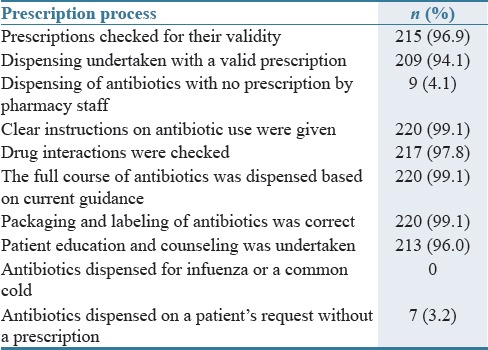
Ambulatory care clinics were the most predominant source of antimicrobials (64%) followed by hospitals (20.3%), while those generated in pharmacies were only 12.6%. 3.1% of prescriptions had an unknown source. Table 2 contains further details of the prescribers by the site. There was no statistically significant association between patient gender, their age (adult or child), and the antimicrobial prescribed and dispensed. However, there was a statistically significant difference between the antimicrobials prescribed [Figure 1] and whether these were from a hospital (P = 0.019) or clinic (P = 0.017). As seen [Table 2], different cadres of professionals prescribed the various antibiotics. There were intra-site differences with regard to the prescribers, and these were statistically significant (P = 0.000). Site 3 received most of the prescriptions from qualified personnel. The source of ten antibiotic prescriptions was unknown.
Table 2.
Prescribers of antibiotics by site (n=213)
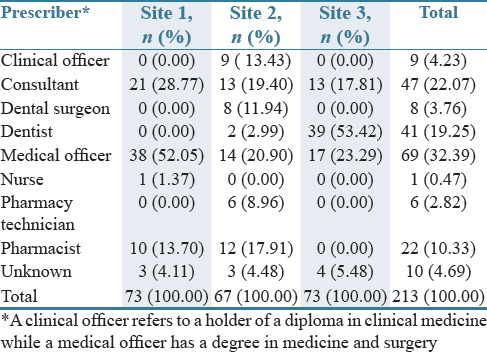
Figure 1.
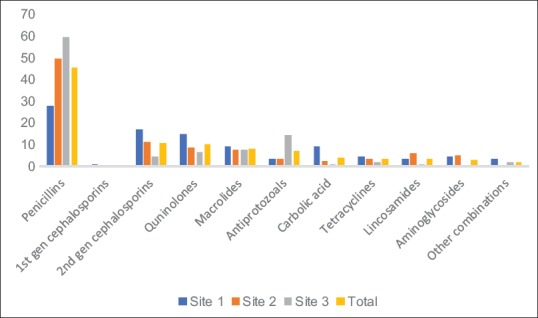
Antimicrobial classes prescribed during the study in percentages (n = 254)
NB: 86, 78 and 90 antibiotics were dispensed in Sites 1, 2, and 3, respectively
The most commonly prescribed antibiotics were the penicillins (J01C) (n = 117, 46.1%), followed by the cephalosporins (overall n = 29, 11.41%), fluoroquinolones (n = 26, 10.24%), and macrolides (n = 21, 8.27%) [Figure 1]. There were statistically significance differences in the dispensing of antibiotic classes among the three sites (P = 0.000). There was though no substitution of a prescribed antibiotic.
Consultants and medical officers were more likely to prescribe third-generation cephalosporins, with half of the aminoglycosides prescribed by medical officers. Quinolones and macrolides were also more likely to be prescribed by medical officers.
Figure 2 describes antibiotics dispensed including those dispensed without a prescription against agreed quality indicators and against a number of former Soviet Union Republics and Turkey.[4,15]
Figure 2.
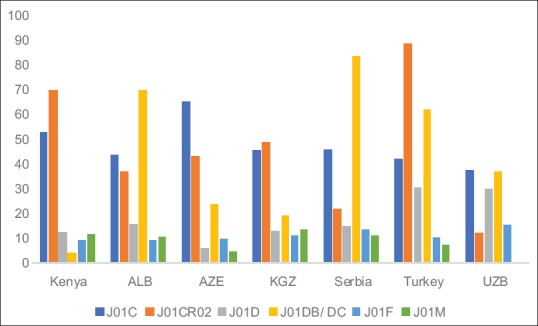
Quality indicators for antibiotics based on percentages
NB: J01C = % Penicillins versus total antibiotics; J01CR02 = % combination penicillins versus % penicillins including amoxicillin; J01D = % cephalosporins versus total antibiotics; J01DB/DC = % first- and second-generation cephalosporins versus all cephalosporins; J01F = % macrolides versus all antibiotics and J01M = % fluoroquinolones versus all antibiotics. ALB = Albania, AZE = Azerbaijan, KGZ = Kyrgyzstan; UZB = Uzbekistan
Dispensing process
Of the 222 antibiotic prescriptions, 84% were dispensed by technicians, with the remainder by a pharmacist. Table 1 gives details of the dispensing process itself against acknowledged quality standards, with high levels of adherence to agreed quality standards. A valid prescription refers to one which meets the legal requirements for a prescription, is clearly written, can be understood and comes from a credible prescriber.
All community pharmacies documented all antibiotic prescriptions as soon as they were dispensed. Two out of the three pharmacies had their prescriptions stapled and placed in files, while the other filed their prescriptions. They also had a handwritten version in a counter book for back-up. Among the prescriptions analyzed, only 4.5% were not well documented.
With respect to the level of pharmacy prescriber, pharmacy technicians did not miss out any steps, while pharmacists missed some steps (P = 0.01). Amoxicillin, co-amoxiclav, ampiclox, flucloxacillin, and the tetracyclines were the antibiotics dispensed following patient requests with no prescription (n = 7).
DISCUSSION
Our study reported low levels of self-medication with antibiotics in Kenya [Table 1], comparing favorably against previous studies in Kenya and other African countries with their high levels of self-medication with antibiotics, ranging from over 30% up to 100%.[2,6,7,8] The low level may be explained by pharmacies in Nairobi being run by qualified personnel with high levels of training, which could be different to rural areas in Kenya. Having said this, the training of pharmacy technicians in Kenya focuses mainly on dispensing while pharmacist training in Kenya is largely clinical. Consequently, most dispensing is undertaken by trained technicians in Kenya, who while in theory are not legally allowed to educate patients and make consultations do so commonly in community pharmacies. Encouragingly, for patients without a prescription, history taking by the pharmacists or pharmacy technicians typically informed the subsequent dispensing of antibiotics. This compares favorably with a recent study in the Republic of Srpska.[9]
Encouragingly as well, at no time were antibiotics prescribed or dispensed patients with for a common cold or influenza [Table 1]. Where patients reported these symptoms, remedies such as cough and cold syrups and lozenges were often dispensed. This is contrary to Saudi Arabia where 68.4% of antibiotics dispensed without a prescription were for coughs and colds, in Vietnam where nearly one-third of all antibiotics sold at the pharmacy were for a cough,[23,24] as well as Uganda.[7]
In addition, there was high use of penicillins as well as relatively low use of cephalosporins, macrolides, and fluoroquinolones among the prescribers [Table 2 and Figure 1]. This compares well with a number of former Soviet Union republics and Turkey [Figure 2]. However, there were areas of concern. These included high levels of prescribing of co-amoxiclav as well as third- and fourth-generation cephalosporins (where cephalosporins were prescribed), which we believe can be attributed to pharmaceutical marketing and its influence in Kenya. This needs to be addressed in the future studies.
Overall, there was a high level of adherence to quality standards for the dispensing process for antibiotics, including appropriate antibiotic use. This is similar to studies in Saudi Arabia and Zambia[8,23] and could be attributed to the fact that the pharmacies chosen are certified internship centers which have qualified well-trained personnel. This compares with Tanzania were the variable training of personnel in private pharmacies and medicine outlets resulted in concerns with irrational dispensing of self-medicated treatments for uncomplicated malaria.[25] All the prescribed antibiotics were given as per the prescription, and no substitution was performed, largely because prescriptions were sourced from qualified medical personnel. The prescription and dispensing processes were carried out in the suggested way, and patients were typically given clear instructions on how to take their antibiotics [Table 1]. This is in contrast with a recent study in the UAE where only 1.4% of the patients were given instructions on how to use antibiotics and none were informed of their potential side effects[26] and the Republic of Srpska.[9]
Encouragingly as well, all antibiotics dispensed were documented in an antibiotic register and all prescriptions stapled together and stored in accordance with the Pharmacy and Poisons Act.[27] However, all the documentation was manual, and there were no electronic records, despite this being a worldwide trend.[28]
The limitations were that only three pharmacies were sampled, which could introduce selection bias especially as certified internship centers should typically adhere to good practice. However, randomization was used to minimize this. Dispensing practices could also be different in rural Kenya. Finally, there is a concern that pharmacists and their technicians might alter their practices if they know they are being monitored. However, this was minimized by the researcher (MMM) working randomly dispensing in the three pharmacies so the staff became used to her presence. The use of pseudopatients,[9] as well as extending the study to include rural pharmacists, are ways to address this as Kenya progresses with its National Antibiotic Action Plan since the findings cannot be extended to all Kenya.
It is encouraging to see low levels of self-medication with antibiotics and high levels of adherence to quality dispensing standards aided by comprehensive training. However, educational initiatives are needed to reduce prescribing of co-amoxiclav in favor of amoxicillin as well as increase prescribing of first- and second-generation cephalosporins. We also recommend the production of educational guidelines and booklets to further improve dispensing in pharmacies, especially around antibiotics. Pharmacists should also be educated on electronic records as well as further education on dispensing practices. These suggestions are now being followed up.
AUTHORS' CONTRIBUTION
MMM, SO and MO devised the research, MMM carried out the research and undertook the initial analysis, MMM, SO, FO and BBG wrote the initially draft manuscript. All authors reviewed subsequent drafts and agreed the content of the manuscripts before submission.
Financial support and sponsorship
This write-up was in part supported by a Newton Institutional Links grant awarded to Margaret Oluka by the Academy of Medical Sciences, through the UK Government's Newton Fund program.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.McKay R, Mah A, Law MR, McGrail K, Patrick DM. Systematic review of factors associated with antibiotic prescribing for respiratory tract infections. Antimicrob Agents Chemother. 2016;60:4106–18. doi: 10.1128/AAC.00209-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ocan M, Obuku EA, Bwanga F, Akena D, Richard S, Ogwal-Okeng J, et al. Household antimicrobial self-medication: A systematic review and meta-analysis of the burden, risk factors and outcomes in developing countries. BMC Public Health. 2015;15:742. doi: 10.1186/s12889-015-2109-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Llor C, Bjerrum L. Antimicrobial resistance: Risk associated with antibiotic overuse and initiatives to reduce the problem. Ther Adv Drug Saf. 2014;5:229–41. doi: 10.1177/2042098614554919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abilova V, Kurdi A, Godman B. Ongoing initiatives in Azerbaijan to improve the use of antibiotics; findings and implications. Expert Rev Anti Infect Ther. 2018;16:77–84. doi: 10.1080/14787210.2018.1417835. [DOI] [PubMed] [Google Scholar]
- 5.Dyar OJ, Beović B, Vlahović-Palčevski V, Verheij T, Pulcini C. on behalf of ESGAP (the ESCMID [European Society of Clinical Microbiology and Infectious Diseases] Study Group for Antibiotic Policies). How can we improve antibiotic prescribing in primary care? Expert Rev Anti Infect Ther. 2016;14:403–13. doi: 10.1586/14787210.2016.1151353. [DOI] [PubMed] [Google Scholar]
- 6.Godman B, Fadare J, Kibuule D, Irawati L, Mubita M, Ogunleye O. Initiatives across countries to reduce antibiotic utilization and resistance patterns; impact and implications. In: Arora G, Sajid A, Kalia V, editors. Drug Resistance in Bacteria, Fungi, Malaria, and Cancer. Switzerland: Springer International Publishing; 2017. [Google Scholar]
- 7.Kibuule D, Kagoya HR, Godman B. Antibiotic use in acute respiratory infections in under-fives in Uganda: Findings and implications. Expert Rev Anti Infect Ther. 2016;14:863–72. doi: 10.1080/14787210.2016.1206468. [DOI] [PubMed] [Google Scholar]
- 8.Kalungia AC, Burger J, Godman B, Costa JO, Simuwelu C. Non-prescription sale and dispensing of antibiotics in community pharmacies in Zambia. Expert Rev Anti Infect Ther. 2016;14:1215–23. doi: 10.1080/14787210.2016.1227702. [DOI] [PubMed] [Google Scholar]
- 9.Marković-Peković V, Grubiša N, Burger J, Bojanić L, Godman B. Initiatives to reduce nonprescription sales and dispensing of antibiotics: Findings and implications. J Res Pharm Pract. 2017;6:120–5. doi: 10.4103/jrpp.JRPP_17_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weller TM, Jamieson CE. The expanding role of the antibiotic pharmacist. J Antimicrob Chemother. 2004;54:295–8. doi: 10.1093/jac/dkh327. [DOI] [PubMed] [Google Scholar]
- 11.Fleming N, Barbar S, Ashiru-Oredope D. Pharmacists have a critical role in the conservation of effective antibiotics. Pharm J. 2011;287:465. [Google Scholar]
- 12.Karambu I. Abuse of ‘Prescription Only’ Drugs on the Rise. [Last accessed on 2017 Aug 25]. Available from: http://www.businessdailyafrica.com/Corporate-News/-/539550/1116580/-/vra01v/-/index.html .
- 13.Pharmacy Board of Australia. Guidelines for Dispensing of Medicines. [Last accessed on 2017 Aug 25]. Available from: http://www.apps.who.int/medicinedocs/documents/s17807en/s17807en.pdf .
- 14.Eslami N, Eshraghi A, Vaseghi G, Mehdizadeh M, Masjedi M, Mehrpooya M, et al. Pharmacists' knowledge and attitudes towards upper respiratory infections (URI) in Iran: A cross sectional study. Rev Recent Clin Trials. 2016;11:342–5. doi: 10.2174/1574887111666160908170618. [DOI] [PubMed] [Google Scholar]
- 15.WHO. Antimicrobial Medicines Consumption (AMC) Network. 2017. [Last accessed on 2017 Sep 05]. Available from: http://www.euro.who.int/en/publications/abstracts/antimicrobial-medicines-consumption-amc-network-amc-data-20112014-2017 .
- 16.de Bie S, Kaguelidou F, Verhamme KM, De Ridder M, Picelli G, Straus SM, et al. Using prescription patterns in primary care to derive new quality indicators for childhood community antibiotic prescribing. Pediatr Infect Dis J. 2016;35:1317–23. doi: 10.1097/INF.0000000000001324. [DOI] [PubMed] [Google Scholar]
- 17.WHO Collaborating Centre for Drug Statistics Methodology. ATC/DDD Index. [accessed on 2017 Aug 20]. Available from: https://www.whocc.no/
- 18.NHS Scotland. National Therapeutic Indicators 2014/2015. [Last accessed 2017 Sep 07]. Available from: http://www.sehd.scot.nhs.uk/publications/DC20141201nti.pdf .
- 19.Dancer SJ. The problem with cephalosporins. J Antimicrob Chemother. 2001;48:463–78. doi: 10.1093/jac/48.4.463. [DOI] [PubMed] [Google Scholar]
- 20.NICE. Clostridium difficile Infection: Risk with Broad Spectrum Antibiotics. [Last accessed on 2017 Sep 05]. Available from: https://www.nice.org.uk/guidance/esmpb1/resources/clostridium-difficile-infe ction-risk-with-broadspectrum-antibiotics-1502609568697285 .
- 21.Charan J, Biswas T. How to calculate sample size for different study designs in medical research? Indian J Psychol Med. 2013;35:121–6. doi: 10.4103/0253-7176.116232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al-Azzam SI, Al-Husein BA, Alzoubi F, Masadeh MM, Al-Horani MA. Self-medication with antibiotics in Jordanian population. Int J Occup Med Environ Health. 2007;20:373–80. doi: 10.2478/v10001-007-0038-9. [DOI] [PubMed] [Google Scholar]
- 23.Hadi MA, Karami NA, Al-Muwalid AS, Al-Otabi A, Al-Subahi E, Bamomen A, et al. Community pharmacists' knowledge, attitude, and practices towards dispensing antibiotics without prescription (DAwP): A cross-sectional survey in Makkah province, Saudi Arabia. Int J Infect Dis. 2016;47:95–100. doi: 10.1016/j.ijid.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 24.Nga do TT, Chuc NT, Hoa NP, Hoa NQ, Nguyen NT, Loan HT, et al. Antibiotic sales in rural and urban pharmacies in northern Vietnam: An observational study. BMC Pharmacol Toxicol. 2014;15:6. doi: 10.1186/2050-6511-15-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mwita S, Jande M, Marwa K, Hamasaki K, Katabalo D, Burger J. Medicines dispenser's knowledge on the implementation of an artemisinin-based combination therapy policy for the treatment of uncomplicated malaria in Tanzania. J Pharm Health Serv Res. 2017;8:227–33. [Google Scholar]
- 26.Akshar SA, Shamssain M, Metwaly Z. Pharmacists' perceptions of community pharmacy practice in UAE: An overview. IOSR J Pharm. 2014;4:47–56. [Google Scholar]
- 27.National Council for Law Reporting with the Authority of the Attorney-General of Kenya. Pharmacy and Poisons Act. Ch. 244. Revised Edition. 2012. [Last accessed on 2017 Sep 05]. Available from: http://www.kenyalaw.org/kl/fileadmin/pdfdownloads/Acts/PharmacyandPoisonsAct_Cap.244.pdf .
- 28.Fung KW, Kayaalp M, Callaghan F, McDonald CJ. Comparison of electronic pharmacy prescription records with manually collected medication histories in an emergency department. Ann Emerg Med. 2013;62:205–11. doi: 10.1016/j.annemergmed.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]


