Abstract
Objective:
Mouth dryness is one of the most prevalent problems in Intensive Care Units (ICUs). It facilitates dental plaque formation. The aim of this study was to analyze the effects of Aloe vera-Peppermint (Veramin) moisturizing gel on mouth dryness and oral health among patients hospitalized in ICUs.
Methods:
This triple-blind two-group randomized placebo-controlled clinical trial was undertaken in 2016–2017 on a convenient sample of 80 patients. Patients were randomly allocated to an intervention and a placebo group. Oral care for patients in the intervention and the placebo groups was provided for 5 successive days using Veramin moisturizing gel and a placebo gel, respectively. Data were collected at the 1st, 3rd, and 5th days of the study using a demographic and clinical characteristics questionnaire, the Challacombe scale (for mouth dryness assessment), and the Mucosal-Plaque Index (for oral health assessment). The Chi-square, Fisher Exact, Mann–Whitney U, and Friedman tests were used for data analysis.
Findings:
In the 5th day, the mean score of mouth dryness in the intervention group was significantly lower than the placebo group (P = 0.0001). On the other hand, in the third and the 5th days, the oral health mean score in the intervention group was significantly lower than the placebo group (P = 0.0001).
Conclusion:
Veramin moisturizing gel is effective in significantly relieving mouth dryness, preventing dental plaque formation, and improving oral health. Thus, it can be used for improving oral care outcomes in ICUs.
KEYWORDS: Aloe vera, Mentha piperita, oral health, xerostomia
INTRODUCTION
Patients hospitalized in Intensive Care Units (ICUs) may develop mouth dryness[1,2] due to a wide range of factors. For example, transoral endotracheal or orogastric tubes not only cause the mouth to remain open and go dry but also make oral care difficult.[3] Moreover, mouth dryness is accelerated by restricted fluid intake brought about by respiratory, cardiac, or renal problems[4] as well as decreased salivation brought about by factors such as fever, diarrhea, burns, and narcotic therapy.[5]
Mouth dryness causes oral mucositis and gingivitis, dental caries and plaque, and bacterial colonization in the mouth and the throat.[6] Bacterial colonization, in turn, is associated with local and systemic complications such as stomatitis, dental caries, periodontitis, systemic infection, bacteremia, and even arthritis and endocarditis.[7] Moreover, decreased salivation, mouth dryness, mucositis, and bacterial colonization in the mouth and the throat predispose patients to pneumonia.[8]
Oral moisturizing is among the key components of different oral care protocols for intubated ICU patients.[9] There are different techniques for oral moisturizing and mouth dryness prevention.[10] Saliva substitutes or artificial saliva and synthetic gels have been used for many years to moisturize oral mucosa, even though patients are reluctant to use them because of their high costs.[11] Pure water is also used for mouth dryness management.[12] However, water is a suitable environment for pseudomonas growth.[13] Moreover, previous studies reported contradictory results respecting the moisturizing effects of water.[8] Another study also indicated that oral care using water facilitates bacterial movement from the mouth toward the throat and thus increases the risk for ventilator-associated pneumonia.[14] Therefore, some studies noted that oral moisturizers are more effective in protecting oral cavity, removing dental plaque, and preventing bacterial colonization provided that they are prepared and used in jelly forms.[15,16]
In ICUs in Iran, oral moisturizing is performed using normal saline or lemon juice-glycerin preparation.[17] Normal saline has positive effects on oral lesions. Nonetheless, it dries oral mucosa.[13] On the other hand, although lemon juice-glycerin preparation is effective in stimulating salivation, its frequent use may cause mouth dryness. In addition, due to its acidity, it irritates mucosa and accelerates dental decay.[18]
Another treatment option for mouth dryness is medicinal plants. Aloe vera is one of the widely used medicinal plants for the management of health problems.[7] More than 75 active ingredients from inner gel have been identified including vitamins, minerals, enzymes, sugars, anthraquinones or phenolic compounds, lignin, saponins, sterols, amino acids, and salicylic acid.[19] About 99% of the inner jelly of A. vera leaf is water and therefore, it has strong moisturizing effects.[20] Mucopolysaccharides help in binding moisture into the skin and mucosa. It was proposed that the A. vera gel containing products improve skin and mucosa hydration through humectant mechanism.[19] The results of a study showed that A. vera along with other ingredients (like salivary substitute) is effective in relieving xerostomia associated symptoms.[21]
A. vera has wound healing effect due to the presence of Glucomannan, a mannose-rich polysaccharide and gibberellin, a growth hormone, interacts with growth factor receptor on the fibroblast, thereby stimulating its activity and proliferation, which in turn increases collagen synthesis after topical and oral application.[4,22] In addition, the presence of glucomannan and acemannan activate macrophages, stimulate immune system, so A. vera has antibacterial and antiviral effects.[23] Moreover, it has anti-inflammatory effect and inhibits the cyclooxygenase pathway and reduces prostaglandin E2.[24] Therefore, A. vera has been used for the treatment of disorders such as lichen planus, burning mouth syndrome, and mucositis.[25]
The results of a study showed that oral A. vera mouthwash has anti-inflammatory and wound healing properties, thus preventing the development of radiation-induced mucositis. In addition, it has antifungal and immunomodulatory properties, which prevents oral candidiasis in the patients undergoing head-and-neck radiotherapy.[26] However, contradicting this, Su et al., in their study concluded that oral A. vera was not was not effective in eliminating mucositis or improving theses patients' well-being.[27] A study also showed the positive effects of A. vera gel on oral submucous fibrosis.[28] Some other studies also revealed that A. vera has the same antiplaque effects as chlorhexidine.[29] However, a study reported that it had no significant effects on oral health.[30]
Peppermint essential oil is another herbal preparation with strong antibacterial and cooling effects.[31] As a safe herbal preparation,[32] peppermint essential oil has been found to be effective in alleviating the pain associated with aphthous stomatitis[33] and managing dental plaque.[34]
To the best of our knowledge, no study has yet investigated the effects of A. vera on mouth dryness among intubated patients. Moreover, the results of previous studies respecting the effects of A. vera on mouth dryness and oral health are contradictory. Consequently, this study was undertaken to fill these gaps. The purpose of this study was to analyze the effects of Veramin moisturizing gel on mouth dryness and oral health among ICU patients.
METHODS
This was a triple-blind two-group randomized placebo-controlled clinical trial. Study population consisted of all intubated patients hospitalized from November 2016 to August 2017 in the ICU of a teaching hospital in Isfahan, Iran. The ICU was a mixed one with 21 beds. Sampling was done conveniently. Inclusion criteria were an age of 18–65, an endotracheal tube in place through the mouth, ICU hospitalization of <24 h, no hospitalization in other hospital wards before ICU admission, no history of autoimmune disorders, pneumonia, or sepsis, no pregnancy, no known sensitivity to herbal ingredients, no denture, and no obvious oral or perioral lesions. Patients were excluded if they died, were transferred from ICU to other settings, developed severe oral lesions, or their legal guardian chose to withdraw from the study.
Using the results of an earlier study[2] and with a Type II error of 0.05 and a power of 0.80, necessary number of patients for each study group was calculated to be 35. Given an attrition rate of 10%, 40 patients were recruited to each group. Randomization was performed using simple randomization based on random numbers tables by an independent person who was unaware of study. All randomization numbers were concealed in separate envelopes that were sealed, opaque, serially numbered, and kept by the hospital radiologist.
Data collection tools were a demographic and clinical characteristics questionnaire, the Challacombe Scale and the Mucosal-Plaque Index. The items of the first tool were age, gender, underlying condition, Acute Physiologic Assessment and Chronic Health Evaluation II (APACHE II) score, medications, and smoking history. This questionnaire was completed in the 1st day of the study.
The primary outcome was mouth dryness which was assessed by a dentist in the first (this assessment was baseline assessments before the first session intervention), 3rd, and 5th days of the study (T1–T3) using the Challacombe Scale. The scale was developed in the King's College London Dental Institute. As a visual scale, the Challacombe scale assesses mouth dryness and treatment outcomes over time. It is scored 1–10, with higher scores representing severer dryness.[35] The scale was translated into Persian and 10 critical care nursing faculty members and critical care subspecialists assessed and approved its content validity. Moreover, its reliability was assessed using the interobserver method, in which the first author and the same dentist simultaneously used the scale to rate mouth dryness of 10 patients. Interobserver correlation coefficient was 0.94, denoting the high reliability of the scale.
The secondary outcome was oral health as measured using the Mucosal-Plaque Index by the same dentist at T1–T3. This index consists of a mucosal and a plaque score. The mucosal score includes four items on the degrees of mucosal inflammation and erythema. This score ranges from 1 to 4. The plaque score also includes four items on the amount of dental plaque and ranges from 1 to 4. The sum score of these two components is 2–8. Higher scores stand for poorer oral health. The validity and reliability of this index had been approved in previous studies.[3]
Study intervention was an oral care protocol which was developed based on the protocols used in previous studies.[2,3] At the beginning of the study, three workshops held for the nurses of the study setting to teach them about the oral care protocol. Moreover, an oral care-related educational video clip and a PowerPoint file were given to the nurses. Finally, a poster, depicted the oral care protocol, was hanged in the study setting. All nurses were asked to provide oral care based on educations provided to them in the workshop, video clip, PowerPoint file, and poster. The steps of oral care protocol were as follows: set the pressure of the endotracheal tube cuff at 20–25 mm Hg; Raise the head of the bed by 30°–45°; Perform mouth and throat suction (repeat every 4 h); brush the inner and the outer surfaces of the teeth and the tongue for 2 min using a kids toothbrush and a 0.12% chlorhexidine solution (repeat every 12 h); apply the Veramin gel to all surfaces of the oral mucosa, the gum, and the tongue after brushing (repeat every 4 h); lubricate the lips with Vaseline (repeat every 4 h); remove and clean the oropharyngeal airway, if any, and then place it in the mouth. This protocol was used for all patients in both groups. The only difference between the groups was related to the oral care gel. In other words, the oral care gel for patients in the intervention group was a moisturizing gel containing 100% A. vera jelly, 3% peppermint essential oil, carboxymethyl cellulose, 10% propylene glycol, and 0.1% potassium sorbate, while the gel for the placebo group contained carboxymethyl cellulose, 10% propylene glycol, 0.1% potassium sorbate, and water up to 100%. In addition, the smell of placebo gel is provided with food grade mint flavor, which does not have any therapeutic effect. Both gels were produced by Barij Essence Pharmaceutical Company, Kashan, Iran, and had similar appearance, color, smell, and weight. The manufacturing company had labeled a series of the gels A, and a series B. The authors, the nurses of the study setting, the dentist, and the statistical analyst were blind to the contents of the gel tubes until final data analysis.
Two research assistants were involved in the study after being fully trained regarding the oral care protocol with principal investigator monitored the performance of nurses in different shifts. The nurses adhere to all the steps of the interventions for each patient because the researchers designed an oral care sheet that information documented. To minimize the diffusion of the treatment, we explained and asked staff nurses to cooperate with this study.
Ethical approval and necessary permissions for the study were obtained from the Ethics Committee of Isfahan University of Medical Sciences, Isfahan, Iran (with the codes 2.141 and 295141, respectively). It was also registered in the Iranian Registry of Clinical Trials (with the number IRCT2016120331200N1). Study aim was explained to patients' legal guardians and they were ensured that the data would be handled confidentially and withdrawal from the study would be voluntarily. All of them signed the informed consent form.
Data analysis was carried out through the SPSS software (version 16, SPSS Inc., Chicago, IL, USA). Between-group comparisons respecting demographic and clinical characteristics were done by running the Chi-square, Independent t-test, and the Fisher Exact tests. Moreover, the Mann–Whitney U and the Friedman tests were run to compare the groups respecting mouth dryness and oral health.
RESULTS
The CONSORT diagram of the clinical trial is showed in Figure 1. In total, 80 patients were recruited. Two were excluded from the intervention group due to experiencing death and weaning from ventilator. Moreover, two were excluded from the placebo group due to experiencing death. Therefore, data analysis was done on the data retrieved from 76 patients.
Figure 1.
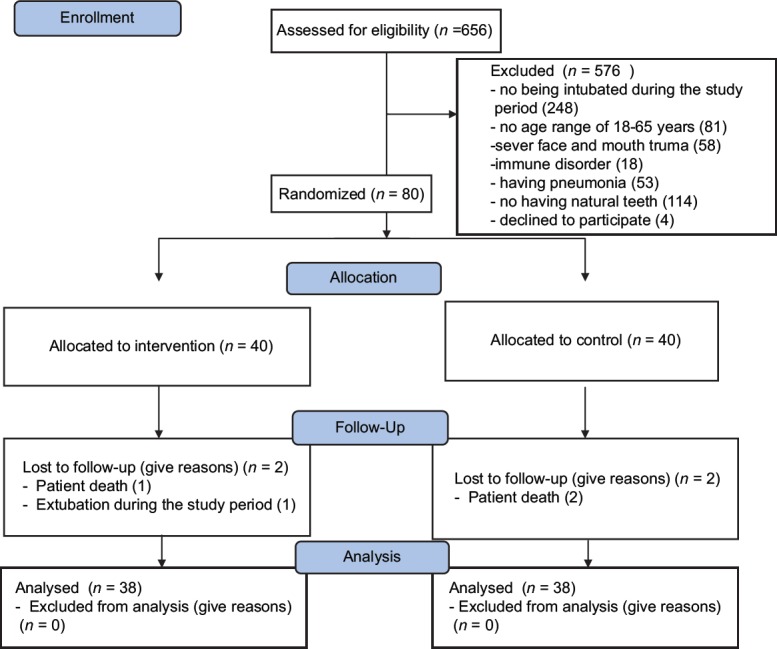
Flow diagram of the study participants
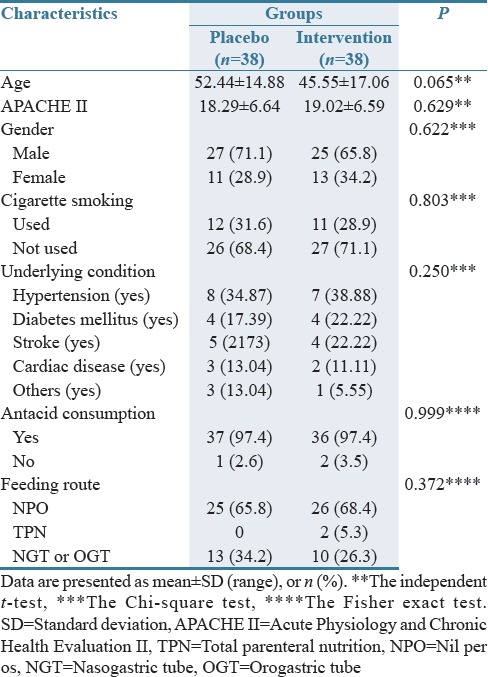
Most participants were male (68%). The Chi-square, Independent t-test, and the Fisher Exact tests revealed no significant differences between the groups respecting patients' age, gender, APACHE II score, antacid consumption, underlying condition, cigarette smoking, and feeding route [P > 0.05; Table 1].
Table 1.
Basic characteristics of the study population
The results of the Mann–Whitney U test revealed that at T1, mouth dryness mean score in the intervention group was significantly greater than the placebo group (Z = 4.48; P = 0.0001). However, the difference was not statistically significant at T2 (Z = 0.596; P = 0.551), while at T3, the mean score of mouth dryness in the intervention group was significantly lower than the placebo group [Z = 6.08; P = 0.0001; Table 2]. Moreover, the Friedman test indicated significant decrease in the mean score of mouth dryness in the intervention group (P = 0.0001) and no significant change in the placebo group (P = 0.325).
Table 2.
Comparison of mouth dryness between two groups at the first, third, and fifth days of the study
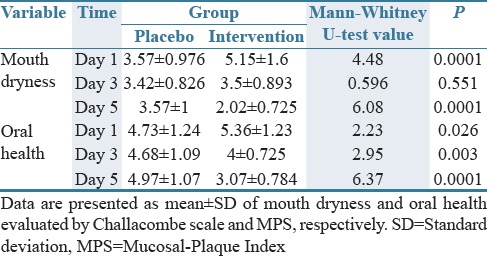
In addition, the results of the Mann–Whitney U test illustrated the significantly greater oral health mean score in the intervention group at T1 compared with the placebo group (Z = 2.23; P = 0.026). However, the oral health mean score in the intervention group was significantly lower than the placebo group both at T2 (Z = 2.95; P = 0.003) and T3 [Z = 6.37; P = 0.0001; Table 2]. In addition, the Friedman test showed that although within-group changes in the mean score of oral health in the placebo group was not statistically significant (P = 0.017), this score in the intervention group significantly decreased from T1 to T3 (P = 0.0001).
Based on these findings, Veramin moisturizing gel alleviates mouth dryness and improves oral health among ICU patients. Moreover, none of the participants reported any adverse effects after receiving Veramin moisturizing gel.
DISCUSSION
The aim of this study was to analyze the effects of Veramin moisturizing gel on mouth dryness and oral health among ICU patients. Findings indicated that the gel had significant effects on mouth dryness and oral health. Water constitutes the largest proportion of the inner jelly of A. vera leaf. Moreover, the jelly contains mucopolysaccharides and amino acids which help moisturize oral mucosa. Therefore, it is used to produce mouthwash preparations. Most previous studies also showed the positive effects of A. vera on oral health. For instance, Chhina et al. found that A. vera mouthwash solution was more effective than chlorhexidine in improving oral health.[24] Gupta et al. also reported that A. vera mouthwash solution was as effective as chlorhexidine in reducing dental plaque severity among students.[29] The findings of these two studies confirm that the anti-plaque effects of A. vera mouthwash solutions are at least the same as those of chlorhexidine. Both studies concluded that due to its cost-effectiveness and safety, A. vera mouthwash can be a good substitute for chlorhexidine. However, in contradiction to our findings, Sahgal et al. reported the ineffectiveness of A. vera gel in reducing plaque severity.[30] This contradiction may be due to the fact that they used 50% A. vera gel for 4 days while we implemented our intervention using 100% A. vera gel and in 5 days. Laboratory evaluations showed that the greatest antiplaque effects of A. vera are at its 100% concentration.[29]
Another component of the gel used in the present study was peppermint essential oil. The previous studies reported that peppermint essential oil has antibacterial, antifungal, and antiviral effects. Yayla also reported that peppermint mouthwash significantly reduced the incidence of chemotherapy-induced oral mucositis. They finally concluded that peppermint mouthwash can be a good option for reducing the incidence and the severity of mucositis.[32] This study faced some limitations including its short follow-up period. Moreover, it was conducted in a single healthcare setting. Future studies are suggested to replicate this study on larger samples of patients and with longer follow-up periods.
Study findings suggest that Veramin moisturizing gel is effective in significantly relieving mouth dryness, preventing dental plaque formation, and improving oral health. Given the cost-effectiveness of A. vera and peppermint harvesting and processing, Veramin gel can be used for improving oral care outcomes in ICUs. To the best of our knowledge, this was the first study into the effects of Veramin gel on mouth dryness and oral health. Therefore, further studies are needed for more conclusive evidence concerning the effectiveness of Veramin moisturizing gel.
AUTHORS' CONTRIBUTION
All authors participated in design, experiments, and gathering information and all of them have read and approved the content of the manuscript. Study design: (VA), (S GH) and (AY); data collection: (VA); data analysis: (HY), (AY) and (VA), manuscript preparation: (HM), (S GH) and (RB).
Financial support and sponsorship
This study is funded by Isfahan University of Medical Science.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
Our sincere appreciation goes to the Research Deputy of Isfahan University of Medical Sciences, president of the School of Nursing and Midwifery and its Research Deputy. The authors want to warmly thank the patients for their participation and the nurses for their collaboration.
REFERENCES
- 1.Puntillo K, Arai SR, Cooper BA, Stotts NA, Nelson JE. A randomized clinical trial of an intervention to relieve thirst and dry mouth in Intensive Care Unit patients. Intensive Care Med. 2014;40:1295–302. doi: 10.1007/s00134-014-3339-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jang CS, Shin YS. Effects of combination oral care on oral health, dry mouth and salivary pH of intubated patients: A randomized controlled trial. Int J Nurs Pract. 2016;22:503–11. doi: 10.1111/ijn.12460. [DOI] [PubMed] [Google Scholar]
- 3.Haghighi A, Shafipour V, Bagheri-Nesami M, Gholipour Baradari A, Yazdani Charati J. The impact of oral care on oral health status and prevention of ventilator-associated pneumonia in critically ill patients. Aust Crit Care. 2017;30:69–73. doi: 10.1016/j.aucc.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Browne JA, Evans D, Christmas LA, Rodriguez M. Pursuing excellence: Development of an oral hygiene protocol for mechanically ventilated patients. Crit Care Nurs Q. 2011;34:25–30. doi: 10.1097/CNQ.0b013e318204809b. [DOI] [PubMed] [Google Scholar]
- 5.Munro CL, Grap MJ. Oral health and care in the Intensive Care Unit: State of the science. Am J Crit Care. 2004;13:25–33. [PubMed] [Google Scholar]
- 6.Klompas M, Speck K, Howell MD, Greene LR, Berenholtz SM. Reappraisal of routine oral care with chlorhexidine gluconate for patients receiving mechanical ventilation: Systematic review and meta-analysis. JAMA Intern Med. 2014;174:751–61. doi: 10.1001/jamainternmed.2014.359. [DOI] [PubMed] [Google Scholar]
- 7.Sahu PK, Giri DD, Singh R, Pandey P, Gupta S, Shrivastava AK, et al. Therapeutic and medicinal uses of Aloe vera: A review. Pharmacol Pharm. 2013;4:599. [Google Scholar]
- 8.Berry AM, Davidson PM. Beyond comfort: Oral hygiene as a critical nursing activity in the Intensive Care Unit. Intensive Crit Care Nurs. 2006;22:318–28. doi: 10.1016/j.iccn.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 9.Booker S, Murff S, Kitko L, Jablonski R. Mouth care to reduce ventilator-associated pneumonia. Am J Nurs. 2013;113:24–30. doi: 10.1097/01.NAJ.0000435343.38287.3a. [DOI] [PubMed] [Google Scholar]
- 10.Epstein JB, Beier Jensen S. Management of hyposalivation and xerostomia: Criteria for treatment strategies. Compend Contin Educ Dent. 2015;36:600–3. [PubMed] [Google Scholar]
- 11.Erdem O, Güngörmüş Z. The effect of royal jelly on oral mucositis in patients undergoing radiotherapy and chemotherapy. Holist Nurs Pract. 2014;28:242–6. doi: 10.1097/HNP.0000000000000033. [DOI] [PubMed] [Google Scholar]
- 12.Kusahara DM, Peterlini MA, Pedreira ML. Oral care with 0.12% chlorhexidine for the prevention of ventilator-associated pneumonia in critically ill children: Randomised, controlled and double blind trial. Int J Nurs Stud. 2012;49:1354–63. doi: 10.1016/j.ijnurstu.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 13.Ames NJ, Sulima P, Yates JM, McCullagh L, Gollins SL, Soeken K, et al. Effects of systematic oral care in critically ill patients: A multicenter study. Am J Crit Care. 2011;20:e103–14. doi: 10.4037/ajcc2011359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takeyasu Y, Yamane GY, Tonogi M, Watanabe Y, Nishikubo S, Serita R, et al. Ventilator-associated pneumonia risk decreased by use of oral moisture gel in oral health care. Bull Tokyo Dent Coll. 2014;55:95–102. doi: 10.2209/tdcpublication.55.95. [DOI] [PubMed] [Google Scholar]
- 15.Kiyoshi-Teo H, Blegen M. Influence of institutional guidelines on oral hygiene practices in Intensive Care Units. Am J Crit Care. 2015;24:309–18. doi: 10.4037/ajcc2015920. [DOI] [PubMed] [Google Scholar]
- 16.Liao YM, Tsai JR, Chou FH. The effectiveness of an oral health care program for preventing ventilator-associated pneumonia. Nurs Crit Care. 2015;20:89–97. doi: 10.1111/nicc.12037. [DOI] [PubMed] [Google Scholar]
- 17.Andrews T, Steen C. A review of oral preventative strategies to reduce ventilator-associated pneumonia. Nurs Crit Care. 2013;18:116–22. doi: 10.1111/nicc.12002. [DOI] [PubMed] [Google Scholar]
- 18.Fields LB. Oral care intervention to reduce incidence of ventilator-associated pneumonia in the neurologic Intensive Care Unit. J Neurosci Nurs. 2008;40:291–8. doi: 10.1097/01376517-200810000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Gupta VK, Malhotra S. Pharmacological attribute of Aloe vera: Revalidation through experimental and clinical studies. Ayu. 2012;33:193–6. doi: 10.4103/0974-8520.105237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hur MH, Park J, Maddock-Jennings W, Kim DO, Lee MS. Reduction of mouth malodour and volatile sulphur compounds in intensive care patients using an essential oil mouthwash. Phytother Res. 2007;21:641–3. doi: 10.1002/ptr.2127. [DOI] [PubMed] [Google Scholar]
- 21.Morales-Bozo I, Rojas G, Ortega-Pinto A, Espinoza I, Soto L, Plaza A, et al. Evaluation of the efficacy of two mouthrinses formulated for the relief of xerostomia of diverse origin in adult subjects. Gerodontology. 2012;29:e1103–12. doi: 10.1111/j.1741-2358.2012.00626.x. [DOI] [PubMed] [Google Scholar]
- 22.Subhash AV, Suneela S, Anuradha C, Bhavani S, Babu MS. The role of Aloe vera in various fields of medicine and dentistry. J Orofac Sci. 2014;6:5. [Google Scholar]
- 23.Radha MH, Laxmipriya NP. Evaluation of biological properties and clinical effectiveness of Aloe vera: A systematic review. J Tradit Complement Med. 2015;5:21–6. doi: 10.1016/j.jtcme.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chhina S, Singh A, Menon I, Singh R, Sharma A, Aggarwal V, et al. Arandomized clinical study for comparative evaluation of Aloe vera and 0.2% chlorhexidine gluconate mouthwash efficacy on de novo plaque formation. J Int Soc Prev Community Dent. 2016;6:251–5. doi: 10.4103/2231-0762.183109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nair GR, Naidu GS, Jain S, Nagi R, Makkad RS, Jha A, et al. Clinical effectiveness of Aloe vera in the management of oral mucosal diseases – A systematic review. J Clin Diagn Res. 2016;10:ZE01–7. doi: 10.7860/JCDR/2016/18142.8222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahmadi A. Potential prevention: Aloe vera mouthwash may reduce radiation-induced oral mucositis in head and neck cancer patients. Chin J Integr Med. 2012;18:635–40. doi: 10.1007/s11655-012-1183-y. [DOI] [PubMed] [Google Scholar]
- 27.Su CK, Mehta V, Ravikumar L, Shah R, Pinto H, Halpern J, et al. Phase II double-blind randomized study comparing oral Aloe vera versus placebo to prevent radiation-related mucositis in patients with head-and-neck neoplasms. Int J Radiat Oncol Biol Phys. 2004;60:171–7. doi: 10.1016/j.ijrobp.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 28.Shanbhag VK. Medical management of oral submucous fibrosis. Asia Pac J Oncol Nurs. 2015;2:51. doi: 10.4103/2347-5625.143766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gupta RK, Gupta D, Bhaskar DJ, Yadav A, Obaid K, Mishra S, et al. Preliminary antiplaque efficacy of Aloe vera mouthwash on 4 day plaque re-growth model: Randomized control trial. Ethiop J Health Sci. 2014;24:139–44. doi: 10.4314/ejhs.v24i2.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sahgal A, Chaturvedi SS, Bagde H, Agrawal P, Suruna R, Limaye M. A randomized control trial to evaluate efficacy of anti-bacterial and anti-inflammatory effect of Aloe vera, pomegranate and chlorhexidine gel against periodontopathogens. J Int Oral Health. 2015;7:33. [Google Scholar]
- 31.McKay DL, Blumberg JB. A review of the bioactivity and potential health benefits of peppermint tea (Mentha piperita L.) Phytother Res. 2006;20:619–33. doi: 10.1002/ptr.1936. [DOI] [PubMed] [Google Scholar]
- 32.Mutluay Yayla E, Izgu N, Ozdemir L, Aslan Erdem S, Kartal M. Sage tea-thyme-peppermint hydrosol oral rinse reduces chemotherapy-induced oral mucositis: A randomized controlled pilot study. Complement Ther Med. 2016;27:58–64. doi: 10.1016/j.ctim.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 33.Kang HY, Na SS, Kim YK. Effects of oral care with essential oil on improvement in oral health status of hospice patients. J Korean Acad Nurs. 2010;40:473–81. doi: 10.4040/jkan.2010.40.4.473. [DOI] [PubMed] [Google Scholar]
- 34.Singh A, Daing A, Dixit J. The effect of herbal, essential oil and chlorhexidine mouthrinse on de novo plaque formation. Int J Dent Hyg. 2013;11:48–52. doi: 10.1111/j.1601-5037.2012.00556.x. [DOI] [PubMed] [Google Scholar]
- 35.Challacombe SJ, Osailan SM, Proctor GB. Clinical scoring scales for assessment of dry mouth. In: Carpenter G, editor. Dry Mouth. Springer, Berlin, Heidelberg: Springer Nature; 2015. [Last accessed on 2018 Jan]. Available from: https://doi.org/10.1007/978-3-642-55154-3_8 . [Google Scholar]


