Abstract
Our 1992 paper, ‘The neural substrates of sensorimotor gating of the startle reflex: a review of recent findings and their implications’, reviewed a series of (then) new and preliminary findings from cross-species studies of prepulse inhibition of the startle reflex, and commented on their implications. At the time that the report was composed, PubMed listed about 40 citations for studies using the search term ‘prepulse inhibition’. In the ensuing 25 years, the field has added about 2700 such reports, reflecting the substantial growth in interest in prepulse inhibition and its utility across a number of different experimental applications. The 30th anniversary of the Journal of Psychopharmacology provides an opportunity to comment briefly on what was described in that 1992 report, how the field has progressed in the subsequent decades, and the paths forward for studies of prepulse inhibition and its use as an operational measure of sensorimotor gating. Among these future paths, we highlight the use of prepulse inhibition as: an endophenotype for genomic studies, and a biomarker for healthy brain circuitry, which may predict sensitivity to psychotherapeutics. Our 1992 report was highly speculative and based on paper-thin empirical data, yet viewed in a certain light, it appears to have contained a basic roadmap for a journey spanning the next 25 years of prepulse inhibition research… and ‘what a long, strange trip it’s been’.
Keywords: Antipsychotic, dopamine, prepulse inhibition, schizophrenia, sensorimotor gating, startle reflex
Introduction
As part of this issue marking the occasion of the 30th anniversary of the Journal of Psychopharmacology, we were invited to comment on our 1992 publication, ‘The neural substrates of sensorimotor gating of the startle reflex: a review of recent findings and their implications’ (Swerdlow et al., 1992b) – on its importance to the field, and on how the field has developed over the past quarter century. With this opportunity, we now revisit the content of that 1992 report, and the three major themes developed within it, including: (a) the concept that sensory, motor or cognitive ‘gating’ might be a ‘domain of function’ that is impaired across neuropsychiatric disorders that otherwise had been viewed as categorically distinct clinical entities – including schizophrenia, obsessive compulsive disorder (OCD), Tourette syndrome (TS), and Huntington’s disease (HD), among others; (b) evidence that an operational measure of sensorimotor gating – prepulse inhibition (PPI) – could be demonstrated in cross-species studies to be regulated by specific neurochemical and anatomical substrates within the limbic cortex, striatum and pallidum, and the pontine tegmentum (limbic ‘CSPP’ circuitry); and (c) the observation that this limbic–motor circuitry appeared to be relevant to disorders characterized by impaired sensory, motor or cognitive ‘gating’. Next, we review the PPI literature that has emerged subsequent to our 1992 publication, and identified areas of convergence and divergence between the perspectives and predictions raised in our report and the findings and directions of the PPI-relevant science that has taken place in these 25 years. Three introductory observations provide relevant context for this ‘review and update’ process.
First, our 1992 review described a substantial amount of unpublished data, from studies ‘in progress’. In fact, at the time that this review was composed, the published neuropsychiatric literature related to PPI totaled about 40 papers, many of which addressed topics that were not directly related to the thematic focus of our review – i.e. brain mechanisms and neuropsychiatric disorders. Thus, much of the experimental ‘evidence’ described in our review – which spanned human and rodent studies, with complex pharmacological and neural circuit-based manipulations – was very preliminary. How we were able to publish a ‘review’ with so much speculation, based on very preliminary data, remains somewhat mysterious to us. But it happened nonetheless, and we are happy to attribute this fact to the wisdom and foresight of the editorial leadership of this journal.
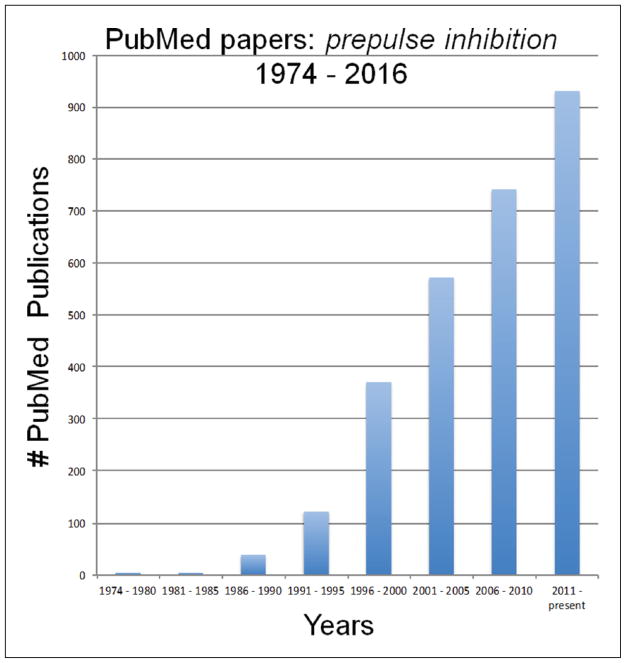
Second, since the submission of our 1992 review, the PPI literature has added approximately 2700 new PubMed articles (Figure 1), at a rate that accelerated in the five years after the publication of this review, and that has remained robust and increasing at a linear pace since that time. Many of these subsequent publications address topics that are thematically related to those described in our 1992 paper, while others address areas that were unanticipated at the time of our report – particularly those related to the genetic regulation of PPI, reflecting the genetic and molecular ‘revolution’ in the neurosciences that has transpired over the past 25 years. We can only comment selectively on this extensive literature; more comprehensive reviews can be found elsewhere (e.g. Powell, 2010; Powell and Geyer, 2002; Powell et al., 2009, 2012; Swerdlow et al., 2008).
Figure 1.
PubMed publications using the search term ‘prepulse inhibition’.
Third, it is impossible to try to credit any single report as the ‘first’ or ‘original’ source of specific ideas or conceptualizations in our field, but our 1992 review – like many reports that we have written in the ensuing decades – took great care in its introductory sections to acknowledge the formative studies of the startle reflex and the ‘primary startle circuit’, conducted and reported by Michael Davis and colleagues in the 1970s and early 1980s (see Davis, 1984). While our 1992 focus on PPI, its regulation by the basal forebrain, and the broader relevance of deficient gating mechanisms to human brain disorders, represented a departure from the literature of that day, it was only possible to make such conceptual advances because of the solid foundational work from Davis and colleagues, related to the neurophysiology of mammalian startle. With this foundation as a ‘leaping point’, the wild speculation within our 1992 review can mostly be assigned to three broad themes.
Theme 1: PPI is impaired across categorically distinct neuropsychiatric disorders
While it is now clear that PPI deficits are not clinically specific, the real catalyst behind the intense investigation of PPI came from the initial reports of PPI deficits in schizophrenia patients, from studies conducted in the laboratory of Enoch Callaway (Braff et al., 1978). Our review presented data both from this original 1978 report, and also from the second report of PPI deficits in schizophrenia patients, which was published contemporaneously with our review (Braff et al., 1992). Findings of deficient PPI in schizophrenia spectrum patients have since been replicated in almost 40 reports in the literature (cf. Swerdlow et al., 2014), despite the emergence and nearly ubiquitous use of PPI-enhancing antipsychotics during the ensuing decades – a clinical reality that makes PPI deficits more difficult to detect and quantify (e.g. Kumari et al., 1999; Weike et al., 2000).
Our 1992 review, however, made the new suggestion that a loss of automatic inhibitory mechanisms might underlie symptoms not only in schizophrenia, but also in other brain disorders, such as those characterized by intrusive thoughts and images (e.g. OCD), sensations (e.g. TS) and movements (e.g. HD). We speculated that such deficient inhibition might be accompanied by reduced levels of PPI. The basis for such speculation, also developed in our review, was the evolving evidence for the role of limbic cortico-striato-pallidothalamic (CSPT) circuitry in both the regulation of PPI, and in the pathophysiology of these other brain disorders (e.g. Swerdlow and Koob, 1987). At the time of the 1992 review, our only ‘hard’ evidence for PPI deficits in patient groups came from patients with schizophrenia, although our 1992 paper also included unpublished and nascent PPI data from just nine HD patients.
Over the ensuing decades, a number of studies have tested our speculation of PPI deficits across multiple brain disorders and confirmed that – in addition to the reduction of PPI in schizophrenia populations, PPI is also impaired in cohorts of patients with OCD (Ahmari et al., 2012; Hoenig et al., 2005; Kohl et al., 2015; Swerdlow et al., 1993b), TS (Buse et al., 2016; Castellan Baldan et al., 2014; Castellanos et al., 1996; Swerdlow et al., 2001b; Zebardast et al., 2013), and HD (Munoz et al., 2003; Swerdlow et al., 1995; Valls-Solé et al., 2004). In addition, PPI deficits have been identified in other patient populations, including individuals with nocturnal enuresis (Ornitz et al., 1992), Asperger’s syndrome (Howlin and Murphy, 2002; McAlonan et al., 2002), 22q11 syndrome (Sobin et al., 2005), Kleinfelter syndrome (van Rijn et al., 2011), fragile-X syndrome (Frankland et al., 2004; Renoux et al., 2014; Yuhas et al., 2011), and blepharospasm (Gomez-Wong et al., 1998). Some of these studies have used PPI as a quantitative phenotype to understand better the genetics (e.g. Castellan Baldan et al., 2014; Greenwood et al., 2012, 2013), neuropathology (e.g. McAlonan et al., 2002), and treatment (e.g. Kohl et al., 2015) of these disorders, often engaging a range of cross-species models of PPI in the process (e.g. Angelov et al., 2014; Baldan Ramsey et al., 2011; Brooks et al., 2012; Carter et al., 1999; Shilling et al., 2008). The role of psychotropic medications and other demographic and clinical variables in some of these clinical phenotypes has also been tested in many reports (e.g. Ahmari et al., 2012; Swerdlow et al., 2006).
As these advances in the PPI literature were emerging, the fact that PPI deficits were being identified in so many different brain disorders was viewed by some as ‘problematic’ evidence that reduced PPI was ‘non-specific’ for a particular clinical diagnosis. In fact, what has become clear via a preponderance of evidence is that PPI is regulated by descending forebrain circuitry, and – as discussed below – disturbances throughout this circuitry accompany a wide range of diagnostically diverse psychiatric disorders – something that was proposed well before our 1992 review (e.g. Swerdlow and Koob, 1987). However, it is also critical to note that PPI (and presumably its underlying neural regulation) appears to remain relatively intact, or at least functional, in a number of other serious brain disorders, including attention deficit disorder (ADHD; Castellanos et al., 1996; Conzelmann et al., 2010; Feifel et al., 2009; Hanlon et al., 2012; Ornitz et al., 1992, 1999), high functioning autism (Kohl et al. 2014), bipolar disorder (in euthymic states; Barrett et al., 2005; Carroll et al., 2007; but see Sanchez-Morla et al., 2016), and major depressive disorder (Ludewig and Ludewig, 2003; Perry et al., 2004; Quednow et al., 2006), while evidence from chronic substance use disorders is mixed and likely to be substance specific (e.g. Quednow et al., 2004; Schellekens et al., 2012). We assume that other ‘negative’ findings of intact PPI in other patient groups have gone unreported.
Theme 2: Limbic CSPP circuitry regulates PPI
Prior to our 1992 review, a handful of studies had investigated the neural regulation of PPI (e.g. Groves et al., 1974). A focus on the role of the ventral striatum/nucleus accumbens (NAC) emerged from evidence that startle inhibition by pulsating tactile tail pressure was eliminated after NAC ablation (Sorenson and Swerdlow 1982). This focus on the NAC has been substantiated by numerous subsequent reports, and 30+ years later, the NAC remains a crucial structure in current models for the regulation and dys-regulation of PPI (e.g. Bikovsky et al., 2016; Ma and Leung, 2016; Vadnie et al., 2016). But a key concept introduced by our 1992 review was that of a regulation of PPI by inter-connected CSPT circuitry – of which the NAC was one component – and which accessed pontine startle circuitry via descending efferent projections from the ventral pallidum into the pedunculopontine nucleus (PPTg; hence cortico-striato-pallidopontine, or ‘CSPP’ circuitry); it was this latter structure, we proposed, that served to transmit the regulatory ‘tone’ established within the forebrain circuit to the primary startle circuit, and thereby alter the inhibitory impact of the lead stimulus (prepulse) on the startle reflex. As with our speculation about PPI in human brain disorders, this speculation about neural circuitry was based on thin ‘circuit-level’ evidence, consisting of a few published reports (Caine et al., 1991, 1992; Swerdlow et al., 1986, 1990a, 1990b, 1990c), and some unpublished data described in this 1992 review (e.g. 10 rats with PPTg lesions).
Over the subsequent decades, compelling evidence for CSPP and related circuit involvement in the regulation of PPI has come through many different levels of experimental manipulations, as reviewed elsewhere (e.g. Swerdlow et al., 2001a, 2008). Elegant scientific strategies have added cellular – (e.g. Ma and Leung, 2016; Takahashi et al., 2007) and molecular (e.g. Culm et al., 2004) levels of resolution to PPI circuit models. Space constraints preclude a full review of this extensive literature. Nevertheless, we will comment on three points.
First, the apparent overlap in the neural substrates regulating PPI with those implicated in the pathophysiology of human brain disorders is part of the support for the etiological validity of animal models for impaired PPI in these disorders, and has been used in an iterative cross-species strategy. In this strategy, PPI changes after neural circuit manipulations in laboratory animals have been used to develop and then test hypotheses about specific circuit disturbances in patients (e.g. Kumari et al., 2003), and in some cases, circuit-based therapeutics are being modeled based on PPI deficits in rats (e.g. Angelov et al., 2014; Ma and Leung, 2014). Often, when substrates have been demonstrated to regulate PPI in rodents, the fact that PPI is deficient in patients has been used as the basis for justifying a fine grain analysis of those substrates in rats, in terms of their anatomical, neurochemical, and molecular properties. In turn, information about the detailed characteristics of this circuitry derived from studies in rodents has been used to support, develop, or test hypotheses regarding the nature of neural circuit disturbances in human brain disorders (e.g. Hines et al., 2013; Miller et al., 2010). This iterative process of cross-species translation follows a bench-to-bedside model that is espoused across our field, and which is made feasible in the case of PPI based on the closely analogous, if not homologous, aspects of the experimental paradigm across mammalian species.
Second, much of the focus of the neural circuit-based translational models of PPI has been on the regulation of PPI by brain dopamine systems, and the potential utility of this neural mechanism in predictive models for antipsychotic medications. The focus on the PPI-regulatory role of NAC dopaminergic systems (Swerdlow et al., 1986) and dopamine activity more broadly (Mansbach et al., 1988) was initially motivated by the prevailing hypothesis of a causative role of dopamine hyperfunction in the etiology of schizophrenia. The finding that PPI was disrupted in rodents by dopamine agonists (Mansbach et al., 1988; Swerdlow et al., 1986) was applied in a manner prescribed for animal models of that era, by assessing the ability of this pharmacological effect to predict the antipsychotic potential and potency of established and novel compounds (cf. Mansbach et al., 1988; Swerdlow and Geyer, 1993a, 1993b; Swerdlow et al., 1991, 1994). This approach differed from pre-existing predictive models, such as apomorphine-induced canine emesis (Janssen and Niemegeers, 1959), primarily because the behavior being measured (PPI) as a predictive index was very similar, if not homologous, across species. Our 1992 review included a partial listing of antipsychotics and (largely unpublished) data supporting the ability of these antipsychotics to prevent the apomorphine-induced disruption of PPI in Sprague–Dawley rats, and showed their relative clinical potency in schizophrenia (see Table 1 in Swerdlow et al., 1992b). These data were expanded and reported soon thereafter (Swerdlow et al., 1994), showing an expanded list of known antipsychotic compounds that prevented the PPI-disruptive effects of apomorphine, with their potency in this assay correlating highly (R=0.99) with their clinical antipsychotic potency. This compelling relationship was a catalyst for the use of PPI within a model with predictive validity, and led to the identification or validation of compounds with novel antipsychotic properties (e.g. ICI 204,636 (quetiapine; Swerdlow et al., 1994)).
This predictive model was expanded significantly by the observation that putative antipsychotics with novel chemical properties were distinguished by their ability to block the PPI-disruptive effects of NMDA antagonists (Bakshi et al., 1994; Johansson et al., 1994) coupled with the inability of most typical antipsychotics to prevent these effects of NMDA antagonists (Keith et al., 1991). Indeed, the prevailing wisdom of the 1990s was that the ability to prevent the PPI-disruptive effects of NMDA antagonists such as phencyclidine and ketamine might predict properties unique to ‘atypical’ or second generation antipsychotics (SGAPs), and thereby identify agents that would be both more clinically effective and better tolerated than first generation antipsychotics. Over time, this approach ran into some experimental and clinical headwind. First, the ability to prevent NMDA antagonist-induced PPI deficits was not always specific to SGAPs (e.g. chlorpromazine blocks the PPI-disruptive effects of ketamine (Swerdlow et al., 1998)) or particularly sensitive to SGAPs (e.g. several studies reported either marginal or no ability of clozapine to prevent the PPI-disruptive effects of phencyclidine in rats). Second, and more importantly, clinical experience revealed that the benefits of SGAPs over older, first generation antipsychotics were not robust, and in fact SGAPs carried a new and non-trivial list of adverse properties. Thus, while the predictive validity of these PPI models for antipsychotics was further extended in many informative ways as reviewed previously (e.g. Geyer et al., 2001; Swerdlow et al., 2008), they ultimately must be seen in the more humbling context of the clinical reality that antipsychotics of any generation are not well tolerated, and have palpable but limited ability to enhance real world function and improve the quality of life of schizophrenia patients (e.g. Lieberman et al., 2005).
A third comment on the ‘PPI-regulatory neural circuit’ introduced in this 1992 review, is that the proposed ‘circuit’ was based on studies conducted largely in rats. Among the most dramatic and influential shifts in preclinical studies of PPI to emerge in the ensuing decades has been the preponderance of studies conducted in other species – particularly mice – driven largely by the utility of mice for studies with molecular and genetic levels of analysis (e.g. Francis et al., 2003). While the neurochemical and neuroanatomical substrates of PPI in rats translated broadly to studies in mice (e.g. the involvement of forebrain monoamine and NMDA systems, the hippocampus and ventral forebrain, etc.), it became evident early in studies of ‘mouse PPI’ that finer grain analyses revealed distinctions and even opposite roles for specific receptor subtypes and circuit elements in the regulation of PPI across these two rodent species (cf. Geyer et al., 2002). In particular, convergent pharmacological and genetic assessments of the influences of dopamine receptor subtypes on PPI demonstrated substantial differences between rats and mice (Ralph-Williams et al., 2002; 2003). Hence, caution is warranted before extrapolating to mice the well-validated rat PPI model using dopamine agonists to identify antipsychotic treatments.
The biology (and particularly the genetics) of mouse PPI has evolved into a complex and powerful science beyond the scope of this review, but two points deserve comment: (a) the fact that the neurochemical regulation of PPI differed even among rats from different genetic backgrounds was recognized as early as 1990 (Rigdon, 1990), has in some cases been tracked down to its underlying molecular and neural circuit substrates (e.g. Palmer et al., 2000; Qu et al., 2009; Shilling et al., 2008; Swerdlow et al., 2011), and is recapitulated among mice with different genetic backgrounds (e.g. Ralph and Caine, 2005, 2007); (b) the fact that some aspects of the neural regulation of PPI can differ between two strains of mice or rats presents daunting challenges when trying to extrapolate findings from rodents to humans (Dulawa et al., 2000).
At the same time, the fact that some PPI-regulatory neural mechanisms are conserved across species, from zebrafish (in whom PPI is disrupted by apomorphine and restored by antipsychotics; Burgess and Granato, 2007), mice, rats, guinea pigs (Sipes and Geyer, 1996; Vaillancort and Boksa, 2000), pigs (Lind et al., 2004), lower primates (Linn et al., 2003) and higher primates (Talledo et al., 2009), continues to make PPI an appealing measure for cross-species analyses of neural circuit connectivity.
Theme 3: PPI-regulatory circuitry is relevant to disorders characterized by impaired sensory, motor or cognitive ‘gating’
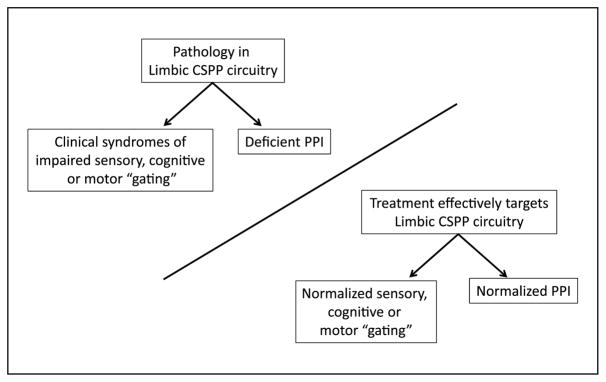
The third area of speculation within our 1992 ‘review’ was that the neural substrates of PPI are relevant to disorders of impaired sensory, cognitive or motor ‘gating’. Implicit in this concept is the notion that PPI might be useful as a ‘read-out’ of CSPP function and dysfunction, and thus could be used to guide the development of interventions for these brain disorders (Figure 2). Over the past 25 years, this concept has, on occasion, been misinterpreted to suggest that deficient PPI ‘causes’ clinical symptoms, to which our standard response is that no patient, to our knowledge, has ever complained that their startle is not inhibited enough by prepulses.
Figure 2.
Schematic relationship of limbic cortico-striato-pallidopontine (CSPP) pathology, deficient prepulse inhibition (PPI) and clinical syndromes of impaired sensory, cognitive or motor ‘gating’.
Rather, we have viewed PPI as a convenient pontine ‘portal’ from which to observe descending forebrain activity that, in addition to regulating PPI, may be relevant to the clinical syndromes associated with a group of disorders. We specifically proposed this notion in our 1992 review, in a section entitled, ‘Are these substrates relevant to disorders of deficient cognitive and sensorimotor gating?’ The information applied towards this question in the ensuing decades has taken a bidirectional route, from ‘bedside’ – for example, neuroimaging studies of OCD (Schwartz et al., 1996) and neuropathological studies of schizophrenia (Lewis et al., 2005) and TS (Kataoka et al., 2010) – to ‘bench’, and from ‘bench’ – for example, neurodevelopmental models of schizophrenia (Lipska et al., 1995), kindling models of temporal lobe epilepsy (TLE; Wolf et al., 2016) and gene ‘knock-out’ models of TS (Castellan Baldan et al., 2014) – to bedside.
The clinical evidence for CSPP and CSPT dysfunction in psychiatric disorders is now extensive, and since our original hypothesis paper in 1987 (Swerdlow and Koob, 1987), has been reviewed numerous times (e.g. most recently by Gunaydin and Kreitzer, 2016). We will touch briefly on the findings that have since flowed from the ‘benchside’, supporting the hypothesis that this ‘gating circuitry’ is relevant to human brain disorders.
Because the first (and for some years, the only) evidence for human PPI deficits came from studies in schizophrenia patients, early neural circuit manipulation in animal studies of PPI focused on substrates implicated in this disorder, for example, forebrain dopamine mechanisms (Sorenson and Swerdlow, 1982; Swerdlow et al., 1986). Starting in the early 1990s, this focus expanded to include the prefrontal cortex (PFC) and mesial temporal lobe regions, in addition to ventral pallidum and thalamic structures, that figure prominently in models of schizophrenia neuropathology (cf. Swerdlow et al., 1992a, 1992b; 2001a, 2001b; 2008; Rohleder et al., 2014). The apparent overlap in the neural substrates regulating PPI, with those implicated in the pathophysiology of schizophrenia, supported the etiological validity of animal models for impaired PPI in schizophrenia; this etiological validity is strengthened by the fact that experimental manipulations in rodents that are thought to model some of the suspected pathogenic insults responsible for schizophrenia also produce adult rodents with deficient PPI.
For example, in schizophrenia patients, the integrity of the hippocampal–PFC connection is disrupted, and the level of this deficiency predicts both neurocognitive and functional impairment (e.g. Hanlon et al., 2012). Lesions of the ventral hippocampus (VH) in neonatal rats recreate a number of deficits associated with schizophrenia (Lipska et al., 1993; Marquis et al., 2006; cf. O’Donnell, 2012), including deficient PPI (Daenen et al., 2003; Le Pen and Moreau, 2002; Le Pen et al., 2003; Lipska et al., 1995; Swerdlow et al., 2012). The use of deficient PPI as a validating ‘phenotype’ in this model has been expanded to several different early developmental insults of the mesial temporal lobe that produce PPI deficits in adulthood, including immune/inflammatory activation of the VH (e.g. Ribeiro et al., 2013; Zhu et al., 2014a), neonatal pilocarpine-induced seizures (Labbate et al., 2014), cell-type-specific inhibition of the VH (Nguyen et al., 2014) and neonatal lesions of the basolateral amygdala (Vázquez-Roque et al., 2012).
Other in utero or neonatal manipulations also produce PPI deficits in adult rats, including social isolation rearing (Geyer et al., 1993), methylazoxymethanol exposure (Le Pen et al., 2006), and neonatal administration of NMDA antagonists (Uehara et al., 2010). Presumably, the failure to develop normal levels of PPI in these developmental models could reflect many different underlying mechanisms. In some cases, the expression of PPI deficits induced by these early developmental manipulations can be blocked by acute treatments during adulthood, using antipsychotics (e.g. clozapine; Ribeiro et al., 2013), putative neuroprotective agents (e.g. minocycline; Zhu et al. 2014b) and glycinergic agents (Le Pen et al., 2003).
While the past 25 years has produced substantial ‘bidirectional’ support that PPI-regulatory circuitry is relevant to disorders such as schizophrenia, OCD, TS, TLE and others, it has also yielded clear evidence that the absolute level of PPI does not suggest either the presence or absence of pathology in this circuitry. Thus, among healthy humans, there is a wide range of basal levels of PPI; conversely, PPI can be ‘normalized’ in severely ill schizophrenia patients by SGAPs, which clearly do not substantially ‘normalize’ their clinical state or CSPP dysfunction. Perhaps the clearest evidence that reduced PPI suggests neither circuit nor clinical dysfunction comes from findings of sex differences (Swerdlow et al., 1993a) and menstrual cyclicity (Jovanovic et al., 2004; Kask et al., 2008; Swerdlow et al., 1997) of PPI in healthy humans.
Future directions?
Predicting the future directions for PPI research in 1992 – when PPI science was largely a ‘tabula rasa’ – was a lot easier than it is today, with the myriad directions that this field has taken. Perhaps it would be safest to claim, as Professor Marvel told Dorothy in The Wizard of Oz, ‘That’s it – the crystal’s gone dark’. Twenty-five years after our 1992 review, there are reasons to be less sanguine about the utility of PPI as a tool to understand the neural circuit disturbance in psychopathology.
First, it has become clear over the past 25 years that the neuropathology of many brain disorders – and schizophrenia is a prime example – is widely distributed throughout different levels of CSPT circuitry, and highly heterogeneous across patients (see Swerdlow, 2011). Even a high resolution ‘pontine portal’ cannot provide the level of anatomical resolution needed to generate an orderly map of such variable and dispersed neural disturbances. Second, specifically because PPI is regulated by circuitry that – in its essence – defines much of what constitutes the complex, variable and individualized features of human consciousness, studies over the past 25 years have shown that PPI is very sensitive to a long list of ‘individualizing’ factors, even within healthy populations. These factors include subject demographics (age, sex, race), state-defining normal physiological variables (reproductive hormones, stress, fatigue, resting blink rate), substance use (smoking), more complex variables such as personality, and a long list of experimental parameters and conditions that require substantial oversight (reviewed in Swerdlow et al., 2008). Even with the strictest inclusion/exclusion criteria for clinically impaired and healthy comparison populations, using PPI to characterize circuitry differences between these populations – particularly in an era with increasingly sophisticated and accessible neurophysiological and brain imaging tools – may not be the best use of this reflex measure.
On the other hand, there are many new uses of PPI that are indirectly related to its underlying neural circuitry, which we did not anticipate at the time of our 1992 paper, and which may represent future ‘growth areas’ for this science. First, PPI has had increasing use as an ‘endophenotype’ to identify risk genes and polygenic risk load in clinical populations (especially schizophrenia, e.g. Greenwood et al., 2012, 2013), and – in concert with other quantitative physiological and neurocognitive measures – it may ultimately serve this role for disorders other than schizophrenia.
Second, there is increasing evidence that PPI – perhaps as a marker of ‘intact’ CSPT resources in clinical populations – may be useful as a biomarker predicting clinical response to therapies ranging from cognitive behavioral therapy (Kumari et al., 2012) to stimulants (Swerdlow et al., 2013, 2016). We have written previously that ‘biomarkers of spared function’ rather than ‘bio-markers of disease’ can be the strongest predictors of a positive therapeutic response to various clinical interventions (Light and Swerdlow, 2015). In a simple model, ‘healthier’ (higher) levels of PPI, for example, suggest the integrity of CSPT circuitry that can serve as a neural resource for neuroplasticity-based gains and their augmentation by medications. Ironically, the strategy of targeting healthy neural/psychological resources in order to correct disease-based deficits has been a longstanding tenet of interventions ranging from psychodynamic psychotherapy to stroke rehabilitation, yet it departs significantly from the failed strategy of trying to use drugs to ‘undo’ the neuropathology of schizophrenia that has dominated the past 60 years of schizophrenia psychopharmacology. Perhaps measures of ‘healthy’ CSPT function, such as PPI, will serve more prominent roles as predictive biomarkers in future treatment models for brain disorders.
Conclusion
The PPI literature has grown dramatically since the publication of our 1992 review, adding about one new PPI-related paper every 3.25 days during this time. Our review focused on the inter-relationships between: (a) disorders characterized by deficiencies within a domain of automatic inhibitory ‘gating’ processes; (b) PPI deficits among patients with these disorders; and (c) a limbic CSPP circuitry that we proposed might regulate PPI in rodents and humans, and might be central to the pathophysiology of ‘gating’ disorders in humans. Our speculation was based on very thin empirical data, but within those faint, tentative pencil strokes seems to have been a reasonable hint of the complex portrait that has emerged from 25 years of intensive investigation across our field. How to use this now mature body of empirical science to actualize its goal – to relieve suffering in individuals afflicted with mental illness – remains a major unanswered challenge for our field.
Acknowledgments
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The authors disclosed receipt of the following financial lsupport for the research, authorship, and/or publication of this article: The work was supported by MH059803 (NRS), MH094320 (NRS), MH093453 (NRS), MH42228 (NRS, MAG, DLB), MH065571 (NRS, DLB), MH071916 (MAG), Veteran’s Administration VISN 22 Mental Illness Research, Education, and Clinical Center (MAG, DLB).
Footnotes
Declaration of conflicting interests
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: In the past three years, Dr Swerdlow has received consulting compensation from Genco, Inc., and Dr Geyer has received consulting compensation from Lundbeck, Omeros, Otsuka and Sunovion, and holds an equity interest in San Diego Instruments.
References
- Ahmari SE, Risbrough VB, Geyer MA, et al. Impaired sensorimotor gating in unmedicated adults with obsessive-compulsive disorder. Neuropsychopharmacology. 2012;37:1216–1223. doi: 10.1038/npp.2011.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelov SD, Dietrich C, Krauss JK, et al. Effect of deep brain stimulation in rats selectively bred for reduced prepulse inhibition. Brain Stimulation. 2014;7(4):595–602. doi: 10.1016/j.brs.2014.03.013. [DOI] [PubMed] [Google Scholar]
- Bakshi VP, Swerdlow NR, Geyer MA. Clozapine antagonizes phencyclidine-induced deficits in sensorimotor gating of the startle response. The Journal of Pharmacology and Experimental Therapeutics. 1994;271:787–794. [PubMed] [Google Scholar]
- Baldan Ramsey LC, Xu M, et al. Lesions of the dorsomedial striatum disrupt prepulse inhibition. Neuroscience. 2011;180:222–228. doi: 10.1016/j.neuroscience.2011.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett SL, Kelly C, Watson DR, et al. Normal levels of prepulse inhibition in the euthymic phase of bipolar disorder. Psychological Medicine. 2005;35:1737–1746. doi: 10.1017/S0033291705005702. [DOI] [PubMed] [Google Scholar]
- Bikovsky L, Hadar R, Soto-Montenegro ML, et al. Deep brain stimulation improves behavior and modulates neural circuits in a rodent model of schizophrenia. Experimental Neurology. 2016 doi: 10.1016/j.expneurol.2016.06.012. Epub ahead of print 11 June 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braff DL, Grillon C, Geyer M. Gating and habituation of the startle reflex in schizophrenic patients. Archives of General Psychiatry. 1992;49:206–215. doi: 10.1001/archpsyc.1992.01820030038005. [DOI] [PubMed] [Google Scholar]
- Braff D, Stone C, Callaway E, et al. Prestimulus effects on human startle reflex in normals and schizophrenics. Psychophysiology. 1978;15:339–343. doi: 10.1111/j.1469-8986.1978.tb01390.x. [DOI] [PubMed] [Google Scholar]
- Brooks S, Higgs G, Jones L, et al. Longitudinal analysis of the behavioural phenotype in Hdh(CAG)150 Huntington’s disease knock-in mice. Brain Research Bulletin. 2012;88(2–3):182–188. doi: 10.1016/j.brainresbull.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Burgess HA, Granato M. Sensorimotor gating in larval zebrafish. Journal of Neuroscience. 2007;27(18):4984–4994. doi: 10.1523/JNEUROSCI.0615-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buse J, Beste C, Herrmann E, et al. Neural correlates of altered sensorimotor gating in boys with Tourette syndrome: a combined EMG/fMRI study. The World Journal of Biological Psychiatry. 2016;17(3):187–197. doi: 10.3109/15622975.2015.1112033. [DOI] [PubMed] [Google Scholar]
- Caine SB, Geyer MA, Swerdlow NR. Carbachol infusion into the dentate gyrus disrupts sensorimotor gating of startle in the rat. Psychopharmacology. 1991;105:347–354. doi: 10.1007/BF02244429. [DOI] [PubMed] [Google Scholar]
- Caine SB, Geyer MA, Swerdlow NR. Hippocampal modulation of acoustic startle and prepulse inhibition in rats. Pharmacology, Biochemistry and Behavior. 1992;43:1201–1208. doi: 10.1016/0091-3057(92)90503-8. [DOI] [PubMed] [Google Scholar]
- Carroll CA, Vohs JL, O’Donnell BF, et al. Sensorimotor gating in manic and mixed episode bipolar disorder. Bipolar Disorders. 2007;9:221–229. doi: 10.1111/j.1399-5618.2007.00415.x. [DOI] [PubMed] [Google Scholar]
- Carter RJ, Lione LA, Humby T, et al. Characterization of progressive motor deficits in mice transgenic for the human Huntington’s disease mutation. Journal of Neuroscience. 1999;19:3248–3257. doi: 10.1523/JNEUROSCI.19-08-03248.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellan Baldan L, Williams KA, Gallezot JD, et al. Histidine decarboxylase deficiency causes tourette syndrome: parallel findings in humans and mice. Neuron. 2014;81:77–90. doi: 10.1016/j.neuron.2013.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX, Fine EJ, Kaysen DL, et al. Sensorimotor gating in boys with Tourette’s syndrome and ADHD: preliminary results. Biological Psychiatry. 1996;39:33–41. doi: 10.1016/0006-3223(95)00101-8. [DOI] [PubMed] [Google Scholar]
- Conzelmann A, Pauli P, Mucha RF, et al. Early attentional deficits in an attention-to-prepulse paradigm in ADHD adults. Journal of Abnormal Psychology. 2010;119(3):594–603. doi: 10.1037/a0019859. [DOI] [PubMed] [Google Scholar]
- Culm KE, Lugo-Escobar N, Hope BT, et al. Repeated quinpirole treatment increases cAMP-dependent protein kinase activity and CREB phosphorylation in nucleus accumbens and reverses quin-pirole-induced sensorimotor gating deficits in rats. Neuropsychopharmacology. 2004;29:1823–1830. doi: 10.1038/sj.npp.1300483. [DOI] [PubMed] [Google Scholar]
- Daenen EW, Wolterink G, Van Der Heyden JA, et al. Neonatal lesions in the amygdala or ventral hippocampus disrupt prepulse inhibition of the acoustic startle response; implications for an animal model of neurodevelopmental disorders like schizophrenia. European Neuropsychopharmacology. 2003;13:187–197. doi: 10.1016/s0924-977x(03)00007-5. [DOI] [PubMed] [Google Scholar]
- Davis M. The Mammalian Startle Response. In: Eaton RC, editor. Neural Mechanisms of Startle Behavior. New York: Springer Science; 1984. pp. 287–351. [Google Scholar]
- Dulawa SC, Gross C, Stark KL, et al. Knockout mice reveal opposite roles for serotonin 1A and 1B receptors in prepulse inhibition. Neuropsychopharmacology. 2000;22:650–659. doi: 10.1016/S0893-133X(99)00164-5. [DOI] [PubMed] [Google Scholar]
- Feifel D, Minassian A, Perry W. Prepulse inhibition of startle in adults with ADHD. Journal of Psychiatric Research. 2009;43(4):484–489. doi: 10.1016/j.jpsychires.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis DD, Szegda K, Campbell G, et al. Epigenetic sources of behavioral differences in mice. Nature Neuroscience. 2003;6:445–446. doi: 10.1038/nn1038. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Wang Y, Rosner B, et al. Sensorimotor gating abnormalities in young males with fragile X syndrome and Fmr1-knockout mice. Molecular Psychiatry. 2004;9:417–425. doi: 10.1038/sj.mp.4001432. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Krebs-Thomson K, Braff DL, et al. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology. 2001;156:117–154. doi: 10.1007/s002130100811. [DOI] [PubMed] [Google Scholar]
- Geyer MA, McIlwain KL, Paylor R. Mouse genetic models for prepulse inhibition: an early review. Molecular Psychiatry. 2002;7:1039–1053. doi: 10.1038/sj.mp.4001159. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Wilkinson LS, Humby T, et al. Isolation rearing of rats produces a deficit in prepulse inhibition of acoustic startle similar to that in schizophrenia. Biological Psychiatry. 1993;34:361–372. doi: 10.1016/0006-3223(93)90180-l. [DOI] [PubMed] [Google Scholar]
- Gomez-Wong E, Marti MJ, Tolosa E, et al. Sensory modulation of the blink reflex in patients with blepharospasm. Archives of Neurology. 1998;55:1233–1237. doi: 10.1001/archneur.55.9.1233. [DOI] [PubMed] [Google Scholar]
- Greenwood TA, Light GA, Swerdlow NR, et al. Association analysis of 94 candidate genes and schizophrenia-related endophenotypes. PLoS One. 2012;7(1):e29630. doi: 10.1371/journal.pone.0029630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood TA, Swerdlow NR, Gur RE, et al. Genome-wide linkage analyses of 12 endophenotypes for schizophrenia from the Consortium on the Genetics of Schizophrenia. American Journal of Psychiatry. 2013;170:521–532. doi: 10.1176/appi.ajp.2012.12020186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves PM, Boyle RD, Welker RL, et al. On the mechanism of prepulse inhibition. Physiology and Behavior. 1974;12(3):367–375. doi: 10.1016/0031-9384(74)90111-5. [DOI] [PubMed] [Google Scholar]
- Gunaydin LA, Kreitzer AC. Cortico-basal ganglia circuit function in psychiatric disease. Annual Review of Physiology. 2016;78:327–350. doi: 10.1146/annurev-physiol-021115-105355. [DOI] [PubMed] [Google Scholar]
- Hanlon FM, Houck JM, Klimaj SD, et al. Frontotemporal anatomical connectivity and working-relational memory performance predict everyday functioning in schizophrenia. Psychophysiology. 2012;49(10):1340–1352. doi: 10.1111/j.1469-8986.2012.01448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines RM, Hines DJ, Houston CM, et al. Disrupting the clustering of GABAA receptor a2 subunits in the frontal cortex leads to reduced-power and cognitive deficits. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(41):16628–16633. doi: 10.1073/pnas.1308706110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoenig K, Hochrein A, Quednow BB, et al. Impaired prepulse inhibition of acoustic startle in obsessive-compulsive disorder. Biological Psychiatry. 2005;57:1153–1158. doi: 10.1016/j.biopsych.2005.01.040. [DOI] [PubMed] [Google Scholar]
- Howlin P, Murphy DG. Brain anatomy and sensorimotor gating in Asperger’s syndrome. Brain. 2002;125(Pt 7):1594–1606. doi: 10.1093/brain/awf150. [DOI] [PubMed] [Google Scholar]
- Janssen PAJ, Niemegeers CJE. Chemistry and pharmacology of compounds related to 4-(4-hydroxy-Cphenyl-piperidino)-butyrophenone. Part II. Inhibition of apomorphine vomiting in dogs. Arzneimittel-Forsch. 1959;9:765–767. [PubMed] [Google Scholar]
- Johansson C, Jackson DM, Svensson L. The atypical antipsychotic, remoxipride, blocks phencyclidine-induced disruption of prepulse inhibition in the rat. Psychopharmacology (Berl) 1994;116(4):437–442. doi: 10.1007/BF02247475. [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Szilagyi S, Chakravorty S, et al. Menstrual cycle phase effects on prepulse inhibition of acoustic startle. Psychophysiology. 2004;41:401–406. doi: 10.1111/1469-8986.2004.00166.x. [DOI] [PubMed] [Google Scholar]
- Kask K, Bäckström T, Gulinello M, et al. Lower levels of prepulse inhibition of startle response in pregnant women compared to postpartum women. Psychoneuroendocrinology. 2008;33:100–107. doi: 10.1016/j.psyneuen.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Kataoka Y, Kalanithi PS, Grantz H, et al. Decreased number of parvalbumin and cholinergic interneurons in the striatum of individuals with Tourette syndrome. The Journal of Comparative Neurology. 2010;518:277–291. doi: 10.1002/cne.22206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith VA, Mansbach RS, Geyer MA. Failure of haloperidol to block the effects of phencyclidine and dizocilpine on acoustic startle prepulse inhibition. Biological Psychiatry. 1991;30:557–566. doi: 10.1016/0006-3223(91)90025-h. [DOI] [PubMed] [Google Scholar]
- Kohl S, Gruendler TO, Huys D, et al. Effects of deep brain stimulation on prepulse inhibition in obsessive–compulsive disorder. Translational Psychiatry. 2015;5:e675. doi: 10.1038/tp.2015.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl S, Wolters C, Gruendler TO, et al. Prepulse inhibition of the acoustic startle reflex in high functioning autism. PLoS One. 2014;9(3):e92372. doi: 10.1371/journal.pone.0092372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari V, Gray JA, Geyer MA, et al. Neural correlates of tactile prepulse inhibition: a functional MRI study in normal and schizophrenic subjects. Psychiatry Research. 2003;122(2):99–113. doi: 10.1016/s0925-4927(02)00123-3. [DOI] [PubMed] [Google Scholar]
- Kumari V, Premkumar P, Fannon D, et al. Sensorimotor gating and clinical outcome following cognitive behaviour therapy for psychosis. Schizophrenia Research. 2012;134:232–238. doi: 10.1016/j.schres.2011.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari V, Soni W, Sharma T. Normalization of information processing deficits in schizophrenia with clozapine. American Journal of Psychiatry. 1999;156:1046–1051. doi: 10.1176/ajp.156.7.1046. [DOI] [PubMed] [Google Scholar]
- Labbate GP, da Silva AV, Barbosa-Silva RC. Effect of severe neonatal seizures on prepulse inhibition and hippocampal volume of rats tested in early adulthood. Neuroscience Letters. 2014;568:62–66. doi: 10.1016/j.neulet.2014.03.019. [DOI] [PubMed] [Google Scholar]
- Le Pen G, Moreau JL. Disruption of prepulse inhibition of startle reflex in a neurodevelopmental model of schizophrenia: reversal by clozapine, olanzapine and risperidone but not by haloperidol. Neuropsychopharmacology. 2002;27:1–11. doi: 10.1016/S0893-133X(01)00383-9. [DOI] [PubMed] [Google Scholar]
- Le Pen G, Gourevitch R, Hazane F, et al. Peripubertal maturation after developmental disturbance: a model for psychosis onset in the rat. Neuroscience. 2006;143:395–405. doi: 10.1016/j.neuroscience.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Le Pen G, Kew J, Alberati D, et al. Prepulse inhibition deficits of the startle reflex in neonatal ventral hippocampallesioned rats: reversal by glycine and a glycine transporter inhibitor. Biological Psychiatry. 2003;54:1162–1170. doi: 10.1016/s0006-3223(03)00374-3. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nature Reviews. Neuroscience. 2005;6(4):312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of anti-psychotic drugs in patients with chronic schizophrenia. New England Journal of Medicine. 2005;353:1209–1223. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- Light GA, Swerdlow NR. Future clinical uses of neurophysiological biomarkers to predict and monitor treatment response for schizophrenia. Annals of the New York Academy of Sciences. 2015;1344:105–119. doi: 10.1111/nyas.12730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind NM, Arnfred SM, Hemmingsen RP, et al. Prepulse inhibition of the acoustic startle reflex in pigs and its disruption by d-amphetamine. Behavioural Brain Research. 2004;155:217–222. doi: 10.1016/j.bbr.2004.04.014. [DOI] [PubMed] [Google Scholar]
- Linn GS, Negi SS, Gerum SV, et al. Reversal of phencyclidine-induced prepulse inhibition deficits by clozapine in monkeys. Psychopharmacology. 2003;169:234–239. doi: 10.1007/s00213-003-1533-8. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Jaskiw GE, Weinberger DR. Postpubertal emergence of hyperresponsiveness to stress and to amphetamine after neonatal excitotoxic hippocampal damage: a potential animal model of schizophrenia. Neuropsychopharmacology. 1993;9(1):67–75. doi: 10.1038/npp.1993.44. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Swerdlow NR, Geyer MA, et al. Neonatal excito-toxic hippocampal damage in rats causes postpubertal changes in prepulse inhibition of startle and its disruption by apomorphine. Psychopharmacology. 1995;122:35–43. doi: 10.1007/BF02246439. [DOI] [PubMed] [Google Scholar]
- Ludewig S, Ludewig K. No prepulse inhibition deficits in patients with unipolar depression. Depression and Anxiety. 2003;17(4):224–225. doi: 10.1002/da.10109. [DOI] [PubMed] [Google Scholar]
- Ma J, Leung LS. Deep brain stimulation of the medial septum or nucleus accumbens alleviates psychosis-relevant behavior in ketamine-treated rats. Behavioural Brain Research. 2014;266:174–182. doi: 10.1016/j.bbr.2014.03.010. [DOI] [PubMed] [Google Scholar]
- Ma J, Leung LS. Dual effects of limbic seizures on psychosis-relevant behaviors shown by nucleus accumbens kindling in rats. Brain Stimulation. 2016;9:762–769. doi: 10.1016/j.brs.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlonan GM, Daly E, Kumari V, et al. Brain anatomy and sensorimotor gating in Asperger’s syndrome. Brain. 2002;125:1594–1606. doi: 10.1093/brain/awf150. [DOI] [PubMed] [Google Scholar]
- Mansbach RS, Geyer MA, Braff DL. Dopaminergic stimulation disrupts sensorimotor gating in the rat. Psychopharmacology. 1988;94:507–514. doi: 10.1007/BF00212846. [DOI] [PubMed] [Google Scholar]
- Marquis JP, Goulet S, Doré FY. Neonatal lesions of the ventral hippocampus in rats lead to prefrontal cognitive deficits at two maturational stages. Neuroscience. 2006;140(3):759–767. doi: 10.1016/j.neuroscience.2006.02.048. [DOI] [PubMed] [Google Scholar]
- Miller EJ, Saint Marie LR, Breier MR, et al. Pathways from the ventral hippocampus and caudal amygdala to forebrain regions that regulate sensorimotor gating in the rat. Neuroscience. 2010;165(2):601–611. doi: 10.1016/j.neuroscience.2009.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz E, Cervera A, Valls-Solé J. Neurophysiological study of facial chorea in patients with Huntington’s disease. Clinical Neurophysiology. 2003;114(7):1246–1252. doi: 10.1016/s1388-2457(03)00076-2. [DOI] [PubMed] [Google Scholar]
- Nguyen R, Morrissey MD, Mahadevan V, et al. Parvalbumin and GAD65 interneuron inhibition in the ventral hippocampus induces distinct behavioral deficits relevant to schizophrenia. Journal of Neuroscience. 2014;34:14948–14960. doi: 10.1523/JNEUROSCI.2204-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell P. Cortical disinhibition in the neonatal ventral hippocampal lesion model of schizophrenia: new vistas on possible therapeutic approaches. Pharmacology and Therapeutics. 2012;133(1):19–25. doi: 10.1016/j.pharmthera.2011.07.005. [DOI] [PubMed] [Google Scholar]
- Ornitz EM, Hanna GL, de Traversay J. Prestimulation-induced startle modulation in attention deficit hyperactivity disorder and nocturnal enuresis. Psychophysiology. 1992;29:437–451. doi: 10.1111/j.1469-8986.1992.tb01717.x. [DOI] [PubMed] [Google Scholar]
- Ornitz EM, Russell AT, Hanna GL, et al. Prepulse inhibition of startle and the neurobiology of primary nocturnal enuresis. Biological Psychiatry. 1999;45(11):1455–1466. doi: 10.1016/s0006-3223(98)00205-4. [DOI] [PubMed] [Google Scholar]
- Palmer AA, Dulawa SC, Mottiwala AA, et al. Prepulse startle deficit in the Brown–Norway rat. A potential genetic model. Behavioral Neuroscience. 2000;114:374–388. doi: 10.1037//0735-7044.114.2.374. [DOI] [PubMed] [Google Scholar]
- Perry W, Minassian A, Feifel D. Prepulse inhibition in patients with non-psychotic major depressive disorder. Journal of Affective Disorders. 2004;81:179–184. doi: 10.1016/S0165-0327(03)00157-5. [DOI] [PubMed] [Google Scholar]
- Powell SB. Models of neurodevelopmental abnormalities in schizophrenia. Current Topics in Behavioral Neuroscience. 2010;4:435–481. doi: 10.1007/7854_2010_57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell SB, Geyer MA. Developmental markers of psychiatric disorders as identified by sensorimotor gating. Neurotoxicity Research. 2002;4:489–502. doi: 10.1080/10298420290030578. [DOI] [PubMed] [Google Scholar]
- Powell SB, Weber M, Geyer MA. Genetic models of sensorimotor gating: relevance to neuropsychiatric disorders. Current Topics in Behavioral Neuroscience. 2012;12:251–318. doi: 10.1007/7854_2011_195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell SB, Zhou X, Geyer MA. Prepulse inhibition and genetic mouse models of schizophrenia. Behavioural Brain Research. 2009;204:282–294. doi: 10.1016/j.bbr.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Y, Saint Marie RL, Breier MR, et al. Neural basis for a heritable phenotype: differences in the effects of apomorphine on startle gating and ventral pallidal GABA efflux in male Sprague Dawley and Long Evans rats. Psychopharmacology. 2009;207:271–280. doi: 10.1007/s00213-009-1654-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quednow BB, Quednow KU, Kuhn K, et al. Prepulse inhibition and habituation of acoustic startle response in male MDMA (‘ecstasy’) users, cannabis users, and healthy controls. Neuropsychopharmacology. 2004;29:982–990. doi: 10.1038/sj.npp.1300396. [DOI] [PubMed] [Google Scholar]
- Quednow BB, Wagner M, Westheide J, et al. Sensorimotor gating and habituation of the startle response in schizophrenic patients randomly treated with amisulpride or olanzapine. Biological Psychiatry. 2006;59:536–545. doi: 10.1016/j.biopsych.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Ralph RJ, Caine SB. Dopamine D1 and D2 agonist effects on prepulse inhibition and locomotion: comparison of Sprague–Dawley rats to Swiss–Webster, 129X1/SvJ, C57BL/6J, and DBA/2J mice. The Journal of Pharmacology and Experimental Therapeutics. 2005;312:733–741. doi: 10.1124/jpet.104.074468. [DOI] [PubMed] [Google Scholar]
- Ralph RJ, Caine SB. Effects of selective dopamine D1-like and D2-like agonists on prepulse inhibition of startle in inbred C3H/ HeJ, SPRET/EiJ, and CAST/EiJ mice. Psychopharmacology. 2007;191:731–739. doi: 10.1007/s00213-006-0511-3. [DOI] [PubMed] [Google Scholar]
- Ralph-Williams RJ, Lehmann-Masten V, Geyer MA. Dopamine D1 rather than D2 receptor agonists disrupt prepulse inhibition of startle in mice. Neuropsychopharmacology. 2003;28:108–118. doi: 10.1038/sj.npp.1300017. [DOI] [PubMed] [Google Scholar]
- Ralph-Williams RJ, Lehmann-Masten V, Otero-Corchon V, et al. Differential effects of direct and indirect dopamine agonists on prepulse inhibition: a study in D1 and D2 receptor knockout mice. Journal of Neuroscience. 2002;22:9604–9611. doi: 10.1523/JNEUROSCI.22-21-09604.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renoux AJ, Sala-Hamrick KJ, Carducci NM, et al. Impaired sensorimotor gating in Fmr1 knock out and Fragile X premutation model mice. Behavioural Brain Research. 2014;267:42–45. doi: 10.1016/j.bbr.2014.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro BM, do Carmo MR, Freire RS, et al. Evidence for a progressive microglial activation and increase in iNOS expression in rats submitted to a neurodevelopmental model of schizophrenia: reversal by clozapine. Schizophrenia Research. 2013;151:12–19. doi: 10.1016/j.schres.2013.10.040. [DOI] [PubMed] [Google Scholar]
- Rigdon GC. Differential effects of apomorphine on prepulse inhibition of acoustic startle reflex in two rat strains. Psychopharmacology (Berl) 1990;102(3):419–421. doi: 10.1007/BF02244115. [DOI] [PubMed] [Google Scholar]
- Rohleder C, Jung F, Mertgens H, et al. Neural correlates of sensorimotor gating: a metabolic positron emission tomography study in awake rats. Frontiers in Behavioral Neuroscience. 2014;8:178. doi: 10.3389/fnbeh.2014.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Morla EM, Mateo J, Aparicio A, et al. Prepulse inhibition in euthymic bipolar disorder patients in comparison with control subjects. Acta Psychiatrica Scandinavica. 2016;134:350–359. doi: 10.1111/acps.12604. [DOI] [PubMed] [Google Scholar]
- Schellekens AF, Mulders PC, Ellenbroek B, et al. Early-onset alcohol dependence increases the acoustic startle reflex. Alcoholism, Clinical and Experimental Research. 2012;36:1075–1083. doi: 10.1111/j.1530-0277.2011.01700.x. [DOI] [PubMed] [Google Scholar]
- Schwartz JM, Stoessel PW, Baxter LR, Jr, et al. Systematic changes in cerebral glucose metabolic rate after successful behavior modification treatment of obsessive-compulsive disorder. Archives of General Psychiatry. 1996;53:109–113. doi: 10.1001/archpsyc.1996.01830020023004. [DOI] [PubMed] [Google Scholar]
- Shilling PD, Saint Marie RL, Shoemaker JM, et al. Strain differences in the gating-disruptive effects of apomorphine: relationship to gene expression in nucleus accumbens signaling pathways. Biological Psychiatry. 2008;63:748–758. doi: 10.1016/j.biopsych.2007.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipes TE, Geyer MA. Functional behavioral homology between rat 5-HT1B and guinea pig 5-HT1D receptors in the modulation of prepulse inhibition of startle. Psychopharmacology (Berl) 1996;125(3):231–237. doi: 10.1007/BF02247333. [DOI] [PubMed] [Google Scholar]
- Sobin C, Kiley-Brabeck K, Karayiorgou M. Lower prepulse inhibition in children with the 22q11 deletion syndrome. American Journal of Psychiatry. 2005;162:1090–1099. doi: 10.1176/appi.ajp.162.6.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorenson CA, Swerdlow NR. The effect of tail pinch on the acoustic startle response in rats. Brain Research. 1982;247:105–113. doi: 10.1016/0006-8993(82)91032-0. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR. Are we studying and treating schizophrenia correctly? Schizophrenia Research. 2011;130:1–10. doi: 10.1016/j.schres.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA. Prepulse inhibition of acoustic startle in rats after lesions of the pedunculopontine tegmental nucleus. Behavioral Neuroscience. 1993a;107:104–117. doi: 10.1037//0735-7044.107.1.104. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA. Clozapine and haloperidol in an animal model of sensorimotor gating deficits in schizophrenia. Pharmacology, Biochemistry and Behavior. 1993b;44:741–744. doi: 10.1016/0091-3057(93)90193-w. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Koob GF. Dopamine, schizophrenia, mania and depression: toward a unified hypothesis of cortico-striato-pallidothalamic function. The Behavioral and Brain Sciences. 1987;10:197–245. [Google Scholar]
- Swerdlow NR, Auerbach P, Monroe SM, et al. Men are more inhibited than women by weak prepulses. Biological Psychiatry. 1993a;34:253–260. doi: 10.1016/0006-3223(93)90079-s. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Bakshi V, Waikar M, et al. Seroquel, clozapine and chlorpromazine restore sensorimotor gating in ketamine-treated rats. Psychopharmacology (Berl) 1998;140:75–80. doi: 10.1007/s002130050741. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Benbow CH, Zisook S, et al. A preliminary assessment of sensorimotor gating in patients with obsessive compulsive disorder. Biological Psychiatry. 1993b;33:298–301. doi: 10.1016/0006-3223(93)90300-3. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Bhakta SG, Talledo JA, et al. Sensorimotor gating predicts sensitivity to pro-attentional effects of amphetamine in healthy adults. Society for Neuroscience; San Diego: 2013. Nov 9–13, [Google Scholar]
- Swerdlow NR, Braff DL, Geyer MA. GABAergic projection from nucleus accumbens to ventral pallidum mediates dopamine-induced sensorimotor gating deficits of acoustic startle in rats. Brain Research. 1990a;532:146–150. doi: 10.1016/0006-8993(90)91754-5. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Braff DL, Geyer MA, et al. Central dopamine hyperactivity in rats mimics abnormal acoustic startle response in schizophrenics. Biological Psychiatry. 1986;21:23–33. doi: 10.1016/0006-3223(86)90005-3. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Braff DL, Masten VL, et al. Schizophrenic-like sensorimotor gating abnormalities in rats following dopamine infusion into the nucleus accumbens. Psychopharmacology. 1990b;101:414–420. doi: 10.1007/BF02244063. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Braff DL, Taaid N, et al. Assessing the validity of an animal model of deficient sensorimotor gating in schizophrenic patients. Archives of General Psychiatry. 1994;51:139–154. doi: 10.1001/archpsyc.1994.03950020063007. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Breier MR, Saint Marie RL. Probing the molecular basis for an inherited sensitivity to the startle-gating disruptive effects of apomorphine in rats. Psychopharmacology. 2011;216:401–10. doi: 10.1007/s00213-011-2228-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Caine SB, Braff DL, et al. The neural substrates of sensorimotor gating of the startle reflex: a review of recent findings and their implications. Journal of Psychopharmacology. 1992a;6:176–190. doi: 10.1177/026988119200600210. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Caine SB, Geyer MA. Regionally selective effects of intracerebral dopamine infusion on sensorimotor gating of the startle reflex in rats. Psychopharmacology. 1992b;108:189–195. doi: 10.1007/BF02245306. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA, Braff DL. Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacology. 2001a;156:194–215. doi: 10.1007/s002130100799. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Hartman PL, Auerbach PP. Changes in sensorimotor inhibition across the menstrual cycle: Implications for neuropsychiatric disorders. Biological Psychiatry. 1997;41:452–460. doi: 10.1016/S0006-3223(96)00065-0. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Karban B, Ploum Y, et al. Tactile prepuff inhibition of startle in children with Tourette’s Syndrome: In search of an “fMRI-friendly” startle paradigm. Biological Psychiatry. 2001b;50:578–585. doi: 10.1016/s0006-3223(01)01164-7. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Keith VA, Braff DL, et al. Effects of spiperone, raclopride, SCH 23390 and clozapine on apomorphine inhibition of sensorimotor gating of the startle response in the rat. The Journal of Pharmacology and Experimental Therapeutics. 1991;256:530–536. [PubMed] [Google Scholar]
- Swerdlow NR, Light GA, Breier MR, et al. Sensory and sensorimotor gating deficits after neonatal ventral hippocampal lesions in rats. Developmental Neuroscience. 2012;34:240–249. doi: 10.1159/000336841. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Light GA, Cadenhead KS, et al. Startle gating deficits in a large cohort of patients with schizophrenia: Relationship to medications, symptoms, neurocognition, and level of function. Archives of General Psychiatry. 2006;63:1325–1335. doi: 10.1001/archpsyc.63.12.1325. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Light GA, Sprock J, et al. Deficient prepulse inhibition in schizophrenia detected by the multi-site COGS. Schizophrenia Research. 2014;152:503–512. doi: 10.1016/j.schres.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Mansbach RS, Geyer MA, et al. Amphetamine disruption of prepulse inhibition of acoustic startle is reversed by depletion of mesolimbic dopamine. Psychopharmacology. 1990c;100:413–416. doi: 10.1007/BF02244616. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Paulsen J, Braff DL, et al. Impaired prepulse inhibition of acoustic and tactile startle response in patients with Huntington’s disease. Journal of Neurology, Neurosurgery, and Psychiatry. 1995;58:192–200. doi: 10.1136/jnnp.58.2.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Tarasenko M, Bhakta SG, et al. Pharmacologic augmentation of cognitive training (PACT): amphetamine enhances gains in auditory discrimination training in adult schizophrenia patients. Society for Neuroscience 46th annual meeting; San Diego. 12–16 November 2016; Washington, DC: Society for Neuroscience; 2016. [Google Scholar]
- Swerdlow NR, Weber M, Qu Y, et al. Realistic expectations of prepulse inhibition in translational models for schizophrenia research. Psychopharmacology. 2008;199:331–388. doi: 10.1007/s00213-008-1072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Zisook D, Taaid N. Seroquel (ICI 204,636) restores prepulse inhibition of acoustic startle in apomorphine-treated rats: similarities to clozapine. Psychopharmacology. 1994;114:675–678. doi: 10.1007/BF02245001. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Nagai T, Kamei H, et al. Neural circuits containing pallidotegmental GABAergic neurons are involved in the prepulse inhibition of the startle reflex in mice. Biological Psychiatry. 2007;62(2):148–157. doi: 10.1016/j.biopsych.2006.06.035. [DOI] [PubMed] [Google Scholar]
- Talledo JA, Sutherland Owens AN, et al. Amphetamine effects on startle gating in normal women and female rats. Psychopharmacology. 2009;204:165–175. doi: 10.1007/s00213-008-1446-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara T, Sumiyoshi T, Seo T, et al. Neonatal exposure to MK-801, an N-methyl-D-aspartate receptor antagonist, enhances methamphetamine-induced locomotion and disrupts sensorimotor gating in pre- and postpubertal rats. Brain Research. 2010;1352:223–230. doi: 10.1016/j.brainres.2010.07.013. [DOI] [PubMed] [Google Scholar]
- Vadnie CA, Ayers-Ringler J, Oliveros A, et al. Antipsychotic-like effects of a neurotensin receptor type 1 agonist. Behavioural Brain Research. 2016;305:8–17. doi: 10.1016/j.bbr.2016.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaillancourt C, Boksa P. Birth insult alters dopamine-mediated behavior in a precocial species, the guinea pig. Implications for schizophrenia. Neuropsychopharmacology. 2000;23:654–666. doi: 10.1016/S0893-133X(00)00164-0. [DOI] [PubMed] [Google Scholar]
- Valls-Sole J, Munoz JE, Valldeoriola F. Abnormalities of prepulse inhibition do not depend on blink reflex excitability: a study in Parkinson’s disease and Huntington’s disease. Clinical Neurophysiology. 2004;115:1527–1536. doi: 10.1016/j.clinph.2004.02.014. [DOI] [PubMed] [Google Scholar]
- van Rijn S, Swaab H, Magnée M, et al. Psychophysiological markers of vulnerability to psychopathology in men with an extra X chromosome (XXY) PLoS One. 2011;6:e20292. doi: 10.1371/journal.pone.0020292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez-Roque RA, Solis O, Camacho-Abrego I, et al. Dendritic morphology of neurons in prefrontal cortex and ventral hippocampus of rats with neonatal amygdala lesion. Synapse. 2012;66:373–382. doi: 10.1002/syn.21517. [DOI] [PubMed] [Google Scholar]
- Weike AI, Bauer U, Hamm AO. Effective neuroleptic medication removes prepulse inhibition deficits in schizophrenia patients. Biological Psychiatry. 2000;47:61–70. doi: 10.1016/s0006-3223(99)00229-2. [DOI] [PubMed] [Google Scholar]
- Wolf DC, Bueno-Júnior LS, Lopes-Aguiar C, et al. The frequency of spontaneous seizures in rats correlates with alterations in sensorimotor gating, spatial working memory, and parvalbumin expression throughout limbic regions. Neuroscience. 2016;312:86–98. doi: 10.1016/j.neuroscience.2015.11.008. [DOI] [PubMed] [Google Scholar]
- Yuhas J, Cordeiro L, Tassone F, et al. Brief report: Sensorimotor gating in idiopathic autism and autism associated with fragile X syndrome. Journal of Autism and Developmental Disorders. 2011;41(2):248–253. doi: 10.1007/s10803-010-1040-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zebardast N, Crowley MJ, Bloch MH, et al. Brain mechanisms for prepulse inhibition in adults with Tourette syndrome: initial findings. Psychiatry Research. 2013;214(1):33–41. doi: 10.1016/j.pscychresns.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu F, Zhang L, Ding YQ, et al. Neonatal intrahippocampal injection of lipopolysaccharide induces deficits in social behavior and prepulse inhibition and microglial activation in rats: implication for a new schizophrenia animal model. Brain, Behavior and Immunity. 2014a;38:166–174. doi: 10.1016/j.bbi.2014.01.017. [DOI] [PubMed] [Google Scholar]
- Zhu F, Zheng Y, Ding YQ, et al. Minocycline and risperidone prevent microglia activation and rescue behavioral deficits inducted by neonatal intrahippocampal injection of lipopolysaccharide in rats. PLoS One. 2014b;9:393966. doi: 10.1371/journal.pone.0093966. [DOI] [PMC free article] [PubMed] [Google Scholar]