Abstract
Background:
The impact of adjuvant chemotherapy (CTx) and chemo-radiation therapy (cXRT) in the treatment of resectable gastric cancer remains varied. We sought to define the clinical impact of lymph node ratio (LNR) on the relative benefit of adjuvant CTx or cXRT among patients having undergone curative-intent resection for gastric cancer.
Methods:
Using the multi-institutional U.S. Gastric Cancer Collaborative database, 719 patients with gastric adenocarcinoma who underwent curative-intent resection between 2000 and 2013 were identified. Patients with metastasis or an R2 margin were excluded. The impact of LNR on overall survival (OS) among patients who received CTx or cXRT was evaluated.
Results:
Median patient age was 65 years, and the majority of patients were male (56.2%). The majority of patients underwent either subtotal (40.6%) or total gastrectomy (41.0%), with the remainder undergoing distal gastrectomy or wedge resection (18.4%). On pathology, median tumor size was 4 cm; most patients had a T3 (33.0%) or T4 (27.9%) lesion with lymph node metastasis (59.7%). Margin status was R0 in 92.5% of patients. A total of 325 (45.2%) patients underwent resection alone, 253 (35.2%) patients received 5-FU or capecitabine-based cXRT, whereas the remaining 141 (19.6%) received CTx. Median OS was 40.9 months, and 5-year OS was 40.3%. According to LNR categories, 5-year OS for patients with a LNR of 0, 0.1–0.10, >0.10–0.25, >0.25 were 54.1%, 53.1 %, 49.1 % and 19.8 %, respectively. Factors associated with worse OS included involvement of the gastroesophageal junction (hazard ratio [HR] 1.8), T-stage (3–4: HR 2.1), lymphovascular invasion (HR 1.4), and LNR (>0.25: HR 2.3) (all P<0.05). In contrast, receipt of adjuvant cXRT was associated with an improved OS in the multivariable model (vs. resection alone: HR 0.40; vs. CTx: HR 0.45, both P<0.001). The benefit of cXRT for resected gastric cancer was noted only among patients with LNR >0.25 (vs. resection alone: HR 0.34; vs. CTx: HR 0.45, both P<0.001). In contrast, there was no noted OS benefit of CTx or cXRT among patients with LNR ≤0.25 (all P>0.05).
Conclusion:
Adjuvant CTx or cXRT was utilized in over one-half of patients undergoing curative-intent resection for gastric cancer. LNR may be a useful tool to select patients for adjuvant cXRT, because the benefit of cXRT therapy was isolated to patients with greater degrees of lymphatic spread (i.e., LNR >0.25).
Keywords: lymph node ratio, chemoradiotherapy, gastric cancer, outcomes
Introduction
Gastric cancer is the fifth most common gastrointestinal cancer and the third leading cause of cancer death worldwide.1 The American Cancer Society estimates that in 2016 there will be approximately 26,370 cases of gastric cancer resulting in nearly 11,000 deaths in the United States in.2 While complete operative resection with regional lymph node (LN) dissection remains the mainstay of cure for patients with gastric cancer, the outcome of patients with advanced disease is poor, with 5-year survival ranging from 5% to 83% depending on the stage of disease.3–6 There are discrepancies in the survival outcomes between the Eastern and Western studies, with survivals of 60–70% in Japanese trials compared to 30–40% in Western trials.7 Previous studies reported a high incidence of locoregional or distant recurrence after operative resection of gastric cancer, suggesting the need for additional therapeutic modalities after curative gastrectomy.8–13 While the efficacy and safety of adjuvant chemotherapy (CTx) or chemoradiation (cXRT) therapy for gastric cancer have been studied, results have varied.3,14
Several randomized clinical trials (RCTs) reported improved recurrence-free and overall survival among patients who received adjuvant cXRT over adjuvant CTx or resection only.15–18 In one trial, Park et al. suggested that adjuvant cXRT and CTx were equally beneficial in preventing relapse of gastric cancer.19 Of note, in a subsequent subset analyses, Kim et al. demonstrated that the benefit of adjuvant cXRT for gastric cancer resected with a D2 resection over CTx was most pronounced among patients having a greater degree of lymphatic spread.20 As such, it is important to identify patients at high risk for recurrence, as well as to assess those who most benefit from adjuvant therapy. In addition to the TNM staging system, lymph node ratio (LNR) has been proposed as a promising tool to evaluate prognosis among patients with gastric cancer.21–25 Given this conceptof the importance of the LNR, we sought to evaluate the clinical impact of LNR on the relative benefit of adjuvant CTx or cXRT among patients undergoing curative intent operative resection for gastric cancer using a large, multicenter, national collaborative database. Specifically, the objective of the current study was to define the relation between LNR and overall survival (OS) using propensity-matched analysis of patients undergoing curative intent resection of gastric cancer and adjuvant CTx or cXRT.
Methods
Patient selection
Patients who underwent curative resection for gastric cancer between Jan 1, 2000 and June, 30 2013 were identified from the U.S. Gastric Cancer Collaborative (Johns Hopkins Hospital, Baltimore, MD; Emory University, Atlanta GA; Stanford University, Palo Alto, CA; Washington University, St. Louis, MO; Wake Forest University, Winston-Salem, NC; University of Wisconsin, Madison, WI; and The Ohio State University, Columbus, OH). The institutional review boards at each participating institution approved the study. Only patients who underwent resection with curative-intent were included; patients with metastatic disease and patients with macroscopically positive (R2) margins were excluded. Patients who died or were lost to follow-up within 30 days after operation, and those patients who had missing data on lymph node status were not included (Supplemental Figure 1).
We collected standard demographic and clinicopathologic data, including age, sex, race, body-mass-index (BMI), the physical ststus classificstion system of the American Society of Anesthesiologists (ASA), tumor size, tumor location, Lauren classification, histologic grade, depth of invasion, number of LNs harvested, number of metastatic LNs identified, final stage according to the American Joint Committee on Cancer (AJCC), and presence or absence of lymphovascular invasion (LVI) and perineural invasion (PNI). Treatment and operative details included type and extent of resection (wedge resection, distal gastrectomy, subtotal gastrectomy, or total gastrectomy), and estimated blood loss (EBL). Resection margin status was categorized as microscopically negative (R0) versus microscopically positive (R1); LN status (no metastasis [N0], LN metastasis [N1]) was also ascertained. The extent of lymphadenectomy was defined according to the Japanese Research Society for the Study of Gastric Cancer (D0: No dissection or less than a D1; D1: Perigastic LNs including the left and right pericardial LNs, lesser and greater curvature LNs, and the supra and infrapyloric LNs; D2: D1 plus removal of LNs along the left gastric artery, common hepatic artery, celiac trunk, and splenic artery; D3: D2 plus removal of LNs along the hepatoduodenal ligament and root of the mesentery). LNR was evaluated as a categorical variable using the cut-off values proposed by Marchet et al. to be more comparable with previous studies and categorized further into LNR ≤0.25 and LNR >0.25 after sensitivity analyses.20,23,26–28 Perioperative details including information on CTx and cXRT were collected. Vital status and date of death or last follow-up were also collected.
Statistical analysis
Continuous variables were described as medians with interquartile range (IQR) and categorical variables as frequencies with percentages. Univariable comparisons for continuous variables were assessed using the Kruskal-Wallis test, while categorical variables were assessed using the Chi-squared or the Fisher’s exact test, as appropriate. Univariable and multivariable logistic regression analysis was performed to determine associations of covariates with receipt of adjuvant therapy. Variables that were statistically significant with a P-value <0.10 in univariable analyses were assessed in a multiviariable model. OS was estimated using the Kaplan-Meier method; unadjusted differences in OS were assessed using the log-rank test. The association of relevant clinicopathologic variables with OS was assessed using a Cox proportional hazards model. To account for possible confounding variables, multivariable Cox proportional hazards regression analyses and propensity score methods were utilized.29 Multiple imputation was performed in the multivariable analysis for covariates that were missing at <20%. Statistical analyses were performed with STATA version 14.0 (StataCorp, College Station, TX), and R version 3.0.3 (http://www.r-project.org); all tests were twosided and a P-value <0.05 was considered statistically significant.
Results
Demographic and Clinicopathologic Characteristics
A total of 719 patients underwent resection of gastric cancer and met the inclusion criteria. The demographic and clinical characteristics of the study cohort are presented in Table 1. Median patient age was 65.2 years (IQR: 56.2, 73.8), and most patients were white (n=444, 61.8%) and male (n=404, 56.2%). At the time of resection, the majority of patients underwent either a subtotal (n=292, 40.6%) or total gastrectomy (n=295, 41.0%), with the remainder undergoing a distal gastrectomy or wedge resection (n=132, 18.4%). The majority of patients underwent a D2/3 lymphadenectomy (62.3%). On pathology, median tumor size was 4.0 cm (IQR: 2.2, 6.6); the majority of tumors were AJCC T3 (n=232, 33.0%) or T4 (n=196, 27.9%); approximately two-thirds of lesions (n=478, 69.2%) were moderate- to poorly-differentiated tumors. A median number of 17 (IQR: 11–25) LNs were harvested. LN metastasis was found in 59.7% (n=429) of patients, and a median number of 2 LN (IQR: 0, 7) were involved. According to AJCC 7th edition, approximately one third of patients were N1 (n=124, 17.3%) or N2 (n=125, 17.4%) diseases, while one fourth of patients were N3a (n=126, 17.5%) or N3b (n=54, 7.5%) diseases. On surgical pathology, most patients (n=662, 92.5%) had an R0 margin. Of note, while some demographic factors such as age and race, differed between patients who underwent a D0/D1 and D2D3 resection, most clinicopathologic and tumor characteristics were comparable (Supplemental Table).
Table 1.
Baseline characteristics of the patients
| Characteristic | Total (n=719) | Resection only (n=325) | Resection + CTx (n=141) | Resection + cXRT (n=253) | P | ||||
|---|---|---|---|---|---|---|---|---|---|
| Age, y, median (IQR) | 65.2(56.2–73.8) | 68.8(61.4–77.2) | 63.6(56.0–73.0) | 60.2(53.2–68.6) | |||||
| Male sex | 404 (56.2) | 182 (56.0) | 73 (51.8) | 149 (58.9) | 0.392 | ||||
| Race | 0.372 | ||||||||
| White | 444 (61.8) | 213 (65.5) | 87 (61.7) | 144 (56.9) | |||||
| African-American | 129 (17.9) | 53 (16.3) | 27 (19.1) | 49 (19.4) | |||||
| Asian | 84 (11.7) | 37 (11.4) | 16 (11.3) | 31 (12.3) | |||||
| Others / Unknown | 62 (8.6) | 22 (6.8) | 11 (7.8) | 29 (11.5) | |||||
| BMI, Kg/m2, median (IQR) | 25.3(22.1–29.0) | 25.5(22.3–29.1) | 24.1(21.8–27.9) | 25.6(22.1–29.7) | <0.001 | ||||
| ASA (n=697) | <0.001 | ||||||||
| 1–2 | 243 (34.9) | 83 (26.4) | 55 (40.7) | 105 (42.3) | |||||
| 3–4 | 454 (65.1) | 231 (73.6) | 80 (59.3) | 143 (57.7) | |||||
| Tumor size, cm, median (IQR) | 4.0(2.2– 6.6) | 3.4(1.6– 5.6) | 4.0(3.0– 7.0) | 4.6(3.0– 7.0) | <0.001 | ||||
| Site (n=704) | 0.528 | ||||||||
| Antrum | 275 (39.1) | 123 (39.0) | 58 (41.7) | 94 (37.6) | |||||
| Body | 250 (35.5) | 112 (35.6) | 48 (34.5) | 90 (36.0) | |||||
| Cardia | 63 (8.9) | 30 (9.5) | 11 (7.9) | 22 (8.8) | |||||
| Fundus | 61 (8.7) | 27 (8.6) | 16 (11.5) | 18 (7.2) | |||||
| Gastroesophageal junction | 55 (7.8) | 23 (7.3) | 6 (4.3) | 26 (10.4) | |||||
| Lauren classification (n=480) | 0.001 | ||||||||
| Diffuse | 150 (31.2) | 48 (23.3) | 37 (35.9) | 65 (38.0) | |||||
| Intestinal | 316 (65.8) | 156 (75.7) | 63 (61.2) | 97 (56.7) | |||||
| Mixed | 14 (2.9) | 2 (1.0) | 3 (2.9) | 9 (5.3) | |||||
| Histologic Grade (n=691) | <0.001 | ||||||||
| Well to Moderate | 213 (30.8) | 131 (43.1) | 34 (25.0) | 48 (19.1) | |||||
| Moderate to Poor | 478 (69.2) | 173 (56.9) | 102 (75.0) | 203 (80.9) | |||||
| Depth of Invasion (n=702) | <0.001 | ||||||||
| T1 | 174 (24.8) | 134 (42.9) | 15 (10.9) | 25 (9.9) | |||||
| T2 | 100 (14.2) | 42 (13.5) | 25 (18.1) | 33 (13.1) | |||||
| T3 | 232 (33.0) | 74 (23.7) | 52 (37.7) | 106 (42.1) | |||||
| T4 | 196 (27.9) | 62 (19.9) | 46 (33.3) | 88 (34.9) | |||||
| AJCC Stage (n=708) | <0.001 | ||||||||
| I | 213 (30.1) | 168 (53.0) | 22 (15.9) | 23 (9.1) | |||||
| II | 180 (25.4) | 63 (19.9) | 39 (28.3) | 78 (30.8) | |||||
| III | 315 (44.5) | 86 (27.1) | 77 (55.8) | 152 (60.1) | |||||
| AJCC Nodal Stage | <0.001 | ||||||||
| N0 | 290 (40.3) | 209 (64.3) | 38 (27.0) | 43 (17.0) | |||||
| N1 | 124 (17.3) | 39 (12.0) | 26 (18.4) | 59 (23.3) | |||||
| N2 | 125 (17.4) | 36 (11.1) | 38 (27.0) | 51 (20.1) | |||||
| N3a | 126 (17.5) | 31 (9.5) | 24 (17.0) | 71 (28.1) | |||||
| N3b | 54 (7.5) | 10 (3.1) | 15 (10.6) | 29 (11.5) | |||||
| Number of LN harvested, | 17 (11–25) | 16 (10–23) | 17 (11–26) | 19 (13–26) | <0.001 | ||||
| median (IQR) | |||||||||
| Number of metastatic lymph nodes, median (IQR) | 2 (0–7) | 0 (0–2) | 3 (0–7) | 4 (1–10) | <0.001 | ||||
| LNR, median (IQR) | 0.1 (0–0.4) | 0 (0–0.1) | 0.2 (0–0.5) | 0.3 (0.1–0.5) | <0.001 | ||||
| 0 | 290 (40.3) | 209 (64.3) | 38 (27.0) | 43 (17.0) | <0.001 | ||||
| 0–0.10 | 73 (10.2) | 23 (7.1) | 17 (12.1) | 33 (13.0) | |||||
| >0.10–0.25 | 97 (13.5) | 28 (8.6) | 24 (17.0) | 45 (17.8) | |||||
| >0.25 | 259 (36.0) | 65 (20.0) | 62 (44.0) | 132 (52.2) | |||||
| Margin Status | 0.013 | ||||||||
| R0 | 662 (92.5) | 309 (95.7) | 126 (90.0) | 227 (89.7) | |||||
| R1 | 54 (7.5) | 14 (4.3) | 14 (10.0) | 26 (10.3) | |||||
| Lymphovascular invasion | 277 (43.6) | 90 (32.5) | 65 (48.9) | 122 (54.2) | <0.001 | ||||
| Perineural invasion | 161 (29.9) | 54 (22.0) | 32 (30.5) | 75 (39.9) | <0.001 | ||||
| Operation Type | 0.632 | ||||||||
| Distal and wedge resection | 132 (18.4) | 64 (19.7) | 28 (19.9) | 40 (15.8) | |||||
| Subtotal | 292 (40.6) | 134 (41.2) | 52 (36.9) | 106 (41.9) | |||||
| Total | 295 (41.0) | 127 (39.1) | 61 (43.3) | 107 (42.3) | |||||
| EBL, mL, median (IQR) | 200 (100–400) | 200 (100–400) | 200 (150–400) | 200 (150–400) | 0.619 | ||||
| Extent of Lymphadenectomy | <0.001 | ||||||||
| D0 | 13 (1.8) | 9 (2.8) | 3 (2.1) | 1 (0.4) | |||||
| D1 | 254 (35.4) | 142 (44.0) | 33 (23.4) | 79 (31.2) | |||||
| D2 | 442 (61.6) | 169 (52.3) | 102 (72.3) | 171 (67.6) | |||||
| D3 | 8 (1.1) | 3 (0.9) | 3 (2.1) | 2 (0.8) | |||||
| Duration-of-stay, days, median (IQR) |
8.0(7.0–12.0) | 9.0(7.0–14.0) | 8.0(6.0–11.0) | 8.0(7.0–10.0) | <0.001 | ||||
Factors associated with receipt of adjuvant therapy
Post-operatively, 394 (54.8%) patients received adjuvant therapy (CTx: n=141, 19.6% vs. cXRT: n=253, 35.2%), whereas 325 (45.2%) patients underwent resection only. Among those 330 patients who were available for the data on the type of adjuvant chemotherapy, the majority of patients received 5-FU-based chemotherapy (n=191,57.9%) or capecitabine-based chemotherapy (n=119, 36.1%); a small subset of patients received both 5-FU and capecitabine-based regimen (n=14, 4.2%) or other regimens (n=6,1.8%). On univariable analysis, factors associated with an increased likelihood to receive adjuvant therapy included tumor size, histologic grade, T stage, R1 margin, presence of LVI, PNI, and the extent of lymphadenectomy as well as LNR greater than 0.25 (Table 2). After controlling for competing risk factors on multivariable analysis, tumor size (OR 1.07,95%CI 1.01–1.14), moderate-to-poor grade (OR 1.53, 95%CI 1.05–2.23), T3/T4 disease (OR 1.83, 95%CI 1.20–2.78), and the presence of LVI (OR 1.55, 95%CI 1.042.32) remained associated with an increased likelihood of receiving adjuvant therapy (all P<0.05). Similarly, patients who underwent D2/D3 lymphadenectomy (OR 2.45, 95%CI 1.71–3.51) and had a LNR >0.25 (OR 2.42, 95%CI 1.60–3.66) were associated with an increased odds of receiving adjuvant therapy (both P<0.001). In contrast, elderly patients (>65 years) were less likely to receive adjuvant therapy (OR 0.37, 95%CI 0.27–0.53; P<0.001).
Table 2.
Univariable and multivariable logistic analyses of factors associated with receipt of adjuvant therapy
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| OR | (95% CI) | P value | OR | (95% CI) | P value | |
| Age | ||||||
| ≤65 | Ref | Ref | ||||
| >65 | 0.35 | 0.26–0.47 | <0.001 | 0.37 | 0.27–0.53 | <0.001 |
| Male Sex | 1.01 | 0.75–1.36 | 0.926 | |||
| Tumor Size | 1.17 | 1.11–1.24 | <0.001 | 1.07 | 1.01–1.14 | 0.023 |
| Site | ||||||
| Non-GEJ | Ref | |||||
| Gastro-Esophageal Junction (GEJ) | 1.14 | 0.65–1.99 | 0.65 | |||
| Histologic Grade | ||||||
| Well to Moderate | Ref | Ref | ||||
| Moderate to Poor | 2.82 | 2.02–3.93 | <0.001 | 1.53 | 1.05–2.23 | 0.027 |
| T stage | ||||||
| 1–2 | Ref | Ref | ||||
| 3–4 | 3.86 | 2.80–5.31 | <0.001 | 1.83 | 1.20–2.78 | 0.005 |
| Margin | ||||||
| R0 | Ref | Ref | ||||
| R1 | 2.5 | 1.34–4.68 | 0.004 | 1.27 | 0.62–2.60 | 0.515 |
| LNR | ||||||
| ≤0.25 | Ref | Ref | ||||
| >0.25 | 3.88 | 2.77–5.43 | <0.001 | 2.42 | 1.60–3.66 | <0.001 |
| Lymphovascular Invasion | 2.27 | 1.64–3.15 | <0.001 | 1.55 | 1.04–2.32 | 0.033 |
| Perineural Invasion | 2.03 | 1.39–2.99 | <0.001 | 0.97 | 0.61–1.53 | 0.886 |
| Operation type | ||||||
| Partial resection | Ref | |||||
| Total resection | 1.16 | 0.86–1.56 | 0.334 | |||
| Extent of Lymphadenectomy | ||||||
| D0/D1 | Ref | Ref | ||||
| D2/D3 | 2.1 | 1.55–2.86 | <0.001 | 2.45 | 1.71–3.51 | <0.001 |
Overall survival after resection for gastric cancer
Median OS was 40.9 months (95%CI: 33.9– 49.5) with 1-, 3-and 5-year OS of 78.8%, 52.5%, and 40.3%, respectively. According to LNR categories, 5-year OS of patients with a LNR of 0, 0.01–0.09, >0.10–0.25, >0.25 were 54.1%, 53.1%, 49.1%, and 19.8%, respectively (Supplemental Figure 2). On multivariable analysis, involvement of the gastroesophageal junction (HR 1.79, 95%CI 1.19–2.70; P=0.005), T3/T4 diseases (HR2.8,95%CI 1.54–2.80; P<0.001), a LNR (>0.25: HR 2.33, 95%CI 1.79–3.03; P<0.001), and the presence of LVI (HR 1.4, 95%CI 1.09–1.79; P=0.008) were associated with worse OS (Table 3). After controlling for these factors, adjuvant cXRT (vs. resetion alone: HR 0.40, 95%CI 0.30–0.53; vs. CTx: HR 0.45, 95%CI 0.33–0.62; both P<0.001) remained associated with a decreased risk of death.
Table 3.
Univariable and multivariable Cox proportional analyses of factors associated with OS
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| HR | (95% CI) | P value | HR | (95% CI) | P value | |
| Age | 1.01 | 1.00–1.02 | 0.065 | 1.01 | 1.00–1.02 | 0.08 |
| Male | 0.95 | 0.76–1.19 | 0.669 | |||
| Tumor size | 1.08 | 1.05–1.11 | <0.001 | 1.03 | 0.99–1.06 | 0.127 |
| Site | ||||||
| Non-GEJ | Ref | Ref | ||||
| Gastro-Esophageal Junction (GEJ) | 1.77 | 1.22–2.59 | 0.003 | 1.79 | 1.19–2.70 | 0.005 |
| Histologic Grade | ||||||
| Well to Moderate | Ref | |||||
| Moderate to Poor | 1.19 | 0.93–1.52 | 0.163 | |||
| Depth of Invasion | ||||||
| T1-T2 | Ref | Ref | ||||
| T3-T4 | 2.47 | 1.92–3.18 | <0.001 | 2.08 | 1.54–2.80 | <0.001 |
| Margin | ||||||
| R0 | Ref | Ref | ||||
| R1 | 1.69 | 1.17–2.44 | 0.005 | 0.89 | 0.60–1.31 | 0.547 |
| LNR | ||||||
| ≤0.25 | Ref | Ref | ||||
| >0.25 | 2.58 | 2.07 | <0.001 | 2.33 | 1.79–3.03 | <0.001 |
| Lymphovascular Invasion | 1.98 | 1.56–2.50 | <0.001 | 1.4 | 1.09–1.79 | 0.008 |
| Operation Type | ||||||
| Subtotal | Ref | Ref | ||||
| Total | 1.49 | 1.20–1.86 | <0.001 | 1.25 | 0.98–1.60 | 0.073 |
| Extent of Lymphadenectomy | ||||||
| D0/D1 | Ref | Ref | ||||
| D2/D3 | 0.75 | 0.60–0.93 | 0.01 | 0.8 | 0.63–1.00 | 0.051 |
| Adjuvant Therapy | ||||||
| None | Ref | Ref | ||||
| CTx | 1.34 | 1.00–1.79 | 0.051 | 0.88 | 0.65–1.20 | 0.434 |
| cXRT | 0.8 | 0.63–1.03 | 0.088 | 0.4 | 0.30–0.53 | <0.001 |
The effect of adjuvant cXRT compared with adjuvant CTx or resection alone on OS was most pronounced among patients with greater degrees of LN spread (i.e. LNR >0.25) (Figure 1). In contrast, cXRT did not confer an OS benefit among patients who had a LNR ≤0.25 or patients who had N0 disease (both P>0.05). When stratified by the extent of lymphadenectomy, among patients undergoing an extended (D2/D3) lymphadenectomy, the benefit of adjuvant cXRT therapy was present only among those patients who had LNR >0.25 (Figure 2a). In contrast, among patients undergoing D0/D1 lymphadenectomy, the benefit of adjuvant cXRT versus CTx was noted among patients who had both low (LNR ≤0.25) had high (LNR >0.25) metastatic LN burden; the benefit of adjuvant cXRT versus resection alone was limited to patients with LNR >0.25 (Figure 2b). After excluding patients who received any form of preoperative chemotherapy, the effect of adjuvant therapy was the same. Specifically, the benefit of cXRT for resected gastric cancer was noted only among patients with LNR >0.25 (vs. resection alone: HR 0.34, P<0.001; vs. CTx: HR 0.48, P=0.001). In contrast, there was no OS benefit of CTx or cXRT among patients with LNR ≤0.25 (all P>0.05).
Figure 1.
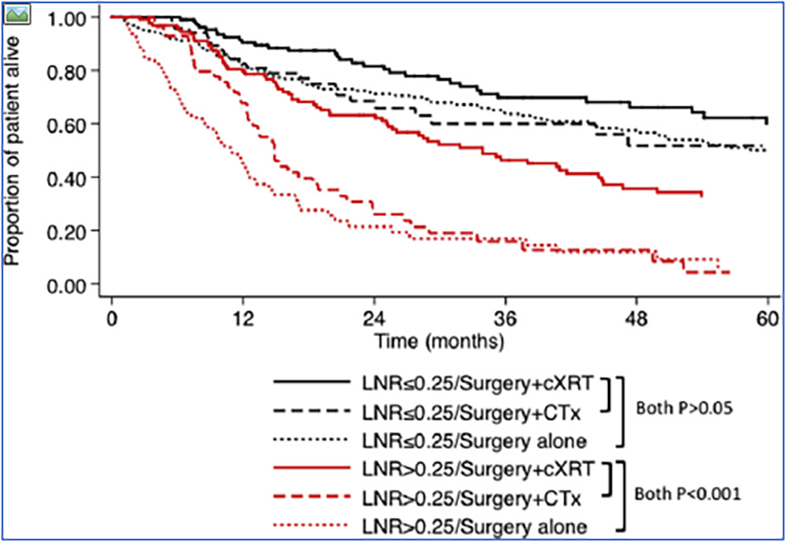
Kaplan-Meier survival curves among patients comparing operative resection plus adjuvant CTx / cXRT to resection alone for gastric cancer: overall survival (OS) stratified by lymph node ratio (LNR) ≤0.25 vs. LNR >0.25.
Figure 2.
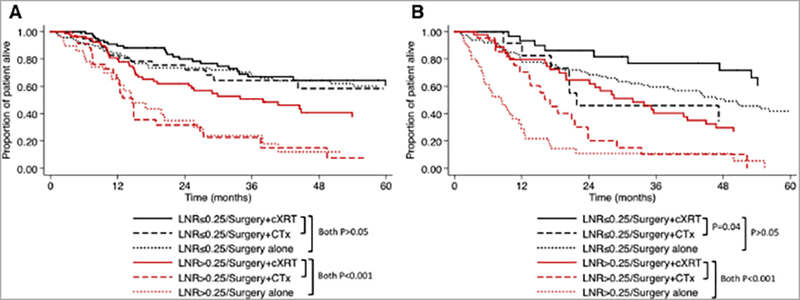
Kaplan-Meier survival curves among patients comparing operative resection plus adjuvant CTx / cXRT to resection alone for gastric cancer: OS stratified by LNR ≤0.25 and >0.25 among patients with (a) D2/D3 lymphadenectomy, and (b) D0/D1 lymphadenectomy.
Propensity-matched analyses were then undertaken to minimize confounding by indication and to create more balanced cohorts of patients who received adjuvant cXRT versus CTx. After propensity-matching for age, sex, tumor size, tumor grade, T stage, surgical margin status, type of operation, and presence/absence of LVI, the propensity-matched cohort included 241 patients who received adjuvant cXRT therapy versus 141 patients who had adjuvant CTx. On propensity-matched analysis, the benefit of adjuvant cXRT over adjuvant CTx therapy alone remained for patients who had undergone D2/D3 lymphadenectomy and had a LNR >0.25 (Figure 3).
Figure 3.
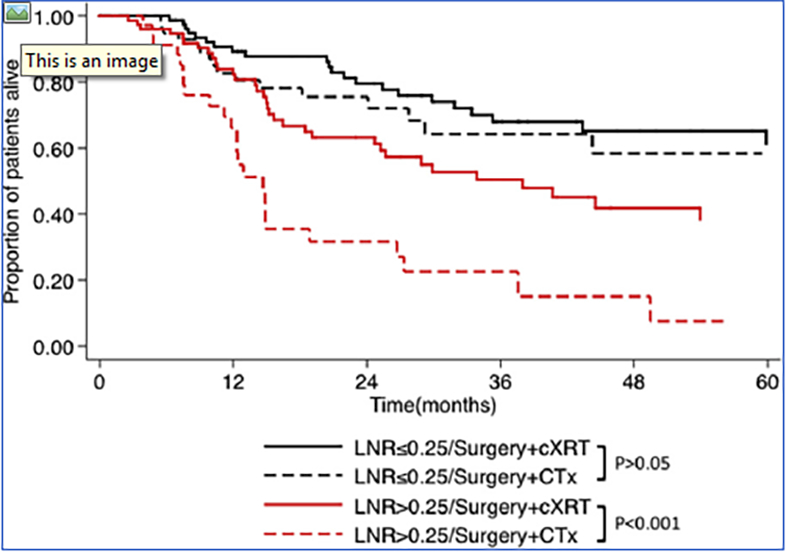
Kaplan-Meier survival curves among propensity-matched cohort comparing adjuvant cXRT to adjuvant CTx for gastric cancer: OSstratified by LNR ≤0.25 and >0.25.
Discussion
Gastric cancer is the third leading cause of cancer deaths worldwide. While resection provides the best chance at cure, recurrence is common, and therefore, longterm cure can be difficult to achieve.1,8,30 Recurrence can range widely from 5% to 90% depending on the stage of the disease, and patterns of recurrence can be varied (e.g. locoregional, hematogenous, peritoneal).8–11 Given the high incidence of recurrence, adjuvant chemotherapy and / or radiation therapy has been considered to improve patient long-term clinical outcomes.12 Several studies have sought to evaluate the efficacy and safety of adjuvant chemotherapy (CTx) or chemoradiation therapy (cXRT) among patients with gastric cancer.12,16–19,31,32,33,34,35 The results of these studies have been controversial as well as limited by small sample size and lack of a cohort receiving contemporary chemotherapy regimens. The current study is important, because it reports the outcomes of adjuvant CTx / cXRT among a contemoporary cohort of patients with gastric cancer using one of the largest, multi-institutional experiences on the surgical management of gastric cancer in the United States. More importantly, using both multivariable and propensity-matched analyses, a beneficial effect of adjuvant cXRT over CTx or resection alone was noted for patients undergoing resection of gastric cancer. Of note, the benefit of adjuvant cXRT was most pronounced among those patients with greater degrees of LN disease (i.e. LNR >0.25) who underwent extended lymphadenectomy (D2/D3).
In the current study, adjuvant therapy was utilized in over one-half of patients undergoing curative-intent resection for gastric cancer. Receipt of adjuvant therapy was associated with certain patient and clinicopathologic factors, including extent of lymphadenectomy and a greater degree of LN spread. Of note, elderly patients (>65years) were 60% less likely to receive adjuvant therapy. Use of adjuvant therapy after resection of gastric in the elderly population remains debated. For example, a recent metaanalysis of adjuvant therapy for gastric cancer reported no benefit of adjuvant chemotherapy among the elderly population.36 In contrast, in a separate study by Jo et al., the authors reported that adjuvant chemotherapy may confer a potential survival benefit in elderly patients with stages II or III gastric cancer after a D2 resection.37 These data suggest that a different strategy and risk stratification may be needed for the elderly patients that considers their functional, social, and mental conditions.
The efficacy and safety of adjuvant therapy for gastric cancer has been investigated in several RCTs.15–19,31 A large, phase III RCT of 556 patients with gastric cancer demonstrated that OS and relapse-free survival was improved among patients undergoing adjuvant cXRT versus resection alone.17 Several other studies have similarly reported that adjuvant cXRT provided an incremental survival benefit over adjuvant CTx or resection alone.5,14–16,18,38 The present data confirmed the survival benefit of adjuvant cXRT therapy compared with resection alone, especially in those patients at greatest risk of recurrence (i.e. LNR > 0.25). Interestingly, in a phase III trial of 458 patients with resected gastric cancer who had a D2 dissection, the Adjuvant Chemo-radiotherapy in Stomach Tumors (ARTIST) trial noted that adjuvant cXRT did not significantly decrease recurrence or risk of death.19,31 Interestingly, in a subset analyses of ARTIST trial, Park et al. reported that adjuvant cXRT was beneficial for patients with N1 disease and intestinal type gastric cancers.19 These data suggested strongly that further risk prediction was need to refine better which patients might benefit from adjuvant therapy.
Rather than stratifying patients as simply N0 versus N1 when assessing extent of nodal disease, the ratio between metastatic and examined lymph nodes (LNR) has been proposed as a better predictor of long-term outcomes ater resection of gastric cancer.20–26,28,39 The present study confirmed that LNR was a strong, independent prognostic indicator of tumor prognosis relative to other risk factors. Two previous studies had also reported the potential role of LNR to select patients for adjuvant cXRT after gastric surgery with D2 lymphadenectomy.20,28 In turn, these findings were consistent with data reported by Kim et al. in the phase III ARTIST trial in which the survival benefit of adjuvant cXRT was most pronounced among patients with LNR >0.25.20 In contrast, Costa Junior and colleagues reported an observational study that found adjuvant cXRT to benefit patients with milder degrees of LN disease (i.e. LNR 0.10–0.25).28 The reasons for these disparate results are likely multifactorial and related to study design and patient selection. For example, unlike the study by Kim et al., the report by Costa Junior et al. was retrospective and had a smaller sample size. In the current study, we noted that the benefit of adjuvant cXRT was most pronounced among patients with a greatser degree of LN disease (i.e. LNR >0.25) who had undergone an extended (D2/D3) lymphadenectomy. Collectively, data from the current study as well as Kim et al. suggest strongly that systemic cXRT may benefit patients differently after gastric resection. In particular, administration of adjuvant cXRT may benefit patients with resected gastric cancer in a subset of patients based on LNR and therefore, help select gastric cancer patients for adjuvant cXRT.
Several limitations should be considered when interpreting our report. While the collaboration among multiple institutions increased the generalizability of the results, this multi-institutional study design limited the ability to standardize treatment criteria including the regimen of adjuvant therapy. While the use of multivariable analyses and propensity-matching allowed us to control for measurable confounders, unmeasured /residual confounders could not be taken into account given the non-randomized observational design. In addition, data on the number of chemotherapy cycles received, as well as any chemotherapy associated toxicity, were not available.
In conclusion, adjuvant CTx or cXRT were utilized in over one-half of patients undergoing curative-intent resection for gastric cancer. After adjusting for varied clinicopathologic factors in the no adjuvant versus adjuvant gastric cancer cohorts using multivariable and propensity-matching, cXRT remained independently associated with a long-term survival benefit. LNR may be a useful tool to select patients for adjuvant cXRT, because the benefit of cXRT therapy was isolated to patients with greater degrees of LN disease (i.e., LNR >0.25).
Supplementary Material
Acknowledgement
None
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA: a cancer journal for clinicians. March 2015;65(2):87–108. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA: a cancer journal for clinicians. Jan-Feb 2016;66(1):7–30. [DOI] [PubMed] [Google Scholar]
- 3.Foo M, Crosby T, Rackley T, Leong T. Role of (chemo)-radiotherapy in resectable gastric cancer. Clinical oncology. September 2014;26(9):541–550. [DOI] [PubMed] [Google Scholar]
- 4.Lasithiotakis K, Antoniou SA, Antoniou GA, Kaklamanos I, Zoras O. Gastrectomy for stage IV gastric cancer. a systematic review and metaanalysis. Anticancer research. May 2014;34(5):2079–2085. [PubMed] [Google Scholar]
- 5.Soon YY, Leong CN, Tey JC, Tham IW, Lu JJ. Postoperative chemo- radiotherapy versus chemotherapy for resected gastric cancer: a systematic review and meta-analysis. J Med Imaging Radiat Oncol. August 2014;58(4):483– 496. [DOI] [PubMed] [Google Scholar]
- 6.Lordick F, Terashima M. Gastric cancer adjuvant therapy. Best Pract Res Clin Gastroenterol. August 2016;30(4):581–591. [DOI] [PubMed] [Google Scholar]
- 7.Bickenbach K, Strong VE. Comparisons of Gastric Cancer Treatments: East vs. West. J Gastric Cancer. June 2012;12(2):55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoo CH, Noh SH, Shin DW, Choi SH, Min JS. Recurrence following curative resection for gastric carcinoma. The British journal of surgery. February 2000;87(2):236–242. [DOI] [PubMed] [Google Scholar]
- 9.Spolverato G, Ejaz A, Kim Y, et al. Rates and patterns of recurrence after curative intent resection for gastric cancer: a United States multi-institutional analysis. Journal of the American College of Surgeons. October 2014;219(4):664– 675. [DOI] [PubMed] [Google Scholar]
- 10.Marrelli D, De Stefano A, de Manzoni G, Morgagni P, Di Leo A, Roviello F. Prediction of recurrence after radical surgery for gastric cancer: a scoring system obtained from a prospective multicenter study. Annals of surgery. February 2005;241(2):247–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gunderson LL. Gastric cancer--patterns of relapse after surgical resection. Seminars in radiation oncology. April 2002;12(2):150–161. [DOI] [PubMed] [Google Scholar]
- 12.Liang JW, Zheng ZC, Yu T, Wang X, Zhang JJ. Is postoperative adjuvant chemoradiotherapy efficacious and safe for gastric cancer patients with D2 lymphadenectomy? A meta-analysis of the literature. European journal of surgical oncology : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. December 2014;40(12):1614–1621. [DOI] [PubMed] [Google Scholar]
- 13.Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet. May 5 2016. [DOI] [PubMed] [Google Scholar]
- 14.Agolli L, Maurizi Enrici R, Osti MF. Adjuvant radiochemotherapy for gastric cancer: Should we use prognostic factors to select patients? World journal of gastroenterology : WJG. January 21 2016;22(3):1131–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. The New England journal of medicine. July 6 2006;355(1):11–20. [DOI] [PubMed] [Google Scholar]
- 16.Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. The New England journal of medicine. September 6 2001;345(10):725–730. [DOI] [PubMed] [Google Scholar]
- 17.Smalley SR, Benedetti JK, Haller DG, et al. Updated analysis of SWOG-directed intergroup study 0116: a phase III trial of adjuvant radiochemotherapy versus observation after curative gastric cancer resection. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. July 1 2012;30(19):2327–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu WG, Xua DF, Pu J, et al. A randomized, controlled, multicenter study comparing intensity-modulated radiotherapy plus concurrent chemotherapy with chemotherapy alone in gastric cancer patients with D2 resection. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. September 2012;104(3):361–366. [DOI] [PubMed] [Google Scholar]
- 19.Park SH, Sohn TS, Lee J, et al. Phase III Trial to Compare Adjuvant Chemotherapy With Capecitabine and Cisplatin Versus Concurrent Chemoradiotherapy in Gastric Cancer: Final Report of the Adjuvant Chemoradiotherapy in Stomach Tumors Trial, Including Survival and Subset Analyses. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. October 1 2015;33(28):3130–3136. [DOI] [PubMed] [Google Scholar]
- 20.Kim Y, Park SH, Kim KM, et al. The Influence of Metastatic Lymph Node Ratio on the Treatment Outcomes in the Adjuvant Chemoradiotherapy in Stomach Tumors (ARTIST) Trial: A Phase III Trial. J Gastric Cancer. June 2016;16(2):105–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bilici A, Seker M, Ustaalioglu BB, et al. Determining of metastatic lymph node ratio in patients who underwent D2 dissection for gastric cancer. Medical oncology. September 2010;27(3):975–984. [DOI] [PubMed] [Google Scholar]
- 22.Inoue K, Nakane Y, Iiyama H, et al. The superiority of ratio-based lymph node staging in gastric carcinoma. Annals of surgical oncology. Jan-Feb 2002;9(1):27–34. [DOI] [PubMed] [Google Scholar]
- 23.Nitti D, Marchet A, Olivieri M, et al. Ratio between metastatic and examined lymph nodes is an independent prognostic factor after D2 resection for gastric cancer: analysis of a large European monoinstitutional experience. Annals of surgical oncology. November 2003;10(9):1077–1085. [DOI] [PubMed] [Google Scholar]
- 24.Wu XJ, Miao RL, Li ZY, et al. Prognostic value of metastatic lymph node ratio as an additional tool to the TNM stage system in gastric cancer. European journal of surgical oncology : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. July 2015;41(7):927– 933. [DOI] [PubMed] [Google Scholar]
- 25.Xiao LB, Yu JX, Wu WH, Xu FF, Yang SB. Superiority of metastatic lymph node ratio to the 7th edition UICC N staging in gastric cancer. World journal of gastroenterology : WJG. December 14 2011;17(46):5123–5130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marchet A, Mocellin S, Ambrosi A, et al. The prognostic value of N-ratio in patients with gastric cancer: validation in a large, multicenter series. European journal of surgical oncology : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. February 2008;34(2):159–165. [DOI] [PubMed] [Google Scholar]
- 27.Marchet A, Mocellin S, Ambrosi A, et al. The ratio between metastatic and examined lymph nodes (N ratio) is an independent prognostic factor in gastric cancer regardless of the type of lymphadenectomy: results from an Italian multicentric study in 1853 patients. Annals of surgery. April 2007;245(4):543–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Costa Junior WL, Coimbra FJ, Batista TP, Ribeiro HS, Diniz AL. Evaluation of N-ratio in selecting patients for adjuvant chemoradiotherapy after d2- gastrectomy. Arq Gastroenterol. Oct-Dec 2013;50(4):257–263. [DOI] [PubMed] [Google Scholar]
- 29.Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate behavioral research. May 2011;46(3):399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diaz de Liano A, Yarnoz C, Aguilar R, Artieda C, Ortiz H. Rationale for gastrectomy with D2 lymphadenectomy in the treatment of gastric cancer. Gastric cancer : official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association. 2008;11(2):96–102. [DOI] [PubMed] [Google Scholar]
- 31.Lee J, Lim DH, Kim S, et al. Phase III trial comparing capecitabine plus cisplatin versus capecitabine plus cisplatin with concurrent capecitabine radiotherapy in completely resected gastric cancer with D2 lymph node dissection: the ARTIST trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. January 20 2012;30(3):268–273. [DOI] [PubMed] [Google Scholar]
- 32.Norero E, Bustos M, Herrera ME, et al. Postoperative adjuvant treatment for gastric cancer improves long-term survival after curative resection and D2 lymphadenectomy. Results from a Latin American Center. European journal of surgical oncology : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. January 2016; 42(1): 94–102. [DOI] [PubMed] [Google Scholar]
- 33.Costa WL Jr., Coimbra FJ, Fogaroli RC, et al. Adjuvant chemoradiotherapy after d2-lymphadenectomy for gastric cancer: the role of n-ratio in patient selection. results of a single cancer center. Radiation oncology. October 15 2012;7:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quan R, Huang J, Chen H, et al. Comparison of efficacy in adjuvant chemotherapy regimens in patients with radically resected gastric cancer : a propensity-matched analysis. Oncotarget. September 1 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lichthardt S, Kerscher A, Dietz UA, et al. Original article: role of adjuvant chemotherapy in a perioperative chemotherapy regimen for gastric cancer. BMC cancer. August 18 2016;16:650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang SH, Kim SN, Choi HJ, et al. Adjuvant Chemotherapy for Advanced Gastric Cancer in Elderly and Non-Elderly Patients: Meta-Analysis of Randomized Controlled Trials. Cancer Res Treat. July 04 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jo JC, Baek JH, Koh SJ, et al. Adjuvant chemotherapy for elderly patients (aged 70 or older) with gastric cancer after a gastrectomy with D2 dissection: A single center experience in Korea. Asia Pac J Clin Oncol. December 2015;11(4):282– 287. [DOI] [PubMed] [Google Scholar]
- 38.Ejaz A, Spolverato G, Kim Y, et al. Impact of external-beam radiation therapy on outcomes among patients with resected gastric cancer: a multi-institutional analysis. Annals of surgical oncology. October 2014;21(11):3412–3421. [DOI] [PubMed] [Google Scholar]
- 39.Spolverato G, Ejaz A, Kim Y, et al. Prognostic Performance of Different Lymph Node Staging Systems After Curative Intent Resection for Gastric Adenocarcinoma. Annals of surgery. January 5 2015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


