Abstract
Acinar cells in the adult pancreas show high plasticity and can undergo transdifferentiation to a progenitor-like cell type with ductal characteristics. This process, termed acinar-to-ductal metaplasia (ADM), is an important feature facilitating pancreas regeneration after injury. Data from animal models show that cells that undergo ADM in response to oncogenic signalling are precursors for pancreatic intraepithelial neoplasia lesions, which can further progress to pancreatic ductal adenocarcinoma (PDAC). As human pancreatic adenocarcinoma is often diagnosed at a stage of metastatic disease, understanding the processes that lead to its initiation is important for the discovery of markers for early detection, as well as options that enable an early intervention. Here, the critical determinants of acinar cell plasticity are discussed, in addition to the intracellular and extracellular signalling events that drive acinar cell metaplasia and their contribution to development of PDAC.
Of the adult cell lineages of the pancreas, acinar cells show the highest plasticity1,2. Pancreatic acinar cells can dedifferentiate or transdifferentiate to an embryonic progenitor phenotype that expresses ductal markers, in a process termed acinar-to-ductal metaplasia (ADM)3. Multiple factors have been implicated in mediating ADM, including KRAS hyperactivity and increased inflammatory signalling4–7 (FIG. 1). The implication of ADM in the development of pancreatic adenocarcinoma was first demonstrated in mice by transgenic overexpression of transforming growth factor (TGF)-α8. ADM was also demonstrated in vitro in 3D cell culture, in which mouse acinar cell clusters, in the presence of internal or external stress signalling, oncogenic KRAS, inflammatory cytokines or growth factors that activate epidermal growth factor receptor (EGFR), spontaneously transdifferentiate into duct-like structures4,6,9–11. Similar 3D cell culture experiments showed that ADM in human acinar cells can be induced by TGFβ12.
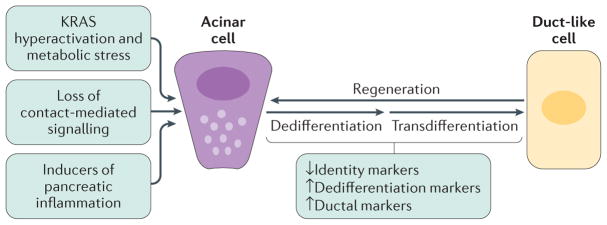
Figure 1. Acinar cell plasticity and metaplasia to duct-like cells in the adult pancreas.
In response to pancreatic injury, the loss of cell–cell and cell–matrix contacts (contact-mediated signalling), loss of polarity, KRAS hyperactivity and increased inflammatory signalling can drive acinar cells to undergo dedifferentiation and transdifferentiation to a duct-like phenotype that is needed for pancreatic regeneration. Acinar-to-ductal metaplasia becomes irreversible in the presence of an oncogenic Kras mutation and persistent growth factor signalling, leading to metabolic and signalling changes that lock the duct-like cells in their transdifferentiated state and initiate further progression to low-grade precancerous lesions.
ADM is a common and reversible process during pancreatic inflammation (pancreatitis) or injury in mouse and human tissue4,13, and the resulting cells are believed to contribute to the regeneration of acinar structures and repopulation of the pancreas. Transgenic mouse models showed that ADM becomes irreversible when cells acquire oncogenic Kras mutations or persistent aberrant growth factor signalling, which prevent redifferentiation and initiate further progression. Oncogenic KRAS in mouse acinar cells alter gene expression profiles and lead to: silencing of acinar genes such as Mist1 (also known as Bhlha15), Cpa1 or those encoding elastase and amylase; the induction of ductal genes encoding cytokeratin 19 (Krt19) and mucin 1 (Muc1); and the upregulated expression of pancreatic and duodenal homeobox 1 (Pdx1) and Sry-related high-mobility group box 9 (Sox9)4,11. Lineage tracing in mice has shown that acinar cells undergoing such changes due to persistent expression of oncogenic KrasG12D transdifferentiate to ADM cells that are incapable of redifferentiating, but instead further progress to duct-like cells that form precancerous pancreatic intraepithelial neoplasia (PanIN) 1A or 1B (early dysplastic) or PanIN2 lesions (increasing levels of dysplasia)14. However, oncogenic KRAS alone does not drive carcinogenesis beyond this initiation level; secondary events are needed for further progression to carcinoma in situ (PanIN3; high-grade dysplasia) and pancreatic ductal adenocarcinoma (PDAC). Such events include additional activation of wild-type KRAS alleles through EGFR signalling6,15–17, inflammation5,18–20 and acquisition of additional gene mutations21. However, comparative studies of human tissues and transgenic mice (using the Pdx1-Cre;KrasLSL-G12D model) suggest that ADM also can give rise to dysplastic lesions other than PanIN22,23.
Although ADM as an initiating event for the development of pancreatic cancer has been demonstrated in mice, the proof that ADM has a role in the development of human cancer is still outstanding. On the basis of knowledge mainly obtained with genetic mouse models, this Review will discuss how acinar cell identity is maintained, how ADM (either reversible or irreversible) is initiated, as well as the currently favoured progression model via PanIN.
Acinar cell identity factors
Several basic helix–loop–helix (bHLH) transcription factors contribute to acinar cell identity and their genetic ablation in mouse models leads to dedifferentiation and ADM. Pancreas transcription factor 1 complex (PTF1) has a central role in not only maintaining the differentiation of acinar cells, but also their function by regulating the production of digestive enzymes24. The PTF1 complex in adult pancreas is a trimeric transcription factor formed by recombining binding protein suppressor of hairless (RBPJ) and a dimer of the bHLH transcription factor pancreas specific transcription factor 1 alpha (PTF1A, also known as p48)25. This complex then recruits p300/CREB (also known as histone acetyltransferase KAT2B), which acetylates PTF1A to further enhance transcriptional activity26. This interaction can be blocked by inhibitor of β-catenin and TCF4 (ICAT; also known as β-catenin-interacting protein 1) with the net effect of negatively-regulating acinar cell differentiation26. Ptf1a has been demonstrated to be epigenetically silenced during inflammation and during oncogenic KRAS-driven ADM in mice27. Furthermore, ablation of Ptf1a in mice is sufficient to induce ADM, potentiate inflammation and accelerate development of invasive PDAC by sensitizing cells to KRAS-mediated transformation28.
Another key regulator of proper development of the exocrine pancreas, as well as maintenance of identity and organization of adult acinar cells, is the bHLH factor MIST1, which functions as a homodimer29. In acinar cells, MIST1 regulates apical–basal polarity, formation of gap junctions, proper positioning of zymogen granules and exocytosis30. Acinar cells in which MIST1 homodimerization is blocked are predisposed to conversion to a duct-like phenotype, which becomes evident by increased expression of SOX9 (REF. 31), as well as upregulation of EGFR and Notch signalling pathways32. Ablation of MIST1 function leads to depletion of gap junctions, loss of polarity, dedifferentiation and ADM31,33, but also acquisition of proliferative potential due to a decrease in p21 gene expression34,35. In the context of KrasG12D mice, these effects, owing to a loss of MIST1, accelerate ADM and the occurrence of PanIN32.
GATA6 is a transcription factor that maintains acinar cell differentiation by suppressing pro-inflammatory and EGFR signalling pathways. Its ablation in mice results in extensive ADM, and in the context of an activating KrasG12V mutation, accelerates tumour development36. Interestingly, smoking is a risk factor for the development of pancreatic cancer and nicotine has been shown to decrease GATA6 promoter activity, leading to loss of its expression37.
Other factors that regulate acinar cell identity in mice are the bHLH transcription factor E47 (also known as TFE2), NR5A2, DICER1 and PAF1 (also known as pancreatic differentiation protein 2)38–41. NR5A2 maintains the mature acinar differentiation state, and in the context of an oncogenic Kras mutation, loss of NR5A2 accelerates the occurrence of ADM and PanIN40,42. Processing of microRNA (miRNA) by DICER1 is required for the maintenance of adult pancreatic acinar cells, and deletion of DICER1 increased acinar cell plasticity owing to a loss of polarity41. Additionally, deletion of DICER1 accelerates KRAS-driven acinar cell dedifferentiation and ADM, but not progression of PanIN43. PAF1 expression is normally restricted to acinar cells in the pancreas, but its depletion promotes ADM, indicating a role in maintenance of acinar cell identity39. Consequently, its expression is gradually lost during PDAC initiation39.
Acinar cell dedifferentiation factors
CDKN1B and SOX9
Loss of cyclin-dependent kinase inhibitor 1B (CDKN1B; also known as p27Kip1) occurs frequently in human PDAC and is associated with decreased survival44. Nuclear CDKN1B suppresses the expression of key factors that regulate acinar cell dedifferentiation and transdifferentiation to a ductal phenotype, such as the transcription factors SOX9 and PDX1 (REF. 45). KRAS activation can decrease nuclear CDKN1B localization, which increases the expression of both SOX9 and PDX1.
In the normal (mouse and human) adult pancreas, SOX9 is expressed in centroacinar cells, at very low levels in acinar cells and in a subpopulation of ductal cells46,47. Under inflammatory conditions, or in the presence of oncogenic KRAS, SOX9 is increasingly expressed in acinar cells and stimulates gene expression that leads to ADM48, development of pre-malignant lesions and initiation of PDAC in mice14. In line with these findings, SOX9 expression in patient tumour samples is elevated at all stages of preneoplastic lesions and PDAC49, correlating with increased expression of EGFR pathway-related genes50. Similarly, in mice, the absence of SOX9 reduces EGFR signalling and pancreatic tumorigenesis50. However, EGFR signalling can also regulate expression of SOX9 through activation of NFATC1 and NFATC4 (REFS 51,52).
PDX1
In the adult mouse pancreas, PDX1 is mainly expressed in islets and only at low levels in acinar cells53,54. As shown by lineage tracing, during mouse development, PDX1-positive cells represent progenitors of all mature pancreatic cell types55. PDX1 is involved in regulation of morphologic changes needed for branching morphogenesis during pancreas organogenesis56,57, but it is also required at a later stage in development for differentiation of islet β cells58 and the formation of acinar tissue59. Transgenic persistent expression of PDX1 in mice leads to smaller pancreata, in which acinar cells are replaced by duct-like structures60. In line with this finding, PDX1 is upregulated during pancreatitis, in all types of precursor lesions including PanIN, intraductal papillary mucinous neoplasms (IPMN) and mucinous cystic neoplasms (MCN), as well as in PDAC, pancreatic endocrine neoplasms and acinar cell carcinoma53,60. PDX1 regulates ADM and the metaplastic phenotype through activation of signal transducer and activator of transcription 3 (STAT3)60. STAT3 is a regulator of stem cell self-renewal and inflammation, and its activity is also upregulated via IL-6 (REF. 61) and KRAS–YAP1/TAZ signalling62,63.
Notch signalling
Human PanIN samples show increased Notch activity64. During mouse and zebrafish pancreas development, expression of Notch1 intracellular domain (NICD; activated Notch1) prevents differentiation of pancreatic acinar cells and endocrine and exocrine development, indicating that it functions to maintain the undifferentiated state of pancreatic precursor cells65,66. In mice, Notch can be activated downstream of both EGFR–KRAS signalling and oncogenic KRAS activation to drive acinar cell dedifferentiation into a duct-like progenitor phenotype9,64,67, but its activation is not sufficient to drive progression of preneoplastic lesions to invasive adenocarcinoma64. Furthermore, NICD induces SOX9 expression68, but SOX9 function is also required for maintaining Notch signalling69, indicating a mechanism for signal amplification.
Other factors
In addition to the previously discussed molecules, MYC and KLF4 are other factors that are required to initiate the ADM process in mice70,71. Moreover, ectopic expression of hepatocyte nuclear factor 6 (HNF6) in mouse or human acinar cells represses acinar genes and upregulates ductal genes48.
Data also indicate that ADM might be induced by alteration of acinar cell polarity or cell–cell contacts72. For example, deletion of liver kinase B1 (LKB1, a regulator of energy homeostasis) in mouse pancreas (Pdx1-Cre;KrasLSL-G12D model) leads to defective acinar cell polarity, cytoskeletal alterations and loss of tight junctions, with all in combination resulting in increased ADM7. Additionally, loss of NUMB, a protein that regulates integrins and cell junctions, results in dedifferentiation of acinar cells and accelerates the ADM process in mice in the presence of oncogenic KRAS73. In addition to cell–matrix connections, E-cadherin-based cell–cell adhesions have important functions in maintaining the acinar cell phenotype. E-cadherin stability in epithelial cells is regulated by p120 catenin and deletion of this protein in epithelial lineages of the developing pancreas in mice leads to ADM and PanIN1A74.
Inflammatory macrophages drive acinar cell dedifferentiation and reversible ADM
Inflammation can be a driver of acinar cell transdifferentiation and the resulting ADM cells might contribute to regeneration after pancreatitis4,75. During pancreatic inflammation, cellular programmes downregulate factors that drive acinar cell identity such as MIST1 (REFS 76,77). Forced expression of MIST1 counteracts ADM and leads to dramatic increases in acinar cell death, organ damage and failure of pancreas repopulation77. In caerulein-induced pancreatitis in mice, the formation of metaplastic ductal intermediates was also associated with increased Hedgehog signalling78, which is necessary to prevent acinar cell damage and to facilitate regeneration. Ablation of macrophages in mice indicated that caerulein-driven ADM is dependent on the presence of macrophages4. Moreover, macrophages were shown to affect acinar cell identity in the absence or presence of an oncogenic Kras mutation4,20.
Inflammatory macrophages initiate the ADM process via secretion of inflammatory mediators (FIG. 2), including IL-6 (REF. 61), TNF and CCL5 (also known as RANTES)4. IL-6 contributes to ADM through activation of JAK–STAT3 signalling61. TNF and CCL5 both activate NF-κB in acinar cells to induce expression of a multitude of genes including those that regulate the degradation of extracellular matrix and ADM, such as matrix metalloproteinase (MMP)-9 (REF. 4). Additionally, macrophage-secreted MMP7 might activate Notch signalling. Consequently, MMP inhibition in mice completely blocked caerulein-induced ADM4. Other transcription factors activated in acinar cells after inflammation that contribute to ADM are NFATC1 and NFATC4 (REFS 51,52).
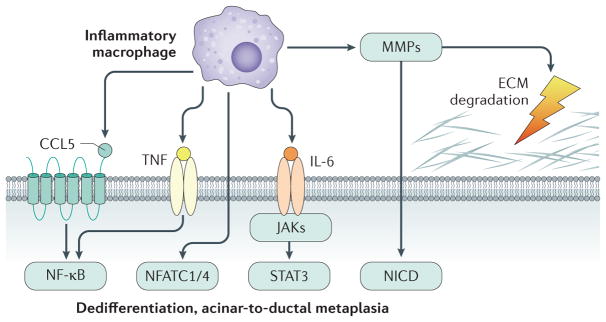
Figure 2. Inflammatory macrophage-driven signalling leading to acinar-to-ductal metaplasia.
Inflammatory macrophages can initiate acinar-to-ductal metaplasia (ADM) through NF-κB activation as caused by secreted inflammatory cytokines such as TNF and the chemokine CCL5. Macrophage-secreted IL-6 contributes to ADM and the development of PDAC through JAK–STAT3 signalling. In addition, macrophage-secreted matrix metalloproteinases (MMPs) contribute to extracellular matrix (ECM) degradation and activate Notch signalling (NICD, Notch intracellular domain). Other transcription factors activated in acinar cells after inflammation and contributing to ADM are NFATC1 and NFATC4.
Oncogene-driven irreversible ADM
Oncogenic KRAS activates transcription factors similar to inflammatory macrophages (FIG. 3), but also facilitates persistent signalling resulting in irreversibility of the ADM process79. Major signalling targets for activated KRAS during ADM are the RAF–MEK–ERK pathway, the phosphatidylinositol 3-kinase (PI3K)–AKT pathway and serine/threonine-protein kinase D1 (PRKD1).
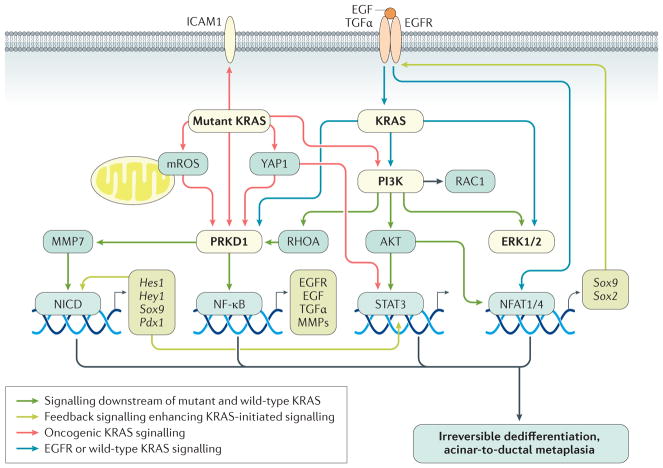
Figure 3. KRAS-driven intrinsic signalling pathways leading to irreversible acinar-to-ductal metaplasia in mice.
To initiate irreversible acinar-to-ductal metaplasia (ADM), both oncogenic KRAS and wild-type KRAS activities need to be increased. This KRAS signalling mediates induction of similar transcription factors to inflammatory macrophages, but facilitates persistent signalling leading to irreversibility of the ADM process. Signalling hubs downstream of wild-type and mutant KRAS that relay signals to activate transcription factors driving ADM are PRKD1 and phosphatidylinositol 3-kinase (PI3K). PRKD1 can be activated by mutant KRAS-initiated metabolic changes and increases in mitochondrial reactive oxygen species (mROS). PRKD1 then initiates NF-κB and Notch (NICD; Notch intracellular domain) signalling and upregulated expression of matrix metalloproteinases (MMPs), epidermal growth factor (EGF), EGF receptor (EGFR) and transforming growth factor (TGF)-α (via NF-κB), and SOX9 and PDX1 (via NCID). Increased intrinsic EGFR signalling leads to further activation of wild-type KRAS and signal amplification. MMPs can contribute to extracellular matrix degradation as well as activation of Notch. PI3K induces cytoskeletal reorganization by activating small GTPases such as RAC1 and RHOA, but also activates ERK1/2 and AKT. STAT3 and NFATC1 or NFATC4 are activated via PI3K or AKT signalling. NFATC1 or NFATC4 mediate upregulation of SOX2 and SOX9. Mutant KRAS also upregulates the expression of intercellular adhesion molecule 1 (ICAM1), a surface molecule that initiates chemoattraction of inflammatory macrophages into the ADM region. Red arrows indicate signalling mediated by oncogenic KRAS. Blue arrows indicate signalling mediated by EGFR or wild-type KRAS. Grey arrows indicate signalling downstream of both mutant and wild-type KRAS. Green arrows indicate feedback signalling that potentiates KRAS-initiated signalling.
The PI3K–AKT pathway
PI3K acts downstream of KRAS and in mice, oncogenic KRAS-induced plasticity of pancreatic cells, formation of preneoplastic lesions and cancer initiation are all dependent on p110α (also known as PIK3CA, the catalytic subunit of PI3K)80,81. In line with this finding, ADM, PanIN and the formation of invasive PDAC can also be observed after transgenic expression of a constitutively-active form of p110α82. PI3K-mediated transdifferentiation of acinar cells is mediated through ERK1/2 signalling82, and small molecule inhibitors targeting the activation of ERK1/2 indicate that these MAP kinases are involved in KrasG12D-driven dedifferentiation of acinar cells, ADM and PanIN formation11,83. To drive these processes, PI3K also initiates actin reorganization processes that are orchestrated by Rho GTPases80,81,84. Pancreas-specific deletion of phosphatase and tensin homolog (PTEN), which negatively regulates PI3K signalling, leads to ductal metaplasia and malignant transformation in mice85. In the context of an oncogenic Kras mutation, loss of PTEN leads to even more accelerated formation of PDAC86,87. Similarly, expression of a constitutively-active allele of Akt1, one of the downstream targets for PI3K signalling, induces ADM88 and cooperates with Kras oncogenic mutations to drive the onset and progression of PDAC89. However, only a subset (2–3%) of human patients with pancreatic cancer carry activating mutations in PIK3CA90, which suggests that increased PI3K activity might mainly be achieved by signalling through oncogenic KRAS in patients.
The PRKD1 pathway
Another emerging signalling pathway that drives ADM and progression to PanIN in mice (p48-Cre;KrasLSL-G12D mouse model) is regulated by PRKD1. This enzyme converges signalling initiated by oncogenic KRAS and wild-type KRAS downstream of EGFR6,9, and increases Notch1 activity to upregulate SOX9 and PDX1. This process is mediated through PRKD1-induced downregulation of suppressors of Notch signalling, such as SEL1L and CBL9, and upregulation of inducers of Notch activation, such as ADAM10, ADAM17 and MMP7 (REFS 9,91). PRKD1 also links oncogenic KRAS signalling to activation of NF-κB6, and activation of the PRKD1–NF-κB pathway is driven by metabolic changes initiated by KRAS that lead to an increase in mitochondrial reactive oxygen species (ROS)6. ROS–PRKD1–NF-κB signalling in acinar cells then upregulates expression of EGFR and its ligands, TGFα and EGF, further potentiating the oncogenic effects of mutant KRAS in a feedback loop6. Notch and NF-κB signalling pathways can cooperate to mediate formation of preneoplastic lesions92. Thus, PRKD1 brings together two important pathways that drive the formation of precancerous lesions. Notch can also act synergistically with other transcription factors such as STAT3 and the combined inhibition of Notch and JAK2–STAT3 signalling in KrasLSL-G12D/+;Trp53−/+;Pdx1-Cre (KPC) mice has been shown to impair ADM and its progression93.
Oncogene-driven microinflammation
Acinar cells with an oncogenic KrasG12D mutation have also been shown to produce chemoattractants for inflammatory macrophages18. This process causes a persistent microinflammation that contributes to acinar cell transdifferentiation. One of the factors released by acinar cells is intracellular adhesion molecule 1 (ICAM1, also known as CD54), which can be shed as a soluble form. Blocking ICAM1 using neutralization antibodies has been shown to substantially reduce the occurrence and progression of KrasG12D-driven preneoplastic lesions in mice20. However, such microinflammation is not sufficient to drive the progression to PDAC, and additional inflammatory insults and genetic alterations are needed for acceleration of the oncogenic process18,19,94.
ADM as a precursor lesion
Cancer initiation
Mutations in the KRAS proto-oncogenes are the earliest events leading to development of human PDAC95. Data from genetic mouse models have shown that transgenic expression of oncogenic KRAS in acinar cells initiates ADM and locks them into a transdifferentiated duct-like state. To progress from ADM to PanIN and pancreatic cancer, the activities of endogenous and mutant alleles of Kras need to be further increased16 (FIG. 4). Such increases in KRAS activity can be achieved by additional activation of growth factor signalling or chronic inflammation15,17–20. EGFR signalling, for example, not only further activates oncogenic KRAS but also activates the wild-type allele9,15,96. Upregulation of inflammatory and EGFR signalling pathways can also be achieved by loss of GATA6 (REFS 16,36,37).
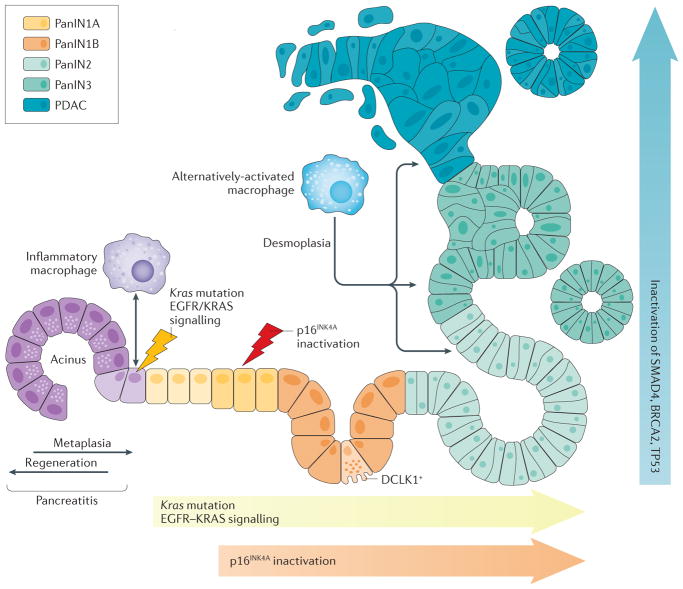
Figure 4. Oncogenic KRAS and inflammation as drivers of acinar-to-ductal metaplasia and clonal expansion.
Schematic showing how macrophage subtypes and genetic mutations contribute to acinar-to-ductal metaplasia (ADM), clonal expansion and progression to pancreatic cancer. During pancreatitis ADM is a reversible process, but becomes irreversible when an oncogenic Kras mutation is present. The accumulation of KRAS activity as caused by oncogenic Kras mutations and epidermal growth factor receptor (EGFR)–wild-type KRAS signalling, as well as loss of senescence due to an additional inactivation of cyclin-dependent kinase inhibitor 2A (CDKN2A, also known as p16INK1A), is needed for progression. Further progression to pancreatic intraepithelial neoplasia (PanIN)-2, carcinoma in situ (PanIN3) and pancreatic ductal adenocarcinoma (PDAC) occurs after acquisition of additional gene mutations inTp53 (p53), Brca2 and Smad4. The progression to cancerous lesions occurs with an increase in desmoplasia. Cells positive for the serine/threonine-protein kinase DCLK1 are of acinar origin, are formed mainly in low-grade PanIN lesions (PanIN1A, PanIN1B and PanIN2) and have cancer stem cell functions.
During the process of cancer initiation, crosstalk between acinar cells with Kras mutations and inflammatory macrophages contributes to ADM and formation of early lesions20. However, during progression to PDAC, the tumour microenvironment becomes immuno-suppressive with a predominance of myeloid-derived suppressor cells and regulatory T cells97. Furthermore, desmoplasia increases with progression98. Pancreatic cancers can have different stromal subtypes, such as stroma characterized by stellate cell expression profiles, or more aggressive stroma characterized by activated fibroblasts and alternatively-activated macrophages99.
Further progression
In the current model for PDAC development, ADM cells can progress to PanIN1A or PanIN1B lesions and PanIN2 lesions, which are high in senescence markers. Clonal expansion and progression to PanIN3 and PDAC requires additional signalling and mutational events to overcome oncogene-induced senescence, such as loss of cyclin-dependent kinase inhibitor 2A (CDKN2A, also known as p16INK4A). Eventually, additional inactivating mutations of tumour suppressor genes, such as Tp53, Brca2 and Smad4, occur during PanIN2 or PanIN3 progression, but at reduced rates (a detailed review on pancreatic cancer biology and genetics and photomicrographs of different lesions can be found elsewhere100). However, comparative studies of human tissues and transgenic mice (Pdx1-Cre;KrasLSL-G12D model) suggest that dysplastic lesions other than PanIN can also arise from ADM. Such atypical flat lesions might indicate pancreatic cancer development directly from ADM without the intermediate step of PanIN22,23.
Early dissemination and stemness
An interesting aspect during development of PDAC is that cells can disseminate from low-grade lesions with inflammatory foci, with circulating pancreatic epithelial cells present in the blood stream of mice and patients before the development of cancer101,102. Some cells in PanIN1 or PanIN2 lesions were shown to undergo epithelial-to-mesenchymal transition (EMT)101, a programme that enables cells to gain invasive properties. In addition, ADM and PanIN1 or PanIN2 lesions contain a subpopulation of cells positive for the serine/threonine-protein kinase DCLK1 (also known as doublecortin-like kinase 1), and the acinar origin of these DCLK-positive cells has been demonstrated by lineage tracing103. A majority of the circulating pancreatic epithelial cells express DCLK1 as a marker; interestingly, DCLK1 expression has also been linked to EMT104.
EMT not only leads to increased invasiveness of cells, but it can also induce stem cell formation105. In low-grade PanIN, DCLK1-positive cells were also shown to characterize a subpopulation with cancer stem cell properties106,107. These cells are characterized by upregulation of Notch and EGFR signalling103,106. However, the signalling pathways that drive the development of DCLK1-positive cells from a clonal population within ADM or PanIN cells, as well as their functions, are not yet well characterized.
Is ADM an initiating event for human PanIN and pancreatic cancer?
Although mouse data obtained with different model systems point to oncogene-driven ADM as an initiating event for the formation of PanIN lesions, the role of ADM in the development of human PDAC is still undefined. That ADM occurs in human pancreatic cancer specimens is generally accepted, as it can be observed in proximity to neoplastic precursor lesions108,109. Attempts have been made to investigate if human acinar cells that underwent ADM can be precursors to PanIN. Analyses of human ADM lesions for KRAS mutations indicated that sections with ADM associated with PanIN lesions harboured the same KRAS gene mutation. By contrast, ADM lesions that were not associated with PanIN had wild-type KRAS. The conclusion was that the ADM lesion associated with PanIN might represent retrograde extension of the PanIN95. With the knowledge that inflammation and macrophage-released cytokines can lead to ADM independent of KRAS mutations6, the detection of ADM lesions that are KRAS wild-type is not surprising. As human PDAC often has pancreatitis associated, one would expect both ADM lesions that express wild-type KRAS and ADM lesions that express mutant KRAS95. Thus, these data can also be interpreted differently: PanIN and ADM lesions associated with PanIN have the same mutations because ADM is a precursor for PanIN; and some of the ADM have progressed to PanIN owing to additional signalling or mutations that other ADM did not have. As discussed earlier, it is also possible that the development of human PDAC from ADM might not follow the PanIN progression model, but rather might lead to occurrence of flat lesions22.
Conclusions
Although this Review focuses on acinar cell transdifferentiation as an initiating step, data does exist that supports other pancreas cell types, including duct cells or centroacinar cells, as the tumour-initiating population. For example, centroacinar cell markers were detected in patient PanINs, which led to a progression model with centroacinar cells as the origin for PDAC110.
In humans, PanIN are the most common of the precursor lesions for pancreatic cancer and are usually found in medium-sized ducts, whereas IPMN and MCN are found in the main duct and its major branches111. With respect to acinar cells as potential progenitors for pancreatic lesions, accumulated conclusive evidence obtained from genetic animal models shows that mature acinar cells in the presence of an oncogenic Kras mutation transdifferentiate to the duct-like cells that form PanIN lesions112. This finding was demonstrated with lineage tracing experiments113–115, but was also shown in different animal models in which expression of oncogenic KRAS under acinar cell-specific promoters, such as PTF1A, elastase or MIST1, all induce ADM and PanIN in mice116. The high plasticity of acinar cells, needed for regeneration processes after pancreatic injury, also makes them vulnerable for persistent transdifferentiation to PanIN cells in the presence of an oncogenic insult.
One could argue that, with increasing age, ADM and low-grade PanIN are relatively common in humans and rarely progress to pancreatic cancer117. Additionally, PanIN are small, clinically difficult to detect (in contrast to IPMN) and the main focus should be on developing new treatment strategies for metastatic disease. However, unexpected findings suggest that early (low-grade) lesions produce cancer stem cells103,107, and that epithelial cells might disseminate into the blood stream at a time point at which no primary tumour is formed in the pancreas101. If seeding potentially occurs at an early stage of PanIN progression, the development of an efficient treatment strategy for the time of diagnosis will be difficult, and a focus should be on detecting and intervening with these early events118. With respect to early detection, circulating factors that have been released by oncogenic KRAS-expressing PanIN cells might be detected in pancreatic juice or blood. The challenge in identifying reliable markers, however, is that they need to be indicative for developing cancer and distinct from factors released during pancreatitis. Eventually, understanding the crosstalk between precancerous and/or cancerous cells with cells of the desmoplastic microenvironment could be of importance to reprogramming pro-tumorigenic signalling into antitumorigenic signalling119.
Key points.
Adult pancreatic acinar cells show high plasticity that enables a change in their differentiation commitment
Acinar-to-ductal metaplasia (ADM) is a mechanism needed for regeneration after inflammation or injury
ADM is a result of epigenetic silencing of markers of acinar cell identity, activation of drivers of acinar cell dedifferentiation or loss of acinar cell organization
ADM is driven by intrinsic and extrinsic signalling
ADM in the presence of oncogenic KRAS signalling is irreversible and leads to a duct-like cell type that forms pancreatic intraepithelial neoplasia
Acknowledgments
The author thanks H. R. Döppler and V. Pandey in the Storz laboratory for critical reading of the manuscript. The author apologizes to colleagues whose papers, although important contributions to the field, have not been cited because of the scope of this Review. This work was supported by grants from the NIH (CA200572 and CA102701-12DRP3).
Footnotes
Competing interests statement
The author declares no competing interests.
References
- 1.Stanger BZ, Hebrok M. Control of cell identity in pancreas development and regeneration. Gastroenterology. 2013;144:1170–1179. doi: 10.1053/j.gastro.2013.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pinho AV, et al. Adult pancreatic acinar cells dedifferentiate to an embryonic progenitor phenotype with concomitant activation of a senescence programme that is present in chronic pancreatitis. Gut. 2011;60:958–966. doi: 10.1136/gut.2010.225920. [DOI] [PubMed] [Google Scholar]
- 3.Rooman I, Real FX. Pancreatic ductal adenocarcinoma and acinar cells: a matter of differentiation and development? Gut. 2012;61:449–458. doi: 10.1136/gut.2010.235804. [DOI] [PubMed] [Google Scholar]
- 4.Liou GY, et al. Macrophage-secreted cytokines drive pancreatic acinar-to-ductal metaplasia through NF-kappaB and MMPs. J Cell Biol. 2013;202:563–577. doi: 10.1083/jcb.201301001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Logsdon CD, Ji B. Ras activity in acinar cells links chronic pancreatitis and pancreatic cancer. Clin Gastroenterol Hepatol. 2009;7:S40–S43. doi: 10.1016/j.cgh.2009.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liou GY, et al. Mutant KRas-induced mitochondrial oxidative stress in acinar cells upregulates EGFR signaling to drive formation of pancreatic precancerous lesions. Cell Rep. 2016;14:2325–2336. doi: 10.1016/j.celrep.2016.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hezel AF, et al. Pancreatic LKB1 deletion leads to acinar polarity defects and cystic neoplasms. Mol Cell Biol. 2008;28:2414–2425. doi: 10.1128/MCB.01621-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sandgren EP, Luetteke NC, Palmiter RD, Brinster RL, Lee DC. Overexpression of TGF alpha in transgenic mice: induction of epithelial hyperplasia, pancreatic metaplasia, and carcinoma of the breast. Cell. 1990;61:1121–1135. doi: 10.1016/0092-8674(90)90075-p. [DOI] [PubMed] [Google Scholar]
- 9.Liou GY, et al. Protein kinase D1 drives pancreatic acinar cell reprogramming and progression to intraepithelial neoplasia. Nat Commun. 2015;6:6200. doi: 10.1038/ncomms7200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Means AL, et al. Pancreatic epithelial plasticity mediated by acinar cell transdifferentiation and generation of nestin-positive intermediates. Development. 2005;132:3767–3776. doi: 10.1242/dev.01925. [DOI] [PubMed] [Google Scholar]
- 11.Shi G, et al. Maintenance of acinar cell organization is critical to preventing Kras-induced acinar-ductal metaplasia. Oncogene. 2013;32:1950–1958. doi: 10.1038/onc.2012.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu J, et al. TGF-beta1 promotes acinar to ductal metaplasia of human pancreatic acinar cells. Sci Rep. 2016;6:30904. doi: 10.1038/srep30904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Houbracken I, et al. Lineage tracing evidence for transdifferentiation of acinar to duct cells and plasticity of human pancreas. Gastroenterology. 2011;141:731–741.e4. doi: 10.1053/j.gastro.2011.04.050. [DOI] [PubMed] [Google Scholar]
- 14.Kopp JL, et al. Identification of Sox9-dependent acinar-to-ductal reprogramming as the principal mechanism for initiation of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;22:737–750. doi: 10.1016/j.ccr.2012.10.025. Using lineage tracing of pancreatic cell populations, the authors show that ductal and centroacinar cells are refractory to transformation by oncogenic KRAS, whereas acinar cells undergo metaplasia to a duct-like state and form precursors for PDAC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ardito CM, et al. EGF receptor is required for KRAS-induced pancreatic tumorigenesis. Cancer Cell. 2012;22:304–317. doi: 10.1016/j.ccr.2012.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ji B, et al. Ras activity levels control the development of pancreatic diseases. Gastroenterology. 2009;137:1072–1082.e6. doi: 10.1053/j.gastro.2009.05.052. Part of a series of papers from the Logsdon laboratory showing that acquisition of an oncogenic form of KRAS alone is not sufficient to drive development of PDAC. To progress to PDAC, the activities of wild-type and mutant KRAS need to be further increased. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Navas C, et al. EGF receptor signaling is essential for k-ras oncogene-driven pancreatic ductal adenocarcinoma. Cancer Cell. 2012;22:318–330. doi: 10.1016/j.ccr.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guerra C, et al. Pancreatitis-induced inflammation contributes to pancreatic cancer by inhibiting oncogene-induced senescence. Cancer Cell. 2011;19:728–739. doi: 10.1016/j.ccr.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guerra C, et al. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell. 2007;11:291–302. doi: 10.1016/j.ccr.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 20.Liou GY, et al. Mutant KRAS-induced expression of ICAM-1 in pancreatic acinar cells causes attraction of macrophages to expedite the formation of precancerous lesions. Cancer Discov. 2015;5:52–63. doi: 10.1158/2159-8290.CD-14-0474. The authors show that acquisition of an oncogenic KRAS mutation in acinar cells can induce the expression of chemoattractants for inflammatory macrophages, which then contribute to the ADM process. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bardeesy N, DePinho RA. Pancreatic cancer biology and genetics. Nat Rev Cancer. 2002;2:897–909. doi: 10.1038/nrc949. [DOI] [PubMed] [Google Scholar]
- 22.Aichler M, et al. Origin of pancreatic ductal adenocarcinoma from atypical flat lesions: a comparative study in transgenic mice and human tissues. J Pathol. 2012;226:723–734. doi: 10.1002/path.3017. [DOI] [PubMed] [Google Scholar]
- 23.Wagner M, Luhrs H, Kloppel G, Adler G, Schmid RM. Malignant transformation of duct-like cells originating from acini in transforming growth factor transgenic mice. Gastroenterology. 1998;115:1254–1262. doi: 10.1016/s0016-5085(98)70098-8. [DOI] [PubMed] [Google Scholar]
- 24.Rodolosse A, et al. PTF1alpha/p48 transcription factor couples proliferation and differentiation in the exocrine pancreas [corrected] Gastroenterology. 2004;127:937–949. doi: 10.1053/j.gastro.2004.06.058. [DOI] [PubMed] [Google Scholar]
- 25.Masui T, et al. Replacement of Rbpj with Rbpjl in the PTF1 complex controls the final maturation of pancreatic acinar cells. Gastroenterology. 2010;139:270–280. doi: 10.1053/j.gastro.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campos ML, et al. ICAT is a novel Ptf1a interactor that regulates pancreatic acinar differentiation and displays altered expression in tumours. Biochem J. 2013;451:395–405. doi: 10.1042/BJ20120873. [DOI] [PubMed] [Google Scholar]
- 27.Benitz S, et al. Polycomb repressor complex 1 promotes gene silencing through H2AK119 mono-ubiquitination in acinar-to-ductal metaplasia and pancreatic cancer cells. Oncotarget. 2016;7:11424–11433. doi: 10.18632/oncotarget.6717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krah NM, et al. The acinar differentiation determinant PTF1A inhibits initiation of pancreatic ductal adenocarcinoma. eLife. 2015;4:e07125. doi: 10.7554/eLife.07125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pin CL, Rukstalis JM, Johnson C, Konieczny SF. The bHLH transcription factor Mist1 is required to maintain exocrine pancreas cell organization and acinar cell identity. J Cell Biol. 2001;155:519–530. doi: 10.1083/jcb.200105060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Direnzo D, et al. Induced Mist1 expression promotes remodeling of mouse pancreatic acinar cells. Gastroenterology. 2012;143:469–480. doi: 10.1053/j.gastro.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson CL, et al. Activation of protein kinase Cδ leads to increased pancreatic acinar cell dedifferentiation in the absence of MIST1. J Pathol. 2012;228:351–365. doi: 10.1002/path.4015. [DOI] [PubMed] [Google Scholar]
- 32.Shi G, et al. Loss of the acinar-restricted transcription factor Mist1 accelerates Kras-induced pancreatic intraepithelial neoplasia. Gastroenterology. 2009;136:1368–1378. doi: 10.1053/j.gastro.2008.12.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu L, et al. Inhibition of Mist1 homodimer formation induces pancreatic acinar-to-ductal metaplasia. Mol Cell Biol. 2004;24:2673–2681. doi: 10.1128/MCB.24.7.2673-2681.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jia D, Sun Y, Konieczny SF. Mist1 regulates pancreatic acinar cell proliferation through p21 CIP1/WAF1. Gastroenterology. 2008;135:1687–1697. doi: 10.1053/j.gastro.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grabliauskaite K, et al. p21(WAF1) (/Cip1) limits senescence and acinar-to-ductal metaplasia formation during pancreatitis. J Pathol. 2015;235:502–514. doi: 10.1002/path.4440. [DOI] [PubMed] [Google Scholar]
- 36.Martinelli P, et al. The acinar regulator Gata6 suppresses KrasG12V-driven pancreatic tumorigenesis in mice. Gut. 2016;65:476–486. doi: 10.1136/gutjnl-2014-308042. [DOI] [PubMed] [Google Scholar]
- 37.Hermann PC, et al. Nicotine promotes initiation and progression of KRAS-induced pancreatic cancer via Gata6-dependent dedifferentiation of acinar cells in mice. Gastroenterology. 2014;147:1119–1133. doi: 10.1053/j.gastro.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 38.Kim S, et al. The basic helix-loop-helix transcription factor E47 reprograms human pancreatic cancer cells to a quiescent acinar state with reduced tumorigenic potential. Pancreas. 2015;44:718–727. doi: 10.1097/MPA.0000000000000328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dey P, Rachagani S, Vaz AP, Ponnusamy MP, Batra SK. PD2/Paf1 depletion in pancreatic acinar cells promotes acinar-to-ductal metaplasia. Oncotarget. 2014;5:4480–4491. doi: 10.18632/oncotarget.2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.von Figura G, Morris JP, IV, Wright CV, Hebrok M. Nr5a2 maintains acinar cell differentiation and constrains oncogenic Kras-mediated pancreatic neoplastic initiation. Gut. 2014;63:656–664. doi: 10.1136/gutjnl-2012-304287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang YJ, et al. Dicer is required for maintenance of adult pancreatic acinar cell identity and plays a role in Kras-driven pancreatic neoplasia. PLoS ONE. 2014;9:e113127. doi: 10.1371/journal.pone.0113127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Flandez M, et al. Nr5a2 heterozygosity sensitises to, and cooperates with, inflammation in KRas(G12V)-driven pancreatic tumourigenesis. Gut. 2014;63:647–655. doi: 10.1136/gutjnl-2012-304381. [DOI] [PubMed] [Google Scholar]
- 43.Morris JP, IV, et al. Dicer regulates differentiation and viability during mouse pancreatic cancer initiation. PLoS ONE. 2014;9:e95486. doi: 10.1371/journal.pone.0095486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu CD, et al. Loss of p27Kip1 expression independently predicts poor prognosis for patients with resectable pancreatic adenocarcinoma. Cancer. 1999;85:1250–1260. [PubMed] [Google Scholar]
- 45.Jeannot P, et al. Loss of p27Kip(1) promotes metaplasia in the pancreas via the regulation of Sox9 expression. Oncotarget. 2015;6:35880–35892. doi: 10.18632/oncotarget.5770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kopp JL, et al. Sox9+ ductal cells are multipotent progenitors throughout development but do not produce new endocrine cells in the normal or injured adult pancreas. Development. 2011;138:653–665. doi: 10.1242/dev.056499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Furuyama K, et al. Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nat Genet. 2011;43:34–41. doi: 10.1038/ng.722. [DOI] [PubMed] [Google Scholar]
- 48.Prevot PP, et al. Role of the ductal transcription factors HNF6 and Sox9 in pancreatic acinar-to-ductal metaplasia. Gut. 2012;61:1723–1732. doi: 10.1136/gutjnl-2011-300266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shroff S, et al. SOX9: a useful marker for pancreatic ductal lineage of pancreatic neoplasms. Hum Pathol. 2014;45:456–463. doi: 10.1016/j.humpath.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grimont A, et al. SOX9 regulates ERBB signalling in pancreatic cancer development. Gut. 2015;64:1790–1799. doi: 10.1136/gutjnl-2014-307075. [DOI] [PubMed] [Google Scholar]
- 51.Chen NM, et al. NFATc1 links EGFR signaling to induction of Sox9 transcription and acinar-ductal transdifferentiation in the pancreas. Gastroenterology. 2015;148:1024–1034.e9. doi: 10.1053/j.gastro.2015.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hessmann E, et al. NFATc4 regulates Sox9 gene expression in acinar cell plasticity and pancreatic cancer initiation. Stem Cells Int. 2016;2016:5272498. doi: 10.1155/2016/5272498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Park JY, et al. Pdx1 expression in pancreatic precursor lesions and neoplasms. Appl Immunohistochem Mol Morphol. 2011;19:444–449. doi: 10.1097/PAI.0b013e318206d958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rose SD, Swift GH, Peyton MJ, Hammer RE, MacDonald RJ. The role of PTF1-P48 in pancreatic acinar gene expression. J Biol Chem. 2001;276:44018–44026. doi: 10.1074/jbc.M106264200. [DOI] [PubMed] [Google Scholar]
- 55.Gu G, Dubauskaite J, Melton DA. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development. 2002;129:2447–2457. doi: 10.1242/dev.129.10.2447. [DOI] [PubMed] [Google Scholar]
- 56.Wescott MP, et al. Pancreatic ductal morphogenesis and the Pdx1 homeodomain transcription factor. Mol Biol Cell. 2009;20:4838–4844. doi: 10.1091/mbc.E09-03-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marty-Santos L, Cleaver O. Pdx1 regulates pancreas tubulogenesis and E-cadherin expression. Development. 2016;143:101–112. doi: 10.1242/dev.126755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oliver-Krasinski JM, et al. The diabetes gene Pdx1 regulates the transcriptional network of pancreatic endocrine progenitor cells in mice. J Clin Invest. 2009;119:1888–1898. doi: 10.1172/JCI37028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hale MA, et al. The homeodomain protein PDX1 is required at mid-pancreatic development for the formation of the exocrine pancreas. Dev Biol. 2005;286:225–237. doi: 10.1016/j.ydbio.2005.07.026. [DOI] [PubMed] [Google Scholar]
- 60.Miyatsuka T, et al. Persistent expression of PDX-1 in the pancreas causes acinar-to-ductal metaplasia through Stat3 activation. Genes Dev. 2006;20:1435–1440. doi: 10.1101/gad.1412806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Corcoran RB, et al. STAT3 plays a critical role in KRAS-induced pancreatic tumorigenesis. Cancer Res. 2011;71:5020–5029. doi: 10.1158/0008-5472.CAN-11-0908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gruber R, et al. YAP1 and TAZ control pancreatic cancer initiation in mice by direct up-regulation of JAK-STAT3 signaling. Gastroenterology. 2016;151:526–539. doi: 10.1053/j.gastro.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang W, et al. Downstream of mutant KRAS, the transcription regulator YAP is essential for neoplastic progression to pancreatic ductal adenocarcinoma. Sci Signal. 2014;7:ra42. doi: 10.1126/scisignal.2005049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.De La OJ, et al. Notch and Kras reprogram pancreatic acinar cells to ductal intraepithelial neoplasia. Proc Natl Acad Sci USA. 2008;105:18907–18912. doi: 10.1073/pnas.0810111105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Esni F, et al. Notch inhibits Ptf1 function and acinar cell differentiation in developing mouse and zebrafish pancreas. Development. 2004;131:4213–4224. doi: 10.1242/dev.01280. [DOI] [PubMed] [Google Scholar]
- 66.Hald J, et al. Activated Notch1 prevents differentiation of pancreatic acinar cells and attenuate endocrine development. Dev Biol. 2003;260:426–437. doi: 10.1016/s0012-1606(03)00326-9. [DOI] [PubMed] [Google Scholar]
- 67.Avila JL, Troutman S, Durham A, Kissil JL. Notch1 is not required for acinar-to-ductal metaplasia in a model of Kras-induced pancreatic ductal adenocarcinoma. PLoS ONE. 2012;7:e52133. doi: 10.1371/journal.pone.0052133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hosokawa S, et al. Impact of Sox9 dosage and Hes1-mediated Notch signaling in controlling the plasticity of adult pancreatic duct cells in mice. Sci Rep. 2015;5:8518. doi: 10.1038/srep08518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Delous M, et al. Sox9b is a key regulator of pancreaticobiliary ductal system development. PLoS Genet. 2012;8:e1002754. doi: 10.1371/journal.pgen.1002754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Grippo PJ, Sandgren EP. Acinar-to-ductal metaplasia accompanies c-myc-induced exocrine pancreatic cancer progression in transgenic rodents. Int J Cancer. 2012;131:1243–1248. doi: 10.1002/ijc.27322. [DOI] [PubMed] [Google Scholar]
- 71.Wei D, et al. KLF4 is essential for induction of cellular identity change and acinar-to-ductal reprogramming during early pancreatic carcinogenesis. Cancer Cell. 2016;29:324–338. doi: 10.1016/j.ccell.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hall PA, Lemoine NR. Rapid acinar to ductal transdifferentiation in cultured human exocrine pancreas. J Pathol. 1992;166:97–103. doi: 10.1002/path.1711660203. [DOI] [PubMed] [Google Scholar]
- 73.Greer RL, Staley BK, Liou A, Hebrok M. Numb regulates acinar cell dedifferentiation and survival during pancreatic damage and acinar-to-ductal metaplasia. Gastroenterology. 2013;145:1088–1097.e8. doi: 10.1053/j.gastro.2013.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hendley AM, et al. p120 Catenin is required for normal tubulogenesis but not epithelial integrity in developing mouse pancreas. Dev Biol. 2015;399:41–53. doi: 10.1016/j.ydbio.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Criscimanna A, Coudriet GM, Gittes GK, Piganelli JD, Esni F. Activated macrophages create lineage-specific microenvironments for pancreatic acinar- and beta-cell regeneration in mice. Gastroenterology. 2014;147:1106–1118.e11. doi: 10.1053/j.gastro.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 76.Kowalik AS, et al. Mice lacking the transcription factor Mist1 exhibit an altered stress response and increased sensitivity to caerulein-induced pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1123–G1132. doi: 10.1152/ajpgi.00512.2006. [DOI] [PubMed] [Google Scholar]
- 77.Karki A, et al. Silencing Mist1 gene expression is essential for recovery from acute pancreatitis. PLoS ONE. 2015;10:e0145724. doi: 10.1371/journal.pone.0145724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fendrich V, et al. Hedgehog signaling is required for effective regeneration of exocrine pancreas. Gastroenterology. 2008;135:621–631. doi: 10.1053/j.gastro.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kong B, et al. Dynamic landscape of pancreatic carcinogenesis reveals early molecular networks of malignancy. Gut. 2016 doi: 10.1136/gutjnl-2015-310913. [DOI] [PubMed]
- 80.Baer R, et al. Pancreatic cell plasticity and cancer initiation induced by oncogenic Kras is completely dependent on wild-type PI 3-kinase p110alpha. Genes Dev. 2014;28:2621–2635. doi: 10.1101/gad.249409.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wu CY, et al. PI3K regulation of RAC1 is required for KRAS-induced pancreatic tumorigenesis in mice. Gastroenterology. 2014;147:1405–1416.e7. doi: 10.1053/j.gastro.2014.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Payne SN, et al. PIK3CA mutations can initiate pancreatic tumorigenesis and are targetable with PI3K inhibitors. Oncogenesis. 2015;4:e169. doi: 10.1038/oncsis.2015.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Collins MA, Yan W, Sebolt-Leopold JS, Pasca di Magliano M. MAPK signaling is required for dedifferentiation of acinar cells and development of pancreatic intraepithelial neoplasia in mice. Gastroenterology. 2014;146:822–834.e7. doi: 10.1053/j.gastro.2013.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Heid I, et al. Early requirement of Rac1 in a mouse model of pancreatic cancer. Gastroenterology. 2011;141:719–730.e7. doi: 10.1053/j.gastro.2011.04.043. [DOI] [PubMed] [Google Scholar]
- 85.Xu HN, Nioka S, Chance B, Li LZ. Heterogeneity of mitochondrial redox state in premalignant pancreas in a PTEN null transgenic mouse model. Adv Exp Med Biol. 2011;701:207–213. doi: 10.1007/978-1-4419-7756-4_28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hill R, et al. PTEN loss accelerates KrasG12D-induced pancreatic cancer development. Cancer Res. 2010;70:7114–7124. doi: 10.1158/0008-5472.CAN-10-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Eser S, et al. Selective requirement of PI3K/PDK1 signaling for Kras oncogene-driven pancreatic cell plasticity and cancer. Cancer Cell. 2013;23:406–420. doi: 10.1016/j.ccr.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 88.Elghazi L, et al. Regulation of pancreas plasticity and malignant transformation by Akt signaling. Gastroenterology. 2009;136:1091–1103. doi: 10.1053/j.gastro.2008.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Albury TM, et al. Constitutively active Akt1 cooperates with KRasG12D to accelerate in vivo pancreatic tumor onset and progression. Neoplasia. 2015;17:175–182. doi: 10.1016/j.neo.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Weiss GA, et al. Evaluation of phosphatidylinositol-3-kinase catalytic subunit (PIK3CA) and epidermal growth factor receptor (EGFR) gene mutations in pancreaticobiliary adenocarcinoma. J Gastrointest Oncol. 2013;4:20–29. doi: 10.3978/j.issn.2078-6891.2012.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sawey ET, Johnson JA, Crawford HC. Matrix metalloproteinase 7 controls pancreatic acinar cell transdifferentiation by activating the Notch signaling pathway. Proc Natl Acad Sci USA. 2007;104:19327–19332. doi: 10.1073/pnas.0705953104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Maniati E, et al. Crosstalk between the canonical NF-kappaB and Notch signaling pathways inhibits Pparγ expression and promotes pancreatic cancer progression in mice. J Clin Invest. 2011;121:4685–4699. doi: 10.1172/JCI45797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Palagani V, et al. Combined inhibition of Notch and JAK/STAT is superior to monotherapies and impairs pancreatic cancer progression. Carcinogenesis. 2014;35:859–866. doi: 10.1093/carcin/bgt394. [DOI] [PubMed] [Google Scholar]
- 94.Carriere C, Young AL, Gunn JR, Longnecker DS, Korc M. Acute pancreatitis accelerates initiation and progression to pancreatic cancer in mice expressing oncogenic Kras in the nestin cell lineage. PLoS ONE. 2011;6:e27725. doi: 10.1371/journal.pone.0027725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shi C, et al. KRAS2 mutations in human pancreatic acinar-ductal metaplastic lesions are limited to those with PanIN: implications for the human pancreatic cancer cell of origin. Mol Cancer Res. 2009;7:230–236. doi: 10.1158/1541-7786.MCR-08-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Huang H, et al. Oncogenic K-Ras requires activation for enhanced activity. Oncogene. 2014;33:532–535. doi: 10.1038/onc.2012.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Clark CE, Beatty GL, Vonderheide RH. Immunosurveillance of pancreatic adenocarcinoma: insights from genetically engineered mouse models of cancer. Cancer Lett. 2009;279:1–7. doi: 10.1016/j.canlet.2008.09.037. [DOI] [PubMed] [Google Scholar]
- 98.Rosati A, et al. BAG3 promotes pancreatic ductal adenocarcinoma growth by activating stromal macrophages. Nat Commun. 2015;6:8695. doi: 10.1038/ncomms9695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Moffitt RA, et al. Virtual microdissection identifies distinct tumor- and stroma-specific subtypes of pancreatic ductal adenocarcinoma. Nat Genet. 2015;47:1168–1178. doi: 10.1038/ng.3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Makohon-Moore A, Iacobuzio-Donahue CA. Pancreatic cancer biology and genetics from an evolutionary perspective. Nat Rev Cancer. 2016;16:553–565. doi: 10.1038/nrc.2016.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rhim AD, et al. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148:349–361. doi: 10.1016/j.cell.2011.11.025. The authors show that previously tagged pancreatic epithelial cells invade and enter the blood stream at a stage when malignancy could not be detected by histological means. A majority of the circulating pancreatic epithelial cells express DCLK1 as a marker. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rhim AD, et al. Detection of circulating pancreas epithelial cells in patients with pancreatic cystic lesions. Gastroenterology. 2014;146:647–651. doi: 10.1053/j.gastro.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bailey JM, et al. DCLK1 marks a morphologically distinct subpopulation of cells with stem cell properties in preinvasive pancreatic cancer. Gastroenterology. 2014;146:245–256. doi: 10.1053/j.gastro.2013.09.050. In this paper, Bailey et al. show that precancerous lesions contain a subpopulation of cells positive for DCLK1. Using lineage tracing they demonstrate the acinar origin of these DCLK-positive cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Qu D, et al. Doublecortin-like kinase 1 is elevated serologically in pancreatic ductal adenocarcinoma and widely expressed on circulating tumor cells. PLoS ONE. 2015;10:e0118933. doi: 10.1371/journal.pone.0118933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mani SA, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Delgiorno KE, et al. Identification and manipulation of biliary metaplasia in pancreatic tumors. Gastroenterology. 2014;146:233–244.e5. doi: 10.1053/j.gastro.2013.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Westphalen CB, et al. Dclk1 defines quiescent pancreatic progenitors that promote injury-induced regeneration and tumorigenesis. Cell Stem Cell. 2016;18:441–455. doi: 10.1016/j.stem.2016.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Basturk O, et al. A revised classification system and recommendations from the Baltimore consensus meeting for neoplastic precursor lesions in the pancreas. Am J Surg Pathol. 2015;39:1730–1741. doi: 10.1097/PAS.0000000000000533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Newman K, et al. Pancreatic carcinoma with multilineage (acinar, neuroendocrine, and ductal) differentiation. Int J Clin Exp Pathol. 2009;2:602–607. [PMC free article] [PubMed] [Google Scholar]
- 110.Esposito I, et al. Hypothetical progression model of pancreatic cancer with origin in the centroacinar-acinar compartment. Pancreas. 2007;35:212–217. doi: 10.1097/mpa.0b013e31805d0190. [DOI] [PubMed] [Google Scholar]
- 111.Tanaka M, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183–197. doi: 10.1016/j.pan.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 112.Strobel O, et al. Beta cell transdifferentiation does not contribute to preneoplastic/metaplastic ductal lesions of the pancreas by genetic lineage tracing in vivo. Proc Natl Acad Sci USA. 2007;104:4419–4424. doi: 10.1073/pnas.0605248104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Grippo PJ, Nowlin PS, Demeure MJ, Longnecker DS, Sandgren EP. Preinvasive pancreatic neoplasia of ductal phenotype induced by acinar cell targeting of mutant Kras in transgenic mice. Cancer Res. 2003;63:2016–2019. [PubMed] [Google Scholar]
- 114.Sanchez-Munoz A, et al. Lack of evidence for KRAS oncogenic mutations in triple-negative breast cancer. BMC Cancer. 2010;10:136. doi: 10.1186/1471-2407-10-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tuveson DA, et al. Mist1-KrasG12D knock-in mice develop mixed differentiation metastatic exocrine pancreatic carcinoma and hepatocellular carcinoma. Cancer Res. 2006;66:242–247. doi: 10.1158/0008-5472.CAN-05-2305. [DOI] [PubMed] [Google Scholar]
- 116.Habbe N, et al. Spontaneous induction of murine pancreatic intraepithelial neoplasia (mPanIN) by acinar cell targeting of oncogenic Kras in adult mice. Proc Natl Acad Sci USA. 2008;105:18913–18918. doi: 10.1073/pnas.0810097105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cornish TC, Hruban RH. Pancreatic intraepithelial neoplasia. Surg Pathol Clin. 2011;4:523–535. doi: 10.1016/j.path.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 118.Real FX. A “catastrophic hypothesis” for pancreas cancer progression. Gastroenterology. 2003;124:1958–1964. doi: 10.1016/s0016-5085(03)00389-5. [DOI] [PubMed] [Google Scholar]
- 119.Ohlund D, Elyada E, Tuveson D. Fibroblast heterogeneity in the cancer wound. J Exp Med. 2014;211:1503–1523. doi: 10.1084/jem.20140692. [DOI] [PMC free article] [PubMed] [Google Scholar]