Summary
Purpose
Gamma Knife radiosurgery (RS) may be an alternative to open surgery for mesial temporal lobe epilepsy (MTLE), but morbidities and the anticonvulsant mechanisms of RS are unclear. Examination of visual field defects (VFD) after RS may provide evidence of the extent of a postoperative fixed lesion. VFD occur in 52-100% of patients following open surgery for MTLE.
Methods
This multicenter prospective trial of RS enrolled patients with unilateral hippocampal sclerosis and concordant video-EEG findings. Patients were randomized to low (20Gy) or high (24Gy) doses delivered to the amygdala, hippocampal head, and parahippocampal gyrus. Postoperative perimetry were obtained at 24 months after RS. Visual field defect ratios (VFDR) were calculated to quantify the degree of VFD. Results were contrasted with age, RS dose and 50% isodose volume, peak volume of radiation-induced change at the surgical target, quality of life measurements, and seizure remission.
Key Findings
No patients reported visual changes and no patients had abnormal bedside visual field examinations. 15 of 24 patients (62.5%) had postoperative VFD, all homonymous superior quadrantanopsias. None of the VFDs were consistent with injury to the optic nerve, chiasm or tract. Clinical diagnosis of VFD correlated significantly with VFDR (p=0.0005). Patients with seizure remission had smaller (more severe) VFDR (p=0.04). No other variables had significant correlations.
Significance
VFD appeared after RS in proportions similar to historical comparisons from open surgery for MTLE. The nature of VFD was consistent with lesions of the optic radiations. The findings support the hypothesis that the mechanism of RS involves some degree of tissue damage and is not confined entirely to functional changes in neuromodulation.
Keywords: Visual field defects, gamma knife, radiosurgery, mesial temporal lobe epilepsy, epilepsy surgery, partial seizures
Introduction
Visual field defects (VFD) are a common side effect of temporal lobe resections for patients with medically intractable mesial temporal lobe epilepsy (MTLE). The frequency and distribution of VFD after resection ranges from a low of 52% (Tecoma, et al. 1993) to a high of nearly 100% (Hughes, et al. 1999). More recent studies attempt to correlate VFD with surgical technique. Nilsson et al. found that more restricted resections that spared the superior most aspect of the superior temporal gyrus yielded less VFD (Nilsson, et al. 2004). Mengesha et al. found that points along the horizontal meridian of the visual fields tended to be spared with selective amygdalohippocampectomy compared to standard anterior temporal lobectomy (Mengesha, et al. 2009).
Gamma knife radiosurgery (RS) may be an alternative to open resection in the treatment of MTLE (Quigg, et al. 2012). Seizure remission rates in the U.S. Multicenter Pilot Study are comparable to that historically seen after temporal lobe resection (Barbaro, et al. 2009). Additionally, VFD following radiosurgery for MTLE approximate that seen after standard surgery, with the European prospective trial reporting an incidence of 50% (Régis, et al. 2004). RS offers a unique opportunity to study VFD in temporal lobe surgery. Unlike open resection, RS targets deep structures without involvement of surrounding tissue. The radiosurgical target in the U.S. Multicenter Pilot Study was standardized in terms of specific anatomic targets with limits of dose, volume, and safety factors; therefore, neurosurgical variations – a relatively underreported variable in epilepsy surgery – were minimized.
The mechanism of the anticonvulsant effects of RS remains controversial (Quigg, et al. 2012). Animal models of limbic epilepsy show that improvement in seizure frequency is not dependent upon destruction of the epileptic focus, implying a neuromodulatory effect of RS (Chen, et al. 2001). However, spectroscopic data of human subjects suggests that a radiodestructive (rather than neuromodulatory) lesion is important in seizure-free patients (Chang, et al. 2010).
In this study, we examined the incidence and severity of VFD in patients who underwent RS for intractable MTLE. Our hypothesis was that the extent of the VFD was correlated to radiosurgical dose or outcome. We also reasoned that examination of the visual fields would provide evidence of destruction to the optic radiations, which would in turn provide evidence of a destructive antiepileptic lesion. Finally, the type of VFD would provide evidence for or against damage to nearby structures (optic nerve, chiasm of tract) related to radiation exposure.
Methods
Participants and RS protocol
Study design, protocols, and patient demographics are discussed in detail elsewhere (Barbaro, et al. 2009). This prospective study was approved by the Institutional Review Boards of our multicenter group with patients providing informed consent. Briefly, patients had unilateral MTLE defined as complex partial seizures arising from a single temporal focus determined by video-EEG concordant to unilateral hippocampal sclerosis on MRI. Patients with a pre-existing VFD were excluded, as were those with cataracts, glaucoma, diabetes mellitus, or other significant ocular diseases.
Patients were randomized to treatment with either a 20 Gy (low dose) or 24 Gy (high dose) protocol of Gamma Knife™ RS (Elekta AB, Stockholm, Sweden) containing a 50% isodose volume ranging from 5.5-7.5 ml comprising the amygdala, anterior hippocampus, and parahippocampal gyrus. Radiation safety factors limited dose to a maximum of 10 Gy to the nearby brainstem and 8 Gy to the optic nerves and chiasm. Each treatment plan was reviewed electronically by members of the Study Center (UCSF) and approved prior to initiating treatment at individual treatment centers. RS is an outpatient procedure in which patients are placed in a stereotactic frame, undergo mild conscious sedation to insure proper registration, and usually are exposed for a total treatment time of 2-4 hours. The postoperative clinical and neuroimaging course has been described previously (Barbaro, et al. 2009, Chang, et al. 2010). The beneficial RS effects are not immediate and take >6 months to develop.
Visual field measurements
Neurological examination and automated visual field perimetry were performed at pre-operative baseline and at 24-month follow-up. VFD were assessed either with Goldmann perimetry or Humphrey perimetry (including 24-2, 30-2 and 120 full field).
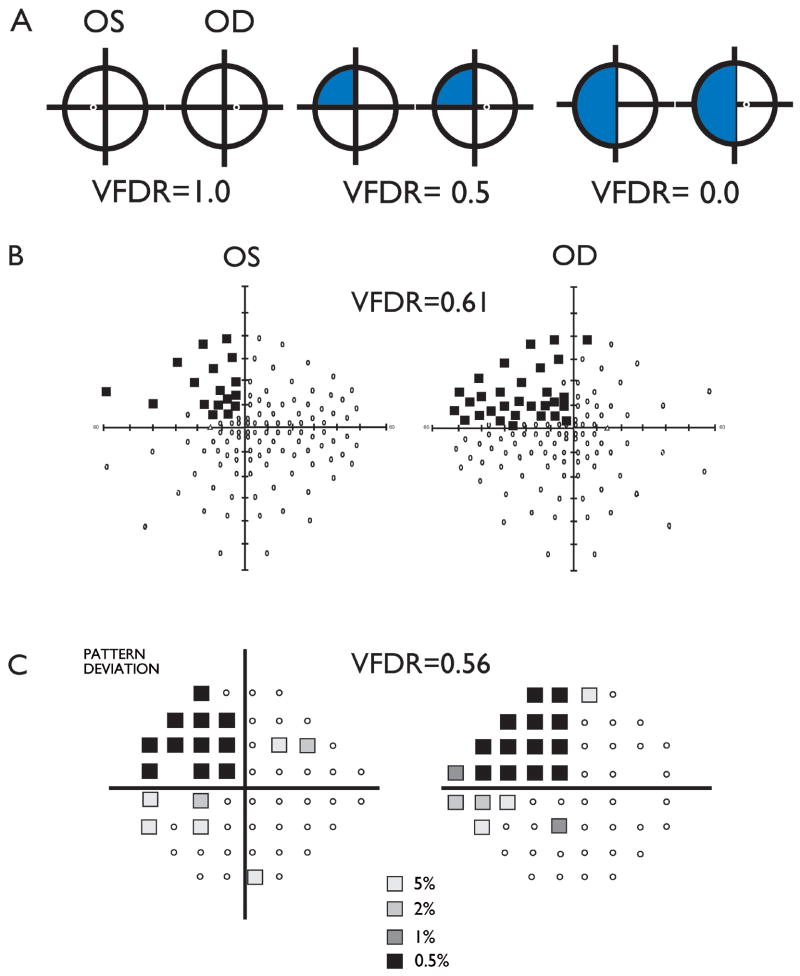
VFD were first categorized as present or absent through blinded interpretation of perimetry (H.H-J.). In order to evaluate VFD in an objective fashion, to allow measurement of severity of VFD beyond just presence or absence, and to allow comparison of VFD obtained by different perimetry methods, we calculated a quantitative measure of visual field loss. We defined the visual field defect ratio (VFDR) as the summed area of intact vision on the hemi-visual field contralateral to RS divided by that ipsilateral to surgery:
As Figure 1A illustrates, a complete homonymous superior visual field defect will have a VFDR = 0.5; a full homonymous hemifield loss will equal zero. Test points in Humphrey perimetry with standard p value <0.5% were deemed normal. Figures 1B and 1C demonstrate the calculated VFDR for representative patients tested with Humphrey 120 and Humphrey 24-2 techniques, respectively. Areas from Goldman perimetry were calculated by measuring visual field areas with the use of ImageJ image analysis software (NIH) from scanned perimetry plots.
Figure 1.
(A) VFDR calculations for a left superior homonymous quadrantanopia. (B) Representative Humphrey 120 program visual field defects of left homonymous quadrantanopia and calculated VFDR calculation. (C) Representative patient Humphrey 24-2 program visual field defects of left homonymous quadrantanopia. VFDR data were calculated from P<0.05% data points where available.
Data Analysis
We used nonparametric statistical methods throughout to conservatively evaluate our data set. To validate our measure of the severity of visual field defects, we grouped VFDR by presence or absence of qualitative VFD and compared mean values with the Mann-Whitney rank-sum test. We then evaluated relationships between the VFDR, which was our primary outcome variable, to the demographic parameter of patient age at RS, RS treatment parameters of dose (high versus low) and 50% isodose volume, and clinical outcome variables of radiosurgery-induced change (RIC) on MRI, quality of life measurements, and seizure remission status. RIC was determined from MRI obtained at 12 months after RS and consisted of the volume of T2-weighted inflammatory edema induced by radiation. Quality of life was measured with the use of a self-administered questionnaire, the Quality of Life in Epilepsy-10 (QOLIE-10 (Cramer, et al. 2000)). Patients designated as seizure free had no complex partial seizures (Engel class IB or better) between months 24 and 36. Categorical variables were tested with the Mann-Whitney rank-sum tests, and continuous variables with Spearman’s correlations. P-values < 0.05 were accepted for statistical significance.
Results
Postoperative visual field testing was available for 24 of the 30 patients enrolled in the trial. Three subjects did not complete the 36 month study: one subject was lost to follow up; one required urgent temporal lobe surgery fifteen months following RS (high dose); and one subject was followed for 24 months, was not seizure-free, and requested temporal lobectomy (low dose). These three patients had no postoperative VF testing. The remaining three patients with no VF testing completed the trial but did not comply with testing.
None of the remaining 24 subjects had abnormal bedside pupillary, visual acuity, or visual field abnormalities, and no patient complained of subjective visual changes. Blinded examination of perimetry results determined that 15 patients (62.5%) developed VFD, all consisting of homonymous superior quadrantanopias. None of the VFDs was consistent with injury to the optic nerve or chiasm. These interpretations of VFD provided by perimetry correlated significantly with VFDR (mean ± standard deviation: mean VFDR in patients with interpreted VFD = 0.64±0.08 vs those without VFD = 0.95±0.06, Mann-Whitney U P-value = 0.0001).
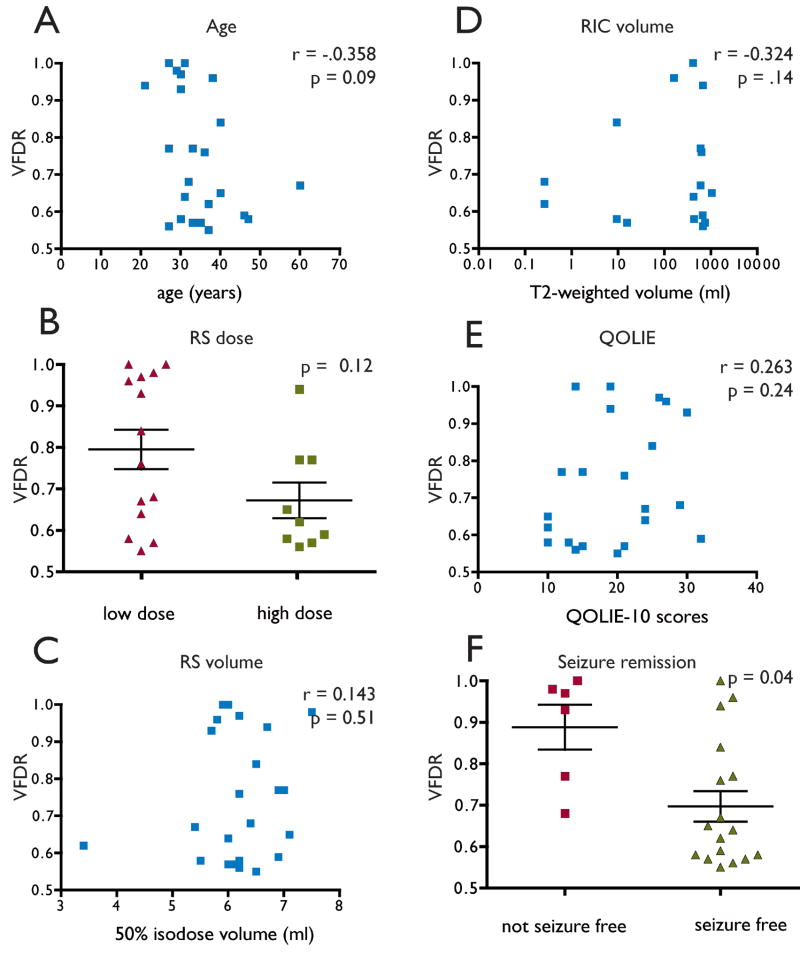
There was no correlation between age and VFDR (Figure 2A). Neither the dose category (low or high, Figure 2B) nor the isodose volume into which it was delivered (Figure 2C) varied with VFDR. Neither RIC (Figure 2D) nor QOLIE-10 scores (Figure 2E) varied with VFDR. Finally, patients with seizure remission had smaller (more severe) VFDR compared to patients whose seizures continued (Figure 2F).
Figure 2.
VFDR compared to (A) patient age, (B) dose of radiation, (C) 50% of isodose volume, (D) volume of T2-weighted radiation induced change on MRI, (E) quality of life scale, (F) seizure remission.
Discussion
The main finding of this prospective study of VFD following RS for MTLE was that the incidence and severity of VFD after RS were similar to those reported for open temporal lobe resection. We conclude that the RS protocol used in the Barbaro et al. US Multicenter Pilot Study is not only appropriate for its anticonvulsant effect (Barbaro, et al. 2009), but the restrictions placed on dose-volume and ophthalmic nerve exposures are effective in preventing excessive morbidity to the visual system. Secondly, the severity of VFD correlated with seizure remission, indicating that the optic radiations lay within the destructive zone of RS. We infer an important anticonvulsant mechanism of RS lies in its focal destruction of tissue within the epileptogenic limbic circuit (Bertram 2009).
In this study, post-operative VF were measured using automated perimetry methods. The VFDR enables normalization among these methods as it allows comparison of VF abnormalities from side to side in an intrapatient comparison. Use of the VFDR to quantify VFD is also important in that it provides a quantified method of documenting the severity of VFD following surgery involving portions of the visual system. This approach avoids problems with interrater reliability and allows for multiple pools of data to be compared. Additionally, the VFDR was validated by comparing it to clinical examination of VF patterns provided by standard VF testing.
The present study is the first to show that quality of life is not adversely affected by the presence of postsurgical VFD when defects are limited to the upper quadrant. This measurement effect agrees with our clinical experience that patients after standard temporal lobe surgery rarely complain of visual symptoms. The lack of clinical findings and the fact that no patient self-reported a visual loss suggests that the morbidity of postoperative VFD when confined to the superior quadrant of vision is minimal, especially compared to the benefits of successful epilepsy surgery. The study emphasizes the value of quantitative visual field measurements, given the absence of abnormalities by standard clinical examination.
The severity of VFD after epilepsy surgery, regardless of surgical technique, is important given that vision may independently limit the ability to drive. Data on driving status was not collected during the study, the quality of life measure used (the QOLIE-10) includes a scale on improvements in driving. It is unknown whether any of the patients who were seizure free following the procedure were denied a driving license. Although visual acuity limits are present in all countries and states, requirements for visual fields vary widely and are empirically derived. In the European Union, binocular field requirements generally require ≥120° of intact vision across the 180° hemifield, with no encroachment of vision within 20-30° of the horizontal meridian. Whereas some US states specify no requirements, the majority define limits of 110-140° (Bron, et al. 2010). Only one study specifically evaluated driving eligibility related to post-surgical VFD (Manji and Plant 2000). Of a sample of 24 patients, 11 to 13 patients (depending on perimetry technique) had “failing” VFD according to the European standard after temporal lobectomy; 3 (~30%) of those patients could not drive despite seizure-freedom (Manji and Plant 2000). Under the European standard (and converting between VFD definitions), we estimate that 10/23 (43%) of our patients would have VFD≥120° (~VFDR<0.67); all these patients were seizure-free. The ongoing ROSE Trial (Radiosurgery or Open Surgery for Epilepsy), a randomized comparison of temporal lobectomy versus RS, may be able to better provide direct and consistent comparisons across surgical techniques.
Limitations of this study included the small sample size, which was difficult to avoid as RS for MTLE is not widely performed. However, this study includes the largest group of patients treated with RS for MTLE reported thus far.
No association was seen between dose/isodose volume and VFD. We have no comparisons in other studies of RS; the European prospective study did not evaluate dose effects (Régis, et al. 2004).
Studies of open surgery show small effects of surgical technique on postoperative VFD. For example, selective amygdalohippocampectomy, a smaller volume technique than anterior temporal lobectomy, tends to spare vision near the horizontal meridian (Mengesha, et al. 2009). Subtle differences were found using slightly different surgical techniques (Nilsson, et al. 2004). The discrepancies between the present study and open surgery reports attest that “surgical volumes” may not be comparable between RS and resection.
No association was seen between presence of VFD and volume of T2 lesions on MRI at 12 months post-operatively. This emphasizes that the RIC seen in terms of T2 lesion size is transient and has no long-lasting effect on visual function.
One implication of our findings is that it comments on the possible mechanisms of RS in epilepsy surgery. Whereas experimental models of epilepsy and limited human data suggest a neuromodulatory effect [see reviews: (Regis, et al. 2010), (Quigg, et al. 2012)], in the present study the correlation between radiation dose and VFD, as well as improved seizure freedom with more severe VFD, would support permanent tissue destruction as the mechanism of effective RS. The present findings support the spectroscopic findings (MRS) performed by our group that found evidence of ischemia within the radiosurgical target (Chang, et al. 2010). An alternative argument would be that the effect on seizure remission is neuromodulatory while the effect on visual fibers is destructive. While possible, the more parsimonious explanation is that the radiosurgical effect is similar, that is destructive, to all tissues in the treatment volume.
In summary, this study found that radiation dose in RS was not significantly associated with severity of VFD and confirmed that the incidence and severity of VFD were similar to that of open surgery for MTLE. Based on these findings, we speculate that the mechanism of RS involves some degree of tissue damage and is not confined entirely to changes in neuromodulation. In addition, existing tissue tolerance limits, as used in this protocol, are sufficient to protect other, important, components of the visual system.
Acknowledgments
The study was supported by the NIH (National Institute of Neurological Diseases and Stroke, R01 NS39280-03; all authors) and Elekta AB (clinical care supplement).
Footnotes
Disclosures
MQ has been sponsored by Elekta AB for a speaking engagement. DK is a consultant for Elekta AB. No other authors have any conflict of interest to disclose.
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines
References
- Barbaro NM, Quigg M, Broshek DK, Ward MM, Lamborn KR, Laxer KD, Larson DA, Dillon W, Verhey L, Garcia P, Steiner L, Heck C, Kondziolka D, Beach R, Olivero W, Witt TC, Salanova V, Goodman R. A multicenter, prospective pilot study of gamma knife radiosurgery for mesial temporal lobe epilepsy: seizure response, adverse events, and verbal memory. Ann Neurol. 2009;65:167–175. doi: 10.1002/ana.21558. [DOI] [PubMed] [Google Scholar]
- Bertram EH. Temporal lobe epilepsy: where do the seizures really begin? Epilepsy Behav. 2009;14(Suppl 1):32–37. doi: 10.1016/j.yebeh.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bron AM, Viswanathan AC, Thelen U, Natale R, Ferreras A, Gundgaard J, Schwartz G, Buchholz P. international vision requirements for driver licensing and disability pensions: using a milestone approach in characterization of progressive eye disease. Clinical Ophthalmology. 2010;4:1361–1369. doi: 10.2147/OPTH.S15359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang EF, Quigg M, Oh MC, Dillon WP, Ward MM, Laxer KD, Broshek DK, Barbaro NM. Predictors of efficacy after stereotactic radiosurgery for medial temporal lobe epilepsy. Neurology. 2010;74:165–172. doi: 10.1212/WNL.0b013e3181c9185d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZF, Kamiryo T, Henson SL, Yamamoto H, Bertram EH, Schottler F, Patel F, Steiner L, Prasad D, Kassell NF, Shareghis S, Lee KS. Anticonvulsant effects of gamma surgery in a model of chronic spontaneous limbic epilepsy in rats. J Neurosurgery. 2001;94:270–280. doi: 10.3171/jns.2001.94.2.0270. [DOI] [PubMed] [Google Scholar]
- Cramer JA, Arrigo C, Van Hammee G, Bromfield EB. Comparison between the QOLIE-31 and derived QOLIE-10 in a clinical trial of levetiracetam. Epilepsy Research. 2000;41:29–38. doi: 10.1016/s0920-1211(00)00127-3. [DOI] [PubMed] [Google Scholar]
- Hughes TS, Abou-Khalil B, Lavin PJ, Fakhoury T, Blumenkopf B, Donahue SP. Visual field defects after temporal lobe resection: a prospective quantitative analysis. Neurology. 1999;53:167–172. doi: 10.1212/wnl.53.1.167. [DOI] [PubMed] [Google Scholar]
- Manji H, Plant GT. Epilepsy surgery, visual fields, and driving: a study of the visual field criteria for driving in patients after temporal lobe epilepsy surgery with a comparison of Goldmann and Esterman perimetry. J Neurol Neurosurg Psychiatry. 2000;68:80–82. doi: 10.1136/jnnp.68.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengesha T, Abu-Ata M, Haas KF, Lavin PJ, Sun DA, Konrad PE, Pearson M, Wang L, Song Y, Abou-Khalil BW. Visual field defects after selective amygdalohippocampectomy and standard temporal lobectomy. J Neuroophthalmol. 2009;29:208–213. doi: 10.1097/WNO.0b013e3181b41262. [DOI] [PubMed] [Google Scholar]
- Nilsson D, Malmgren K, Rydenhag B, Frisen L. Visual field defects after temporal lobectomy -- comparing methods and analysing resection size. Acta Neurol Scand. 2004;110:301–307. doi: 10.1111/j.1600-0404.2004.00331.x. [DOI] [PubMed] [Google Scholar]
- Quigg M, Rolston J, Barbaro NM. Radiosurgery for epilepsy: clinical experience and potential antiepileptic mechanisms. Epilepsia. 2012;53:7–15. doi: 10.1111/j.1528-1167.2011.03339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Régis J, Carron R, Park M. Is radiosurgery a neuromodulation therapy? : A 2009 Fabrikant award lecture. J Neurooncol. 2010;98:155–162. doi: 10.1007/s11060-010-0226-5. [DOI] [PubMed] [Google Scholar]
- Régis J, Rey M, Bartolomei F, Vladyka V, Liscak R, Schrottner O, Pendl G. Gamma knife surgery in mesial temporal lobe epilepsy: a prospective multicenter study. Epilepsia. 2004;45:504–515. doi: 10.1111/j.0013-9580.2004.07903.x. [DOI] [PubMed] [Google Scholar]
- Tecoma ES, Laxer KD, Barbaro NM, Plant GT. Frequency and characteristics of visual field deficits after surgery for mesial temporal sclerosis. Neurology. 1993;43:1235–1238. doi: 10.1212/wnl.43.6.1235. [DOI] [PubMed] [Google Scholar]