Abstract
Introduction
Measurement of symptoms domains and their response to treatment in relative isolation from diagnosed mental disorders has gained new urgency, as reflected by the National Institute of Mental Health’s introduction of the Research Domain Criteria (RDoC). The Snaith Hamilton Pleasure Scale (SHAPS) and the Motivation and Energy Inventory (MEI) are two scales measuring positive valence symptoms. We evaluated the effect of exercise on positive valence symptoms of Major Depressive Disorder (MDD).
Methods
Subjects in the Treatment with Exercise Augmentation for Depression (TREAD) study completed self-reported SHAPS and MEI during 12 weeks of exercise augmentation for depression. We evaluated the effect of exercise on SHAPS and MEI scores, and whether the changes were related to overall MDD severity measured with the Quick Inventory of Depression Symptomatology (QIDS).
Results
SHAPS and MEI scores significantly improved with exercise. MEI score change had larger effect size and greater correlation with change in QIDS score. MEI also showed significant moderator and mediator effects of exercise in MDD.
Limitations
Generalizability to other treatments is limited. This study lacked other bio-behavioral markers that would enhance understanding of the relationship of RDoC and the measures used.
Conclusions
Positive valence symptoms improve with exercise treatment for depression, and this change correlates well with overall outcome. Motivation and energy may be more clinically relevant to outcome of exercise treatment than anhedonia.
Keywords: Depression, Exercise, RDoC, Motivation, Anhedonia
1. Introduction
Given the high prevalence and associated morbidity of depression (Ustun et al., 2004), much effort has been made to meaningfully subtype patients for targeted therapy (Goldberg, 2011). However, this strategy has failed to improve our understanding of mental disorders or to direct treatment for patients in practice. Most recently the National Institute of Mental Health tried to address this issue through the introduction of the Research Domain Criteria (RDoC) model (Insel et al., 2010). RDoC is designed to examine biological correlates of behavior and to define meaningful groups and subgroups. This means examining the relationships among brain circuits, cells, intracellular components and particular behaviors – relationships that are understood best currently in animal models – rather than on how symptoms are experienced subjectively in human beings (Cuthbert and Insel, 2013). There is an increasing recognition that no single global clinical or bio-behavioral marker is likely to universally explain the variable presentations of Mood Disorders or assist with treatment selection (Trivedi, 2013; Trivedi et al., 2016). The need to evaluate patient presentations according to a more precise bio-behavioral model necessarily requires evaluation of specific symptoms in addition to overall severity.
Although patients with depression present with symptoms clearly mapping onto specific RDoC domains, these are rarely assessed routinely with focused measurement tools. While there is a need to develop new assessments based on RDoC, there are standardized rating instruments already available that bear face validity with RDoC domains and constructs. Routine measurement with these tools is needed to clarify the relationships between the core Major Depressive Disorder (MDD) symptoms as defined by the Diagnostic and Statistical Manual of Mental Disorders (upon which current diagnoses are based) and RDoC constructs. This will link bio-behavioral results to more traditional outcomes and serve as a basis for the development of RDoC based trials. For example, differential effect of treatment modality (e.g. medication, psychotherapy, exercise) on symptom constructs cannot be determined without a measurement that parses individual constructs from the overall picture of depression. With this in mind we examined symptoms associated with the RDoC Positive Valence Domain using the Snaith Hamilton Pleasure Scale (SHAPS) and Motivation and Energy Inventory (MEI) - two scales that were used in the Treatment with Exercise Augmentation of Depression (TREAD) study.
The positive valence domain contains five constructs: 1) approach motivation, 2) initial reward responsiveness, 3) sustained reward responsiveness, 4) reward learning, and 5) habit (National Institutes of Mental Health, 2011). While all positive valence constructs may conceivably be impaired in depression, approach motivation may be one of the most relevant. Approach motivation is a complex construct that itself includes four sub-constructs – reward valuation, effort valuation, reward expectation, and preference. Anhedonia, a core positive valence symptom of depression, is often parsed in animal studies as a state in which preferences are intact but motivation is lacking (Berridge and Kringelbach, 2015; Treadway and Zald, 2013). Following this lead, RDoC, deemphasizes the experience – or lack – of pleasure in positive valence, replacing it with a model in which consummatory behaviors are taken as the outcome of the interplay of underlying constructs. For example, hunger may increase the reward value of food sources in general but prior experience (learning) may direct behavioral responses to particular types of food based on effort required or taste preference. On the other hand, when hunger is low, even though preferences are intact, motivation is decreased and consumption of even preferred foods declines. Other than the emphasis on behavior over experience, the description of anhedonia in the Diagnostic and Statistical Manual for Mental Disorders (DSM) (American Psychiatric Association, 2013), as lack of enjoyment specifically of things for which one has preference, is clearly analogous. However, it is also important to consider that positive valence may apply to other traditional symptoms of depression. For example, fatigue may be viewed as dysfunction in positive valence systems, a conceptualization consistent with literature examining fatigue as a medical, more than psychiatric, symptom (Dantzer et al., 2014). The SHAPS and MEI by measuring specific components of deficits in positive valence are well suited to assessment of this domain in a way that bridges the traditional concept of anhedonia with RDoC.
Trials of exercise therapy for depression are well suited to the application of RDoC principles because they utilize complex bio-behavioral treatment (Schuch et al., 2016). In the Treatment with Exercise Augmentation for Depression (TREAD) study, adults who failed to respond to a Selective Serotonin Reuptake Inhibitor (SSRI) were treated with an augmentation regimen of aerobic exercise for 12 weeks (Trivedi et al., 2011). Half of the subjects were randomized to each of two dose groups while all continued to take the antidepressant prescribed prior to study entry. To assess the effect of exercise on anhedonia, decreased motivation and other positive valence domain symptoms, subjects in TREAD were assessed with domain specific measures including the SHAPS and MEI.
2. Methods
Complete details of the TREAD study have been previously published (Trivedi et al., 2011, 2006). Briefly, this was a 12-week comparison of a public health dose of exercise to a low dose in patients with MDD who were eligible for augmentation treatment due to partial or no response to an SSRI.
2.1. Subjects
TREAD enrolled adults, ages 18–70, with MDD who were taking a stable adequate dose of an approved SSRI for at least 6 weeks (and up to 6 months) at screening. Included subjects reported no more than partial response as defined by a screening Hamilton Depression Rating Scale (HDRS) score of ≥14 and reported being sedentary for at least the last month. Subjects with medical illness contraindicating exercise were excluded, as were those with lifetime history of psychosis. History of two or more failed anti-depressant trials in the current episode was also exclusionary. All subjects provided voluntary informed consent and remained on their prescribed antidepressant throughout the trial.
2.2. Exercise treatment
At baseline, subjects were randomized to receive one of two doses of exercise: a high dose of 16 kcal/kg/week or a low dose of 4 kcal/kg/week. The high dose was chosen based on public health recommendations for weekly exercise requirements and had previously been shown to be effective as an antidepressant treatment (Dunn et al., 2002). Subjects met with trainers at the Cooper Institute in Dallas, TX to develop exercise plans meeting the prescribed dose using aerobic exercise; for example, walking, jogging, or running on a treadmill. Subjects were able to choose the intensity of exercise and received instruction on time needed to meet the prescribed dose. After the first two weeks of the study period in which trainers assisted in the development of the exercise prescription, subjects returned for one supervised session a week (at which weekly assessment occurred) and completed the remaining exercise at home, using a web-based tracker to log exercise completed.
2.3. Assessments
All subjects had inclusionary and exclusionary diagnoses validated using the Structured Clinical Interview for DSM-IV (SCID) at screening. They also received maximal oxygen consumption (VO2 max) testing to determine baseline level of fitness and confirm medical suitability for exercise.
The primary outcome measure used in TREAD was the Inventory of Depression Symptomatology – Clinician Rated (IDS-C). From each of the 30 item IDS-C and the corresponding self-rated IDS (IDS-SR), item scores for each depression symptom domain were extracted to form a Quick Inventory of Depression Symptomatology – Clinician (QIDS-C) and self-rated (QIDS-SR) score respectively (Rush et al., 2000, 2003). The IDS includes items designed to assess non-core symptoms of depression (such as diurnal variation and mood reactivity) that are associated with subtypes as identified in the DSM. The QIDS measures only the core depression symptoms, and therefore, unlike the IDS, the QIDS is uni-dimensional. For this reason we used extracted QIDS-C and -SR scores as the measures of outcome in this analysis. The self-reported SHAPS and MEI were given at baseline, week 6 and week 12.
2.4. SHAPS
The SHAPS was developed in the mid-1990s to measure ‘hedonic tone” (Snaith et al., 1995). At the time, Snaith et al. were concerned that previous assessments were too dependent on personal preferences. The items were created by surveying healthy adults about pleasurable experiences, and the scale was tested on healthy and mentally ill samples to establish norms. A key feature of the scale is the use of the Montgomery Asberg Depression Rating Scale (MADRS) item “inability to feel” (Montgomery and Asberg, 1979) as the index by which presence of anhedonia was established. The SHAPS contains 14 items, which ask subjects to strongly agree, agree, disagree or strongly disagree with statements beginning “I would enjoy…” followed by an activity or experience. Any agreement with the statement is scored zero points, with one point for any disagreement, yielding a maximum possible score of 14. Receiver Operating Characteristic (ROC) analysis suggested that a score of greater than 2 on the SHAPS indicates clinically abnormal results (Snaith et al., 1995). It is well validated and has been analyzed for psychometric properties, including using the TREAD sample data (Nakonezny et al., 2010, 2015). By asking directly about the experience of pleasure, the SHAPS items are associated closely with the DSM conceptualization of anhedonia as well as RDoC approach motivation and reward responsiveness.
2.5. MEI
When developing the MEI, Fehnel et al. (2004) intentionally incorporated three sub-areas: physical energy, mental energy, and social motivation. There are 27 items, each scored from zero to six, consisting of a stem “During the past 4 weeks, how often did you…” or “to what extent did you…” and responses ranging from “never” to “everyday” or “all of the time,” for a total range of 0–162. Higher scores are reflective of high motivation and energy; depressed subjects would be expected to have lower scores than healthy controls. The physical subscale contains seven items, which ask about energy level, fatigue, physical activity and general motivation. The mental and social subscales each contain ten items. Mental energy items address primarily cognitive complaints such as ability to focus but also ask about feelings of successful accomplishment of daily tasks. Finally, the social subscale focuses on level of interest in social activities but also asks about the frequency of social engagement. The conceptual structure of the MEI applies well to positive valence as outlined in RDoC; it asks subjects to report on behaviors rather than the subjective experience of enjoyment. It also attempts to parse aspects of motivation by division into subscales which may be useful for RDoC based analyses.
2.6. Statistical analysis
To assess change in SHAPS and MEI score over 6 and 12 week intervals, mixed models performed with SAS PROC MIXED (Hamlett and Wolfinger 2006) were used for the entire study sample and by dose group. Models contained a random intercept. Effect sizes for each group and the entire sample were also calculated for each time point from the same mixed model using the method of Cortina (2000). We reran the model with an adjustment for change in QIDS-C score to determine whether and to what extent changes in SHAPS, MEI, and MEI subscales could be accounted for by change in overall depression level. Spearman’s correlations were calculated between QIDS-C scores and SHAPS and MEI scores at baseline and Week 12 and for the change from baseline to Week 12.
Moderator analyses were performed using a mixed effects model with QIDS-C as the dependent variable, time as the within subjects factor, treatment group as the between subjects factor, and the following covariates: baseline score of SHAPS or MEI, baseline value of the QIDS-C, baseline Short Form Health Survey 36 score, recurrent MDD status, family history, and gender. Intercepts were random effects and all other effects were fixed.
Finally, we performed a path analysis that included MEI, SHAPS and QIDS-C scores at baseline, week 6 and week 12. A path analysis in an extension of multiple linear regression where correlations between variables (i.e., paths) are computed with adjustment for all other variables in the model (Streiner, 2005). As is the case with multiple linear regression, it is unnecessary to adjust for multiple tests of coefficients performed within the same model. Our aim was to determine whether scores on scales at earlier time points affected scores latter in the study and thus acted as mediators. A cross-lagged panel model was fit using MPLUS Version 7.3. Missing data were handled by using full information maximum likelihood. The covariates used in Trivedi et. al. in the original TREAD analysis (Trivedi et al., 2011) (SF-36 mental, gender, family history, race, recurrent MDD, and treatment group) were included if significant. Alpha of 0.05 was used as the threshold of significance for all analyses.
3. Results
Descriptive statistics for the TREAD sample, showing that the dose groups did not differ in demographic or illness measures at baseline is shown in Table 1. At baseline, SHAPS mean score for the TREAD sample was 3.1 ± 3.0, and did not differ between the low and high dose groups. At week 6, scores dropped by 0.81 points in the entire sample (p=0.005), and 1.19 points from baseline (p=0.001) by week 12 (Table 2). The whole sample effect size for the change in SHAPS after 6 weeks of exercise was 0.311, this increased to 0.406 after 12 weeks. Change in SHAPS score did not differ significantly over the study period when analyzing the dose groups individually, nor was the difference significant when comparing the two dose groups at week 6 and 12.
Table 1.
Baseline Clinical and demographic features of the sample as a whole and by dose group.
| All mean (std) or N (%)(N=119) | High dose mean (std) or N (%)(N=58) | Low dose mean (std) or N (%)(N=61) | T or χ2 | p | |
|---|---|---|---|---|---|
| Age (yrs) | 46.9 (10.1) | 45.3(10.6) | 48.5(9.4) | 1.7 | 0.086 |
| Female (%) | 81.5 | 84.5 | 78.7 | 0.7 | 0.416 |
| Race | 1.4 | 0.703 | |||
| White (%) | 87.4 | 86.2 | 88.5 | ||
| Black (%) | 10.1 | 12.1 | 8.2 | ||
| Hisp (%) | 3.4 | 5.2 | 1.6 | ||
| Other (%) | 4.2 | 1.7 | 6.6 | ||
| Age of Onset (yrs) | 27.1(11.1) | 27.0(10.2) | 27.1(12.0) | 0.0 | 0.988 |
| Current Episode (Months) | 81.5(96.8) | 73.1(95.0) | 89.6(98.5) | 0.9 | 0.354 |
| Weeks on SSRIa | 7.9(6.7) | 9.1(8.4) | 6.7(4.1) | −1. 8 | 0.075 |
| SSRI | 4.0 | 0.552 | |||
| Citalopram (%) | 3.4 | 3.4 | 3.3 | ||
| Escitalopram (%) | 73.9 | 75.9 | 72.1 | ||
| Fluoxetine (%) | 3. | 1.7 | 4.9 | ||
| Paroxetine CR (%) | 3.4 | 5.2 | 1.6 | ||
| Paroxetine (%) | 1.7 | 0.0 | 3.3 | ||
| Sertraline (%) | 14.3 | 13.8 | 14.8 | ||
| QIDS-C | 14.0(2.6) | 14.0(2.5) | 14.0(2.6) | 0.0 | 0.972 |
| QIDS-SR | 12.6(4.0) | 12.2(3.6) | 13.0(4.3) | 1.1 | 0.276 |
| Weight (kg) | 87.3(20.2) | 86.8(22.7) | 87.8(17.7) | 0.3 | 0.777 |
| BMI | 30.9 (6.0) | 30.3(6.5) | 31.4(5.5) | 1.0 | 0.325 |
| VO2 max (lmin) | 1.7 (0.6) | 1.7(0.5) | 1.7(0.7) | 0.6 | 0.569 |
| SHAPS total score | 3.1(3.0) | 2.8(3.1) | 3.4(3.0) | 1.0 | 0.329 |
| MEI total score | 34.7(14.4) | 34.4(12.5) | 34.9(16.0) | 0.2 | 0.854 |
| MEI mental energy | 19.0(8.6) | 18.9(7.9) | 19.1(9.3) | 0.1 | 0.916 |
| MEI social Motivation | 9.9(5.3) | 9.7(5.0) | 10.0(5.7) | 0.3 | 0.768 |
| MEI physical Energy | 4.9(3.3) | 5.0(2.9) | 4.8(3.7) | −0.2 | 0.809 |
Length of SSRI trial at baseline. Subjects maintained SSRI treatment during the 12 week study.
Table 2.
Mixed model results for change in scale scores for the entire sample and by dose group, with corresponding effect sizes.
| Mean (std) change all | p all | Effect size | Mean (std) change 4 KKW | p 4 KKW | Effect size | Mean (std) change 16 KKW | p 16 KKW | Effect size | p between groups | |
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline to week 6 | ||||||||||
| SHAPS | −0.81(2.61) | 0.005 | 0.311 | −1.02 (2.83) | 0.020 | 0.361 | −0.58 (2.35) | 0.130 | 0.244 | 0.434 |
| MEI total | 9.96(15.99) | < 0.001 | 0.623 | 9.59(14.49) | < 0.001 | 0.662 | 10.41(17.77) | < 0.001 | 0.586 | 0.803 |
| MEI ME | 4.74(8.30) | < 0.001 | 0.570 | 4.57 (7.48) | < 0.001 | 0.612 | 4.93 (9.28) | 0.001 | 0.531 | 0.832 |
| MEI PE | 1.82(4.12) | < 0.001 | 0.442 | 1.71 (4.15) | 0.003 | 0.412 | 1.96 (4.12) | 0.003 | 0.474 | 0.768 |
| MEI SM | 2.96(5.41) | < 0.001 | 0.547 | 2.83 (4.90) | 0.001 | 0.490 | 3.11 (6.02) | 0.001 | 0.516 | 0.801 |
| Baseline to week 12 | ||||||||||
| SHAPS | −1.19 (2.93) | 0.001 | 0.406 | −1.21 (2.78) | 0.017 | 0.400 | −1.17 (2.88) | 0.013 | 0.406 | 0.958 |
| MEI total | 16.08 (19.49) | < 0.001 | 0.825 | 15.10 (16.06) | < 0.001 | 0.807 | 17.14 (20.48) | < 0.001 | 0.837 | 0.616 |
| MEI ME | 7.56 (9.73) | < 0.001 | 0.778 | 6.92 (8.33) | < 0.001 | 0.773 | 8.27 (10.56) | < 0.001 | 0.782 | 0.505 |
| MEI PE | 2.87 (4.86) | < 0.001 | 0.591 | 2.67 (3.88) | 0.001 | 0.521 | 3.09 (4.58) | < 0.001 | 0.675 | 0.809 |
| MEI SM | 4.81 (6.44) | < 0.001 | 0.746 | 4.65 (5.37) | < 0.001 | 0.699 | 4.98 (6.28) | < 0.001 | 0.793 | 0.685 |
The sample entered the study with MEI mean score of 34.7 ± 14.4 with no difference between the treatment groups. The entire sample experienced a significant change (increase) in scores (by 9.96 at week 6 and 16.08 at week 12, both p < 0.001). Effect sizes for the total MEI score were 0.623 after 6 weeks and 0.825 after 12 weeks. While the scores for the high dose group changed more at each time point than those for the lose dose group, the differences were not significant (Table 2). Similarly, in the high dose group the effect sizes were slightly higher (0.620 at 6 week and 1.035 at 12 weeks) than in the low dose group (0.537 at 6 weeks and 0.933 at 12 weeks). MEI subscales showed change in a pattern similar to the scale as a whole and are also given in Table 2. Effect sizes by dose group assignment are given in Table 2 but no significant between group differences were found. Adjustment for change in QIDS-C scores over the course of the study resulted in non-significance for all analyses. Effect sizes also shrank, with none remaining over 0.1 (not shown).
When examining the correlation between score on the QIDS-C with the SHAPS (Table 3), we found that the QIDS-C/SHAPS relationship had small positive rho values at baseline for the whole sample (0.153) and at week 12 for the whole sample, the low dose group and the high dose group (0.205, 0.186, 0.209, respectively), but none of these relationships reached statistical significance. Similarly, when correlating change from baseline to week 12 between the QIDS-C and the SHAPS, no correlation achieved significance. For the MEI moderate negative correlations were found between total score and QIDS-C score at baseline and week 12. Change in MEI score showed similar moderate negative correlation with change in QIDS-C over the 12-week study period. All of these correlations (Table 3) reached statistical significance. The results of the same analysis repeated for each of the three MEI subscales also showed moderate negative correlations with QIDS-C scores with p values < 0.05 in all cases.
Table 3.
Correlations between QIDS-C and SHAPS, MEI and MEI subscales. Correlations are reported for baseline and week 12 scores, and for change in scores across the 12 week study period.
| Group | Period | Scale | Rho | Lower 95% CI | Upper 95% CI | p |
|---|---|---|---|---|---|---|
| All | Baseline | SHAPS | 0.153 | −0.041 | 0.335 | 0.1200 |
| MEI total | −0.499 | −0.622 | −0.348 | < 0.001 | ||
| MEI mental | −0.472 | −0.600 | −0.317 | < 0.001 | ||
| MEI social | −0.341 | −0.490 | −0.169 | < 0.001 | ||
| MEI physical | −0.356 | −0.503 | −0.187 | < 0.001 | ||
| All | Week 12 | SHAPS | 0.205 | −0.004 | 0.395 | 0.054 |
| MEI total | −0.740 | −0.818 | −0.630 | < 0.001 | ||
| MEI mental | −0.671 | −0.767 | −0.540 | < 0.001 | ||
| MEI social | −0.465 | −0.608 | −0.289 | < 0.001 | ||
| MEI physical | −0.684 | −0.777 | −0.558 | < 0.001 | ||
| 4KKW | Week 12 | SHAPS | 0.186 | −0.119 | 0.456 | 0.228 |
| MEI total | −0.712 | −0.825 | −0.534 | < 0.001 | ||
| MEI mental | −0.640 | −0.778 | −0.431 | < 0.001 | ||
| MEI social | −0.428 | −0.630 | −0.163 | 0.002 | ||
| MEI physical | −0.740 | −0.843 | −0.575 | < 0.001 | ||
| 16KKW | Week 12 | SHAPS | 0.209 | −0.093 | 0.471 | 0.170 |
| MEI total | −0.757 | −0.857 | −0.593 | < 0.001 | ||
| MEI mental | −0.697 | −0.819 | −0.504 | < 0.001 | ||
| MEI social | −0.513 | −0.696 | −0.256 | < 0.001 | ||
| MEI physical | −0.648 | −0.787 | −0.435 | < 0.001 | ||
| All | WK12 - BL | SHAPS | 0.213 | −0.010 | 0.413 | 0.0560 |
| MEI total | −0.625 | −0.734 | −0.480 | < 0.001 | ||
| MEI mental | −0.518 | −0.651 | −0.349 | < 0.001 | ||
| MEI social | −0.520 | −0.653 | −0.352 | < 0.001 | ||
| MEI physical | −0.640 | −0.745 | −0.500 | < 0.001 | ||
| 4KKW | WK12 - BL | SHAPS | 0.272 | −0.056 | 0.542 | 0.098 |
| MEI total | −0.462 | −0.657 | −0.200 | < 0.001 | ||
| MEI mental | −0.327 | −0.557 | −0.044 | 0.023 | ||
| MEI social | −0.378 | −0.595 | −0.101 | 0.008 | ||
| MEI physical | −0.570 | −0.731 | −0.339 | < 0.001 | ||
| 16KKW | WK12 - BL | SHAPS | 0.172 | −0.145 | 0.454 | 0.283 |
| MEI total | −0.757 | −0.857 | −0.591 | < 0.001 | ||
| MEI mental | −0.705 | −0.825 | −0.513 | < 0.001 | ||
| MEI social | −0.629 | −0.776 | −0.406 | < 0.001 | ||
| MEI physical | −0.707 | −0.826 | −0.516 | < 0.001 |
For moderator analysis of the QIDS-C, MEI total score and MEI-ME scores at baseline significantly predicted differences in decrease in QIDS-C score by group. Both analyses showed a similar pattern. While subjects close to the middle of the score range of total had similar outcome regardless of group assignment, those at the high and low ends experienced opposite effects (p=0.015, MEI total, p=0.005, MEI-ME). For those with low MEI score at baseline, assignment to the high dose group was associated with more improvement over time on the QIDS-C. However, for those with higher total score at baseline, the low dose group improved more than the high dose group. The same pattern was repeated for the mental energy subscale score alone; change in QIDS-C at week 12 by dose assignment for the 25th and 75th percentiles of MEI-ME score are shown in Fig. 1 to illustrate the effect. No other associations for the QIDS-C were significant.
Fig. 1.
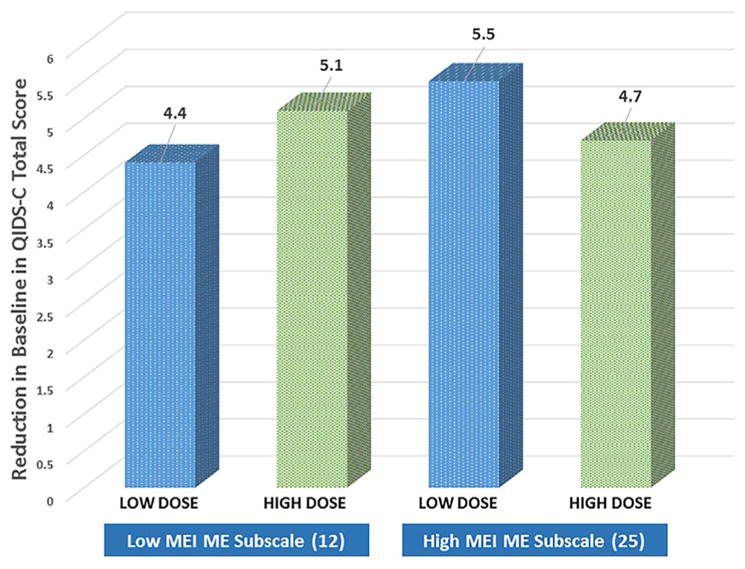
Change in QIDS-C score at week 12 by treatment group for low and high* levels of MEI at study entry. *Low=12 (25th percentile), High=25 (75th percentile).
Path analysis for the SHAPS showed significant pathways between SHAPS scores across each time interval (baseline to week 6 p < 0.001, week 6 to week 12 p=0.003) and QIDS-C scores across each time interval (both p < 0.001) as well as a significant bi-directional connection at week 12 (p=0.025) (Fig. 2). However, there was no significant connection between SHAPS and QIDS-C scores at earlier time points or across time points. The MEI analysis resulted in significant pathways between MEI scores over each interval (baseline to week 6 and week 6 to week 12 p < 0.001) as well as QIDS-C scores over each interval (both p < 0.001). There were significant pathways between QIDS-C and MEI scores at all three time points (baseline p=0.003, week 6 and week 12 p < 0.001), as well as a forward directed relationship between MEI at week 6 with week 12 (final) QIDS-C score (p=0.042) (Fig. 3). The physical energy subscale of the MEI also showed a significant path from week 6 score to final QIDS-C (p=0.008), and its pattern of significant paths was identical to that of the scale as a whole except that baseline MEI-PE score was uncorrelated to its score at week 6. The MEI-ME subscale analysis showed significant pathways between each scale with itself across time points (all p < 0.001) and across the two scales within each time point (baseline p=0.004, week 6 and week 12 p < 0.001), but no significant pathways were found across time. Finally the MEI-SM subscale was not significantly related to QIDS-C score at baseline, but was at week 6 (p < 0.001) and 12 (p=0.028). Week 6 QIDS-C score showed a significant pathway to week 12 MEI-SM, however (p=0.048). SF36 mental health subscale score at baseline was significantly correlated with baseline scale scores in all analyses, and gender was significantly correlated with MEI total, MEI-SM and MEI-PE scores, but not MEI-ME score with a direction indicating that men had higher scores than women.
Fig. 2.
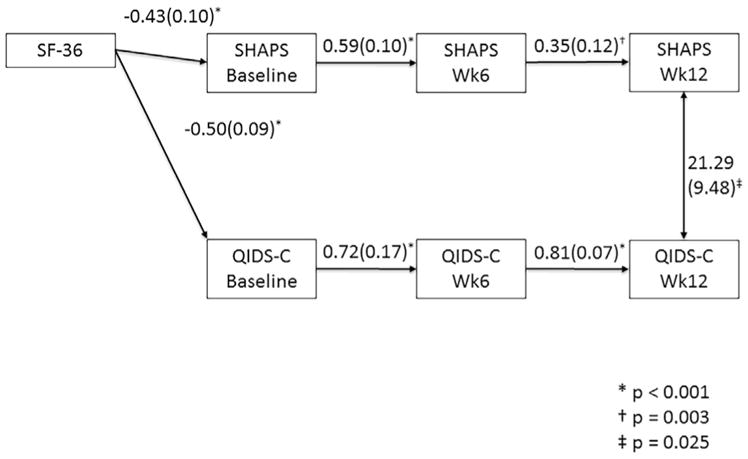
Pathway analysis results for SHAPS. Only significant pathways are shown, with the path coefficient and error. Of the covariates entered into the model, only SF-36 mental health sub-score showed significant association, being significantly related to baseline SHAPS and MEI scores. Not shown are, gender, race, history of recurrent depression and family history of depression..
Fig. 3.
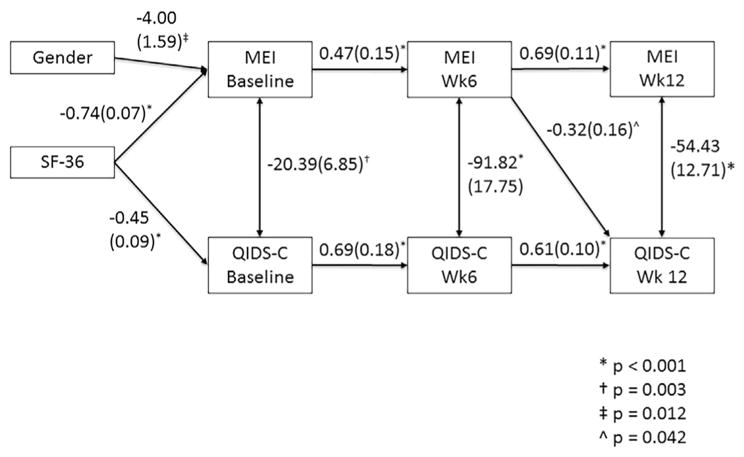
Pathway analysis results for the MEI total score. Only significant pathways are shown, with the path coefficient and error. Of the covariates entered into the model, SF-36 mental health sub-score and gender showed significant association. SF-36 was significantly related to baseline SHAPS and MEI scores. Gender was associated with baseline MEI score, with the direction indicating that men had higher baseline MEI scores. Not shown are race, history of recurrent depression and family history of depression.
4. Discussion
We found that the positive valence symptoms of anhedonia and lack of motivation and energy changed significantly over the course of exercise augmentation treatment of depression; changes in motivation and energy were larger, as measured by effect size, than changes in anhedonia. Change in motivation also was more tightly correlated with change in overall depression severity than change in hedonic capacity. Results controlling for change in depression severity suggest that neither hedonic capacity, motivation nor energy are independent of depression. However, we found a complex relationship between MEI and the MEI-ME subscale at baseline and outcome of treatment. Pathway analysis revealed that changes in the MEI score and the physical energy subscale in response to exercise treatment preceded and help predict improvement in overall symptoms. Interestingly the relationship between social motivation and depression severity over time was reversed, so that improvement in depression by the midpoint of the study led to more social motivation at week 12. Overall our results suggest that exercise affects motivated behavior (the focus of the MEI) more than subjective anhedonia as measured by the SHAPS but that both types of symptoms are effectively treated as part of the antidepressant effect of exercise. An alternate but related interpretation is that exercise affects neuro-vegetative symptoms more than other depression symptoms and that the MEI is more sensitive to this change. In fact, exercise has been used as a treatment specifically for fatigue (Meneses-Echavez et al., 2015); the MEI may therefore simply measure effects specific to exercise treatment more so than the SHAPS.
Our finding that the MEI and its mental energy subscale at baseline affected outcome by dose group on the QIDS-C deserves further attention. Both analyses showed the same pattern. Subjects in the middle of the MEI (or subscale) distribution experienced the same outcome regardless of dose group assignment. Low baseline MEI total or mental energy score was associated with a better outcome for a higher dose of exercise. This is consistent with the idea that exercise is a specific therapy for fatigue and other neuro-vegetative symptoms as reflected in MEI score. However, those subjects with high MEI total or mental energy subscale score at baseline had the opposite pattern, which is more difficult to explain. It is possible that depressed subjects with higher motivation at baseline represent a subgroup that is, for unknown reasons, not significantly helped by more vigorous exercise. The fact that this finding was significant only for the clinician rated QIDS may reflect an interaction between poor cognitive function and overall self-reported symptoms; although this is essentially speculative. A sub-sample of the TREAD study was tested more thoroughly with a cognitive battery (Greer et al., 2015) and it was found that high dose exercise was more effective in improving cognition in TREAD subjects than low dose across the range of tests, although performance in some cognitive areas improved in all subjects regardless of treatment assignment. However, Greer et al. found that change in cognitive performance did not correlate with change in overall depression severity. To the extent that the mental energy subscale corresponds to self-reported cognition, it appears that it may be better correlated to overall outcome than performance. Since it is fairly well established that subjective and objective cognition in depression are often discrepant, the confusion of findings is not that surprising, and perhaps deserves more investigation (Svendsen et al., 2012).
The path analysis results suggest that exercise may improve motivation and energy generally and physical energy specifically, deficits of which would present at baseline as fatigue, earlier in the course of treatment and that this improvement may drive eventual improvement in depression as a whole with further exercise. This is consistent with the use of exercise to treat fatigue but also extends that result to show that addressing fatigue is a critical component of overall depression treatment response. Our finding that, conversely social motivation may require improvements in other areas of depression follows nicely upon the previous finding. Strategies such as behavioral activation are built upon the idea that increases in activity will lead to improvements in other domains (Ekers et al., 2008), a concept nicely supported by these results. Since motivation has been shown to be an important factor associated with physical activity engagement in patients with mood disorders (Vancampfort et al., 2015a) one possible factor behind our results is that those with more motivation were better able to participate in the recommended exercise dose, and therefore benefitted more. There is a lack of overall understanding of factors that improve engagement in exercise in patients with depression (Vancampfort et al., 2015b) despite clear evidence of its efficacy and of low rates of adequate exercise in this population.
4.1. Limitations
TREAD was designed primarily with treatment of overall depression severity in mind, this analysis involves secondary outcomes for which power and other statistical considerations were not determined a priori and may account for the findings associated with effect size which did not reach threshold for significance. Similarly, we have not adjusted analyses for multiple comparisons. Drop-out is a significant factor in exercise treatment for depression (Stubbs et al., 2016) which we did not consider in effecting the results. Generalizability to other treatment scenarios is limited since exercise augmentation is a unique treatment. Also, our sample was predominantly white and had a high percentage of women, even considering the gender discrepancy in depression prevalence. Replication of these results in different samples may be helpful. In addition, TREAD did not include bio-behavioral measures of physiology, such as neuroimaging, which would provide stronger links between domain-based conceptualizations and the clinical concepts measured by the SHAPS and MEI.
4.2. Conclusions
These results reflect opportunities in the application of RDoC concepts to clinical research. We have demonstrated here that measurement of specific positive valence symptoms is feasible and produces data that are clinically and scientifically relevant. These results may help facilitate routine measurement of symptomatic behaviors as valuable adjuncts to core depression assessments. Further work will continue to rethink how questions about neurobehavioral systems are asked and answered. Harmonization of models and even vocabulary – motivation or wanting, anticipation or reward sensitivity - is still needed to effectively isolate positive valence deficits in depression and other mental illnesses, and advance their translation to treatment. This continuing definition of positive valence can serve as a model process for other neurobiological domains less fully developed in their application to mood disorders, establishing a foundation for production of more translational clinical research.
Acknowledgments
Role of funding source
This work was supported by NIMH Grant no. R01-MH067692 (Dr. Trivedi, PI), and by a NARSAD (now called the Brain and Behavior Research Foundation) Independent Investigator Award (Dr Trivedi, PI), Young Investigator Award (Dr. Greer, PI) and the Hersh Foundation (Dr. Trivedi PI). It is registered on clinicaltrials.gov with identifier NCT00076258. Neither NIMH nor NARSAD had any role in the design of the study, or in the analysis and publication of these results.
We would like to acknowledge the editorial assistance of Savitha Kalidas, PhD. in preparing the manuscript.
Abbreviations
- MDD
Major Depressive Disorder
- RDoC
Research Domain Criteria
- TREAD
Treatment with Exercise Augmentation for Depression
- SHAPS
Snaith Hamilton Pleasure Scale
- MEI
Motivation and Energy Inventory
- QIDS
Quick Inventory of Depression Symptomatology
- SSRI
Selective Serotonin Reuptake Inhibitor
Footnotes
Contributors
Dr. Toups drafted, and formatted the manuscript; all authors contributed to the manuscript and approved its contents. Dr. Carmody provided the statistical analysis plan and performed the analysis. Dr. Rethorst provided comments/revisions on the paper. Drs. Trivedi and Greer and Mr. Granneman contributed to the design and operation of the TREAD study.
Conflict of interest
Dr. Toups has received fees from Otsuka Pharmaceuticals. Dr. Trivedi has been an advisor/consultant and received fees from: Alkermes, AstraZeneca, Cerecor, Eli Lilly & Company, Lundbeck, Naurex, Neuronetics, Otsuka Pharmaceuticals, Pamlab, Pfizer Inc., SHIRE Development and Takeda. In addition, Dr. Trivedi has received grants/research support from: National Institute of Mental Health (NIMH) and National Institute on Drug Abuse (NIDA). Dr. Greer has received honoraria, speakers or advisory boards and/or consultant fees from H. Lundbeck A/S. Dr. Carmody, Dr. Rethorst and Mr Grannemann report no disclosures and conflicting interests.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5. American Psychiatric Association; Washington, DC: 2013. [Google Scholar]
- Berridge KC, Kringelbach ML. Pleasure systems in the brain. Neuron. 2015;86:646–664. doi: 10.1016/j.neuron.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortina J. Effect Size for ANOVA Designs. Sage Unversity Press; Thousand Oaks, CA: 2000. [Google Scholar]
- Cuthbert BN, Insel TR. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med. 2013;11:126. doi: 10.1186/1741-7015-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, Heijnen CJ, Kavelaars A, Laye S, Capuron L. The neuroimmune basis of fatigue. Trends Neurosci. 2014;37:39–46. doi: 10.1016/j.tins.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn AL, Trivedi MH, Kampert JB, Clark CG, Chambliss HO. The DOSE study: a clinical trial to examine efficacy and dose response of exercise as treatment for depression. Control Clin Trials. 2002;23:584–603. doi: 10.1016/s0197-2456(02)00226-x. [DOI] [PubMed] [Google Scholar]
- Ekers D, Richards D, Gilbody S. A meta-analysis of randomized trials of behavioural treatment of depression. Psychol Med. 2008;38:611–623. doi: 10.1017/S0033291707001614. [DOI] [PubMed] [Google Scholar]
- Fehnel SE, Bann CM, Hogue SL, Kwong WJ, Mahajan SS. The development and psychometric evaluation of the motivation and energy inventory (MEI) Qual Life Res: Int J Qual Life Asp Treat, Care Rehabil. 2004;13:1321–1336. doi: 10.1023/B:QURE.0000037502.64077.4d. [DOI] [PubMed] [Google Scholar]
- Goldberg D. The heterogeneity of “major depression”. World Psychiatry. 2011;10:226–228. doi: 10.1002/j.2051-5545.2011.tb00061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer TL, Grannemann BD, Chansard M, Karim AI, Trivedi MH. Dosedependent changes in cognitive function with exercise augmentation for major depression: results from the TREAD study. Eur Neuropsychopharmacol: J Eur Coll Neuropsychopharmacol. 2015;25:248–256. doi: 10.1016/j.euroneuro.2014.10.001. [DOI] [PubMed] [Google Scholar]
- Hamlett A, RL, Wolfinger R. Proceedings of the Statistics and Data Analysis. SAS Users Group International; 2006. On the use of PROC MIXED to estimate correlation in the presence of repeated measures. [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Sanislow C, Wang P. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Meneses-Echavez JF, Gonzalez-Jimenez E, Ramirez-Velez R. Effects of supervised multimodal exercise interventions on cancer-related fatigue: systematic review and meta-analysis of randomized controlled trials. Biomed Res Int. 2015;2015:328636. doi: 10.1155/2015/328636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Nakonezny PA, Carmody TJ, Morris DW, Kurian BT, Trivedi MH. Psychometric evaluation of the Snaith-Hamilton pleasure scale in adult outpatients with major depressive disorder. Int Clin Psychopharmacol. 2010;25:328–333. doi: 10.1097/YIC.0b013e32833eb5ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakonezny PA, Morris DW, Greer TL, Byerly MJ, Carmody TJ, Grannemann BD, Bernstein IH, Trivedi MH. Evaluation of anhedonia with the snaith-hamilton pleasure scale (SHAPS) in adult outpatients with major depressive disorder. J Psychiatr Res. 2015;65:124–130. doi: 10.1016/j.jpsychires.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institutes of Mental Health. Positive Valence Systems: Workshop Proceedings.2011. [Google Scholar]
- Rush AJ, Carmody TJ, Reimitz PE. The inventory of depressive symptomatology (IDS): clinician (IDS C) and self report (IDS SR) ratings of depressive symptoms. Int J Methods Psychiatr Res. 2000;9:45–59. [Google Scholar]
- Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, Markowitz JC, Ninan PT, Kornstein S, Manber R. The 16-item quick inventory of depressive symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54:573–583. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- Schuch FB, Morres ID, Ekkekakis P, Rosenbaum S, Stubbs B. A critical review of exercise as a treatment for clinically depressed adults: time to get pragmatic. Acta Neuropsychiatr. 2016:1–7. doi: 10.1017/neu.2016.21. [DOI] [PubMed] [Google Scholar]
- Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P. A scale for the assessment of hedonic tone the Snaith-Hamilton pleasure scale. Br J Psychiatry. 1995;167:99–103. doi: 10.1192/bjp.167.1.99. [DOI] [PubMed] [Google Scholar]
- Streiner DL. Finding our way: an introduction to path analysis. Can J Psychiatry. 2005;50:115–122. doi: 10.1177/070674370505000207. [DOI] [PubMed] [Google Scholar]
- Stubbs B, Vancampfort D, Rosenbaum S, Ward PB, Richards J, Soundy A, Veronese N, Solmi M, Schuch FB. Dropout from exercise randomized controlled trials among people with depression: a meta-analysis and meta regression. J Affect Disord. 2016;190:457–466. doi: 10.1016/j.jad.2015.10.019. [DOI] [PubMed] [Google Scholar]
- Svendsen AM, Kessing LV, Munkholm K, Vinberg M, Miskowiak KW. Is there an association between subjective and objective measures of cognitive function in patients with affective disorders? Nord J Psychiatry. 2012;66:248–253. doi: 10.3109/08039488.2011.626870. [DOI] [PubMed] [Google Scholar]
- Treadway MT, Zald DH. Parsing anhedonia: translational models of reward-processing deficits in psychopathology. Curr Dir Psychol Sci. 2013;22:244–249. doi: 10.1177/0963721412474460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi MH. Modeling predictors, moderators and mediators of treatment outcome and resistance in depression. Biol Psychiatry. 2013;74:2–4. doi: 10.1016/j.biopsych.2013.05.009. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, McGrath PJ, Fava M, Parsey RV, Kurian BT, Phillips ML, Oquendo MA, Bruder G, Pizzagalli D, Toups M, Cooper C, Adams P, Weyandt S, Morris DW, Grannemann BD, Ogden RT, Buckner R, McInnis M, Kraemer HC, Petkova E, Carmody TJ, Weissman MM. Establishing moderators and biosignatures of antidepressant response in clinical care (EMBARC): rationale and design. J Psychiatr Res. 2016;78:11–23. doi: 10.1016/j.jpsychires.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi MH, Greer TL, Church TS, Carmody TJ, Grannemann BD, Galper DI, Dunn AL, Earnest CP, Sunderajan P, Henley SS. Exercise as an augmentation treatment for nonremitted major depressive disorder: a randomized, parallel dose comparison. J Clin Psychiatry. 2011;72:677. doi: 10.4088/JCP.10m06743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi MH, Greer TL, Grannemann BD, Church TS, Galper DI, Sunderajan P, Wisniewski SR, Chambliss HO, Jordan AN, Finley C. TREAD: treatment with exercise augmentation for depression: study rationale and design. Clin Trials. 2006;3:291. doi: 10.1191/1740774506cn151oa. [DOI] [PubMed] [Google Scholar]
- Ustun TB, Ayuso-Mateos JL, Chatterji S, Mathers C, Murray CJ. Global burden of depressive disorders in the year 2000. Br J Psychiatry. 2004;184:386–392. doi: 10.1192/bjp.184.5.386. [DOI] [PubMed] [Google Scholar]
- Vancampfort D, Madou T, Moens H, De Backer T, Vanhalst P, Helon C, Naert P, Rosenbaum S, Stubbs B, Probst M. Could autonomous motivation hold the key to successfully implementing lifestyle changes in affective disorders? A multicentre cross sectional study. Psychiatry Res. 2015a;228:100–106. doi: 10.1016/j.psychres.2015.04.021. [DOI] [PubMed] [Google Scholar]
- Vancampfort D, Stubbs B, Sienaert P, Wyckaert S, De Hert M, Rosenbaum S, Probst M. What are the factors that influence physical activity participation in individuals with depression? A review of physical activity correlates from 59 studies. Psychiatr Danub. 2015b;27:210–224. [PubMed] [Google Scholar]


