Abstract
Congenital muscle diseases, such as myopathies or dystrophies, occur relatively frequently, with estimated incidences of up to 4.7 per 100 000 newborns. To diagnose congenital diseases in the early stages of pregnancy, and to interpret the results of increasingly advanced in utero imaging techniques, a profound knowledge of normal human morphological development of the locomotor system and the nervous system is necessary. Muscular development, however, is an often neglected topic or is only described in a general way in embryology textbooks and papers. To provide the required detailed and updated comprehensive picture of embryologic muscular anatomy, three‐dimensional (3D) reconstructions were created based on serial histological sections of a human embryo at Carnegie stage 23 (8 weeks of development, crown–rump length of 23.8 mm), using amira reconstruction software. Reconstructed muscles, tendons, bones and nerves were exported in a 3D‐PDF file to permit interactive viewing. Almost all adult skeletal muscles of the trunk and limbs could be individually identified in their relative adult position. The pectoralis major muscle was divided in three separate muscle heads. The reconstructions showed remarkable highly developed extraocular, infrahyoid and suprahyoid muscles at this age but surprisingly also absence of the facial muscles that have been described to be present at this stage of development. The overall stage of muscle development suggests heterochrony of skeletal muscle development. Several individual muscle groups were found to be developed earlier and in more detail than described in current literature.
Keywords: 3D‐PDF, anatomy, embryology, morphogenesis, muscle development, ontogeny, three‐dimensional reconstruction
Introduction
Congenital muscle diseases, such as myopathies or dystrophies, occur relatively frequently and constitute a heavy burden for society and, firstly, for the affected child and its family. Global incidences of congenital muscular dystrophies are unknown, but described incidences of 4.7 live born per 100 000 inhabitants in north‐eastern Italy between 1979 and 1993 (Mostacciuolo et al. 1996) and a prevalence of 3.95 cases per 100 000 inhabitants in northern England (Norwood et al. 2009) provide an indication of the scale. An epidemiological study of neuromuscular disorders in children under the age of 16 in western Sweden showed a prevalence of 63.1 per 100 000 inhabitants (Darin & Tulinius, 2000). Comprehensive understanding of congenital muscle diseases requires knowledge of muscle anatomy and physiology. In adults, imaging techniques such as ultrasound and magnetic resonance imaging (MRI) are already contributing to diagnosis of the type of muscle disease (Wattjes et al. 2010) and could therefore also be used for in utero imaging. However, little is known about late embryonic morphological development.
As fetal medicine is developing rapidly (Danzer & Johnson, 2014; Moldenhauer, 2014) and prenatal diagnostics are regularly performed in modern medical practice, the need for detailed anatomical knowledge in clinical settings has become more pressing. Because skeletal muscles contribute significantly to the total volume of the human body, they can often be used as anatomical landmarks (De Battista et al. 2011). Accurate knowledge of late embryonic and fetal spatial anatomical relations is therefore crucial for effective interpretation of high resolution prenatal imaging techniques. Procedures such as sonography or fetoscopy during prenatal diagnostics and fetal surgery can even predict embryonic health, as it is already in use to determine fetal health (D'Addario et al. 2005; Hutchinson et al. 2014).
Morphological muscle development is an often neglected or vaguely described topic in embryology textbooks and articles (Schoenwolf et al. 2009; Sadler, 2012; Deries & Thorsteinsdottir, 2016; Pu et al. 2016). Based on our experience gained in creating the 3D Atlas of Human Embryology (de Bakker et al. 2016), we hypothesized that human embryonic muscle anatomy is more advanced than would be concluded from descriptions in literature. To provide a more detailed and comprehensive picture of embryonic muscular anatomy, we used amira reconstruction software to create three‐dimensional (3D) reconstructions based on serial histological sections of a human embryo at Carnegie stage 23 (CS23, 8 weeks of development), the final stage of embryonic development, with a crown–rump length of 23.8 mm. Reconstructed muscles, tendons, bones and nerves were exported in a 3D‐PDF file to permit interactive investigation of the morphology of skeletal muscles at CS23. To our knowledge comprehensive muscle reconstructions are new and have not been included in previous publications from our research group.
Materials and methods
3D reconstructions
Image acquisition and alignment was done as previously described by de Bakker et al. (2012, 2016). Digital images were captured from the series of sections of a CS23 specimen number 950 of the Carnegie collection in Silver Spring, MD, USA. This male specimen was collected after a miscarriage in 1914 and has been graded by specialists of the Carnegie collection as ‘good’ (O'Rahilly & Müller, 1987). Compared with the other stage 23 specimens in the Carnegie collection, this specimen showed an excellent conservation of histological quality. It has also been incorporated in the 3D Atlas and Database of Human Embryology as published in Science (de Bakker et al. 2016) and revealed no deviations from normal anatomy in any of the studied organ systems compared with the other studied specimens. Therefore, although it was collected after a miscarriage, this specimen is considered to be normal. The specimen was fixed in formalin, transversally sectioned at 42.71 μm and then stained with an aluminum cochineal staining (O'Rahilly & Müller, 1987; de Bakker et al. 2016). Based on the section thickness and number of slices, we calculated an approximate crown–rump length of 23.8 mm. Labeling of the 557 serial sections was accomplished by trained medical students, under the supervision of a physical therapist and four experienced embryologists. Skeletal muscles and tendons were manually segmented, with a Bamboo tablet and pen (http://www.wacom.com) based on digital images of the serial sections. Segmentation of structures on the serial sections was performed on gray‐scale images in amira® software (version 5.3–5.6, http://www.amira.com), and the high‐resolution full color dataset was displayed on a second computer screen. This way, every muscle that could histologically have been identified in this specimen, including its tendons, was traced from origin to insertion. The nerves and bones of this specimen were already reconstructed in previous studies (de Bakker et al. 2012, 2016) and could therefore provide information about the topographic position of muscles relative to bones or nerves. The use of bones and nerves as anatomical landmarks allowed a distinction to be made between different types of tissues and provided topographical orientation throughout the body. If demarcation between individual muscles was not possible, muscles were labeled as muscle groups. When counting all reconstructed muscles, grouped muscles were counted as one muscle.
Structure identification
After reconstructing, muscles and tendons were identified using adult human anatomy atlases (Gilroy et al. 2009; Drake et al. 2014; Netter, 2014) by establishing their origin and insertion and topographic relation to bones, nerves and surrounding organs. English names of the muscles were used, based on Terminologia Anatomica (Baud et al. 1998).
Interactive 3D‐PDF
amira surface files of the 3D reconstructions were converted to a .u3d file with Fiji (imagej, https://imagej.net). deep exploration (version 6.5 CSE, part of Corel DESIGNER Technical Suite ×5 http://www.corel.com) was used for grouping and labeling of structures. Finally, use of Adobe acrobat xi pro (http://www.adobe.com) allowed an interactive 3D‐PDF file to be created containing all reconstructed structures and a custom user interface. A 3D‐PDF can be viewed with a recent version of Adobe reader® (X or higher, freeware, http://www.adobe.com) on MS Windows or MacOS systems, with javascript and playing of 3D content enabled.
The Adobe reader program allows users to not only view the 3D‐PDF file, but also to zoom in or out on a structure, to hide it or to show it transparently. It provides the user with a clear view of the embryo in a 3D interactive fashion, making it possible to obtain a better understanding of the spatial relations between segregated organs and structures – in this case the musculoskeletal system in the embryo. The usefulness of this technique has been proved in earlier publications (van den Berg et al. 2009; van den Berg & Moorman, 2011; de Boer et al. 2011; Sizarov et al. 2011, 2012; de Bakker et al. 2012, 2016).
Results
The reader is encouraged to read the results along with the interactive 3D‐PDF file in the Supporting Information Data S1 (accessible at website https://onlinelibrary.wiley.com/doi/abs/10.1111/joa.12819). A total of 318 individual muscles or muscle groups and 48 individual tendons were reconstructed; bilateral muscles were counted individually. We describe here the most remarkable findings, in addition to a representative illustration of skeletal muscle development at CS23. An overview of muscles that were not identified is presented in Table 1.
Table 1.
Overview of unidentified muscles
| Region | Subregion | Muscle |
|---|---|---|
| Head | Depressor anguli oris | |
| Levator anguli oris | ||
| Epicranius | ||
| Epimysium | ||
| Depressor septi nasi | ||
| Levator labii superioris alaeque nasi | ||
| Levator labii superioris | ||
| Auricularis posterior | ||
| Face | Buccinator | |
| Corrugator supercilii | ||
| Depressor labii inferioris | ||
| Depressor septi nasi | ||
| Depressor supercilii | ||
| Zygomaticus major | ||
| Mentalis | ||
| Transversus menti | ||
| Zygomaticus minor | ||
| Orbicularis oculi | ||
| Orbicularis oris | ||
| Platysma | ||
| Procerus | ||
| Risorius | ||
| Neck | Rectus capitis anterior | |
| Thyrohyoid | ||
| Laryngeal muscles | All | |
| Pharyngeal muscles | All | |
| Torso | Chest | Levator costarum |
| Subcostales | ||
| Abdomen | Cremaster | |
| Pyramidalis | ||
| Back | Psoas minor | |
| Perineal muscles | All | |
| Upper limb | Hand/Thenar | Palmaris brevis |
| Lower limb | Thigh/Medial | Adductor minimus |
Stage 23 human embryo (56–60 days of development), specimen number 950, has been used for this table. The largest group of muscles that were not present in this specimen were muscles of the head, neck and torso. Reconstructed muscles were compared with a list of all muscles from the Terminologia Anatomica (Baud et al. 1998).
Head and neck
Head and neck muscles showed differing degrees of development. All extraocular muscles were identified except for the levator palpebrae muscle. Three extrinsic tongue muscles were identified: genioglossus, hyoglossus and styloglossus. The origin of the styloglossus muscle was at the first pharyngeal cartilage and the insertion was at the tip and medial sides of the tongue. The levator veli palatine was located craniomedially from the medial pterygoid muscle. The semispinalis capitis and cervicis muscles appeared relatively large when compared with the surrounding small muscles such as the rotatores and multifidi muscles (Fig. 1), possibly due to a craniocaudal developmental gradient that causes craniocaudal differences in size. The small longus capitis and longus colli muscles were already at their adult location and also had the similar relative shape and position on vertebrae and bone landmarks as in adults (Fig. 2).
Figure 1.
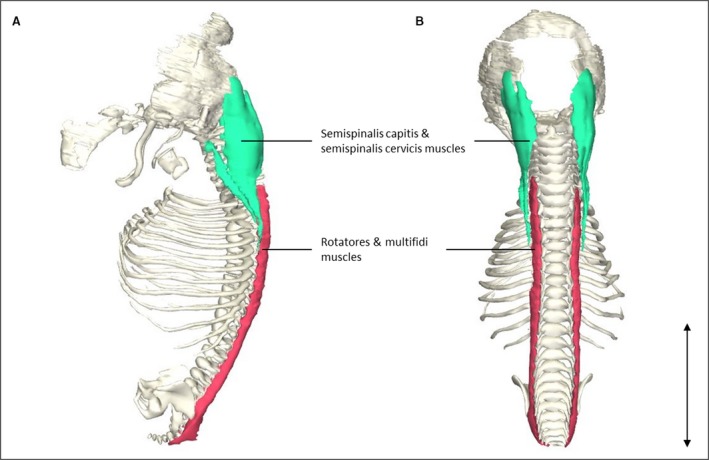
Transversospinal muscle reconstrunctions. (A) Left lateral view of the head and vertebral column of a stage 23 human embryo (56–60 days of development), specimen number 950. Note that the semispinalis capitis and semispinalis cervicis muscles (green) are depicted as one structure because they were histologically inseparable. The same holds for the rotatores and multifidi muscles (red). (B) Dorsal view of the same reconstruction as in (A). The semispinalis capitis and semispinalis cervicis muscles are relatively large in size compared with the rotatores and multifidi muscles, whereas in adults the differences in size between these muscles are minimal, possibly due to a craniocaudal developmental gradient that causes craniocaudal differences in size. Scale bar: ~5 mm.
Figure 2.
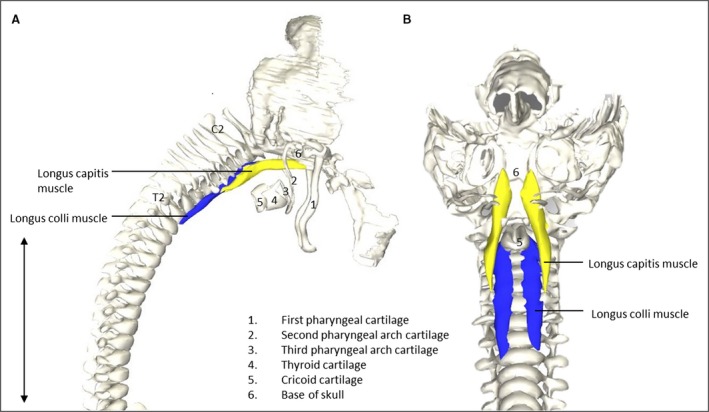
Vertebrate muscles of the neck. (A) Right lateral view of the head region of a stage 23 human embryo (56–60 days of development), specimen number 950. Longus capitis (Lca, yellow) and longus colli (Lco, blue) muscles depicted on vertebrate column, head bones and neck cartilage. (B) Caudofrontal view of head and neck region of the same specimen as in (A). LCa and LCi depicted on vertebrate column, head bones and cartilage. To increase clarity, the first pharyngeal arch, hyoid and thryoid bone were excluded. Note that the relative size and shape of the muscles to the skull and vertebrae are identical, as would be expected in mature humans (Gilroy et al. 2009). Scale bar: ~5 mm.
The suprahyoid muscles (anterior and posterior digastric, stylohyoid, mylohyoid and geniohyoid muscles) and infrahyoid muscles (sternohyoid, sternothyroid and omohyoid muscles) had the same relative shape as in adults, and origins and insertions were also as in adulthood. The temporalis and masseter muscles were relatively small compared with other muscles in this region such as head and neck muscles (Fig. 3). Extraocular muscles were already similar in size and shape to mature anatomy relative to the rest of the head.
Figure 3.
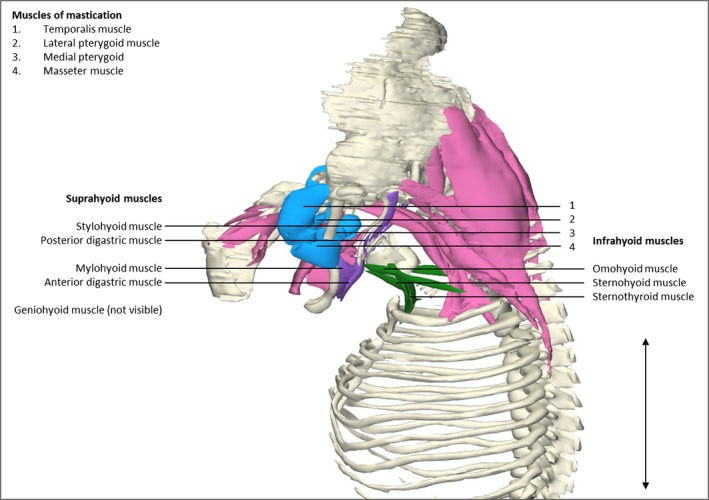
Head and neck muscles. Left lateral view of the head and neck region of a stage 23 human embryo (56–60 days of development), specimen number 950. Skeletal structures are depicted in white, eye and neck musculature in pink, suprahyoid muscles in blue, infrahyoid muscles in green and muscles of mastication in purple. Suprahyoid and infrahyoid muscles show a high degree of segregation. Note that the muscles of mastication have a small size relative to the bones and surrounding muscles when compared with the relative adult sizes. Suprahyoid and infrahyoid muscles are already individually partitioned from the surrounding muscles and have the same position as in adult anatomy (Gilroy et al. 2009). The sternocleidomastoid muscles are excluded. Scale bar: ~5 mm.
Trunk
The erector spinae muscle was the second most profound muscle layer of the back, positioned superficially compared with the transversospinal muscles; the separate erector spinae muscles could be distinguished from each other. The iliocostalis, longissimus and spinalis muscles had differently oriented fibers and were separated by fasciae which allowed a distinction between the three muscles (Fig. 4). The transversospinal muscles were present at their adult location but were only identified as a muscle group because of their relatively small size (Fig. 1).
Figure 4.
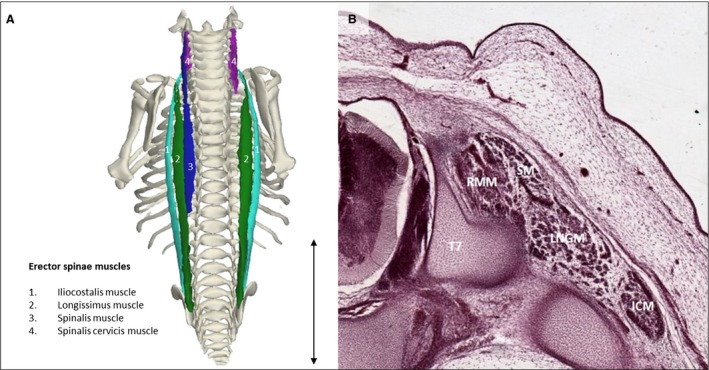
Erector spinae muscles. (A) Dorsal view of the back of a stage 23 human embryo (56–60 days of development), specimen number 950. Erector spinae muscles are depicted along with the bones of the body. The different muscles are the iliocostalis muscle (light green), longissimus muscle (green), spinalis muscle (blue) and spinalis cervicis muscle (pink). On the right side the spinalis muscle is excluded, as are other muscles. (B) Transversal serial section view of the left erector spinae muscles of the same specimen as in (A) at the level of T7. Note that the erector spinae muscles have separated bodies and hence are already individually recognizable at this stage of development. LNGM, longissimus muscle; ICM, iliocostalis muscle; RMM, rotatores and multifidi muscles; SM, spinalis muscle. Scale bar: ~5 mm.
In contrast to the above‐mentioned trunk muscles, abdominal muscles do not appear in their final position and shape in this specimen. The rectus abdominis muscles showed a widened rectal diastasis, resulting from the relatively large diameter of the umbilical cord due to physiological herniation of the gut, which is at this stage in the process of disappearing (Mekonen et al. 2015). The internal and external oblique muscles defined the abdominal outlines of the embryo (Fig. 5). The quadratus lumborum muscle still has a cylindrical shape which will grow out towards a quadrilateral appearance when mature (Gilroy et al. 2009), underlining the difference in relative size favoring cranial structures over caudal structures, as seen in the neck region.
Figure 5.
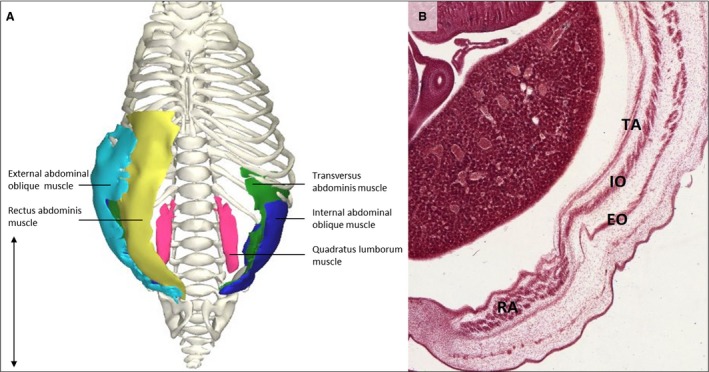
Abdominal muscles. (A) Ventral view of the trunk of a stage 23 human embryo (56–60 days of development), specimen number 950. External abdominal oblique muscle (light blue), internal abdominal oblique muscle (dark blue), rectus abdominis muscle (yellow), transversus abdominis muscle (green), quadratus lumborum muscle (pink) and ribcage, vertebrae and pelvic bones (white). All other muscles and bones are excluded. (B) Detail of a transversal serial section superior view of the abdominal muscles of the same specimen as in (A). Thin muscles such as the transversus abdominis and internal oblique muscles are already distinctly separated from each other, as can be seen on the serial section. Left side of the abdomen illustrates the rectus abdominis muscle (RA), the external oblique abdominal muscle (RO), the internal oblique abdominal muscle (IO) and the transversus abdominis muscle (TA). Scale bar: ~5 mm.
Limbs
Upper limb
Differences in size between upper and lower limbs are apparent; the upper limbs appear larger in size than the lower limbs, according to the craniocaudal developmental gradient. Muscles of the vertebral column that are part of the upper limb (Baud et al. 1998) stood out as the largest muscles in size, relative to other muscles of the embryo. The trapezius muscle as the most superficial dorsal upper limb muscle covered a wide area, from the skull to T9. The latissimus dorsi muscle had its origin on thin fascia‐like fibers at the level of T7–L3 and the insertion was in a common tendon of the three muscle parts on the humeral intertubercular groove. The left serratus posterior superior was not in contact with the spine or the nuchal ligament, which is the usual origin. Thoracic wall muscles, which are also part of the upper limb muscles (Baud et al. 1998), were also large in size and close to their final position compared with other muscles in this region. The serratus anterior muscle fanned out from the medial margin of the scapula to the first eight ribs, as in adult anatomy according to Gilroy and co‐workers (2009). The pectoralis major muscle, which is known to be one large muscle body with different origins that are interconnected in adults (Gilroy et al. 2009), had three distinct muscle heads clearly separated from each other starting at their origins and converging only just before their insertion on the humerus. The 3D reconstruction shows origins as expected from adult anatomy: cranially at the clavicle, intermediate on the sternum and inferior on the lower part of the sternum, the common insertion was at the crista of the major tubercle on the humerus (Fig. 6. The reconstruction of the triceps brachii muscle showed three heads with origins and insertions as in adults.
Figure 6.
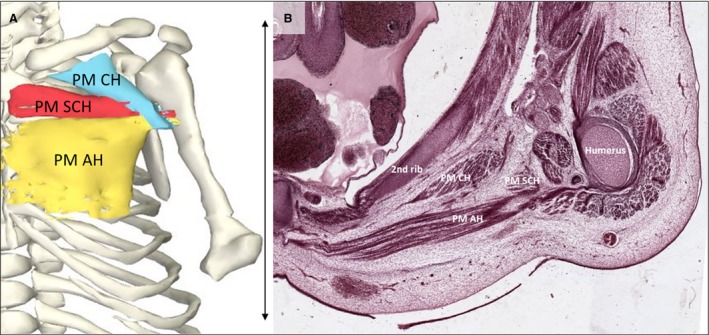
Pectoralis major muscle. (A) Frontal view of the reconstructed pectoralis major portions of a stage 23 human embryo (56–60 days of development), specimen number 950. All other muscles are hidden. Depicted are three heads of the pectoralis major: clavicular head (light blue), sternocostal head (red) and abdominal head (yellow) and bones (white). (B) Transverse section of the left shoulder region. The pectoralis major muscle is divided into three heads at this stage and is therefore even more partitioned than in adults (Gilroy et al. 2009). The tree heads of the left pectoralis muscle and the second rib and humerus of the same specimen as in (A) are tagged. AH, abdominal head; CH, clavicular head; PM, pectoralis major muscle; SCH, sternocostal head. Scale bar: ~5 mm.
All muscles of the forearm could be recognized individually and were positioned as in adults. The palmaris longus muscle, for example, had its origin at the medial epicondyle of the humerus and the insertion at the flexor carpi radialis tendon, as other forearm muscles, and was already positioned as in adult anatomy. The belly of the muscle formed a small oval corps and had a tendon similar to the palmaris longus.
Not all muscles of the hand could be individually identified as some were too small to distinguish. Two thenar muscular structures were seen, one of which was identified as the adductor pollicis muscle. The other one was a common muscle belly at the location of the opponens pollicis, flexor pollicis brevis and abductor pollicis brevis muscles. Three lumbrical muscle bellies were found palmar to the third, fourth and fifth metacarpal bones.
Lower limb
Spatial organization and orientation of the muscles of the lower limbs were recognizable as in adult anatomy (Gilroy et al. 2009). Muscles of the iliac region reached from T12 to the lesser trochanter of the femur. From origin to insertion the major psoas and iliacus muscles were clearly separated from each other. This distinction is not easily made in adult anatomy, where the psoas major and the iliacus muscles meet at their inferior ends to form the iliopsoas muscle. The gluteus maximus muscle already appears in the typical round shape. The lateral rotator muscles are small and appeared mostly in an elongated shape, possibly because of the flexed position of the femur in the hip joint.
Discussion
The results of our study differ from what we expected based on the available literature. Except for the two studies of Mekonen et al. (2015 2016, the state of muscle development at 8 weeks of development is described at the level of muscle fibers (Musumeci et al. 2015) rather than discernible anatomical structures. Textbooks provide only brief information on muscular ontogeny and mention that all skeletal muscles are present after 8 weeks of development (Schoenwolf et al. 2009; Moore et al. 2013) but they do not go into morphological detail. The state of muscular development is graphically depicted as grossly arranged groups of muscles which are still in the process of separation into smaller individual muscles (Schoenwolf et al. 2009; Moore et al. 2013). However, our reconstructions show that individual segregation of most muscle groups is already completed when the embryo measures less than 3 cm. In fact, some muscles, e.g. the pectoralis major or iliopsoas muscles, are even more partitioned than in adult anatomy and will probably converge later in development. In contrast, facial muscles that were thought to be present at CS23 (Gasser, 1967) were not yet differentiated enough to be distinguished in our specimen.
Skeletal muscles are derived from paraxial mesoderm and early muscle development has been broadly described in the literature (Musumeci et al. 2015). In the post‐otic region, mesodermal segments of paraxial mesoderm form somites (Musumeci et al. 2015). These somites differentiate into dermomyotome, myotome and sclerotome. Migratory muscle precursors arise from the lateral dermomyotomal lips at distinct axial levels to give rise to the hypopharyngeal‐hypoglossal, diaphragm, limb and cloacal musculature (Buckingham, 2001). The ultimate stage of myogenesis can be histologically identified by the presence of transverse striation, a sign of muscle fiber maturation. This ultimate step marks myotube formation. The first myotubes in humans are apparent during week 5 and by the end of week 8, most myotubes are differentiated into muscle fibers (Musumeci et al. 2015).
Some studies describe the 3D morphology of selected muscle groups such as epaxial muscles (Mekonen et al. 2016) or muscles of the ventral body wall (Mekonen et al. 2015) but the general state of muscular development at the late embryonic stage is currently not described. Therefore, we investigated the 3D morphological presence and appearance of all embryonic skeletal muscles at 8 weeks of development.
We studied the morphological state of the skeletal muscles in one 8‐week‐old human embryo. This highlights the main limitation of this study. More research is necessary to gain more profound knowledge about morphological development and to allow for generalization of results to all human embryos.
Head and neck
Most extraocular muscles and muscles of mastication were individually identified. Gasser (1975) wrote that muscles of mastication and facial muscles begin to differentiate laterally in the lower region of the face in week 8 of development. This early progress in head and neck muscle development around CS23 is also seen in other species. The cucullaris muscle in chicks is already clearly visible at Hamburger‐Hamilton stage (HH) 30 and this stage is comparable to CS19 in humans (Theis et al. 2010). The sternocleidomastoid and trapezius muscles have been described as homologues of the cucullaris muscle in mammals. These muscles are individually recognizable in mice around 14 days of gestation (E14) and E14.5, respectively, which is comparable to CS19 and 20 (Theis et al. 2010). Except for the stylohyoid muscle and the posterior belly of the digastric muscle, none of the muscles innervated by the seventh cranial nerve (facial nerve) were found in our human specimen.
All extraocular muscles appear in their relative adult position and shape by 8 weeks of development, except for the levator palpebrae muscle, which was not present at this stage. The levator palpebrae muscle in fetuses has been described as having numerous anatomical variations such as accessory medial muscle bellies or broad lateral insertions of the aponeurosis towards the tissue adjacent to the lacrimal glands (Plock et al. 2005). However, literature about its embryonic development is absent. Therefore, we can only speculate that the levator palpebrae muscle develops in a later stage than CS23. As seen in Fig. 3, most head and neck muscles are already discernible at this point, but differ in position from adulthood in relation to other structures. For example, muscles of mastication, e.g. the temporalis and masseter muscles, have to fan out cranially and reach the parietal and sphenoid bone and zygomatic arch and maxilla from the coronoid process, respectively (Gilroy et al. 2009). The individual presence of the temporalis and masseter muscles has also been described in chick embryos at E15.5, which is comparable to CS22 (Buckingham & Vincent, 2009). The exact origin and insertion of these two muscles have not been described and therefore it is not clear whether the temporalis and masseter muscles are already in their final position in chicks at CS22. Generally, most muscles in our human specimen which are innervated by the third and fifth cranial nerve are perfectly recognizable at CS23 (Table 2).
Table 2.
Overview of muscle presence classified per cranial nerve
| Cranial nerve | Muscle | Present Fully | Partly | Not |
|---|---|---|---|---|
| III (Oculomotor) | Levator palpebrae superioris | x | ||
| Superior rectus | x | |||
| Medial rectus | x | |||
| Inferior rectis | x | |||
| Inferior oblique | x | |||
| IV (Trochlear) | Superior oblique | x | ||
| V (Trigeminal) | Masseter | x | ||
| Temporal | x | |||
| Medial pterygoid | x | |||
| Lateral pterygoid | x | |||
| Tensor veli palatini | n | |||
| Mylohyoid | x | |||
| Anterior belly of digastric | x | |||
| Tensor tympani | n | |||
| VI (Abducens) | Lateral rectus | x | ||
| VII (Facial) | Posterior belly of digastric | x | ||
| Stylohyoid | x | |||
| Stapedius | n | |||
| Occipitofrontalis muscle | n | |||
| Temporoparietalis muscle | n | |||
| Procerus muscle | n | |||
| Nasalis muscle | n | |||
| Depressor septi nasi muscle | n | |||
| Orbicularis oculi muscle | n | |||
| Corrugator supercilii muscle | n | |||
| Depressor supercilii muscle | n | |||
| Auricular muscles (anterior, superior and posterior) | n | |||
| Orbicularis oris muscle | n | |||
| Depressor anguli oris muscle | n | |||
| Risorius | n | |||
| Zygomaticus major muscle | n | |||
| Zygomaticus minor muscle | n | |||
| Levator labii superioris | n | |||
| Levator labii superioris alaeque nasi muscle | n | |||
| Depressor labii inferioris muscle | n | |||
| Levator anguli oris | n | |||
| Buccinator muscle | n | |||
| Mentalis | n | |||
| IX (Glossopharyngeal) | Stylopharyngeus | n | ||
| X (Vagus) | Cricothyroid muscle | n | ||
| Levator veli palatini muscle | x | |||
| Salpingopharyngeus muscle | n | |||
| Palatoglossus muscle | n | |||
| Palatopharyngeus muscle | n | |||
| Superior, middle and inferior pharyngeal constrictors | n | |||
| Muscles of the larynx (speech) | ||||
| XI (Accessory) | Sternocleidomastoid | x | ||
| Trapezius | x | |||
| XII (Hypoglossal) | Genioglossus | x | ||
| Hyoglossus | x | |||
| Styloglossus | x | |||
| Intrinsic tongue muscles | n | |||
| Geniohyoid | x |
Stage 23 human embryo (56–60 days of development), specimen number 950, has been used for this table. Note the differences in presence of muscles depending on the particular cranial nerve. Most muscles of cranial nerves III, IV, V, VI, XI and XII were present at this stage, whereas most muscles of cranial nerves VII and X were absent.
Trunk
The large trunk muscles show remarkable differences in size based on their position. More cranial situated structures are larger than caudal structures, as can be seen in Fig. 1, probably due to the craniocaudal growth gradient. The semispinalis capitis and cervicis muscles are relatively large in size compared with the rotatores and multifidi muscles (depicted together in Fig. 1), whereas in adults the differences in size between these muscles are minimal (Gilroy et al. 2009).
The existence of the spinalis muscle at CS23 is controversial. Based on the observed location, the muscle could not be found in a study assessing 7‐ to 13‐week‐old fetuses (Sato et al. 2011) but was present at a stage 23 human embryo according to Mekonen et al. (2016). In concordance with Mekonen et al. (2016), the spinalis muscle was also identified (Fig. 4) in our study. Sato et al. (2011) concluded from their findings that the spinalis muscle must be formed out of the interspinalis muscle; this was based on their assumption that parts of the primitive interspinalis muscle detach and make new connections. We cannot confirm this hypothesis because tissue in this region was not sufficiently individually partitioned to identify the interspinalis muscle.
Abdominal muscles were clearly individually recognizable at the cross‐sections of the human specimen used. This has also been described in rat embryos. Fifteen days after gestation (E15), comparable to human CS18, the dorsal mesoderm is already split in three layers, allowing for distinction between the internal and external abdominal oblique muscles and the transversus abdominis muscle. The rectus abdominis is recognizable at E15 or CS18 in a ventral portion and appears as two muscle bellies on either side of the umbilical foramen at E16 or CS20 (Rizk & Adieb, 1982). Even though most muscles are already distinctively recognizable at the end of the embryonic period (CS23), this does not mean that the reconstructed muscles have already reached their final position, relative size and shape as compared with adult anatomy. For example, the serratus posterior inferior muscle is still in a rather lateral position compared with adult anatomy. And as previously described (Mekonen et al. 2015) at 6–9 weeks of development, the abdominal muscles show a widened diastasis, which is precisely how our reconstructed rectus abdominis muscles appear (Fig. 5). In advanced stages of fetal development, the relative diameter of the umbilical cord will decrease (Gasser, 1975). Also, the quadratus lumborum muscle has not yet attained its final shape as in adults. Instead of a quadrilateral appearance, it looks rather cylindrical, implying that the morphological development has not yet been finalized at 8 weeks of development.
Limbs
Muscles of the upper and lower limbs were clearly individually identifiable. Following the craniocaudal developmental gradient, the upper limbs are relatively larger in size and have a relatively more mature morphology compared with the lower limbs (Gilroy et al. 2009). Although this is novel for human embryos, early muscle segregation has already been described in a mice developmental study. Individually recognizable limb muscles are present at mouse embryonic day 14.5 (E14.5), which is comparable to CS20 in humans (Delaurier et al. 2008).
Upper limbs
Although the three different portions of the pectoralis major muscle have a common point of insertion, their origins differ. In our reconstructions, the muscle bellies are spatially separated before they converge just before their insertion (Fig. 6). Since the clavicular and abdominal portion have independent innervation and vascularization (Chaffai & Mansat, 1988; Kim et al. 2015), these portions of the pectoralis muscle can be used for flap surgery independently from other portions (Tobin, 1985; Chaffai & Mansat, 1988; Chomiak & Dungl, 2008). In adults, the three muscle bellies are combined from their origin until their insertion (Gilroy et al. 2009), suggesting that further growth after CS23 results in a connection and eventual fusion of the three muscle heads. Another muscle that will expand in size in the subsequent weeks of development is the latissimus dorsi. At 19 weeks of development this muscle has been reported to extend until the iliac crest (Yahia Ben Hadj & Vacher, 2011); however, in our reconstructions the muscle fibers only reach level L3 and L2 on the left and right side, respectively. The connection of the latissimus dorsi with the spinous processes as described in fetuses and adult humans (Gilroy et al. 2009; Yahia Ben Hadj & Vacher, 2011), is also not yet established. The latissimus dorsi muscle did not reach the spinous processes in the reconstructions, but the serial sections showed connective tissue between the muscle fibers and the spinous processes, identifiable as fascia or aponeurosis. This illustrates that whereas some muscles appear in their relative position and shape as in adult anatomy by this time, others still have to grow out after CS23.
The triceps brachii muscle is reported to attain the typical three‐headed shape at 10 weeks of development (Grzonkowska et al. 2014). Our reconstructions now show that around week 8 of development this particular shape is already established.
Anatomical variations are described for some muscles of the forearm; even ipsilateral absence of the palmaris longus muscle has been described (Sater et al. 2010; Albay et al. 2013). However, we did not identify noticeable differences between the left and right side in our specimen. Lumbrical muscles were identified earlier than previously described. The earliest description of development of lumbrical muscles to our knowledge is that muscle tissue is starting to be recognized in week 8 and by week 13 of development the lumbrical muscles are easily individually recognizable (Cho et al. 2012). Muscle reconstructions in the hands of our 8‐week‐old specimen showed three distinct lumbrical muscles, again suggesting earlier development of the lumbrical muscles than previously thought.
Lower limbs
Gluteal muscles are very illustrative for our hypothesis of earlier partitioning of adult muscle morphology than is described in current literature. Despite their caudal location, gluteal muscles are already highly segregated and developed at week 8 of development. Previous research described the gluteus maximus recognizable in its final adult shape and position after 17 weeks of development (Kedzia et al. 2014). Our 3D reconstructions show separated gluteal muscles (gluteus maximus, medius and minimus) in mature morphology already in an embryo with a 23.8‐mm crown–rump length, indicating earlier segregation than described so far. For an overview of earlier segregated muscles see Table 3.
Table 3.
Overview of earlier segregated muscles compared with the literature
| Region | Muscle | Earlier segregation | Source |
|---|---|---|---|
| Head and neck | Extraocular (except levator palpebrae muscle) | x | NS |
| Facial nervea | Gasser (1975) | ||
| Trunk | Spinalisb | x | Sato et al. (2011) |
| Upper limb | Triceps brachii | x | Grzonkowska et al. (2014) |
| Pectoralis major | x | NS | |
| Forearm | x | NS | |
| Lumbrical | x | NS | |
| Lower limb | Gluteal | x | Kedzia et al. (2014) |
Stage 23 human embryo (56–60 days of development), specimen number 950, has been used for this table. Eight muscle(s) (groups) are notably more segregated at this stage of development than could be expected from the current literature. Sources that indicate earlier development are given in the last column.
NS, no dedicated source was found on the embryonic development of this muscle (group). Thus, the stage of muscle segregation in this specimen appears to be further developed than described in the general literature about embryonic muscle morphogenesis.
Facial nerve muscles: the muscles innervated by the facial nerve were not present in our specimen except for the stylohyoid and posterior belly of digastric muscle.
Conclusion and future outlook
We conclude that the literature lacks detailed and complete descriptions of morphological embryological and fetal skeletal muscle development. Also, descriptions in the currently available literature differ in many respects from our findings. Some muscles are more advanced in their development and may even resemble mature morphology, whereas others are less advanced than described. To understand how skeletal muscles develop over time, reconstructions of several embryos at different stages of development are necessary. Based on these data, an algorithm could be calculated as a noninvasive screening method to predict normal growth and detect congenital muscle disorders and anomalies. This emphasizes the need for more research in this direction and should be the focus of future studies to elucidate the complicated process of morphological development in human embryos.
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this article.
Author contributions
Contributions to the study concept, data interpretation and critical revision of the manuscript: R.J.O. and J.H. Contribution to data interpretation and article approval: J.R. and P.B.B. Study concept, data acquisition, data interpretation, manuscript drafting: B.S.B. and M.V.W.
Supporting information
Data S1. Supplementary 3D‐PDF. Interactive 3D reconstruction of the skeletal muscular system at 8 weeks of development.
Acknowledgements
We are grateful to E. Lockett and E. Wilson of the Human Developmental Anatomy Center at the National Museum of Health and Medicine in Silver Spring, MD, USA for providing access to the Carnegie collection. We thank O. J. G. B. de Bakker for her assistance in photographing the sections. M. Mehta is acknowledged for critical revision of the manuscript. L. Kuil and C. Berends are acknowledged for annotating the nerves and skeleton, respectively. J. M. Ruijter, K. H. de Jong, K. de Bree and Prof. A. F. M. Moorman are gratefully acknowledged for their scientific support.
References
- Albay S, Kastamoni Y, Sakalli B, et al. (2013) Anatomy and variations of palmaris longus in fetuses. Rom J Morphol Embryol 54, 85–89. [PubMed] [Google Scholar]
- de Bakker BS, de Jong KH, Hagoort J, et al. (2012) Towards a 3‐dimensional atlas of the developing human embryo: the Amsterdam experience. Reprod Toxicol, 34, 225–236. [DOI] [PubMed] [Google Scholar]
- de Bakker BS, de Jong KH, Hagoort J, et al. (2016) An interactive three‐dimensional digital atlas and quantitative database of human development. Science 354, 1019. [DOI] [PubMed] [Google Scholar]
- Baud RH, Neumann PE, Sprumont P (1998), Terminologia anatomica. English tree of Terminologia anatomica 1998 on‐line version, Terminologia anatomica from the International Federation of Associations of Anatomists. Available: http://www.unifr.ch/ifaa/ [2016]
- van den Berg G, Moorman AF (2011) Development of the pulmonary vein and the systemic venous sinus: an interactive 3D overview. PLoS ONE 6, e22055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg G, Abu‐Issa R, de Boer BA, et al. (2009) A caudal proliferating growth center contributes to both poles of the forming heart tube. Circ Res 104, 179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer BA, Soufan AT, Hagoort J, et al. (2011) The interactive presentation of 3D information obtained from reconstructed datasets and 3D placement of single histological sections with the 3D portable document format. Development, 138, 159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham M (2001) Skeletal muscle formation in vertebrates. Curr Opin Genet Dev 11, 440–448. [DOI] [PubMed] [Google Scholar]
- Buckingham M, Vincent SD (2009) Distinct and dynamic myogenic populations in the vertebrate embryo. Curr Opin Genet Dev 19, 444–453. [DOI] [PubMed] [Google Scholar]
- Chaffai MA, Mansat M (1988) Anatomic basis for the construction of a musculotendinous flap derived from the pectoralis major muscle. Surg Radiol Anat 10, 273–282. [DOI] [PubMed] [Google Scholar]
- Cho KH, Kim JH, Ha YS, et al. (2012) Development of the deep flexor tendons and lumbricalis muscle in the hand and foot: a histological study using human mid‐term foetuses. Folia Morphol 71, 154–163. [PubMed] [Google Scholar]
- Chomiak J, Dungl P (2008) Reconstruction of elbow flexion in arthrogryposis multiplex congenita type I. Part I: surgical anatomy and vascular and nerve supply of the pectoralis major muscle as a basis for muscle transfer. J Child Orthop 2, 357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Addario V, Pinto V, Di Cagno L, et al. (2005) The midsagittal view of the fetal brain: a useful landmark in recognizing the cause of fetal cerebral ventriculomegaly. J Perinat Med 33, 423–427. [DOI] [PubMed] [Google Scholar]
- Danzer E, Johnson MP (2014) Fetal surgery for neural tube defects. Semin Fetal Neonatal Med 19, 2–8. [DOI] [PubMed] [Google Scholar]
- Darin N, Tulinius M (2000) Neuromuscular disorders in childhood: a descriptive epidemiological study from western Sweden. Neuromuscul Disord 10, 1–9. [DOI] [PubMed] [Google Scholar]
- De Battista JC, Zimmer LA, Rodriguez‐Vazquez JF, et al. (2011) Muller's muscle, no longer vestigial in endoscopic surgery. World Neurosurg 76, 342–346. [DOI] [PubMed] [Google Scholar]
- Delaurier A, Burton N, Bennett M, et al. (2008) The Mouse Limb Anatomy Atlas: an interactive 3D tool for studying embryonic limb patterning. BMC Dev Biol 8, 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deries M, Thorsteinsdottir S (2016) Axial and limb muscle development: dialogue with the neighbourhood. Cell Mol Life Sci 73, 4415–4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake RL, Vogl AW, Mitchell AWM, et al. (2014) Gray's Atlas of Anatomy. 2nd edn Philadelphia: Churchill Livingstone. [Google Scholar]
- Gasser RF (1967) The development of the facial muscles in man. Dev Dyn 120, 357–375. [Google Scholar]
- Gasser RF (1975) Atlas of Human Embryos. Philadelphia: Lippincott Williams and Wilkins. [Google Scholar]
- Gilroy AM, MacPherson BR, Ross LM, et al. (2009) Atlas of Anatomy. Stuttgart: Thieme Medical Publishers. [Google Scholar]
- Grzonkowska M, Badura M, Lisiecki J, et al. (2014) Growth dynamics of the triceps brachii muscle in the human fetus. Adv Clin Exp Med 23, 177–184. [DOI] [PubMed] [Google Scholar]
- Hutchinson EF, Kieser JA, Kramer B (2014) Morphometric growth relationships of the immature human mandible and tongue. Eur J Oral Sci 122, 181–189. [DOI] [PubMed] [Google Scholar]
- Kedzia A, Janeczko M, Miskiewicz K, et al. (2014) Morphometry of human musculus gluteus maximus in foetal period. Adv Clin Exp Med 23, 9–16. [DOI] [PubMed] [Google Scholar]
- Kim DM, Jeon A, Kim KY, et al. (2015) Neurovascular distribution within the abdominal head of the pectoralis major muscle: application to breast and flap surgery. Clin Anat, 28, 520–526. [DOI] [PubMed] [Google Scholar]
- Mekonen HK, Hikspoors JP, Mommen G, et al. (2015) Development of the ventral body wall in the human embryo. J Anat 227, 673–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekonen HK, Hikspoors JP, Mommen G, et al. (2016) Development of the epaxial muscles in the human embryo. Clin Anat, 29, 1031–1045. [DOI] [PubMed] [Google Scholar]
- Moldenhauer JS (2014) In utero repair of spina bifida. Am J Perinatol 31, 595–604. [DOI] [PubMed] [Google Scholar]
- Moore L, Persaud T, Torchia M (2013), Muscular System, Development of Limbs. In The Developing Human: Clinically Oriented Embryology, 9th edn, Philadelphia: Saunders Elsevier, pp. 86–89, 363‐386. [Google Scholar]
- Mostacciuolo ML, Miorin M, Martinello F, et al. (1996) Genetic epidemiology of congenital muscular dystrophy in a sample from north‐east Italy. Hum Genet 97, 277–279. [DOI] [PubMed] [Google Scholar]
- Musumeci G, Castrogiovanni P, Coleman R, et al. (2015) Somitogenesis: from somite to skeletal muscle. Acta Histochem 117, 313–328. [DOI] [PubMed] [Google Scholar]
- Netter F (2014) Atlas of Human Anatomy. 6th edn Philadelphia: Saunders Elsevier. [Google Scholar]
- Norwood FL, Harling C, Chinnery PF, et al. (2009) Prevalence of genetic muscle disease in Northern England: in‐depth analysis of a muscle clinic population. Brain 132, 3175–3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Rahilly R, Müller F (1987) Developmental stages in human embryos. Washington, DC: Carnegie Institution of Washington, pp. 637. [Google Scholar]
- Plock J, Contaldo C, von Ludinghausen M (2005) Levator palpebrae superioris muscle in human fetuses: anatomical findings and their clinical relevance. Clin Anat, 18, 473–480. [DOI] [PubMed] [Google Scholar]
- Pu Q, Huang R, Brand‐Saberi B (2016) Development of the shoulder girdle musculature. Dev Dyn 245, 342–350. [DOI] [PubMed] [Google Scholar]
- Rizk NN, Adieb N (1982) The development of the anterior abdominal wall in the rat in the light of a new anatomical description. J Anat 134, 237–242. [PMC free article] [PubMed] [Google Scholar]
- Sadler TW (2012) Muscular system; limbs.In Langman's Medical Embryology, 12th edn, pp. 145–161. Philadelphia: Lippincott Williams & Wilkins. [Google Scholar]
- Sater MS, Dharap AS, Abu‐Hijleh MF (2010) The prevalence of absence of the palmaris longus muscle in the Bahraini population. Clin Anat, 23, 956–961. [DOI] [PubMed] [Google Scholar]
- Sato T, Koizumi M, Kim JH, et al. (2011) Fetal development of deep back muscles in the human thoracic region with a focus on transversospinalis muscles and the medial branch of the spinal nerve posterior ramus. J Anat 219, 756–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenwolf GC, Bleyl SB, Brauer PR, et al. (2009)Development of the musculoskeletal system.In Larsen's Human Embryology, 4th edn, Philadelphia: Churchill Livingstone Elsevier, pp. 217–244617‐642. [Google Scholar]
- Sizarov A, Devalla HD, Anderson RH, et al. (2011) Molecular analysis of patterning of conduction tissues in the developing human heart. Circ Arrhythm Electrophysiol 4, 532–542. [DOI] [PubMed] [Google Scholar]
- Sizarov A, Lamers WH, Mohun TJ, et al. (2012) Three‐dimensional and molecular analysis of the arterial pole of the developing human heart. J Anat 220, 336–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theis S, Patel K, Valasek P, et al. (2010) The occipital lateral plate mesoderm is a novel source for vertebrate neck musculature. Development 137, 2961–2971. [DOI] [PubMed] [Google Scholar]
- Tobin GR (1985) Pectoralis major segmental anatomy and segmentally split pectoralis major flaps. Plast Reconstr Surg 75, 814–824. [DOI] [PubMed] [Google Scholar]
- Wattjes MP, Kley RA, Fischer D (2010) Neuromuscular imaging in inherited muscle diseases. Eur Radiol 20, 2447–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahia Ben Hadj S, Vacher C (2011) Does the Latissimus dorsi insert on the iliac crest in man? Anatomic and ontogenic study. Surg Radiol Anat 33, 751–754. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supplementary 3D‐PDF. Interactive 3D reconstruction of the skeletal muscular system at 8 weeks of development.


