Abstract
Human neural stem cells (hNSC) derived from induced pluripotent stem cells can be differentiated into neurons that could be used for transplantation to repair brain injury. In this study we dispersed such hNSC in a three‐dimensional artificial extracellular matrix (aECM) and compared their differentiation in vitro and following grafting into the sensorimotor cortex (SMC) of postnatal day (P)14 rat pups lesioned by localised injection of endothelin‐1 at P12. After 10–43 days of in vitro differentiation, a few cells remained as PAX6+ neuroprogenitors but many more resembled post‐mitotic neurons expressing doublecortin, β‐tubulin and MAP2. These cells remained dispersed throughout the ECM, but with extended long processes for over 50 μm. In vivo, by 1 month post grafting, cells expressing human specific markers instead organised into cerebral organoids: columns of tightly packed PAX6 co‐expressing progenitor cells arranged around small tubular lumen in rosettes, with a looser network of cells with processes around the outside co‐expressing markers of immature neurons including doublecortin, and CTIP2 characteristic of corticofugal neurons. Host cells also invaded the graft including microglia, astrocytes and endothelial cells forming blood vessels. By 10 weeks post‐grafting, the organoids had disappeared and the aECM had started to break down with fewer transplanted cells remaining. In vitro, cerebral organoids form in rotating incubators that force oxygen and nutrients to the centre of the structures. We have shown that cerebral organoids can form in vivo; intrinsic factors may direct their organisation including infiltration by host blood vessels.
Keywords: animal model, cerebral organoids, human, neural stem cells, perinatal stroke
Introduction
Stroke in the neonatal period (which occurs mostly within the first week after delivery) is a leading cause of cerebral palsy (Kirton et al. 2011) for which a range of early intervention approaches are under investigation (Basu, 2014). Stem cell therapy is a compelling current approach that is being investigated extensively in neurological disorders; early interventions using stem cells could provide support to the infarcted cortex, or perhaps even replace lost cortical cells. Interventional experiments for cerebral palsy conducted so far suggest that stem cells contribute to repair after early brain injury (Kiasatdolatabadi et al. 2017).
Neural stem cells (NSCs) are multipotent; they are able to self‐renew but ultimately produce neurons, astrocytes and oligodendrocytes, and potentially replace damaged cells (Jendelová et al. 2016). NSCs derived from human induced pluripotent stem cells (hiPSCs) originating from human umbilical cord blood were found to be safe and reliable in interventional studies using in vitro and in vivo adult and neonatal models of neurological disorders (Bużańska et al. 2002; Jablonska et al. 2010). When hiPSC‐derived NSCs are transplanted into neonatal rats, they have the ability to survive, migrate and differentiate into neuronal cells, with no signs of tumour formation (Jablonska et al. 2010).
An infarcted brain area is a hostile environment for intracerebrally transplanted stem cells, often leading to grafted cell death (Bakshi et al. 2005; Bliss et al. 2007). The absence of trophic factors in the infarction cavity, a damaged blood brain barrier and the loss of extracellular matrix (ECM) proteins due to stroke lead to the accumulation of extracellular fluid and leakage of plasma proteins into the infarction cavity (Baeten & Akassoglou, 2011). For these reasons, the development of compatible biomaterials that fill the infarction cavity to provide the grafted cells with a stimulatory environment for survival and enhance the efficacy of stem cell therapy is a crucial aim in treating stroke (Wang et al. 2014).
Recent advances in tissue engineering have shown that hydrogel works as a compatible artificial ECM (aECM) and can support transplanted stem cell survival in the infarction cavity in adult stroke models (Zhong et al. 2010). In vitro and in vivo neuro‐regeneration studies have shown that hydrogel can be used as scaffold for the stem cells (Thonhoff et al. 2008; Zhong et al. 2010; Burdick & Prestwich, 2011; Bible et al. 2012; Liang et al. 2013). However, thus far, stem cell transplantation studies have failed to fill the infarction site or produce a well‐developed, organised formation of regenerated cerebral cells local to the lesion in vivo due to the accumulation of extracellular fluid and proteins in the post‐stroke lesion site (Baeten & Akassoglou, 2011).
In this study we explored the potential for early intervention after perinatal stroke in an animal model by transplanting hNSCs dispersed in aECM at postnatal day 14 into perinatal sensorimotor cortex (SMC) damaged by inducing focal ischaemia at P12. We made the lesion at P12 because this stage of neurodevelopment of the sensorimotor system most closely matches the human at the time of birth (Hagberg et al. 2002; Clowry, 2007; Tucker et al. 2009; Jablonska et al. 2010; Clowry et al. 2014). Grafts were carried out soon after the lesion because corticospinal innervation early in development is crucial to guiding the maturation of the sensorimotor system. Aberrant plasticity, leading to the symptoms of cerebral palsy, occurs when there is removal of corticospinal input at this stage (Clowry, 2007; Eyre, 2007; Kolb & Gibb, 2007; Basu & Clowry, 2015). Furthermore, the immune system is still immature and less able to mount an immunogenic response to xenogeneic transplants in neonate rodents (Englund et al. 2002; Coenen et al. 2005; Jablonska et al. 2010). A study in a P12 mouse stroke model showed that intrastriatal injection of embryonic stem cell‐derived NSCs at P14 attenuated brain atrophy in the longer term (Comi et al. 2008) suggesting that this might be an appropriate age to make the transplant.
Our hypothesis was that the grafted hNSCs, protected by the aECM and by the underdevelopment of the immune system at this stage of maturation, would differentiate into neurons and extend axons along the corticospinal tract, which is still developing and not completely myelinated at this age (Gorgels, 1990; Fallah & Clowry, 1999). However, instead, the transplanted hNSCs organised into structures resembling cerebral organoids that grow in vitro under specific culture conditions (Mariani et al. 2012; Shi et al. 2012; Lancaster et al. 2013; Mason & Price, 2016). However, this did not happen when hNSCs were grown in three‐dimensional cultures in hydrogel aECM in vitro. We suggest that vascularisation of the graft in vivo initially promotes organisation and initial survival of the organoids but eventually sows the seeds of their destruction by exposing the graft to the host immune system.
Materials and methods
Experimental design
In vitro differentiation of hNSCs/aECM in a 3D culture was assessed at 10, 14, 17 and 43 days in vitro (DV) using immunocytochemistry. In parallel with the in vitro experiment, we undertook in vivo transplantation of hNSCc/aECM into ischaemic SMC of 12 rats to study the survival and integration of the hNSCs and the host tissue response 1, 4 and 10 weeks post‐grafting. Animals in a sham group received only aECM transplantation and were studied 4 weeks post‐grafting.
NSCs culture
Human induced pluripotent stem cell‐derived neural stem cells (iPSC‐NSCs) were obtained and reprogrammed from a male newborn cord blood donor (CD34+) and were purchased from Axol Bioscience (Cambridge, UK). The differentiation and the transplantation protocols were adopted from those provided by Axol Bioscience (available online https://www.axolbio.com) and modified according to other published methods (Zhong et al. 2010; Liang et al. 2013).
Under a sterilised hood, hiPSCs‐NSCs were plated in Neural Plating–XF Medium (Axol Bioscience) at high density of 200 000 cells per cm2 on a coated 6‐cm petri dish (Sigma Aldrich, Poole, UK) overnight at 37 °C, in 5% CO2. On the following day, when NSCs reached 70–80% of confluency, cells were rinsed once with 2 mL per 10 cm2 culture surface area of Dulbecco's‐PBS without calcium or magnesium (Life Technologies, Gibco, the Netherlands) and detached by incubating cells for 5 min at 37 °C with 1 mL per 10 cm2 of Unlock‐XF (Axol Bioscience). The Unlock‐XF solution was then diluted by adding four volumes of pre‐warmed, 37 °C, Neural Expansion‐XF Medium followed by centrifugation at 200 g for 5 min at room temperature. After centrifuging, the supernatant was aspirated and the cell pellet was re‐suspended with freshly prepared HyStem®‐C hydrogel scaffold kit (HYSC020, Sigma Aldrich) at 1 × 105 cells per 5 μL hydrogel concentration. The hydrogel components hyaluronan, gelatine and cross‐linker were mixed at a 2 : 2 : 1 molar ratio.
Then, 5 μL of the NSC/aECM complex was plated in each of four micro‐inserts (Culture‐Insert 4‐Well, Ibidi, Germany) placed in a two‐chamber slide (Ibidi) filled with sterilised phosphate‐buffered saline (PBS) to maintain humidity. The slide was placed in a 100‐mL Petri dish and incubated at 37 °C, in 5% CO2 for 1 h to ensure a successful gelling process. Once the appropriate gel texture was achieved, 140 μL of pre‐warmed, 37 °C, Neural Maintenance‐XF Medium with growth factors FGF2 and EGF (Axol Bioscience) was added into each insert and the dish was returned to the warm incubator. The next day, the medium was replaced with fresh, pre‐warmed, 37 °C, Neural Maintenance‐XF Medium without growth factor, and after a further 24 h, two‐thirds of the medium was replaced. Thereafter, half of the medium was replaced every 2 days.
Induction of focal ischaemia and stem cell transplantation
Wistar rats were purchased from Charles River Laboratories and were housed in the Newcastle Comparative Biology Centre at Newcastle University. All animal procedures were approved and performed in accordance with the Newcastle University Animal Welfare and Ethics Review Board and under licence from the UK Government Home Office in accordance with the UK Animals (Scientific Procedures) Act 1986 and European Union directive 2010/63EU. All rats shared the same room with the same care routine and light/dark cycle.
Under general anaesthesia induced by inhalation of isoflurane, 400 pmol in total of endothelin‐1 (ET‐1) (Calbiochem, Nottingham, UK) dissolved in 0.9% sodium chloride was injected into the SMC of P12 rat pups at two sites to cause focal ischaemia (Soleman et al. 2010). ET‐1 solution 1 μL was injected at a depth of 1 mm at each of the following co‐ordinates: +2.00 mm anterior of bregma and +2.00 mm lateral of the midline, and +0.75 mm anterior and +2.00 mm lateral according to the Paxinos and Watson atlas (Paxinos & Watson, 1998).
At P14, hNSCs in aECM were transplanted intracerebrally into the ischaemic SMC of 15 rat pups. Seven pups in a sham group received aECM only and no NSCs. For each rat, a complex of 1 × 105 hNSCs in 2 μL aECM was prepared; only two components of the HyStem‐C (hyaluronan and gelatine) were mixed and added to the NSC cell pellet to begin with, while the cross‐linker was added just before transplantation due to the small time window for gelling. For the hydrogel molar ratio (2 : 2 : 1) that we used, gelling time was found to be 12 min after adding the cross linker. The cells were cultured for 24 h in vitro following thawing before transplantation. This was comparable to the time that hNSCs were cultured prior to induction of differentiation in in vitro experiments (see above).
Under isoflurane‐induced anaesthesia, a flap in the skull bones was cut and hinged at the midline exposing the right SMC. Using a stereotactic frame with inset and ear bars designed for rat pups, hydrogel (with or without cells) was injected with a Hamilton syringe into the ischaemic SMC at co‐ordinates 1.3 mm anterior to bregma and 2 mm lateral of the midline and at a depth of 1.8 mm. The complex was loaded into 26‐gauge autoclaved Hamilton syringes 4 min after adding the linker. Immediately, to avoid cells settling to the syringe bottom while in liquid form, the needle was lowered after breaking the dura with a fine tip needle into the ischaemic SMC at AP1.3, MD2 and DV1.8 co‐ordinates. The gradual injection of the 2 μL of hNSCs/aECM continued for 4 min at a rate of 0.5 μ min−1 using an Ultra micropump with microcontroller (World Precision Instruments, Sarasota, FL, USA). The needle was kept in its place for an additional minute to prevent back flow and was then withdrawn slowly. The skull and scalp were repaired and the animals allowed to recover before returning to their mother's cage for 24 h. The animals received a subcutaneous injection of analgesic such as buprenorphine if any signs of pain or stress were observed.
Tissue processing and immunofluorescence assessments
Rats were perfused transcardially with 4% paraformaldehyde (PFA) in PBS (pH 7.4) at 1, 4 and 10 weeks post‐transplantation. The brains were removed and cryoprotected by immersion in PBS containing 30% sucrose at 4 °C. Parallel sets of eight coronal brain sections (50 μm) from fixed brains were collected from the frontal two‐thirds of the brain (cut using a freezing sliding microtome) as free‐floating sections.
In vitro cells in 3D culture were fixed at four time points: 10, 14, 17 and 43 days after initiation of differentiation. NSCs in the micro‐inserts were rinsed twice with PBS, and then incubated with 4% PFA in PBS for 30 min at room temperature with gentle agitation. Brain sections were incubated at 4 °C overnight for immunohistochemistry and in vitro cells were incubated similarly for 2 h for immunocytochemistry with gentle agitation in a cocktail containing PBS, 0.3% Triton X‐100 for permeabilisation, 3% appropriate blocking serum and primary antibody. Some sections were, instead, incubated with biotinylated isolectin B4 (1 : 1000, Vector Laboratories, Peterborough, UK) overnight to identify microglia and endothelial cells (Benton et al. 2008; Genade & Lang, 2011).
The sections or cells were then washed and incubated for 2 h in the dark with the conjugated secondary antibodies from the appropriate hosts: Alexa Fluor 488 and Alexa Fluor 594 (diluted 1 : 200–500; Abcam, Cambridge, UK) or with avidin conjugated with fluorescent tags (diluted 1 : 200, Vector Labs) to visualise lectin staining. Nuclei were counterstained with 4′,6‐diamidino‐2‐phenylindole (DAPI; Vectashield, Vector Labs). Details for the primary antibodies are listed in Table 1.
Table 1.
Primary antibodies used in this study
| Primary antibody to | Supplier | Description of target | Antibody dilution | Reference | RRID number | |
|---|---|---|---|---|---|---|
| IHC | ICC | |||||
| IBA1, ionised calcium‐binding adapter molecule 1 (gt polyclonal) | Abcam, Cambridge, UK | Microglia | 1 : 500 | – | Ito et al. (1998) | AB_2224402 |
| GFAP, glial fibrillary acidic protein (ms monoclonal) | Sigma‐Aldrich, Poole, UK | Astrocytes | 1 : 1000 | 1 : 200 | Burtrum & Silverstein (1994) | AB_477010 |
| Pax6, paired box protein (rb polyclonal) | Cambridge Bioscience, Cambridge, UK | Proliferating cortical progenitor cells including radial glia | 1 : 500 | 1 : 100 | Bayatti et al. (2008) | AB_2565003 |
| hNCAM, neural cell adhesion molecules [ms monoclonal IgG)] | Santa Cruz Biotechnology, Heidelberg, Germany | Human neural cell membranes | 1 : 1000 | 1 : 100 | Smith et al. (2017) | AB_627128 |
| STEM121 (ms monoclonal) | Stem Cells Inc., Cambridge, UK | Human cell cytoplasm | 1 : 3000 | 1 : 500 | Guzman et al. (2007); Sareen et al. (2014); Tornero et al. (2013) | AB_2632385 |
| STEM123 (ms monoclonal) | Stem Cells, Inc. | hGFAP, human astrocytes and radial glial cells | 1 : 2000 | 1 : 250 | Sareen et al. (2014) | |
| DCX, doublecortin (rb polyclonal) | Abcam | Migrating neuroblasts | 1 : 1000 | 1 : 250 | Lam et al. (2014) | AB_2088478 |
| Map2, Microtubule Associated Protein‐2 (rb polyclonal) | Abcam | Neuron‐specific expression in dendrites and soma | 1 : 500 | 1 : 200 | Guzman et al. (2007) | AB_448205 |
| Synaptophysin | Sigma‐Aldrich | Synaptic vesicle marker | 1 : 5000 | 1 : 200 | Smith et al. (2017) | AB_477523 |
| CTIP2, COUP‐TF‐interacting protein 2 (rb polyclonal) | Abcam | Transcription factor marking corticofugal neurons | 1 : 300 | – | Ip et al. (2011) | AB‐1140055 |
| TUJ1, Beta‐III‐Tubulin (ms polyclonal) | Merck Millipore, Watford, UK | Specific class III β‐tubulin post‐mitotic neuroblasts and neurons | 1 : 500 | 1 : 100 | Stevanato et al. (2015) | AB_570918 |
Image acquisition
Double‐labelled images of coronal brain sections were captured using the upright fluorescent microscope Zeiss AxioImager with an automated stage. To obtain 3D fluorescence images of the double‐labelled sections, a Nikon A1R confocal microscope was used. Inverted light and fluorescent confocal microscopy (Nikon A1R inverted) was used to capture images of fluorescent double‐labelled cells cultured in vitro. Both Nikon microscopes were equipped with a colour digital camera (Nikon, DS‐Fi1 2560 × 1920). Images were viewed and produced in nis‐elements viewer 4.2.
Results
Behaviour of hNSCs in aECM cultured in 3D in vitro
Cultures were immunostained for PAX6 (paired box 6, transcription factor; cortical neuroprogenitor marker) DCX and bTUB (doublecortin and beta‐tubulin; markers for immature neuroblasts), MAP2 (microtubule associated protein 2: marker for more mature neurons), synaptophysin (identifies synaptic vesicles) and GFAP (glial fibrillary associated protein: astrocytic marker). See Table 1 for more details. After 10–14 days of differentiation only a few PAX6+ neural progenitor cells remained, but many DCX+ immature neurons were present (Fig. 1A,B). The number of bTUB+ immature neurons increased by 14 days of differentiation and these neurons were observed to have extended long processes through the gel that were over 200 μm in length (Fig. 1C). Similarly, increasing numbers of MAP2+ neurons were observed with time, with extended processes over 50 μm in length in the 3D culture. Interestingly, anti‐synaptophysin immunoreactivity was observed at points of contact between cells in culture, suggesting early synapse formation (Fig. 1D).
Figure 1.

The iPSC‐derived hNSCs differentiated in vitro. All sections are counterstained with the nuclear marker, DAPI (blue). (A) By 10 days in culture (D10) cells had begun to express the post‐mitotic marker DCX. By D14, a mixture of cells expressed the nuclear neural stem cell marker PAX6 (red), the cytoplasmic neuronal marker bTUB (green) or both (B). At D14, bTUB expressing cells extended long processes in this three‐dimensional culture medium (C). As early as D14, immunoreactivity for the synaptic marker synaptophysin was seen at potential points of contact between cultured neurons (D). By D43, an increased number of cells were immunoreactive for MAP2 (red), a marker of more mature neurons than bTUB (E). Scale bar: 40 μm.
Between 17 and 43 days of differentiation in vitro the proportion of cells expressing DCX gradually decreased and the proportion expressing MAP2 increased. MAP2+ neurons continued to extend long processes in the direction of other MAP2+ neurons or groups of neurons, thus appearing to form an interconnected network (Fig. 1E). Synaptophysin immunoreactivity continued to be detected within the vicinity of cell clusters. Fewer cells expressed GFAP than expressed markers for neurons.
Behaviour of hNSCs in artificial ECM grafted in vivo
Two experimental groups were compared: hNSCs/aECM and aECM‐only, grafted into the SMC of a neonatal rat that had received an ischaemic lesion. The grafting site was investigated by immunohistochemistry on brain sections. In addition to the immunofluorescent stains described above, antibodies to human cell markers hNCAM (human neural cell adhesion molecule; neurons), STEM121 (cytoplasm), STEM123 (hGFAP, astrocytes and radial glia) as well as to CTIP2 (chicken ovalbumin upstream promoter transcription factor‐interacting protein 2; deep layer cortical neuron marker) and IBA 1 (ionised calcium‐binding adapter molecule 1; microglia) were employed along with isolectin B4 (IB4) histochemistry to identify microglia and blood cells (see Table 1 for more details).
One week post‐grafting
Transplanted hNSCs in the gel scaffold remained as masses or arranged themselves into convoluted thin ribbons of hNCAM+ cells (Fig. 2A), which appeared similar to an early stage of cerebral organoid formation (Qian et al. 2016). Although we employed hNCAM antibodies principally to detect neurons of human origin, human radial glial cells also express hNCAM (Mariani et al. 2012). Human grafted cells expressing hNCAM but not PAX6 were found at the graft site but also away from the graft in the striatum (Fig. 2B) and corpus callosum. In addition, Stem123+ presumptive human astrocytes were observed within and away from the graft (Fig. 2C).
Figure 2.
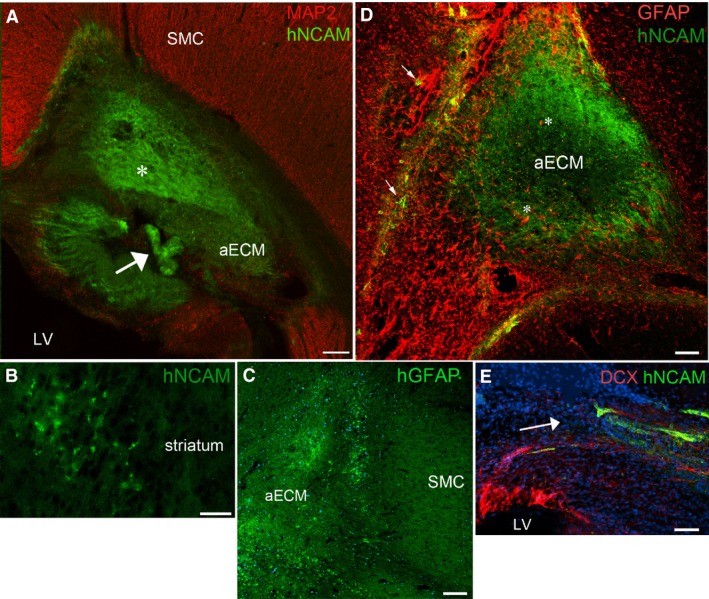
One week post grafting: (A) shows a graft of artificial ECM (aECM) containing hNCAM + cells (green) positioned deep to the host sensorimotor cortex (SMC) close to the lateral ventricle (LV). Some of the hNCAM+ cells were in a disorganised mass (asterisk), whereas some had organised themselves into a convoluted ribbon or tube (arrow). (B) Group of hNCAM + cells that have migrated away from the graft into the nearby striatum. Grafted cells also expressed hGFAP (C) and could be seen around the edges of the graft at the interface with host tissue. (D) Illustrates how host astrocytes (GFAP +, red) infiltrated the grafts (asterisks) mingling with hNCAM + grafted cells (green) in the aECM. Grafted cells also infiltrated the host tissue (arrows). (E) Shows how host DCX + cells (red), presumably newborn neuroblasts, migrated towards and mingled with hNCAM + grafted cells (green). The arrow indicates the direction of migration. Blue staining in (C) and (E) is the nuclear stain DAPI. Scale bars: (A,C,D) 200 μm; (B) 50 μm; (E) 30 μm.
Host cells were attracted towards the graft, regardless of whether it initially contained hNSCs. GFAP+/hNCAM– host astrocytes surrounded and extended long processes into the graft, some appearing to have started to migrate toward the centre (Fig. 2D). Similarly, IB4+ and IBA+ amoeboid microglia accumulated at the boundary of the graft and a few IB4+ blood vessels invaded the transplant (not shown). Host cells also infiltrated the transplanted gel in the aECM‐only group. We found that GFAP+ astrocytes surrounded and invaded the gel but with fewer of them having the long processes seen in astrocytes invading the hNSCs/aECM grafts. Also, a few host DCX immunoreactive neuroblasts migrated from the subventricular zone (SVZ) of both lateral ventricles ipsilateral and contralateral to the graft toward both cell‐containing and aECM‐only grafts. Figure 2E shows DCX+/hNCAM– cells appearing to migrate from close to the ventricle towards, and mingling with, hNCAM+ cells of graft origin.
Four weeks post‐grafting: cerebral organoid formation
In five of the six animals that received grafts and were examined 4 weeks post‐grafting, transplanted hNSCs were seen to form neural tube‐like rosettes with a morphology resembling cerebral organoids previously described to form in vitro under certain culture conditions from neural stem cells derived from hiPSC (Mariani et al. 2012; Lancaster et al. 2013). These aggregations of cells, positive for human cell markers, were contained within the host SMC (Fig. 3A). The sixth brain examined most likely lost its transplant mechanically during brain sectioning. The phenotype of a variety of host cells that infiltrated the xenograft was tested using immunofluorescence. Although both STEM121 and hNCAM were highly expressed in human cells at the graft site, far fewer human cells were observed away from the transplant 4 weeks as compared with 1 week post‐grafting (Fig. 3A).
Figure 3.
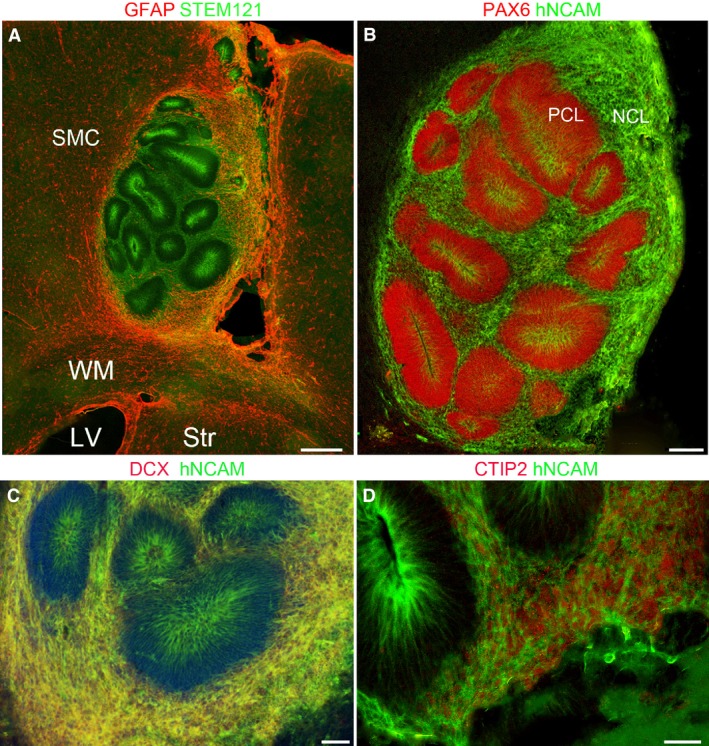
Four weeks post‐grafting. (A) Graft containing cells positive for the human stem cell marker STEM123 (green) within the host sensorimotor cortex (SMC) dorsal to the subcortical white matter (WM), lateral ventricle (LV) and striatum (Str) all positive for GFAP (red). Grafted cells are arranged in rosettes with high expression of STEM121 in cells close to the centre of the rosettes and in the looser arrangement of grafted cells surrounding the rosettes. Host astrocytes (red) have invaded the graft, particularly at the margins. (B) Dense packed layer of progenitor cells (PCL) expressing PAX6 (red) and hNCAM (green). The hNCAM was more strongly expressed in cells at the inner, luminal surface of the PCL (B–D). hNCAM was strongly expressed in the more loosely packed layer of post‐mitotic neurons (NCL; B). (C) These neurons also co‐expressed DCX and hNCAM (yellow) and some co‐expressed CTIP2 (nuclear, red) with hNCAM (green) as shown in (D). Scale bars: (A–B) 200 μm; (C) 100 μm; (D) 50 μm.
The transplanted hNSCs expressing human stem cell markers were present in large numbers and had given rise to both neuroprogenitor cells that were PAX6+/hNCAM+ and post‐mitotic immature neurons which were DCX+/hNCAM+. Columns of tightly packed neuroprogenitor cells uniformly expressing PAX6 formed in rosette structures. These progenitor cells surrounded a lumen that resembled the neural tube or ventricle of a developing brain (Fig. 3). PAX6+ cells expressed hNCAM at the apical surface only (Fig. 3B), as previously described for 3D aggregates formed in vitro (Mariani et al. 2012). hNCAM is a cell‐adhesion molecule (Smith et al. 2017) and may form intercellular junctions holding the structure together. Its expression also indicates that these cells were of transplant origin. No other post‐mitotic markers for neurons or neuroblasts were expressed by these PAX6+ progenitors.
Around this ‘progenitor cell layer’ (PCL) existed a looser network of heterogeneously distributed cells that were positive for the human immature neuron marker hNCAM, indicating that these cells were of human origin (Fig. 3B). Such hNCAM+ cells co‐expressed DCX (Fig. 3C) and bTUB (not shown) confirming they were post‐mitotic neurons forming a ‘neural cell layer’ (NCL). Moreover, some cells in the NCL co‐expressed CTIP2 (Fig. 3D), suggesting some human cells were differentiating towards the phenotype of deep layer cortical neurons including corticofugal neurons (Arlotta et al. 2005; Ip et al. 2011). As was observed at 1 week post‐grafting, a few of the transplanted cells differentiated into astrocytes detected by anti‐STEM123 immunohistochemistry and were located in the NCL close to the PCL (not shown).
Next we examined some of the host cell phenotypes that infiltrated the graft and the cerebral organoid structures. IB4 histochemistry detects both blood vessel endothelial cells and reactive microglia. Host blood vessels were uniformly distributed through the organoids, including both the NCL and PCL (Fig. 4A). Reactive microglia were found around the graft but not inside it (Fig. 4A). Additionally, anti‐IBA1 immunostaining, which detected both quiescent and active host microglia, revealed a much higher density of IBA1‐expressing amoeboid microglia around and in the aECM adjacent to the organoids, whereas within the cerebral organoid there were fewer microglia and these were closer in morphology to the quiescent microglia in the adjacent host tissue (Fig. 4B). Similarly, a dense layer of host cells expressing the astrocyte marker GFAP but not human cell markers, were often observed nested at the boundary between the organoids and the host cortex. They infiltrated the aECM and extended their long processes toward the centre of the graft, with some appearing to have started to migrate toward the centre (Fig. 4C). It is noteworthy that host reactive astrocytes and immunopositive and activated microglia did not always completely surround all the graft boundaries. This suggests that the graft was not totally isolated from the host cortex and that trajectories existed for cells to exchange between the graft and host cortex.
Figure 4.
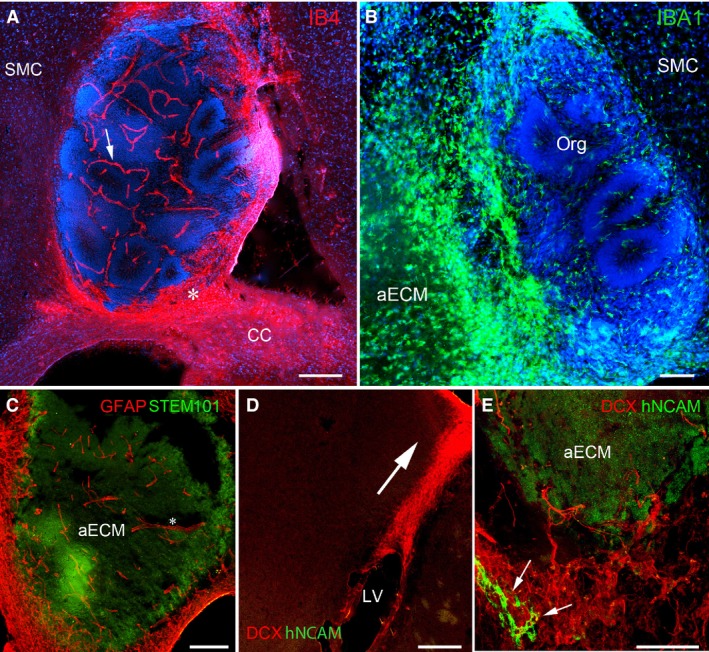
Four weeks post‐grafting. (A) Staining with isolectin B4 (IB4, red) to reveal blood vessels penetrated the organoid graft (arrow) and reactive microglia accumulated around the margins of the graft (e.g. asterisk) close to the corpus callosum (CC). (B) IBA 1 immunoreactivity (green) marking microglia. Reactive microglia accumulated in cell‐free parts of the artificial ECM (aECM), whereas microglia in the organoid (org) more closely resembled the quiescent microglia of the host sensorimotor cortex (SMC). (C) Host astrocytic processes (GFAP +, red, asterisk) penetrated the graft. (D) Host neuroblasts positive for DCX (red) migrated from around the lateral ventricle (LV) towards the graft (arrow). (E) Host DCX + neurons (red) mingled with graft‐derived hNCAM + cells (green). Nuclei are counterstained with DAPI (blue) in (A) and (B). Scale bars: (A,C,D) 200 μm; (B,E) 100 μm.
Finally, we examined some of the host cell phenotypes that infiltrated the aECM but not the cerebral organoid structures. Importantly, we found differences between grafts with and without hNSCs incorporated. Host DCX+/hNCAM– neuroblasts were located in the lateral adult SVZ of both hemispheres and along a migratory trajectory between the SVZ and the graft (Fig. 4D), including DCX+ cells apparently crossing the corpus callosum (not shown). The DCX+/hNCAM– cells surrounded the aECM, forming a dense network at the inferior boundary (Fig. 4E) not far from the adult SVZ. In the presence of grafted hNSCs, some of the host cells intermingled with hNCAM+/DCX+ neurons of graft origin; some had an elongated cell body and extended long processes that penetrated the aECM (Fig. 4E). For aECM‐only transplants, and in regions of hNSC/aECM grafts devoid of organoids, host DCX+ cells extending long processes were also seen along the inferior border of the graft but in smaller numbers (not shown). Generally, host cells were much more likely to enter the graft if transplanted cells were present, and blood vessels were only seem to form in the presence transplanted neural stem cells and their progeny.
Ten weeks post‐grafting
In eight animals examined at 10 weeks post‐grafting, cerebral organoids were no longer observed. At some graft sites the aECM appeared to have disappeared, but at other sites it was still possible to observe hNCAM+ cells within the remaining aECM (Fig. 5A) occasionally along with some associated anti‐synaptophysin immunoreactivity (not shown). DCX+/hNCAM– (Fig. 5A) and PAX6+/hNCAM– host cells still appeared to be migrating from the SVZ to the graft site. Few STEM123+ astrocytes of human origin remained at this stage compared with 1 and 4 weeks post‐grafting. Furthermore, reactive microglia immunopositive for IBA1 surrounded and infiltrated the graft (Fig. 5B).
Figure 5.
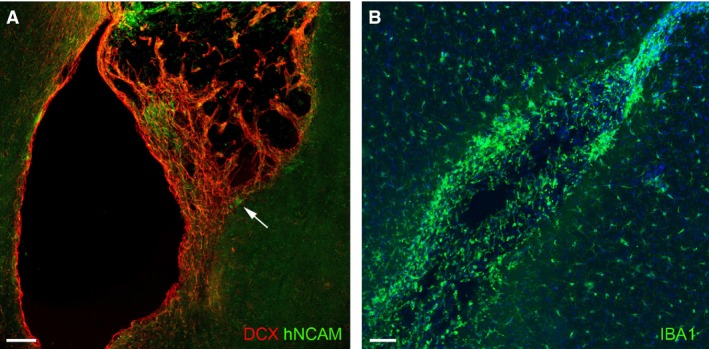
Ten weeks post‐grafting. (A) A lesion cavity no longer filled with aECM. What does remain is infiltrated with DCX + host neuroblasts (red) mingled with hNCAM + transplanted cells, some of which are at the interface with the host tissue (arrow). (B) Reactive microglia positive for IBA1 (green) accumulated around the remants of the graft. Scale bars: 100 μm.
Discussion
This study has demonstrated that human neural stem cells dispersed, suspended and differentiated in a three‐dimensional gel matrix behave quite differently when the gel suspension is grafted into the injured sensorimotor cortex of a rat pup. In vitro the cells remain dispersed (but with some aggregation) but they mostly lose their stem cell identity and differentiate into immature neurons with extended processes. In vivo, over 4 weeks the cells progressively aggregate and self‐organise into cerebral organoids first described in vitro when cells were grown in specialised rotating incubators. These organoids contain a mixture of progenitor cells and post‐mitotic neuroblasts arranged in separate compartments. The greater complexity of these structures 4 weeks compared with 1 week post‐grafting, and the continued presence of PAX6+ progenitors in large numbers, suggests considerable proliferation of the transplanted cells.
In vitro experiment
These experiments confirmed the ability of the hNSCs to survive and differentiate in aECM before proceeding to the grafting experiments. The cells grew well in vitro in this medium, surviving for at least 43 days, the limit of our experiment, and undergoing differentiation with expression of early neuroblast markers such as DCX being replaced with markers of more mature neurons (MAP2). This is agreement with previous studies which suggested that a 3D hydrogel makes a good environment for culturing differentiating neurons (Zhong et al. 2010; Liang et al. 2013). As early as 10 days in culture we observed immature neurons with processes extending 50 μm in length, and by 14 days these processes had extended to over 200 μm, the cells expressed both bTUB and MAP2, and synaptophysin immunoreactivity was observed at what appeared to be discrete points of contact between cells, as it wasn't observed in spaces where cells did not aggregate. These findings support previous observations that a 3D gel scaffold promotes complex neurite outgrowth with synapse formation and upregulation of expression of bTUB and MAP2 (Lam et al. 2014; Carlson et al. 2016; Smith et al. 2017). Conventional 2D culture of hNSC results in around a quarter of surviving cells retaining PAX6 expression after 1 week, indicating they had remained as progenitor cells (Alzu'bi et al. 2017). Here we observed very few PAX6+ cells at 10–14 days in culture and they mostly co‐expressed neuronal markers, suggesting they were transitioning to neuroblasts. Very few cells expressed GFAP immunoreactivity, a marker for older radial glia and astrocytes, supporting previous observations that hydrogel promotes a neuronal fate (Seidlits et al. 2010), unlike other ECM preparations, for instance Matrigel, which promote astrocyte formation (Thonhoff et al. 2008).
In vivo experiment: cerebral organoid formation
The most striking result of our in vivo experiment was the gradual formation, over 1 month, of structures resembling cerebral organoids which have previously been grown under specialised culture conditions from neural stem cells. The defining feature of such organoids is the arrangement of several layers of neural progenitor cells around a central lumen in a rosette formation, mimicking the neural tube of the developing brain, surrounded in turn by a layer of more loosely arranged post‐mitotic neurons (Mariani et al. 2012; Shi et al. 2012; Lancaster et al. 2013; Mason & Price, 2016). The neural tube begins as a single layer of proliferating columnar neuroepithelial cells that eventually gives rise to the entire nervous system. These progenitors first divide symmetrically, generating radial glial cells that later divide asymmetrically and produce daughter progenitors (McConnell, 1995). In human, PAX6 is expressed at the earliest stages of neuroectoderm development and is required for induction of neural identity (Zhang et al. 2010). As development proceeds, PAX6 identifies radial glia that gives rise to dorsal forebrain (Campbell, 2003) and by around 8 post‐conceptional weeks (PCW), the wall of the human telencephalon is characterised by a ventricular zone packed with PAX6+ progenitor radial glia about 10 cells deep (Bayatti et al. 2008; Harkin et al. 2016). This bears a close resemblance in size and gene expression to the progenitor cell layer (PCL) we identified in our graft organoids and was also seen in cerebral organoids grown in vitro (Mariani et al. 2012).
The neural cell layer in our organoid grafts resembled the subventricular (SVZ) and intermediate zone in the developing human neocortex after 8 PCW (Bayatti et al. 2008) in that the cells formed a looser network and many extending processes, and expressed proteins characteristic of migrating neuroblasts and immature neurons (DCX, bTUB and MAP2). Dense, cellular packing characteristic of the cortical plate in the early developing human cortex (Bayatti et al. 2008) was not observed, nor were PAX6+ progenitor cells present, as are found in the human SVZ in particular (Bayatti et al. 2008). Cultured cerebral organoids have been reported to have PAX6+ neuroprogenitors more loosely associated with the PCL but not fully integrated into the NCL (Shi et al. 2012). We also found expression of CTIP2 in neurons of the NCL. This transcription factor is expressed by human deep layer cortical neurons soon after their neurogenesis (Ip et al. 2011). This demonstrated that the grafts have the capacity to produce corticospinal neurons (Arlotta et al. 2005). For cerebral organoids in vitro, after 100 days in culture, it has been reported that progenitor cells become depleted and an outer cortical plate‐like layer containing CTIP2+ neurons expands (Qian et al. 2016). Our grafted cells did not survive long enough for this stage to be reached. Very few grafted cells expressed GFAP, consistent with the observation that neurons are produced before glial cells during corticogenesis (Qian et al. 2000).
Vascularisation of the organoids
One of the most striking features of the grafts was the development of blood vessels visualised by staining with IB4. This is known to bind to endothelial cells and mark newly formed blood vessels in developing or injured central nervous system tissue in particular (Hughes & Chang‐Ling, 2000; Vinores et al. 2003; Benton et al. 2008). Such blood vessels were not observed in aECM‐only grafts, suggesting that the presence of cells was a stimulus to their growth. That these blood vessels did not express human stem cell markers suggests that the endothelial cells were of host origin. Similarly, host astrocytes, which may participate in angiogenesis (Fruttiger, 2002; Scott et al. 2010) were seen to extend processes into the graft. This angiogenesis could have happened due to a limited development of scar tissue at the infarction site because of the use of hydrogel. Some studies using hydrogels, including the one here, as a vehicle for in vivo transplantation have not stimulated angiogenesis (Zhong et al. 2010; Bible et al. 2012); however, other researchers have reported that hydrogels enhanced vascularisation of the graft site (Ju et al. 2014), particularly if they are loaded with cytokines (Peattie et al. 2004). A study has reported that grafted stem cells secrete a vascular endothelial growth factor (VEGF) that stimulates vessel formation (Horie et al. 2011). Neural progenitors of the rodent adult hippocampus also secrete large, biologically relevant quantities of VEGF (Kirby et al. 2015).
Therefore, in our experiments it may be that the presence of neural stem cells stimulated vascularisation of the graft. In vitro, a spinning culture vessel is required to promote organoid survival by forcing the oxygen and the fluid to reach the centre of the organoids physically (Lancaster et al. 2013; Qian et al. 2016). However, our in vivo experiment it appears that the cerebral organoids overcame the limitation in oxygen and nutrient supply by permitting ingrowth of vessels from the host tissue. In our experiments, angiogenesis may have provided a natural alternative to the rotating vessels for the in vitro organoids to keep them nourished. Furthermore, in vitro, in human cerebral organoids it takes 4 weeks for neurons and 10 weeks for cortex‐like structures to appear (Lancaster et al. 2013; Mason & Price, 2016). In our grafted organoids, it appears that this time course is accelerated such that neurons appear within a week and cortex‐like structures within 4 weeks. Thus it appears that the host in some way provides an extra stimulus to the hNSCs to self‐organise and begin asymmetrical division to produce neurons compared with in vitro organoid cultures. Again, perhaps this stimulus comes from vascularisation.
Inflammatory response to grafting
Other host cell types interacting with the graft were microglia. Reactive microglia accumulated at the boundary of the graft and invaded aECM only grafts as well as gel only parts of cellular grafts. However, only quiescent host microglia were found intermingled with the organoid structures after 4 weeks post‐grafting. It has been suggested that encapsulating neural stem cells in hydrogel protects them against an inflammatory reaction when grafted to the adult rodent brain (Zhong et al. 2010). Our observations suggest that it is the grafted cells themselves that have anti‐inflammatory properties rather than the aECM, at least during the first 4 weeks.
The survival rate of the transplanted cells was similar to a previous study in rodent neonate lesioned brain in which grafted human cells (with no aECM) survived for 5 weeks before decreasing (Glezer et al. 2007; Jablonska et al. 2010; Kulbatski, 2010). We did not use immunosuppression, as studies have argued that this can prevent neural repair or lead to deterioration in the underlying disorder and prevent the beneficial role of microglia in the repair of the lesioned brain tissue (Glezer et al. 2007; Kulbatski, 2010). However, the vascularisation of our grafts may have exposed them to a more vigorous immune reaction with time and contributed to the degradation of the grafts. Another factor in the breakdown of the grafts is the fast degradability of the thiolated hydrogel used in our aECM (Hahn et al. 2007; Prestwich et al. 2012) with the transplanted cells themselves, or the host infiltrating cells, potentially contributing to hydrogel dissolution. Moshayedi et al. (2016) reported that grafted hydrogel degraded faster when mixed with neural stem cells than when grafted alone in vivo.
Endogenous repair in response to the graft
In our study host neuroblasts and neural progenitors were located in the SVZ of the lateral ventricles in both hemispheres and along a migratory pathway between the SVZ and the xenograft in the ipsilateral hemisphere at all three time points. It has been previously shown that after a lesion to the rodent sensorimotor cortex, neurogenesis took place in the SVZ and there was migration of new cells to the lesion site guided by blood vessels and reactive astrocytes (Saha et al. 2013), although most of these cells eventually differentiate into glia rather than neurons. In our study, the host DCX+ neurons were being produced, migrating and surviving up to 10 weeks following the lesion/graft. Four weeks post‐grafting, we found that the host neural progenitors and immature neurons surrounded the graft and intermingled with the human grafted neurons at the periphery, but not the centre of, the cerebral organoids. By 10 PCW, host neuroblasts and grafted human neurons were still intermingled at the margins of the graft site. In a similar study that transplanted hydrogel encapsulated NSCs in focal ischaemic stroke model, the host neuroblasts migrated to the graft 2 weeks after transplantation regardless of whether the grafted cells had been injected with or without hydrogel (Zhong et al. 2010). Our experiments suggest that the presence of grafted cells increases the migration of host neuroblasts to the graft site, and in particular, the invasion of aECM by host neuroblasts.
Conclusion
By suspending transplanted cells in an aECM prior to grafting, the intention was to provide a protective environment that would stimulate the neural stem cells to stop dividing and differentiate, producing cortical neurons that could grow axons out of the graft site, or even migrate out of the graft site, and also receive connections from the host tissue. Surprisingly, instead, this environment permitted self‐organisation of the grafted cells into structures resembling cerebral organoids observed in culture, including many neural progenitor cells that retained a proliferative state in an artificial ‘ventricular zone’. Neurons were also produced and showed some limited evidence of integration with host circuitry over the time period examined.
The signal to undergo this transformation could be intrinsic or from the host. Combination of hNSCs with aECM alone is not enough, as we did not observe this in three‐dimensional in vitro cultures. It seems that the medium‐term survival of the organoids is dependent invasion of the graft by host cells, in particular blood vessels and astrocytes, but the extent to which host‐derived morphogens and growth factors drive this process remains to be explored. Some adjustments to the grafting procedure will be required to achieve the original aims of the experiment. Extended immunosuppression, along with neurostatin treatment to suppress microglial activation (Gomez‐Nicola et al. 2010), may help maintain the organoids until they reach the point where neuroproliferation declines and larger numbers of neurons are produced. Alternatively, other approaches, for instance using a cocktail of recently discovered small molecules that halt proliferation and induce cortical neuronal differentiation (Qi et al. 2017) might be employed.
Conflict of interest
The authors have no conflicts of interest to declare.
Acknowledgements
Reem Basuodan was in receipt of a studentship from Princess Nourah Bint Abdulrahman University and the Ministry of Education, Saudi Arabia. We are grateful to Dr Beth Stoll for providing advice and equipment for culturing stem cells, and to the staff of the Newcastle Comparative Biology Centre for their expert assistance and care of the experimental animals. Anna Basu is funded by a Career Development Fellowship award from the National Institute for Health Research. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health.
References
- Alzu'bi A, Lindsay SJ, Harkin LF, et al. (2017) The transcription factors COUP‐TFI and COUP‐TFII have distinct roles in arealisation and GABAergic interneuron specification in the early human fetal telencephalon. Cereb Cortex 27, 4971–4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arlotta P, Molyneaux BJ, Chen J, et al. (2005) Neuronal subtype‐specific genes that control corticospinal motor neuron development in vivo . Neuron 45, 207–221. [DOI] [PubMed] [Google Scholar]
- Baeten KM, Akassoglou K (2011) Extracellular matrix and matrix receptors in blood–brain barrier formation and stroke. Dev Neurobiol 71, 1018–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakshi A, Keck CA, Koshkin VS, et al. (2005) Caspase‐mediated cell death predominates following engraftment of neural progenitor cells into traumatically injured rat brain. Brain Res 1065, 8–19. [DOI] [PubMed] [Google Scholar]
- Basu AP (2014) Early intervention after perinatal stroke: opportunities and challenges. Dev Med Child Neurol 56, 516–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu AP, Clowry G (2015) Improving outcomes in cerebral palsy with early intervention: new translational approaches. Front Neurol 6, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayatti N, Moss JA, Sun L, et al. (2008) A molecular neuroanatomical study of the developing human neocortex from 8 to 17 postconceptional weeks revealing the early differentiation of the subplate and subventricular zone. Cereb Cortex 18, 1536–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton RL, Maddie MA, Minnillo DR, et al. (2008) Griffonia simplicifolia isolectin B4 identifies a specific subpopulation of angiogenic blood vessels following contusive spinal cord injury in the adult mouse. J Comp Neurol 507, 1031–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bible E, Dell'Acqua F, Solanky B, et al. (2012) Non‐invasive imaging of transplanted human neural stem cells and ECM scaffold remodeling in the stroke‐damaged rat brain by (19)F‐ and diffusion‐MRI. Biomaterials 33, 2858–2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss T, Guzman R, Daadi M, et al. (2007) Cell transplantation therapy for stroke. Stroke 38, 817–826. [DOI] [PubMed] [Google Scholar]
- Burdick JA, Prestwich GD (2011) Hyaluronic acid hydrogels for biomedical applications. Adv Mater 23, H41–H56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burtrum D, Silverstein FS (1994) Hypoxic‐ischaemic brain injury stimulates glial fibrillary acidic protein mRNA and protein expression in neonatal rats. Exp Neurol 126, 112–118. [DOI] [PubMed] [Google Scholar]
- Bużańska L, Machaj EK, Zabłocka B, et al. (2002) Human cord blood‐derived cells attain neuronal and glial features in vitro . J Cell Sci 115, 2131–2138. [DOI] [PubMed] [Google Scholar]
- Campbell K (2003) Dorsal‐ventral patterning in the mammalian telencephalon. Curr Opin Neurobiol 13, 50–56. [DOI] [PubMed] [Google Scholar]
- Carlson AL, Bennett NK, Francis NL, et al. (2016) Generation and transplantation of reprogrammed human neurons in the brain using 3D microtopographic scaffolds. Nat Commun 7, 10862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clowry GJ (2007) The dependence of spinal cord development on corticospinal input and its significance in understanding and treating spastic cerebral palsy. Neurosci Biobehav Rev 31, 1114–1124. [DOI] [PubMed] [Google Scholar]
- Clowry GJ, Basuodan R, Chan F (2014) What are the best animal models for testing early Intervention in cerebral palsy? Front Neurol 5, 258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coenen M, Kogler G, Wernet P, et al. (2005) Transplantation of human umbilical cord blood‐derived adherent progenitors into the developing rodent brain. J Neuropathol Exp Neurol 64, 681–688. [DOI] [PubMed] [Google Scholar]
- Comi AM, Cho E, Mulholland JD, et al. (2008) Neural stem cells reduce brain injury after unilateral carotid ligation. Pediatr Neurol 38, 86–92. [DOI] [PubMed] [Google Scholar]
- Englund U, Fricker‐Gates RA, Lundberg C, et al. (2002) Transplantation of human neural progenitor cells into the neonatal rat brain: extensive migration and differentiation with long‐distance axonal projections. Exp Neurol 173, 1–21. [DOI] [PubMed] [Google Scholar]
- Eyre JA (2007) Corticospinal tract development and its plasticity after perinatal injury. Neurosci Biobehav Rev 31, 1136–1149. [DOI] [PubMed] [Google Scholar]
- Fallah Z, Clowry GJ (1999) Gephyrin‐like immunoreactivity is a marker for growing axons in the central nervous system of the immature rat. Dev Neurosci 21, 50–57. [DOI] [PubMed] [Google Scholar]
- Fruttiger M (2002) Development of the mouse retinal vasculature: angiogenesis versus vasculogenesis. Invest Ophthalmol Vis Sci 43, 522–527. [PubMed] [Google Scholar]
- Genade T, Lang DM (2011) Antibody markers for studying neurodegeneration in the Nothobranchius central nervous system. J Cytol Histol 2, 120. [Google Scholar]
- Glezer I, Simard AR, Rivest S (2007) Neuroprotective role of the innate immune system by microglia. Neuroscience 147, 867–883. [DOI] [PubMed] [Google Scholar]
- Gomez‐Nicola D, Valle‐Argos B, Nieto‐Sampedro M (2010) Blockade of IL‐15 activity inhibits microglial activation through the NFκB, p38, and ERK1/2 pathways, reducing cytokine and chemokine release. Glia 58, 264–276. [DOI] [PubMed] [Google Scholar]
- Gorgels TG (1990) A quantitative analysis of axon outgrowth, axon loss, and myelination in the rat pyramidal tract. Brain Res Dev Brain Res 54, 51–61. [DOI] [PubMed] [Google Scholar]
- Guzman R, Uchida N, Bliss TM, et al. (2007) Long‐term monitoring of transplanted human neural stem cells in developmental and pathological contexts with MRI. Proc Natl Acad Sci U S A 104, 10211–10216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg H, Peebles D, Mallard C (2002) Models of white matter injury: comparison of infectious, hypoxic‐ischemic, and excitotoxic insults. Ment Retard Dev Disabil Res Rev 8, 30–38. [DOI] [PubMed] [Google Scholar]
- Hahn SK, Park JK, Tomimatsu T, et al. (2007) Synthesis and degradation test of hyaluronic acid hydrogels. Int J Biol Macromol 40, 374–380. [DOI] [PubMed] [Google Scholar]
- Harkin LF, Gerrelli D, Gold Diaz DC, et al. (2016) Distinct expression patterns for type II topoisomerases IIA and IIB in the early foetal human telencephalon. J Anat 228, 452–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie N, Pereira MP, Niizuma K, et al. (2011) Transplanted stem cell‐secreted vascular endothelial growth factor effects poststroke recovery, inflammation, and vascular repair. Stem Cells 29, 274–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes S, Chang‐Ling T (2000) Roles of endothelial cell migration and apoptosis in vascular remodeling during development of the central nervous system. Microcirculation 7, 317–333. [PubMed] [Google Scholar]
- Ip BK, Bayatti N, Howard NJ, et al. (2011) The corticofugal neuron‐associated genes ROBO1, SRGAP1, and CTIP2 exhibit an anterior to posterior gradient of expression in early fetal human neocortex development. Cereb Cortex 21, 1395–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito D, Imai Y, Ohsawa K, et al. (1998) Microglia‐specific localisation of a novel calcium binding protein, Iba1. Brain Res Mol Brain Res 57, 1–9. [DOI] [PubMed] [Google Scholar]
- Jablonska A, Kozlowska H, Markiewicz I, et al. (2010) Transplantation of neural stem cells derived from human cord blood to the brain of adult and neonatal rats. Acta Neurobiol Exp (Wars) 70, 337–350. [DOI] [PubMed] [Google Scholar]
- Jendelová P, Kubinová Š, Sandvig I, et al. (2016) Current developments in cell‐ and biomaterial‐based approaches for stroke repair. Expert Opin Biol Ther 16, 43–56. [DOI] [PubMed] [Google Scholar]
- Ju R, Wen Y, Gou R, et al. (2014) The experimental therapy on brain ischemia by improvement of local angiogenesis with tissue engineering in the mouse. Cell Transplant 23(Suppl 1), S83–S95. [DOI] [PubMed] [Google Scholar]
- Kiasatdolatabadi A, Lotfibakhshaiesh N, Yazdankhah M, et al. (2017) The role of stem cells in the treatment of cerebral palsy: a review. Mol Neurobiol 54, 4963–4972. [DOI] [PubMed] [Google Scholar]
- Kirby ED, Kuwahara AA, Messer RL, et al. (2015) Adult hippocampal neural stem and progenitor cells regulate the neurogenic niche by secreting VEGF. Proc Natl Acad Sci U S A 112, 4128–4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirton A, Armstrong‐Wells J, Chang T, et al. (2011) Symptomatic neonatal arterial ischemic stroke: the International Pediatric Stroke Study. Pediatrics 128, e1402–e1410. [DOI] [PubMed] [Google Scholar]
- Kolb B, Gibb R (2007) Brain plasticity and recovery from early cortical injury. Dev Psychobiol 49, 107–118. [DOI] [PubMed] [Google Scholar]
- Kulbatski I (2010) Stem/precursor cell‐based CNS therapy: the importance of circumventing immune suppression by transplanting autologous cells. Stem Cell Rev 6, 405–410. [DOI] [PubMed] [Google Scholar]
- Lam J, Lowry WE, Carmichael ST, et al. (2014) Delivery of iPS‐NPCs to the stroke cavity within a hyaluronic acid matrix promotes the differentiation of transplanted cells. Adv Funct Mater 24, 7053–7062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster MA, Renner M, Martin C‐A, et al. (2013) Cerebral organoids model human brain development and microcephaly. Nature 501, 373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Walczak P, Bulte JW (2013) The survival of engrafted neural stem cells within hyaluronic acid hydrogels. Biomaterials 34, 5521–5529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani J, Simonini MV, Palejev D, et al. (2012) Modeling human cortical development in vitro using induced pluripotent stem cells. Proc Natl Acad Sci U S A 109, 12770–12775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason JO, Price DJ (2016) Building brains in a dish: prospects for growing cerebral organoids from stem cells. Neuroscience 334, 105–118. [DOI] [PubMed] [Google Scholar]
- McConnell SK (1995) Constructing the cerebral cortex: neurogenesis and fate determination. Neuron 15, 761–768. [DOI] [PubMed] [Google Scholar]
- Moyashedi P, Nih LR, Llorente IL, et al. (2016) Systematic optimisation of engineered hydrogel allows for selective control of human neural stem cell survival and differentiation after transplantation in the stroke brain. Biomaterials 105, 145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C (1998) A Stereotaxic Atlas of the Rat Brain. New York: Academic Press. [Google Scholar]
- Peattie RA, Nayate AP, Firpo MA, et al. (2004) Stimulation of in vivo angiogenesis by cytokine‐loaded hyaluronic acid hydrogel implants. Biomaterials 25, 2789–2798. [DOI] [PubMed] [Google Scholar]
- Prestwich GD, Erickson IE, Zarembinski TI, et al. (2012) The translational imperative: making cell therapy simple and effective. Acta Biomater 8, 4200–4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Y, Zhang XJ, Renier N, et al. (2017) Combined small‐molecule inhibition accelerates the derivation of functional cortical neurons from human pluripotent stem cells. Nat Biotechnol 35, 154–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X, Shen Q, Goderie SK, et al. (2000) Timing of CNS cell generation: a programmed sequence of neuron and glial cell production from isolated murine cortical stem cells. Neuron 28, 69–80. [DOI] [PubMed] [Google Scholar]
- Qian X, Nguyen HN, Song MM, et al. (2016) Brain‐region‐specific organoids using mini‐bioreactors for modeling ZIKV exposure. Cell 165, 1238–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha B, Peron S, Murray K, et al. (2013) Cortical lesion stimulates adult subventricular zone neural progenitor cell proliferation and migration to the site of injury. Stem Cell Res 11, 965–977. [DOI] [PubMed] [Google Scholar]
- Sareen D, Gowing G, Sahabian A, et al. (2014) Human neural progenitor cells generated from induced pluripotent stem cells can survive, migrate, and integrate in the rodent spinal cord. J Comp Neurol 522, 2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott A, Powner MB, Gandhi P, et al. (2010) Astrocyte‐derived vascular endothelial growth factor stabilizes vessels in the developing retinal vasculature. PLoS ONE 5, e11863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidlits SK, Khaing ZZ, Petersen RR, et al. (2010) The effects of hyaluronic acid hydrogels with tunable mechanical properties on neural progenitor cell differentiation. Biomaterials 31, 3930–3940. [DOI] [PubMed] [Google Scholar]
- Shi Y, Kirwan P, Livesey FJ (2012) Directed differentiation of human pluripotent stem cells to cerebral cortex neurons and neural networks. Nat Protoc 7, 1836–1846. [DOI] [PubMed] [Google Scholar]
- Smith I, Silveirinha V, Stein JL, et al. (2017) Human neural stem cell‐derived cultures in three‐dimensional substrates form spontaneously functional neuronal networks. J Tissue Eng Regen Med 11, 1022–1033. [DOI] [PubMed] [Google Scholar]
- Soleman S, Yip P, Leasure JL, et al. (2010) Sustained sensorimotor impairments after endothelin‐1 induced focal cerebral ischemia (stroke) in aged rats. Exp Neurol 222, 13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevanato L, Hicks C, Sinden JD (2015) Differentiation of a human neural stem cell line on three dimensional cultures, analysis of microRNA and putative target genes. J Vis Exp 12, 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thonhoff JR, Lou DI, Jordan PM, et al. (2008) Compatibility of human fetal neural stem cells with hydrogel biomaterials in vitro . Brain Res 1187, 42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornero D, Wattananit S, Grønning Madsen M, et al. (2013) Human induced pluripotent stem cell‐derived cortical neurons integrate in stroke‐injured cortex and improve functional recovery. Brain 136, 3561–3577. [DOI] [PubMed] [Google Scholar]
- Tucker AM, Aquilina K, Chakkarapani E, et al. (2009) Development of amplitude‐integrated electroencephalography and interburst interval in the rat. Pediatr Res 65, 62–66. [DOI] [PubMed] [Google Scholar]
- Vinores SA, Seo MS, Derevjanik NL, et al. (2003) Photoreceptor‐specific overexpression of platelet‐derived growth factor induces proliferation of endothelial cells, pericytes, and glial cells and aberrant vascular development: an ultrastructural and immunocytochemical study. Brain Res Dev Brain Res 140, 169–183. [DOI] [PubMed] [Google Scholar]
- Wang J, Yang W, Xie H, et al. (2014) Ischemic stroke and repair: current trends in research and tissue engineering treatments. Regen Med Res 2, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Huang CT, Chen J, et al. (2010) Pax6 is a human neuroectoderm cell fate determinant. Cell Stem Cell 7, 90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J, Chan A, Morad L, et al. (2010) Hydrogel matrix to support stem cell survival after brain transplantation in stroke. Neurorehabil Neural Repair 24, 636–644. [DOI] [PMC free article] [PubMed] [Google Scholar]


