Abstract
The infrapatellar pad, a fibro‐adipose tissue with peculiar microscopic and mechanical features, is gaining wide attention in the field of rheumatological research. The purpose of this descriptive review is to summarize the most recent published evidence on the anatomic, physiologic and biomechanical inter‐relationship between the infrapatellar fat pad and the knee synovial membrane. As an extrasynovial tissue, the infrapatellar fat pad does not directly interact with the articular cartilage; based on its location in close contact with the synovial membrane, and due to the metabolic properties of adipose tissue, it may influence the behavior of the synovial membrane. In fact, considering evidence of macroscopic and microscopic anatomy, the infrapatellar fat pad is the site of insertion of the infrapatellar and medial synovial plicae. Also biochemically, there is much evidence highlighting the interaction among these two structures; in the case of inflammation, the mutual interplay is ascribable to the release of pro‐inflammatory mediators stimulating the proliferation of inflammatory cells and promoting tissue modifications in both. All these assumptions could support the emerging idea that the infrapatellar fat pad and the synovial membrane may be considered a morpho‐functional unit.
Keywords: infrapatellar fat pad, knee plicae, osteoarthritis, synovial membrane
Introduction
According to a study published in 2010 evaluating the global burden of disease, hip and knee osteoarthritis (OA) ranked the 11th highest in terms of years lived with disability. Likely, wear and tear of the articular cartilage can greatly influence the quality of life during aging, resulting in the onset of disorders of joints leading to OA (Tiku & Madhan, 2016). Despite the increased prevalence of OA, its pathogenesis is not well understood. To date, there is increasing evidence that synovitis plays an important etiopathogenical role in OA and that inflammation of the synovial membrane seems to be a precursor rather than just a consequence of joint failure (Atukorala et al. 2014). The close topography of the infrapatellar fat pad (IFP) to the synovial membrane has led the Researchers to investigate the possible role of IFP in OA (Eymard et al. 2014). In fact, considering the intra‐articular location of the IFP, the metabolic properties of adipose tissue and the fact that the OA involves all articular tissues, it is likely that the IFP could also be involved in OA of the knee (Clockaerts et al. 2010). Peculiar microscopic changes in OA IFP, such as thickening of interlobular septa, an increase in vascularization and the presence of inflammatory infiltrates, have been described (Favero et al. 2017; Macchi et al. 2017). Importantly, these changes have also been observed in the IFP adjacent synovial membrane, suggesting a possible cross‐talk between these two structures (Eymard et al. 2014), which may support a possible role of IFP in disease progression (Wenham & Conaghan, 2010; Sime et al. 2017). The release of proinflammatory mediators from the synovial membrane, e.g. interleukin (IL)‐1, IL‐6, tumor necrosis factor (TNF)‐α, nitric oxide, neuropeptides and prostaglandins, might in turn promote cartilage degeneration but also cross‐influence the IFP, which contains cells such as immune cells, inflammatory cells and substance P nerve cells, which may play a role in the disease process and could contribute to the development of early OA (Madry et al. 2012).
A complete understanding of the anatomical, biochemical and functional interaction between IFP and synovial membrane may be useful to define the treatment target, for both symptoms and potential structure modification, in order to achieve an optimal therapeutic response in OA.
The aim of this paper is the revision of the literature concerning the macroscopic, microscopic and molecular characteristics of the IFP and synovial membrane. Moreover, evidence of macroscopic features, imaging data, investigations in histopathology and molecular studies supporting the existence of a cross‐talk between the IFP and the synovial membrane will be highlighted. This will suggest a new anatomic‐functional interpretation of this interaction, potentially involved in an active way in the onset of articular diseases.
Methods
To describe the cross‐talk between the IFP and synovial membrane and avoid missing studies, broad search terms were used with no temporal limits or language restrictions; reviews and original articles on the anatomic (gross anatomy, microscopic anatomy), biochemical and functional role of the IFP were considered. In September 2017, we performed a computer‐aided systematic review of the literature by searching PubMed and Scopus for English‐language publications. The infrapatellar fat pad and synovial membrane were considered key search words. More specifically, multiple unrestricted ‘free‐text’ searches were also performed combining the terms [(‘infrapatellar fat pad’ OR ‘IFP’) AND ‘synovial membrane’] and [(‘infrapatellar fat pad’ OR ‘IFP’) AND ‘histology’]. Other references were retrieved from the bibliographies of the articles selected from the search of the databases. In parallel, we refer to our scientific background and most recent experimental evidence (both published and unpublished data).
Results
The combined search in PubMed and Scopus databases retrieved the following results: respectively, 67 and 238 articles for ‘infrapatellar fat pad AND synovial membrane’, 8 and 22 articles for ‘IFP AND synovial membrane’, 276 and 393 papers for ‘infrapatellar fat pad AND histology’, 327 and 619 papers for ‘IFP AND histology’. After removing the duplicates and excluding the non‐relevant records we selected 63 papers. Ten more publications retrieved from the references of the previous article.
New insights into the aetiology of OA
The OA is an important cause of disability, especially in elderly people, and it is expected to be influenced by the population aging process more than other musculoskeletal disorders are (Moradi‐Lakeh et al. 2017).
Even though the aetiology of OA is still not completely understood, imaging techniques have changed the old concept of OA (Mathiessen & Conaghan, 2017). Traditionally, it was primarily considered a disease of hyaline cartilage with associated bone involvement, caused by overload or overuse; conversely, the pathophysiology of OA development is now appreciated to be more complex. In fact, it is not a single disorder but should rather be regarded as a common final stage of joint failure (Martel‐Pelletier & Pelletier, 2010; Mathiessen & Conaghan, 2017). Moreover, it does not only primarily affect the articular cartilage but also involves the entire joint, including the subchondral bone, ligaments, capsule, synovial membrane and periarticular muscles (Madry et al. 2012); for this reason, it should be thought as a multi‐tissue pathology.
Considering the role of the synovial membrane in the development of OA, it is believed that products of cartilage breakdown are phagocytosed by synovial cells, resulting in an inflamed synovium, which then produces proinflammatory mediators (Sellam & Berenbaum, 2010). This is responsible for a further release of proteolytic enzymes that determine the breakdown of cartilage. Thus, inflammation within the OA joint is at least in part the result of a cyclical interaction between damaged cartilage and inflamed synovial membrane (Wenham & Conaghan, 2013).
IFP
Macroscopic and microscopic anatomy of normal IFP
Within the knee joint, several fat pads can be recognized. Each of them is located between the joint capsule and the synovial membrane and, therefore, they are considered as intracapsular and extrasynovial structures (Draghi et al. 2016). The suprapatellar fat pad is composed of the quadriceps fat pad and the pre‐femoral fat pad, which are located above the patella and behind the suprapatellar bursa, respectively. The posterior fat pad is in close contact with the posterior articular capsule behind the menisci (Gallagher et al. 2005). In recent years, the fat pad which has received much attention is the IFP, also known as Hoffa's fat pad (Hoffa, 1903).
Anatomically, the IFP is related to the inferior pole of the patella superiorly, the patellar tendon anteriorly, the anterior tibia and the anterior horns of the menisci postero inferiorly, and the femoral condyles and intercondylar notch posteriorly (Gallagher et al. 2005; Fig. 1Aa). The IFP is composed of lobules of white fibrous adipose tissue (Macchi et al. 2016); typically, this kind of adipose tissue, characterized by a significant prevalence of collagenic stroma, is characteristic of areas subjected to a considerable mechanical stress (Sbarbati et al. 2010; Fig. 1Ab–c). The IFP consists of a central body with medial and lateral extension along with the superior tag (Mace et al. 2016). The ligamentum mucosum is located inferiorly to the central body of the IFP. In the 75% of cases, two recesses are present in the IFP, one horizontal cleft opening posteriorly and one vertical cleft opening superiorly just below the pole of the patella (Gallagher et al. 2005).
Figure 1.
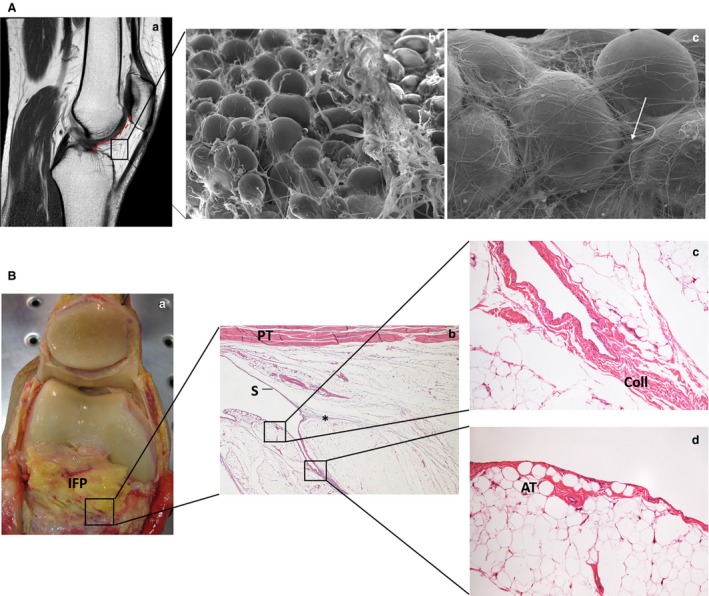
Microscopic characterization of infrapatellar fat pad (IFP) and anatomic evidence of the interplay with synovial membrane. (Aa) Sagittal section of the knee in magnetic resonance imaging showing the localization of the IFP and highlighting the relation with the synovial membrane (red dotted line). (Ab,c) The ultrastructural appearance of the IFP (b), in which the presence of clusters of spherically shaped adipocytes is clearly recognizable. A network of collagen fibres among the adipocytes is visible [c, white arrow; scanning electron micrographs, magnifications: 250× (b); 700× (c)]. (B) Dissection of the knee in partial flexion (with the patella reflected superiorly) showing close relation between the synovial membrane and the deep surface of the IFP (a). The microscopic images show the course of the synovial membrane (S) over the IFP with the presence of a cleft (b); the connection between an interlobular septum and the synovial membrane is evident (*). The subsynovial layer is characterized by collagen (Coll) (c) or adipose tissue (AT) (d). Patellar tendon (PT). Haematoxylin‐eosin, magnifications: 1.25× (b); 10× (c, d). Human tissues were sampled from cadavers managed by the Body Donation Programme of the Section of Human Anatomy, University of Padua (De Caro et al. 2009).
In terms of vascularization, the IFP shows an arterial network supplied by the superior and inferior genicular arteries, connected by three horizontal anastomoses. There is an irregular connection to the medial genicular artery running in the infrapatellar plica (Kohn et al. 1995; Hempfing et al. 2007; Hughes et al. 1998). Moreover, there are many anastomoses with the vessels of menisci and patellar tendon anteriorly and to the tibial periosteum inferiorly (Mace et al. 2016).
The innervation of the IFP predominantly derives from the posterior tibial nerve (Kennedy et al. 1982). In fact, it provides the majority of fibres to the popliteal plexus; fibres course from this innervating the posterior capsule, the cruciate ligaments and anteriorly up to the IFP (Mace et al. 2016). Using immunohistochemistry, the IFP showed free nerve fibres and nerves surrounded by thick multilaminated (three to six layers) connective tissue capsule. The free nerve fibres were recognizable within adipose lobules, whereas the corpuscles were recognizable along the interlobular septa, in the adipose lobules and close to the vessels (Macchi et al. 2016). Moreover, the presence of type VIa free nerve endings is particularly important, as they may initiate afferent signals of pain, pressure and thermic variations to the central nervous system (Biedert & Kernen, 2001). The presence of abundant substance‐P nerve fibres within the IFP has also been highlighted; the number is significantly higher in subjects with anterior knee pain (Bohnsack et al. 2005). Substance‐P causes vasodilation, thereby promoting the recruitment of immune cells and potentially contributing to oedema development within the tissue (Eymard & Chevalier, 2016).
From the functional point of view, the first role attributed to IFP is a biomechanical contribution. The IFP facilitates the distribution of synovial fluid and acts to absorb forces through the knee joint (Platzer, 1999). With numerical meso‐models, the IFP has been reported to have a peculiar mechanical relevance, with respect to other adipose tissues, with a high volumetric stiffness of its adipose lobules and consequent development of tissue straining because of connective septa. Conversely, lobules exhibit low iso‐volumetric stiffness, and shear loadings mainly determine stresses within connective septa (Fontanella et al. 2017). The biomechanical properties of IFP change during OA, with loss of proper stress‐strain behavior under mechanical loads (unpublished data). The second function attributed to IFP is a biochemical role in the development of the anterior knee pain (Clockaerts et al. 2010) and OA (Favero et al. 2017). With reference to OA, there is much evidence highlighting the endocrine‐ paracrine and autocrine‐like properties of the IFP. In fact, immune cells can infiltrate the IFP, which can become an important source of numerous proinflammatory mediators such as adipokines (i.e. leptin, adiponectin, resistin, chemerin), interleukins (ILs; IL‐6 and IL‐8) as well as growth factors [fibroblast growth factor (FGF)2, vascular endothelial growth factor (VEGF), TNF‐α; Clockaerts et al. 2010; Eymard & Chevalier, 2016; Belluzzi et al. 2017; de Jong et al. 2017]. These inflammatory mediators are found in synovial fluid and can influence the metabolism of both cartilage and synovial membrane, causing the onset of structural changes of the IFP, which are clearly identifiable at cellular and histological levels in OA with respect to the IFP of healthy subjects (Distel et al. 2009; Clockaerts et al. 2010; Table 1).
Table 1.
The main features of the IFP. The table summarizes the main characteristics of the infrapatellar fat pad (IFP) considering anatomical and function‐related aspects
| IFP | |
|---|---|
| Gross appearance/tissue organization | A central body of white adipose tissue organized in lobules characterized by collagenic stroma |
| Presence of medial and lateral extensions | |
| Ligamentum mucosum is located inferiorly | |
| Eventually, two clefts (horizontal and vertical, respectively) are identifiable | |
| Vascularization | Superior and inferior genicular arteries connected by anastomosis |
| Anastomoses with the vessels of menisci, patellar tendon and tibial periosteum | |
| Innervation | Nerve fibres from the posterior tibial nerve |
| Free nerve fibres within adipose lobules with corpuscles along the interlobular septa | |
| Function | Biomechanical: promotes synovial fluid distribution absorbing loads of the knee joint |
| Biochemical: secretes proinflammatory mediators (i.e. adipokines, interleukins) and growth factors showing endocrine‐paracrine and autocrine‐like activity on the cartilage and synovial membrane |
Synovial membrane
Macroscopic and microscopic anatomy of normal synovial membrane
The joint internal structures and the musculoskeletal tissues are linked by the synovial membrane; which, by virtue of its cellular composition, is responsible for joint homeostasis (Huang et al. 2017). In fact, from a functional point of view, the synovial membrane promotes skeletal movement by producing synovial fluid; the fluid fills the synovial cavity, lubricating cartilage and tendon surfaces, and facilitating low‐friction and low‐wear articulation (Hui et al. 2012).
Generally, the synovium is composed of two layers including a continuous surface layer of cells (intima) and the underlying tissue (subintima; Hui et al. 2012). Histological and immunohistochemical examinations show that the intima is composed of two to three layers of fibroblast‐like and macrophage‐like synoviocytes. Fibroblast‐like cells express vimentin, CD90 and intracellular adhesion molecules, which are the markers of mesenchymal and fibroblastic lineages; nevertheless, they can be distinguished by high levels of uridine diphosphoglucose dehydrogenase (UDPGD), cadherin 11 and receptor‐type protein tyrosine‐protein phosphatase sigma (R‐PTP‐sigma), and by the constitutive expression of vascular cell adhesion protein 1 (VCAM‐1; Bhattaram & Chandrasekharan, 2017). Fibroblast‐like synoviocytes are involved in production of specialized matrix constituents including collagen, fibronectin, hyaluronan and other proteoglycans of the intimal interstitium; moreover, they secrete the major constituents of synovial fluid, such as the proteoglycan lubricine (Iwanaga et al. 2000; Nio et al. 2002; Bhattaram & Chandrasekharan, 2017). In parallel, the intimal macrophages express typical macrophage lineage markers [i.e. strongly positive for cluster of differentiation (CD)163 and CD68 but less so for CD14; immunoglobulin receptor FcgRIIIa] and show a prominent nonspecific esterase (NSE) activity as well (Smith, 2011). By means of the microvilli and microplicae identifiable on the cell surface, they exert a protective role, helping in clearing bacterial infections and debris resulting from minor joint injuries; this active phagocytosis guarantees a refreshing of the components of the synovial fluid (Nio et al. 2002; Bhattaram & Chandrasekharan, 2017). Macrophages, whose number hugely increases in chronically inflamed synovium, are also responsible for maintaining a fine balance between the levels of proinflammatory and anti‐inflammatory cytokines in the synovial fluid (Kennedy et al. 2011). The subintimal space is made up of fibrous connective tissue, blood vessels and a low content of immune cells, e.g. macrophages, CD3+ T‐cells, including CD4+ and CD8+ cells, B cells, plasma cells and granzyme B‐positive cells. The macrophages compared with those of the intima, are NSE‐weak and strongly CD14+ FcgRI+ (Smith, 2011). The vascular network provides nutrition to the avascular articular cartilage. In the subintimal layer larger venules together with arterioles and lymphatics form an anastomosing network; conversely, capillaries are identifiable in the intima (Wilkinson & Edwards, 1989; Xu et al. 2003). Normal synovial tissue has a rich nerve supply; in the sympathetic nervous system, most of the nerves are found around vascular networks, although they do not extend into the intimal layer (Pereira da Silva & Carmo‐Fonseca, 1990). Typically, in healthy subjects, the intimal layer is 20–40 μm thick in cross‐section and the subintima can be up to 5 mm in thickness. However, at many sites there is no discrete membrane, especially where subintima consists of fibrous tissue or fat pad (Smith, 2011). This evidence may constitute an objective demonstration that the synovial membrane and IFP form a morpho‐functional unit (Table 2).
Table 2.
The main features of the synovial membrane. The table summarizes the main characteristics of the synovial membrane considering anatomical and function‐related aspects
| Synovial membrane | |
|---|---|
| Gross appearance/tissue organization and cells function |
|
| Vascularization |
|
| Innervation |
|
| Function |
|
CD, cluster of differentiation; NSE, nonspecific esterase; R‐PTP sigma, receptor‐type protein tyrosine‐protein phosphatase sigma; UDPGD, uridine diphosphoglucose dehydrogenase; VCAM, vascular cell adhesion protein 1.
Anatomical features, prevalence and incidence of the synovial plicae
Remnants of the mesenchymal tissue, called synovial plicae, are also recognizable in the normal synovial membrane. According to their anatomical location, the plicae have been divided into four major types: the infrapatellar plica – IPP, or ligamentum mucosum; the suprapatellar plica – SPP; the medial patellar plica – MPP; and the medial shelf and the lateropatellar plica – LPP (Kent & Khanduja, 2010).
A normal synovial plica is a soft and elastic structure, whereas a pathological plica is commonly inelastic, tight, thickened, fibrotic and sometimes hyalinized. Bowstringing across the femoral trochlea may cause impingement between the patella and femur in knee flexion and a nonspecific anterior or anteromedial knee pain, which results in synovial plica syndrome (Lee et al. 2017).
Asymptomatic plicae around the knee are a common finding at arthroscopy and are rarely pathological (Kent & Khanduja, 2010). However, chronic inflammation secondary to direct trauma, continuous strenuous exercises or other pathological knee conditions affect the pliability of the synovial folds, which can become symptomatic (Garcia‐Valtuille et al. 2002).
The most common symptomatic plica is the MPP, whereas SPP has rarely been reported to give rise to symptoms. It is generally agreed that the IPP does not cause symptoms (Demirag et al. 2006). In the case of hypertrophy of the plica with consequent thickening, its excision may guarantee good outcomes in the majority of the patients (Kent & Khanduja, 2010).
a. The infrapatellar plica – IPP
Anatomically, the IPP, or ligamentum mucosum, is described as a fan‐shaped structure with a narrow femoral origin at the level of the intercondylar fossa in front of the anterior and lateral to the posterior cruciate ligament. As it descends through the inferior joint space, the IPP proceeds as two fringelike alar folds to attach to the IFP. The attachment to the IFP prevents the fat pad from being displaced anteriorly even in the presence of a large joint effusion (Patel et al. 1998). The shape of the IPP can vary, and according to the morphology it is possible to distinguish five different types: separated (cord‐like) pattern, split pattern, vertical septum pattern, fenestra pattern and none of the above (Kim et al. 1996).
The prevalence of IPP ranges from 61 to 78.3% (Gurbuz et al. 2006; Lee et al. 2012). However, the incidence of IPP is reported to be 85.5% according to Kim et al. (1996), who claimed there is also a decrease of the incidence with aging (Table 3).
Table 3.
Anatomical features, prevalence and incidence of the synovial plicae
| IPP | SPP | MPP | LPP | |
|---|---|---|---|---|
| Origin/localization | Femoral origin, intercondylar fossa in front of the anterior and lateral to the posterior cruciate ligament. It descends through the inferior joint space and proceeds as two fringelike alar folds to attach to the IFP | Between the suprapatellar pouch and the true knee | Originates in the suprapatellar region and passes inferiorly on the medial aspect of the knee joint before inserting into the IFP | Originates from the lateral aspect of the knee and inserts into the synovial membrane |
| Shape/Type | Five patterns:
|
Four types:
|
Four types:
|
– |
| Microscopic characteristics | – | Subsynovial adipose tissue with some dense collagen fibres, some skeletal muscle fibres | Adipose tissue with undulated regular collagen fibres | Subsynovial layer of areolo‐adipose tissue with collagen fibres |
| Prevalence | 61–78.3% | 33–89% | 95% | 20.7% |
| Incidence | 85.5% with an age‐related decrease | Up to 91.2% | 18–72% | <1% |
IFP, infrapatellar fat pad; IPP, infrapatellar plica; LPP, lateropatellar plica; MPP, medial patellar plica; SPP, suprapatellar plica.
b. The suprapatellar plica – SPP
The SPP is a membrane that lies between the suprapatellar pouch and the true knee. Different anatomical variations of the suprapatellar plica have been described (Kent & Khanduja, 2010). In particular, Zidorn (1992) differentiated the terms septum and plica, as he considered the plica an incomplete resorption of a septum (Dupont, 1997). Thus, four types of SPP may be recognized through arthroscopy: type I – the septum completely separates the suprapatellar bursa from the knee joint (septum completum); type II – there are one or more openings in the plica allowing interaction between the suprapatellar bursa and the knee joint cavity (septum perforatum); type III – there is a remaining, usually medially located, fold (septum residuale); type IV – the septum is completely involuted (septum extinctum; Zidorn, 1992).
From the microscopic point of view, the SPP consists of predominantly sub‐synovial adipose tissue with some dense undulated collagenous fibres; the presence of skeletal muscle has also been identified (Geraghty & Spear, 2017).
The prevalence of SPP ranges from 33 to 89% (Harty & Joyce, 1977; Ogata & Uhthoff, 1990; Gurbuz et al. 2006). Most arthroscopic studies have reported an incidence of up to 91.2% (Kim & Choe, 1997; Table 3).
c. The medial patellar plica – MPP
The MPP originates in the suprapatellar region and passes inferiorly on the medial aspect of the knee joint before inserting into the IFP. Based on its morphology, it is classified as type A (cord‐like), type B (shelf‐like, but not covering the medial femoral condyle), type C (covering the medial femoral condyle) and type D (having a double insertion; Boles & Martin, 2001; Al‐Hadithy et al. 2011).
From a microscopic point of view, the MPP consists of adipose tissue surrounded by undulated regular collagen fibres underlying synovial membrane (Geraghty & Spear, 2017).
The prevalence of MPP is 95% (Gurbuz et al. 2006). The reported incidence, however, is extremely variable, ranging from 18 up to 72% (Kim & Choe, 1997; Al‐Hadithy et al. 2011; Table 3).
d. The lateropatellar plica – LPP
The LPP is like a band originating from the lateral aspect of the knee above the popliteal hiatus and inserting into the synovial membrane around the IFP (Kent & Khanduja, 2010). Whether the LPP is a true septal remnant is still controversial; in fact, it seems more likely to be derived from the parapatellar adipose synovial fringe (Al‐Hadithy et al. 2011).
Microscopically, the LPP consists of subsynovial layer of areoloadipose tissue with collagen fibres coursing through (Geraghty & Spear, 2017).
The prevalence LPP 20.7% (Gurbuz et al. 2006). Moreover, as the incidence is less than 1%, it is considered very rare (Boles & Martin, 2001).
Interestingly, Kim & Choe (1997) highlighted that there was no significant correlation in frequencies and distributions of patterns among the plicae according to the age of patients, between men and women, as well as between right and left knee. A significant similarity in patterns of the right and left knee was observed only in the same subject (Table 3).
Morphological and molecular bases of the synovial‐IFP unit
From the review of the literature, the hypothesis of a synovial‐IFP unit is based on the following considerations, summarized in Table 4.
Table 4.
Morphological and molecular evidence of the existence of IFP‐synovial membrane functional unit
| Main evidence | |
|---|---|
| Macroscopic anatomy | Synovial membrane lines the posterior aspect of the IFP |
| Synovial membrane lies beneath the IFP projecting into the joint as two fringers | |
| Imaging | In OA signal alteration in IFP, in front of the IFP and suprapatellar fat pad |
| Synovial thickening within the IFP in early OA | |
| IFP opacity in radiographs is correlated with synovitis grades in MR | |
| Histopathology | Both the IFP and synovial membrane show increase of inflammatory features (i.e. infiltration and vascularization, thickness of interlobular septa and hyperplasia) in OA |
| Loss of IFP adipocytes associated with synovial proliferation and subsynovial fibrosis in experimental models | |
| Molecular biology | In end‐stage OA, IFP inhibits catabolic mediators of the cartilage |
| IFP has a profibrotic effect on synovial membrane exerted by mediators such as prostaglandin F2a |
IFP, infrapatellar fat pad; MR, magnetic resonance; OA, osteoarthritis.
Macroscopic anatomy
The IFP is interposed between the joint capsule and the synovial membrane, which lines its posterior aspect. Where the synovial membrane lies beneath the IFP, it projects into the joint as two fringes, alar folds (Standring et al. 2008; Fig. 1B). The IFP is described to be the largest part of a circumferential extrasynovial fatty ring, which extends around the patellar margins (Newell, 1991). In fact, increased fibrosis, vascularization, leucocytes and mast cell infiltration are present in OA patients not only in IFP but also in the suprapatellar and posterior fat pad (Eymard et al. 2017).
Imaging
In magnetic resonance studies of OA patients, signal alterations are recognizable at the level of IFP and the presence of synovitis is observed in front of the IFP and suprapatellar fat pad (Roemer et al. 2010; Cai et al. 2015). The synovial thickening within the IFP and the multiple synovial projections into IFP can be found in early OA patients on magnetic resonance examination. These features correspond to hyperplasia of synovial membrane, mononuclear cell infiltration, and proliferation of small blood vessels (Fernandez‐Madrid et al. 1995). Moreover, the grade of opacity of IFP at conventional radiographs of the knee is well correlated with most of the synovitis grades observed at magnetic resonance imaging (Yun et al. 2017).
Histopathology
In the IFP of patients with OA undergoing total knee replacement, an increase of inflammatory infiltration, vascularization and thickness of the interlobular septa was found. At the same time the synovial membrane adjacent to IFP presented lymphocytic infiltration, hyperplasia and higher vascularization compared with healthy controls (Favero et al. 2017). In fact, in an experimental model of OA, marked loss of adipocytes in IFP was associated with extensive synovial proliferation (SP) and subsynovial fibrosis (Clements et al. 2009).
Molecular biology
The IFP in the knees of patients with end‐stage OA inhibits catabolic mediators of the cartilage and exerts a profibrotic effect on the synovial membrane, partially due to the presence of prostaglandin F 2a (Bastiaansen‐Jenniskens et al. 2013), which is critical for the functional interaction between IFP and synovium, although other mediators could also be involved (Eymard et al. 2014, 2017).
Conclusions
Exploiting the multidisciplinary skills of our research group (from human anatomy, rheumatology, orthopaedics, radiology, physiotherapy, pharmacotherapy and engineering) and findings on the IFP, a critical revision of the literature has been performed. In accordance with the recent highlights by Eymard et al. (2017) and by Geraghty & Spear (2017), our experience suggests a possible cross‐talk among the IFP and the synovial membrane, supporting the idea that these two structures are actually an anatomo‐functional unit.
To date, therapy of OA is multimodal and requires a combination of pharmacological and non‐pharmacological treatments. Pain and other symptoms of OA profoundly compromise the quality of life of patients, placing a burden on the physical, psychological and economic aspects of life and having a considerable social impact as well. Hence, clarifying and defining the cross‐talk between the IFP and the synovial membrane may be helpful in early identification and treatment of OA.
References
- Al‐Hadithy N, Gikas P, Mahapatra AM, et al. (2011) Review article: Plica syndrome of the knee. J Orthop Surg (Hong Kong) 19, 354–358. [DOI] [PubMed] [Google Scholar]
- Atukorala I, Kwoh CK, Guermazi A, et al. (2014) Synovitis in knee osteoarthritis: a precursor of disease? Ann Rheum Dis 75, 390–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastiaansen‐Jenniskens YM, Wei W, Feijt C, et al. (2013) Stimulation of fibrotic processes by the infrapatellar fat pad in cultured synoviocytes from patients with osteoarthritis: a possible role for prostaglandin f2α. Arthritis Rheum 65, 2070–2080. [DOI] [PubMed] [Google Scholar]
- Belluzzi E, El Hadi H, Granzotto M, et al. (2017) Systemic and local adipose tissue in knee osteoarthritis. J Cell Physiol 232, 1971–1978. [DOI] [PubMed] [Google Scholar]
- Bhattaram P, Chandrasekharan U (2017) The joint synovium: a critical determinant of articular cartilage fate in inflammatory joint diseases. Semin Cell Dev Biol 62, 86–93. [DOI] [PubMed] [Google Scholar]
- Biedert RM, Kernen V (2001) Neurosensory characteristics of the patellofemoral joint: what is the genesis of patello‐femoral pain? Sports Med Arthrosc 9, 295–300. [Google Scholar]
- Bohnsack M, Meier F, Walter GF, et al. (2005) Distribution of substance‐P nerves inside the infrapatellar fat pad and the adjacent synovial tissue: a neurohistological approach to anterior knee pain syndrome. Arch Orthop Trauma Surg 125, 592–597. [DOI] [PubMed] [Google Scholar]
- Boles CA, Martin D (2001) Synovial plicae in the knee. AJR Am J Roentgenol 177, 221–227. [DOI] [PubMed] [Google Scholar]
- Cai J, Xu J, Wang K, et al. (2015) Association between infrapatellar fat pad volume and knee structural changes in patients with knee osteoarthritis. J Rheumatol 42, 1878–1884. [DOI] [PubMed] [Google Scholar]
- Clements KM, Ball AD, Jones HB, et al. (2009) Cellular and histopathological changes in the infrapatellar fat pad in the monoiodoacetate model of osteoarthritis pain. Osteoarthritis Cartilage 17, 805–812. [DOI] [PubMed] [Google Scholar]
- Clockaerts S, Bastiaansen‐Jenniskens YM, Runhaar J, et al. (2010) The infrapatellar fat pad should be considered as an active osteoarthritic joint tissue: a narrative review. Osteoarthritis Cartilage 18, 876–882. [DOI] [PubMed] [Google Scholar]
- De Caro R, Macchi V, Porzionato A (2009) Promotion of body donation and use of cadavers in anatomical education at the University of Padova. Anat Sci Educ 2, 91–92. [DOI] [PubMed] [Google Scholar]
- de Jong AJ, Klein‐Wieringa IR, Kwekkeboom JC, et al. (2017) Lack of high BMI‐related features in adipocytes and inflammatory cells in the infrapatellar fat pad (IFP). Arthritis Res Ther 19, 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirag B, Ozturk C, Karakayali M (2006) Symptomatic infrapatellar plica. Knee Surg Sports Traumatol Arthrosc 14, 156. [DOI] [PubMed] [Google Scholar]
- Distel E, Cadoudal T, Durant S, et al. (2009) The infrapatellar fat pad in knee osteoarthritis: an important source of interleukin‐6 and its soluble receptor. Arthritis Rheum 60, 3374–3377. [DOI] [PubMed] [Google Scholar]
- Draghi F, Ferrozzi G, Urciuoli L, et al. (2016) Hoffa's fat pad abnormalities, knee pain and magnetic resonance imaging in daily practice. Insights Imaging 7, 373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont JY (1997) Synovial plicae of the knee. Controversies and review. Clin Sports Med 16, 87–122. [DOI] [PubMed] [Google Scholar]
- Eymard F, Chevalier X (2016) Inflammation of the infrapatellar fat pad. Joint Bone Spine 83, 389–393. [DOI] [PubMed] [Google Scholar]
- Eymard F, Pigenet A, Citadelle D, et al. (2014) Induction of an inflammatory and prodegradative phenotype in autologous fibroblast‐like synoviocytes by the infrapatellar fat pad from patients with knee osteoarthritis. Arthritis Rheumatol 66, 2165–2174. [DOI] [PubMed] [Google Scholar]
- Eymard F, Pigenet A, Citadelle D, et al. (2017) Knee and hip intra‐articular adipose tissues (IAATs) compared with autologous subcutaneous adipose tissue: a specific phenotype for a central player in osteoarthritis. Ann Rheum Dis 76, 1142–1148. [DOI] [PubMed] [Google Scholar]
- Favero M, EL‐Hadi H, Belluzzi E, et al. (2017) Infrapatellar fat pad features in osteoarthritis: a histopathological and molecular study. Rheumatology 56, 1784–1793. [DOI] [PubMed] [Google Scholar]
- Fernandez‐Madrid F, Karvonen RL, Teitge RA, et al. (1995) Synovial thickening detected by MR imaging in osteoarthritis of the knee confirmed by biopsy as synovitis. Magn Reson Imaging 13, 177–183. [DOI] [PubMed] [Google Scholar]
- Fontanella CG, Carniel EL, Frigo A, et al. (2017) Investigation of biomechanical response of Hoffa's fat pad and comparative characterization. J Mech Behav Biomed Mater 67, 1–9. [DOI] [PubMed] [Google Scholar]
- Gallagher J, Tierney P, Murray P, et al. (2005) The infrapatellar fat pad: anatomy and clinical correlations. Knee Surg Sports Traumatol Arthrosc 13, 268–272. [DOI] [PubMed] [Google Scholar]
- Garcia‐Valtuille R, Abascal F, Cerezal L, et al. (2002) Anatomy and MR imaging appearances of synovial plicae of the knee. Radiographics 22, 775–784. [DOI] [PubMed] [Google Scholar]
- Geraghty RM, Spear M (2017) Evidence for plical support of the patella. J Anat 231, 698–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurbuz H, Calpur OU, Ozcan M, et al. (2006) The synovial plicae in the knee joint. Saudi Med J 27, 1839–1842. [PubMed] [Google Scholar]
- Harty M, Joyce JJ III (1977) Synovial folds in the knee joint. Orthop Rev 7, 91–98. [Google Scholar]
- Hempfing A, Schoeniger R, Koch PP, et al. (2007) Patellar blood flow during knee arthroplasty surgical exposure: intraoperative monitoring by laser doppler flowmetry. J Orthop Res 25, 1389–1394. [DOI] [PubMed] [Google Scholar]
- Hoffa AA (1903) The influence of the adipose tissue with regard to the pathology of the knee joint. JAMA XLIII, 795–796. [Google Scholar]
- Huang YZ, Xie HQ, Silini A, et al. (2017) Mesenchymal stem/progenitor cells derived from articular cartilage, synovial membrane and synovial fluid for cartilage regeneration: current status and future perspectives. Stem Cell Rev 13, 575–586. [DOI] [PubMed] [Google Scholar]
- Hughes SS, Cammarata A, Steinmann SP, et al. (1998) Effect of standard total knee arthroplasty surgical dissection on human patellar blood flow in vivo: an investigation using laser Doppler flowmetry. J South Orthop Assoc 7, 198–204. [PubMed] [Google Scholar]
- Hui AY, McCarty WJ, Masuda K, et al. (2012) A systems biology approach to synovial joint lubrication in health, injury, and disease. Wiley Interdiscip Rev Syst Biol Med 4, 15–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwanaga T, Shikichi M, Kitamura H, et al. (2000) Morphology and functional roles of synoviocytes in the joint. Arch Histol Cytol 63, 17–31. [DOI] [PubMed] [Google Scholar]
- Kennedy J, Alexander I, Hayes K (1982) Nerve supply of the human knee and its functional importance. Am J Sports Med 10, 329–335. [DOI] [PubMed] [Google Scholar]
- Kennedy A, Fearon U, Veale DJ, et al. (2011) Macrophages in synovial inflammation. Front Immunol 2, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent M, Khanduja V (2010) Synovial plicae around the knee. Knee 17, 97–102. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Choe WS (1997) Arthroscopic findings of the synovial plicae of the knee. Arthroscopy 13, 33–41. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Min BH, Kim HK (1996) Arthroscopic anatomy of the infrapatellar plica. Arthroscopy 12, 561–564. [DOI] [PubMed] [Google Scholar]
- Kohn D, Deiler S, Rudert M (1995) Arterial blood supply of the infrapatellar fat pad. Anatomy and clinical consequences. Arch Orthop Trauma Surg 114, 72–75. [DOI] [PubMed] [Google Scholar]
- Lee YH, Song HT, Kim S, et al. (2012) Infrapatellar plica of the knee: revisited with MR arthrographies undertaken in the knee flexion position mimicking operative arthroscopic posture. Eur J Radiol 81, 2783–2787. [DOI] [PubMed] [Google Scholar]
- Lee PYF, Nixion A, Chandratreya A, et al. (2017) Synovial plica syndrome of the knee: a commonly overlooked cause of anterior knee pain. Surg J (N Y) 3, e9–e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macchi V, Porzionato A, Sarasin G, et al. (2016) The infrapatellar adipose body: a histotopographic study. Cells Tissues Organs 201, 220–231. [DOI] [PubMed] [Google Scholar]
- Macchi V, Porzionato A, Rossato M, et al. (2017) Regional differences between perisynovial and infrapatellar adipose tissue depots and their response to class II and III obesity in patients with OA: comment on the article by Harasymowicz et al. Arthritis Rheumatol 70, 146–147. [DOI] [PubMed] [Google Scholar]
- Mace J, Bhatti W, Anand S (2016) Infrapatellar fat pad syndrome: a review of anatomy, function, treatment and dynamics. Acta Orthop Belg 82, 94–101. [PubMed] [Google Scholar]
- Madry H, Luyten FP, Facchini A (2012) Biological aspects of early osteoarthritis. Knee Surg Sports Traumatol Arthrosc 20, 407–422. [DOI] [PubMed] [Google Scholar]
- Martel‐Pelletier J, Pelletier JP (2010) Is osteoarthritis a disease involving only cartilage or other articular tissues? Eklem Hastalik Cerrahisi 21, 2–14. [PubMed] [Google Scholar]
- Mathiessen A, Conaghan PG (2017) Synovitis in osteoarthritis: current understanding with therapeutic implications. Arthritis Res Ther 19, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moradi‐Lakeh M, Forouzanfar MH, Vollset SE, et al. (2017) Burden of musculoskeletal disorders in the Eastern Mediterranean Region, 1990–2013: findings from the Global Burden of Disease Study 2013. Ann Rheum Dis 76, 1365–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell RLM (1991) A complete intra‐articular fat pad around the human patella. J Anat 179, 232. [Google Scholar]
- Nio J, Yokoyama A, Okumura M, et al. (2002) Three‐dimensional ultrastructure of synoviocytes in the knee joint of rabbits and morphological changes in osteoarthritis model. Arch Histol Cytol 65, 189–200. [DOI] [PubMed] [Google Scholar]
- Ogata S, Uhthoff HK (1990) The development of synovial plicae in human knee joints: an embryologic study. Arthroscopy 6, 315–321. [DOI] [PubMed] [Google Scholar]
- Patel SJ, Kaplan PA, Dussault RG, et al. (1998) Anatomy and clinical significance of the horizontal cleft in the infrapatellar fat pad of the knee: MR imaging. AJR Am J Roentgenol 170, 1551–1555. [DOI] [PubMed] [Google Scholar]
- Pereira da Silva JA, Carmo‐Fonseca M (1990) Peptide containing nerves in human synovium: immunohistochemical evidence for decreased innervations in rheumatoid arthritis. J Rheumatol 17, 1592–1599. [PubMed] [Google Scholar]
- Platzer W (1999) Atlas van de Anatomie, vol. 1, Intro, 7th edn Baarn: Thieme. [Google Scholar]
- Roemer FW, Kassim Javaid M, Guermazi A, et al. (2010) Anatomical distribution of synovitis in knee osteoarthritis and its association with joint effusion assessed on non‐enhanced and contrast‐enhanced MRI. Osteoarthritis Cartilage 18, 1269–1274. [DOI] [PubMed] [Google Scholar]
- Sbarbati A, Accorsi D, Benati D, et al. (2010) Subcutaneous adipose tissue classification. Eur J Histochem 54, e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellam J, Berenbaum F (2010) The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat Rev Rheumatol 6, 625–635. [DOI] [PubMed] [Google Scholar]
- Sime K, Choy EH, Williams AS (2017) Alterations to adipose tissue morphology during inflammatory arthritis is indicative of vasculopathology in DBA/1 mice. Adipocyte 6, 87–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MD (2011) The normal synovium. Open Rheumatol J 5, 100–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standring S, Borley NR, Collins P, et al. (2008) Gray's Anatomy, 40th edn pp. 1395, London: Churchill Livingstone. [Google Scholar]
- Tiku ML, Madhan B (2016) Preserving the longevity of long‐lived type II collagen and its implication for cartilage therapeutics. Ageing Res Rev 28, 62–71. [DOI] [PubMed] [Google Scholar]
- Wenham CY, Conaghan PG (2010) The role of synovitis in osteoarthritis. Ther Adv Musculoskelet Dis 2, 349–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenham CY, Conaghan PG (2013) New horizons in osteoarthritis. Age Ageing 42, 272–278. [DOI] [PubMed] [Google Scholar]
- Wilkinson LS, Edwards JCW (1989) Microvascular distribution in normal human synovium. J Anat 167, 129–136. [PMC free article] [PubMed] [Google Scholar]
- Xu H, Edwards J, Banerji S, et al. (2003) Distribution of lymphatic vessels in normal and arthritic human synovial tissues. Ann Rheum Dis 62, 1227–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun SJ, Lim Y, Jin W, et al. (2017) Validity of radiograph‐based infrapatellar fat pad opacity grading for assessing knee synovitis: correlation with contrast‐enhanced MRI. AJR Am J Roentgenol 209, 1–10. [DOI] [PubMed] [Google Scholar]
- Zidorn T (1992) Classification of the suprapatellar septum considering ontogenetic development. Arthroscopy 8, 459–464. [DOI] [PubMed] [Google Scholar]


