Abstract
As a major phytoestrogen of soy, genistein effectively prevents bone loss in both humans and rat models of osteoporosis. However, although the bone‐sparing effects of genistein are achieved directly through estrogen receptors, its mode of action on bone by modulation of other endocrine functions is not entirely clear. Thus, thyroid hormones and calcitonin (CT) have an essential influence on bone metabolism. Besides its action on bones, in this study we examined the effect of genistein on the activity of two different endocrine cell populations, thyroid follicular and C‐cells. Fifteen‐month‐old Wistar rats were either bilaterally orchidectomized (Orx) or sham‐operated (SO). Two weeks after surgery, half of the Orx rats were treated chronically with 30 mg kg−1 b.w. genistein (Orx + G) subcutaneously (s.c.) every day for 3 weeks, while the remaining Orx rats and the SO rats were given the same volume of sterile olive oil to serve as controls. For histomorphometrical analysis of the trabecular bone microarchitecture an imagej public domain image processing programme was used. Thyroid sections were analysed histologically and stereologically after visualization of follicular and C‐cells by immunohistochemical staining for thyroglobulin and CT. Thyroid follicular epithelium, interstitium, colloid and CT‐immunopositive C‐cells were examined morphometrically. Serum concentrations of osteocalcin (OC), triiodothyronine (T3), thyroxine (T4) and CT were determined as well as urinary calcium (Ca2+) concentrations. Genistein treatment significantly increased cancellous bone area (B.Ar), trabecular thickness (TbTh) and trabecular number (TbN) (P < 0.05), but trabecular separation (Tb.Sp) was decreased (P < 0.05) compared with control Orx rats. In the thyroid, genistein treatment significantly elevated the relative volume density (Vv) of the follicular cells (P < 0.05) compared with Orx, whereas Vv of the colloid was lower (P < 0.05) than in the Orx. Evaluation of the biochemical parameters showed significant reductions in serum OC, T3, T4 and urinary Ca2+ concentrations (P < 0.05), compared with Orx rats. These data indicate that genistein treatment improves the trabecular microarchitecture of proximal tibia, induces histomorphometrical changes in thyroid glands, and decreases circulating thyroid hormone levels in orchidectomized rat model of male osteoporosis.
Keywords: bone, genistein, male rats, osteoporosis, thyroid cells, thyroid hormones
Introduction
Although women are more prone to osteoporosis than men, this skeletal disease also affects older males (Penrod et al. 2008). Regardless of gender, bone mass decreases progressively with advancing age due to reduced circulating sex‐hormone levels. Unlike menopausal women, in whom the decline in estrogen level is rapid, the reduction in circulating androgens in men is slow. Therefore, together with the greater skeletal size and bone mass in men than in women, osteoporosis and an increased risk of fractures appear later in life in males (Lunt et al. 1997; Nieves et al. 2005). Clinical findings have comfirmed that osteoporosis and osteoporotic fractures occur more often after therapeutic orchidectomy for prostate cancer (Daniell, 1997). Also, animal studies showed significant bone loss in androgen‐deficient orchidectomized rats (Tuukkanen et al. 1994; Filipović et al. 2007). Although estrogen is the dominant sex hormone in women and testosterone in men, estrogen plays an important role in the regulation of bone mass in both sexes. In men, estrogen is synthesized from circulating testosterone by the action of the enzyme aromatase in gonadal and peripheral tissues including bones (Roselli et al. 1997; Simpson & Dowsett, 2002). As suggested earlier, estrogen regulates bone resorption in men, while both estrogen and testosterone play roles in maintaining bone formation (Falahati‐Nini et al. 2000).
Despite the effectiveness of estrogen as hormonal therapy in prostate cancer, for male‐to‐female transsexuals or male sexual behaviour in patients with aromatase deficiency (Smith et al. 1998; Carani et al. 1999; Tangpricha et al. 2003), its therapeutic use in men is limited. This is due to the possibility of an increased risk of hormone‐related tumours after cross‐sex hormone therapy (Mueller & Gooren, 2008). As an alternative to sex hormone therapy, phytoestrogens that can act as selective estrogen receptor (ER) modulators (SERMs) could be used to treat both male and female osteoporosis. Experimental studies have demonstrated that phytoestrogens, such as the soybean isoflavones, genistein and daidzein, have a bone‐protective effect in rat models of osteoporosis in both sexes (Khalil et al. 2005; Filipović et al. 2010; Miao et al. 2012). These isoflavones are structurally similar to 17 β‐estradiol and are able to bind to ERα and β. ERβ has a higher binding affinity for genistein (Kuiper et al. 1997).
While sex steroids play an important role in maintaining bone mass, other hormones, such as thyroid hormones and calcitonin (CT), are involved in the regulation of bone metabolism. Thyroid hormones affect bone remodelling and an excess is associated with bone loss (Kanatani et al. 2004). On the other hand, the hypocalcaemic hormone CT, produced by thyroid C‐cells, directly inhibits osteoclast‐mediated bone resorption (Warshawsky et al. 1980). Therefore, in addition to recording the effect of genistein on trabecular bone microarchitecture, the purpose of this study was to examine the influence of this isoflavone on the activity of thyroid follicular and C‐cells in a rat model of male osteoporosis. Their hormones may be involved in indirect actions of genistein on maintaining bone mass.
Materials and methods
Animals, diets and experimental design
The study involved 24 male Wistar rats 15 months old, bred at the ‘Siniša Stanković’ Institute for Biological Research, University of Belgrade, Serbia. Animals were housed in groups under standard soy‐free laboratory conditions (at 22 °C, under a 12 h/12 h light/dark cycle), with free access to water. The soy‐free diet was prepared in cooperation with the Department of Nutrition, School of Veterinary Medicine, and INSHRA PKB (Belgrade, Serbia), according to Picherit et al. (2000) with corn oil as the main source of fat. The food contained (per 100 g): casein, 20.3 g; cornstarch, 45 g; sucrose, 20 g; corn oil, 5.2 g; fibre (crystalline cellulose), 3.7 g; vitamin/mineral mix (Ca‐P deficient), 1.5 g; calcium phosphate dibasic, 1.8 g; calcium carbonate, 1 g; and dl‐methionine, 1.5 g; as well as 1.1 mg iodine per kg. Casein and crystalline cellulose were purchased from Alfa Aesar, Johnson Matthey Gmbh & Co. KG (Karlsruhe, Germany). The dl‐methionine was obtained from the Sigma Chemical Company (St. Louis, MO, USA). All other ingredients were from INSHRA PKB. Food and water were available ad libitum. Animals were either bilaterally orchidectomized (Orx, n = 16) or sham‐operated (SO, n = 8) under ketamine anaesthesia (15 mg kg−1 b.w. ketamine hydrochloride, Richter Pharma, Wels, Austria). Genistein was dissolved in a minimal volume of absolute ethanol, then mixed with sterile olive oil. Two weeks after surgery, eight of the Orx animals were treated chronically with 30 mg kg−1 b.w. genistein (Nutraceutica, Monterenzio, Italy) subcutaneously (s.c.) daily for 3 weeks. The control SO (n = 8) and the second Orx group (n = 8) were administered s.c. an equivalent volume of the absolute ethanol and sterile olive oil vehicle. After the last injection, 24‐h urine samples were collected and then all animals were killed. Blood was separated from the trunk. Blood serum and urine samples were frozen at −70 °C until required for biochemical analysis. All animal procedures complied with the EEC Directive (2010/63/EU) on the protection of animals used for experimental and other scientific purposes, and were approved by the Ethical Committee for the Use of Laboratory Animals of the ‘Siniša Stanković’ Institute for Biological Research, University of Belgrade, Serbia.
Histomorphometrical analysis of trabecular bone
The right tibia was collected from each animal, cleaned of soft tissue and fixed immediately in Bouin's solution for 5 days. After fixation, the bone samples were decalcified with 20% EDTA (ethylenediaminetetra‐acetic acid disodium salt), dehydrated with an increasing ethanol concentration and embedded in Paraplast. For structural analysis, 5‐μm‐thick longitudinally sections were cut using a rotatory microtome (RM 2125; Leica, Germany) with microtome blades for hard section (Sakura Finetek, Japan) and stained with Azan as previously described (Filipović et al. 2007).
For histomorphometrical analysis, all variables were expressed and calculated according to the recommendations of the American Society for Bone and Mineral Research nomenclature (Parfitt et al. 1987) using an imagej public domain image processing programme. Data provided by the image analysis system were used to calculate the cancellous bone area (B.Ar) and cancellous bone perimeter (Evans et al. 1994), and trabecular thickness (Tb.Th), trabecular number (Tb.N) and trabecular separation (Tb.Sp) were derived from these parameters as described earlier (Filipović et al. 2007).
Immunohistochemical procedures
After isolation, the thyroid lobes were fixed in Bouin's solution for 48 h, dehydrated through a series of increasing ethanol concentrations and embedded in Paraplast. For histological and stereological analysis, 5‐μm‐thick thyroid longitudinal sections taken from central part of the thyroid lobes were stained by the peroxidase–antiperoxidase immunohistochemical (IHC) method (Sternberger et al. 1970). Thyroglobulin (Tg) in thyroid tissue was characterized using rabbit antisera directed against human Tg (Dakopatts, Glostrup, Denmark; 1 : 500). For IHC localization of CT in the thyroid C‐cells, anti‐human CT antisera (Dakopatts, Copenhagen, Denmark; Lot No. 020; 1:500) served as the primary antibody.
Antigen‐antibody complexes were visualized with diaminobenzidine tetrahydrochloride (DAB; Dakopatts, Glostrup, Denmark). The sections were counterstained with haematoxylin and mounted in DPX medium (Sigma‐Aldrich, Barcelona, Spain).
Stereological analysis
Transversal sections of thyroid gland were analyzed by a point‐counting method (Weibel, 1979). The relative volume densities (Vv) of follicular epithelium, interstitium, colloid and thyroid C‐cells, defined as the ratio of this component in a volume unit of thyroid gland was calculated. The first three parameters were estimated on 50 test fields per section under a Zeiss light microscope (Jena, Germany) at 400× magnification, and C‐cells were measured at a magnification of 1000×, using the multipurpose M42 test system.
Biochemical analyses of serum and urine
Osteocalcin (OC), triiodothyronine (T3), thyroxine (T4) and CT concentrations were determined in the serum samples stored at −70 °C. Serum OC, T3 and T4 were estimated by electrochemiluminescence using a Roche Elecsys 2010 immunoassay analyser (Roche Diagnostics GmbH, Mannheim, Germany). Serum CT levels were measured in an immunochemiluminometric assay (Nichols, USA), using a mouse monoclonal antihuman CT antibody marker with acridium ester. The luminescence was quantified in a semi‐automated MLA 1 chemiluminescence analyzer (Ciba‐Corning). Calcium (Ca2+) levels in 24‐h urine collections were determined using a Hitachi 912 analyzer (Roche Diagnostics GmbH).
Statistical analysis
Statistical analysis of all data was performed using statistica 6.0 software (Statsoft, Tulsa, OK, USA). The Kolmogorov–Smirnov procedure was used to test for deviation from normal distribution, followed by one‐way analysis of variance. Duncan's multiple range test was used for post hoc comparisons. A probability value of P < 0.05 was considered statistically significant for comparisons of mean values between groups. The data are expressed as means ± standard error of the mean.
Results
Trabecular bone morphology and microarchitectural properties
Numerous well‐developed trabeculae were visible on longitudinal sections from the proximal tibiae of control SO animals. After Orx, as previously described (Filipović et al. 2007), significant degradation of the trabecular tissue was evident. This was manifested as reduction of both the number and thickness of trabeculae. The proximal tibia metaphyses of Orx + G animals showed significant recovery of trabecular bone tissue in comparison with those from untreated Orx rats. Thus, as in control SO rats, numerous trabecular spicules were detected in Orx + G rats (Fig. 1A–C). Genistein treatment of Orx rats increased B.Ar, Tb.Th and Tb.N by 115, 26 and 34% (P < 0.05), respectively, but Tb.Sp was 38% lower (P < 0.05) than in the control Orx group. No differences in B.Ar, Tb.Th or Tb.N were seen, whereas Tb.SP had diminished by 21% (P < 0.05) when compared with the value for control SO animals (Table 1).
Figure 1.
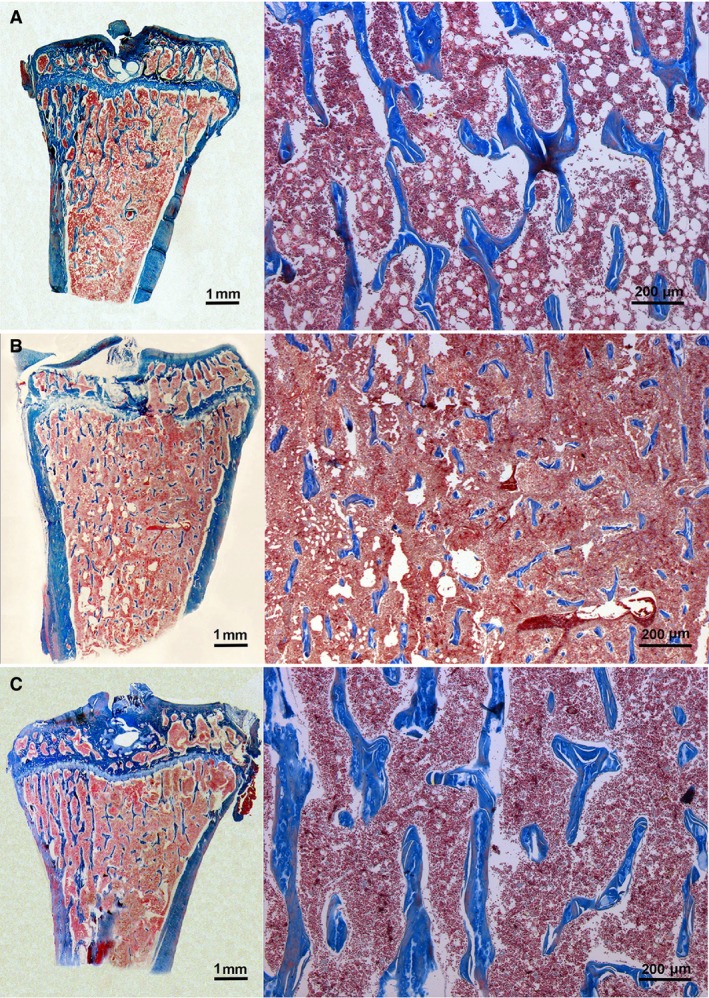
Trabecular bone structure from tibial metaphyses in sham‐operated (SO) (A), orchidectomized (Orx) (B) and orchidectomized rats treated with genistein (Orx + G) (C); Azan method stain.
Table 1.
Histomorphometry of trabecular bone in proximal tibia metaphysis in sham‐operated (SO), orchidectomized (Orx) and genistein‐treated orchidectomized (Orx + G) rats
| SO | Orx | Orx + G | |
|---|---|---|---|
| B.Ar (%) | 12.95 ± 0.74 | 6.25 ± 0.25• | 13.45 ± 1.66* |
| Tb.Th (μm) | 35.80 ± 0.10 | 28.69 ± 1.26• | 36.10 ± 0.40* |
| Tb.N (mm) | 3.62 ± 0.21 | 2.94 ± 0.04• | 3.95 ± 0.15* |
| Tb.Sp (μm) | 242.00 ± 16.50 | 308.00 ± 10.25• | 191.00 ± 18.50•* |
Data expressed as mean ± SEM (n = 8). Filled circle (•): P < 0.05 vs. SO. *P < 0.05 vs. Orx.
Thyroid histomorphometry, Tg and CT immunoreactivity
The thyroid glands of control SO rats were composed of follicles of different size. Distended follicles filled with dense luminal colloid surrounded by cuboidal follicular epithelium were mostly present (Fig. 2A). After Orx, small changes in thyroid parenchyma were evident in comparison with the SO control (Fig. 2D), manifested by smaller amounts of colloid within the follicles and a more prominent capillary network. Greater changes were detected in the Orx + G group thyroid parenchyma, which was mainly composed of small micro‐follicles, characterized by reduced luminal colloid and a well‐developed surrounding capillary network (Fig. 2G). Immunohistochemical analysis of Tg immunopositivity revealed heterogeneity of thyroid follicles in relation to intensity and quantity of IHC in the follicular epithelium and colloid in all experimental groups (Fig. 2B,E,H). In Orx + G rats the micro‐follicles were characterized by strong IHC positivity at the border of the colloid compartment, and some follicles exhibited strong IHC positivity throughout the whole colloid compartment (Fig. 2H). In SO animals, thyroid C‐cells immunostained for CT were numerous and occurred in large clusters (Fig. 2C). After Orx these cells were smaller, present either singly or in small clusters (Fig. 2F). The immunohistochemical features of thyroid C‐cells after genistein treatment were similar to those in the thyroids of Orx animals (Fig. 2I).
Figure 2.
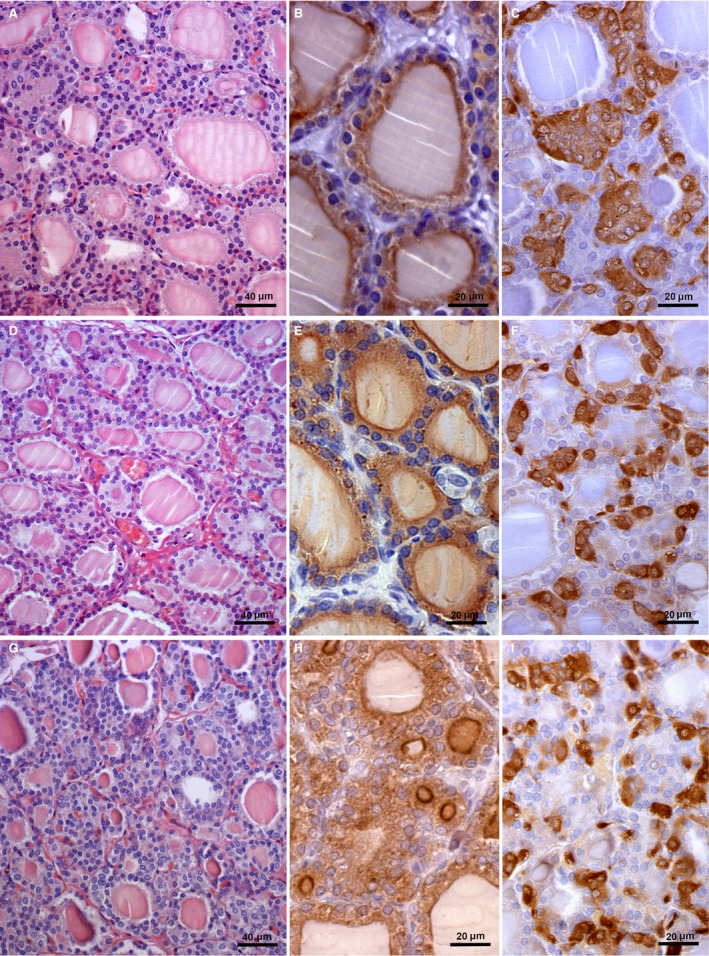
Thyroid gland in sham‐operated (SO) (A–C), orchidectomized (Orx) (D–F) and orchidectomized rats treated with genistein (Orx + G) (G–I); haematoxylin‐eosin stain (A,D,G); immunohistochemical staining of thyroglobulin (Tg) (B,E,H); calcitonin‐immunostained thyroid C‐cells (C,F,I).
Stereological analysis showed that genistein treatment increased relative Vv of the epithelium by 13 and 19% (P < 0.05) compared with Orx and SO animals, respectively. Relative Vv of the colloid was 20 and 25% lower (P < 0.05) in the Orx + G group than in the Orx and SO controls, respectively. Genistein treatment decreased the Vv of thyroid C‐cells by 9% (P < 0.05) in comparison with the value for the SO group (Fig. 3).
Figure 3.
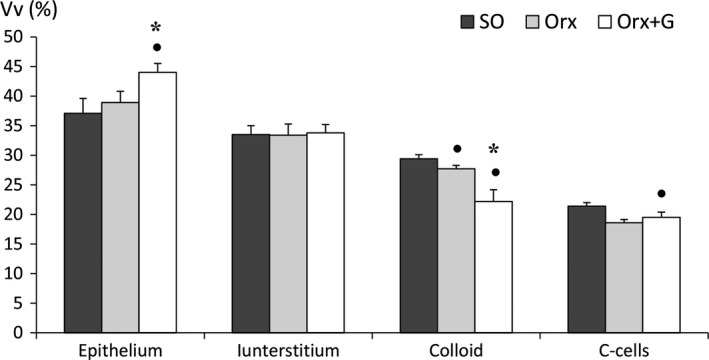
The relative volume density (Vv) of thyroid follicular epithelium, interstitium, colloid and C‐cells in sham‐operated (SO), orchidectomized (Orx) and orchidectomized rats treated with genistein (Orx + G). All values are mean ± SEM. Filled circle (•): P < 0.05 vs. SO. *P < 0.05 vs. Orx.
Biochemical findings in serum and urine
Earlier, we found that Orx markedly increased serum OC and urinary Ca2+ concentrations, whereas serum CT was diminished (Filipović et al. 2007). In genistein‐treated Orx rats, serum OC and urinary Ca2+ concentrations were decreased by 31 and 68% (P < 0.05), respectively, in comparison with the Orx group, while serum OC was 51% (P < 0.05) higher than in the SO group (Fig. 4A–B). After Orx, we detected no significant differences in serum T3 or T4 levels compared with SO values. Genistein administration lowered serum T3 and T4 concentrations by 7 and 32% (P < 0.05), respectively, in comparison with the Orx group and by 7 and 33% (P < 0.05) in relation to SO rats (Fig. 4C,D). No significant differences in serum CT level were detected between the Orx + G and Orx groups, but values for Orx + G rats were 51% lower (P < 0.05) than for the SO animals (Fig. 4E).
Figure 4.
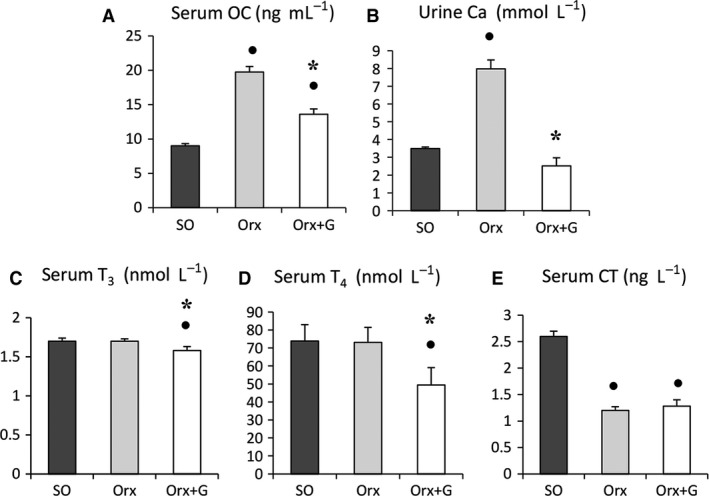
Serum osteocalcin (OC) concentration (A), urine calcium (Ca2+) concentration (B), serum triiodothyronine (T3) concentration (C), serum thyroxine (T4) concentration (D), serum calcitonin (CT) concentration (E) in sham‐operated (SO), orchidectomized (Orx) and orchidectomized rats treated with genistein (Orx + G). All values are mean ± SEM. Filled circle (•): P < 0.05 vs. SO. * P < 0.05 vs. Orx.
Discussion
Age‐related osteoporosis is accompanied by a decrease of available gonadal hormones in elderly men (Gennari et al. 2003) and androgen‐deficient male rats (Erben et al. 2000; Khalil et al. 2005; Filipović et al. 2007). Therefore, orchidectomized rats are the most commonly used animal model for studies of male osteoporosis.
Although estrogens have a protective effect on bone health and may be used to treat some diseases in men (Smith et al. 1998; Carani et al. 1999; Tangpricha et al. 2003; Vanderschueren et al. 2014), their therapeutic use is limited. Thus, certain hormone‐related tumours in transsexuals may be associated with the cross‐sex hormone therapy they had undergone (Mueller & Gooren, 2008). Therefore, important research has focused on examining the action of certain compounds that may serve as alternatives to hormone replacement therapy in the treatment of osteoporosis. The phytoestrogen genistein is a soybean isoflavone that is a known SERM. In this study, we evaluated how it affects trabecular bone and thyroid gland activity in orchidectomized middle‐aged male rats. Thyroid hormones are essential for bone metabolism.
Our results showed marked increases of B.Ar, Tb.Th and Tb.N and decreased Tb.Sp in our Orx rat model after genistein administration. These findings, together with the reduction of biochemical markers of bone metabolism, such as serum OC and urinary Ca2+ concentrations, clearly demonstrates that genistein treatment exerts a protective effect on trabecular bone that is normally reduced after Orx. Protective effects of isoflavones on trabecular bone microstructure and maintenance of bone homeostasis were described earlier in androgen‐deficient male rodents (Ishimi et al. 2002; Khalil et al. 2005; Filipović et al. 2010). Also, isoflavone supplementation reduced bone resorption in men with type 2 diabetes in whom the risk of fractures is increased (Sathyapalan et al. 2017).
The molecular mechanisms through which genistein affects bone metabolism involve ER‐dependent pathways. The family of ERs includes two receptor forms, ERα and Erβ (Dahlman‐Wright et al. 2006), and ERs have been detected in both osteoblasts and osteoclasts (Bord et al. 2001; Crusodé de Souza et al. 2009). Genistein has a higher binding affinity for Erβ and thus induces transcriptional activity (An et al. 2001). The mechanisms by which genistein can have an anabolic effect on bone include ER‐dependent stimulation of cell proliferation. Moreover, it induces ERα gene expression in osteoblasts and stimulates their differentiation and maturation (Pan et al. 2005; Liao et al. 2014). Apart from acting on ERs, as a tyrosine kinase inhibitor genistein may have a suppressive effect on osteoclasts, leading to a reduction in their number (Gao & Yamaguchi, 2000; Sliwinski et al. 2005).
As thyroid hormones have an essential role in bone metabolism, we have simultaneously evaluated the effects of genistein on thyroid follicular structure and function. In our Orx rat model, genistein treatment modulated thyroid parenchyma, which was mainly composed of small follicles containing less colloid. Follicular epithelium volume was increased, whereas colloid volume was reduced after genistein administration. Such findings point to enhanced thyroid activity (Gerber et al. 1981). However, the low serum T3 and T4 levels found after genistein treatment do not support this finding. It has been shown that isoflavones directly inhibit thyroid peroxidase (TPO), a key enzyme in the biosynthesis of thyroid hormones, both in vitro and in vivo, without affecting serum thyroid hormone concentrations in young adult rats (Divi et al. 1997; Chang & Doerge, 2000). As a result of the age‐related decline of thyroid gland activity (Šošić‐Jurjević et al. 2012), TPO enzymatic activity was probably reduced, with consequently decreased levels of circulating thyroid hormones (Šošić‐Jurjević et al. 2010, 2014).
The action of thyroid hormones on bone is mediated by specific receptors which have been detected in osteoblasts and osteoclasts (Allain et al. 1996). Thyroid hormones regulate cell differentiation, synthesis and degradation of bone matrix by stimulating osteoblast activity. T3 stimulates the expression and synthesis of type I collagen, alkaline phosphatase (ALP) and enhances synthesis and secretion of the bone matrix proteins osteopontin and osteocalcin in osteoblasts (Gouveia et al. 2001; Varga et al. 2010). In addition, by acting on osteoblasts, T3 can indirectly affect osteoclast activity by increasing the expression of osteoprotegerin (OPG), which is an inhibitor of bone resorption (Varga et al. 2004). However, although osteoclasts express thyroid receptors, it is not clear whether T3 affects them directly or indirectly through osteoblasts. In support of the latter are findings showing that T3 increases the expression of receptor activator of nuclear factor‐κB ligand (RANKL) in primary osteoblastic cells (Miura et al. 2002), as well as interleukin‐6 and interleukin‐8 in human bone marrow stromal and osteoblast‐like cells in vitro (Siddiqi et al. 2002). It is possible that, in addition to osteoblasts, other bone marrow cells can mediate the stimulatory effect of thyroid hormones on osteoclasts (Siddiqi et al. 2002). By acting on bone cells, thyroid hormones can regulate the bone remodelling cycle. Both bone formation and resorption are increased in conditions of thyroid hormone excess but resorption prevails over bone formation, resulting in reduction of bone mineral density (Waung et al. 2012).
In this study we detected no significant differences in any stereological or biochemical parameters indicating changed activity of thyroid C‐cells after genistein treatment. The main product of these cells is the hypocalcaemic hormone CT, which lowers circulating Ca2+ level and affects bone metabolism through direct inhibition of osteoclast activity (Warshawsky et al. 1980). However, in line with other research (Watanabe et al. 1992), daidzein treatment of Orx middle‐aged rats was found to stimulate C‐cell activity (Filipović et al. 2010). The biological mechanism underlying the different response of C‐cells to genistein and daidzein is uncertain, but membrane bilayer‐mediated perturbations might play a role (Ajdžanović et al. 2014). Also, the differences in the effects of these isoflavones may be either pharmacokinetic, pharmacodynamic, differing dose–response curves for genistein and daidzein, or a combination of these variables.
Moreover, the evidence from this and our earlier study (Pantelic et al. 2013) suggests that genistein affects bone metabolism additionally by acting indirectly on the regulation of thyroid hormone and PTH production, both of which play important roles in calcium homeostasis. It has been shown that genistein lowers serum PTH concentration and stimulates expression of PTHR1 and sodium phosphate cotransporter 2a (NaPi 2a) in the kidney as well as PTHR1 expression in bone (Miao et al. 2012; Pantelic et al. 2013). The anabolic effect of genistein in osteoblasts in vitro is reflected in the stimulation of PTH‐induced ALP activity and inhibition of OPG expression. This weakens osteoclastogenesis induction (Chen & Wong, 2006).
In conclusion, our study has shown that the protective effect of the phytoestrogen genistein on rat trabecular bone, may be achieved not only directly through ERs on bone cells but also indirectly by effects on the activity of thyroid follicular cells. Along with provoking histomorphometrical changes in the thyroid gland, genistein induced a decrease in circulating thyroid hormones. This phytoestrogen had no effect on CT production from thyroid C‐cells in our animal model of osteoporosis, indicating that other bone‐sparing mechanisms, such as changes in thyroid hormone concentrations, might play a more important role.
Acknowledgements
This work was supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia, Grant No. 173009. The authors Vladimir Ajdžanović, Jasmina Živanović and Verica Milošević are participating to the COST Action FA 1403 POSITIVe (Interindividual variation in response to consumption of plant food bioactives and determinants involved), supported by COST (European Cooperation in Science and Technology). The authors wish to express their gratitude to Dr Anne Judith Nikolić, PhD, for language correction of the manuscript.
Author contributions
B.F. and B.Š.‐J., organized and conducted the experiment, processed the experimental material, analysed and interpreted the results, and wrote most of the manuscript. V.A., J.Ž. and M.M.‐S. were involved in setting up the study and in the interpretation of the data. N.N., N.R. and S.T. participated in data collection. V.M. critically revised the manuscript and supplemented the discussion and literature survey.
References
- Ajdžanović VZ, Medigović IM, Pantelić JB, et al. (2014) Soy isoflavones and cellular mechanics. J Bioenerg Biomembr 46, 99–107. [DOI] [PubMed] [Google Scholar]
- Allain TJ, Yen PM, Flanagan AM, et al. (1996) The isoform‐specific expression of the tri‐iodothyronine receptor in osteoblasts and osteoclasts. Eur J Clin Invest 26, 418–425. [DOI] [PubMed] [Google Scholar]
- An J, Tzagarakis‐Foster C, Scharschmidt TC, et al. (2001) Estrogen receptor selective transcriptional activity and recruitment of coregulators by phytoestrogens. J Biol Chem 276, 17808–17814. [DOI] [PubMed] [Google Scholar]
- Bord S, Horner A, Beavan S, et al. (2001) Estrogen receptors alpha and beta are differentially expressed in developing human bone. J Clin Endocrinol Metab 86, 2309–2314. [DOI] [PubMed] [Google Scholar]
- Carani C, Rochira V, Faustini‐Fustini M, et al. (1999) Role of oestrogen in male sexual behavior: insights from the natural model of aromatase deficiency. Clin Endocrinol 51, 517–524. [DOI] [PubMed] [Google Scholar]
- Chang HC, Doerge DR (2000) Dietary genistein inactivates rat thyroid peroxidase in vivo without an apparent hypothyroid effect. Toxicol Appl Pharmacol 168, 244–252. [DOI] [PubMed] [Google Scholar]
- Chen WF, Wong MS (2006) Genistein modulates the effects of parathyroid hormone in human osteoblastic SaOS‐2 cells. Br J Nutr 95, 1039–1047. [DOI] [PubMed] [Google Scholar]
- Crusodé de Souza M, Sasso‐Cerri E, Cerri PS (2009) Immunohistochemical detection of estrogen receptor beta in alveolar bone cells of estradiol‐treated female rats: possible direct action of estrogen on osteoclast life span. J Anat 215, 673–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlman‐Wright K, Cavailles V, Fuqua SA, et al. (2006) International Union of Pharmacology. LXIV. Estrogen receptors. Pharmacol Rev 58, 773–781. [DOI] [PubMed] [Google Scholar]
- Daniell HW (1997) Osteoporosis after orchiectomy for prostate cancer. J Urol 157, 439–444. [PubMed] [Google Scholar]
- Divi RL, Chang HC, Doerge DR (1997) Anti‐thyroid isoflavones from soybean: isolation, characterization, and mechanisms of action. Biochem Pharmacol 54, 1087–1096. [DOI] [PubMed] [Google Scholar]
- Erben RG, Eberle J, Stahr K, et al. (2000) Androgen deficiency induces high turnover osteopenia in aged male rats: a sequential histomorphometric study. J Bone Miner Res 15, 1085–1098. [DOI] [PubMed] [Google Scholar]
- Evans G, Bryant HU, Magee D, et al. (1994) The effects of raloxifene on tibia histomorphometry in ovariectomized rats. Endocrinology 134, 2283–2288. [DOI] [PubMed] [Google Scholar]
- Falahati‐Nini A, Riggs BL, Atkinson EJ, et al. (2000) Relative contributions of testosterone and estrogen in regulating bone resorption and formation in normal elderly men. J Clin Invest 106, 1553–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipović B, Šošić‐Jurjević B, Ajdžanović V, et al. (2007) The effect of orchidectomy on thyroid C cells and bone histomorphometry in middle‐aged rats. Histochem Cell Biol 128, 153–159. [DOI] [PubMed] [Google Scholar]
- Filipović B, Sosić‐Jurjević B, Ajdzanović V, et al. (2010) Daidzein administration positively affects thyroid C cells and bone structure in orchidectomized middle‐aged rats. Osteoporos Int 21, 1609–1616. [DOI] [PubMed] [Google Scholar]
- Gao YH, Yamaguchi M (2000) Suppressive effect of genistein on rat bone osteoclasts: involvement of protein kinase inhibition and protein tyrosine phosphatase activation. Int J Mol Med 5, 261–267. [DOI] [PubMed] [Google Scholar]
- Gennari L, Merlotti D, Martini G, et al. (2003) Longitudinal association between sex hormone levels, bone loss, and bone turnover in elderly men. J Clin Endocrinol Metab 88, 5327–5333. [DOI] [PubMed] [Google Scholar]
- Gerber H, Studer H, Conti A, et al. (1981) Reaccumulation of thyroglobulin and colloid in rat and mouse thyroid follicles during intense thyrotropin stimulation. A clue to the pathogenesis of colloid goiters. J Clin Invest 68, 1338–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouveia CH, Schultz JJ, Bianco AC, et al. (2001) Thyroid hormone stimulation of osteocalcin gene expression in ROS 17/2.8 cells is mediated by transcriptional and post‐transcriptional mechanisms. J Endocrinol 70, 667–675. [DOI] [PubMed] [Google Scholar]
- Ishimi Y, Yoshida M, Wakimoto S, et al. (2002) Genistein, a soybean isoflavone, affects bone marrow lymphopoiesis and prevents bone loss in castrated male mice. Bone 31, 180–185. [DOI] [PubMed] [Google Scholar]
- Kanatani M, Sugimoto T, Sowa H, et al. (2004) Thyroid hormone stimulates osteoclast differentiation by a mechanism independent of RANKL‐RANK interaction. J Cell Physiol 201, 17–25. [DOI] [PubMed] [Google Scholar]
- Khalil DA, Lucas EA, Smith BJ, et al. (2005) Soy isoflavones may protect against orchidectomy‐induced bone loss in aged male rats. Calcif Tissue Int 76, 56–62. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Carlsson B, Grandien K, et al. (1997) Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology 138, 863–870. [DOI] [PubMed] [Google Scholar]
- Liao MH, Tai YT, Cherng YG, et al. (2014) Genistein induces oestrogen receptor‐α gene expression in osteoblasts through the activation of mitogen‐activated protein kinases/NF‐κB/activator protein‐1 and promotes cell mineralisation. Br J Nutr 111, 55–63. [DOI] [PubMed] [Google Scholar]
- Lunt M, Felsenberg D, Reeve J, et al. (1997) Bone density variation and its effects of risk of vertebral deformity in men and women studied in thirteen European centers. The EVOS Study. J Bone Miner Res 12, 1883–1894. [DOI] [PubMed] [Google Scholar]
- Miao Q, Li JG, Miao S, et al. (2012) The bone‐protective effect of genistein in the animal model of bilateral ovariectomy: roles of phytoestrogens and PTH/PTHR1 against post‐menopausal osteoporosis. Int J Mol Sci 13, 56–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura M, Tanaka K, Komatsu Y, et al. (2002) A novel interaction between thyroid hormones and 1,25‐OH 2 D 3 in osteoclast formation. Biochem Biophys Res Commun 291, 987–994. [DOI] [PubMed] [Google Scholar]
- Mueller A, Gooren L (2008) Hormone‐related tumors in transsexuals receiving treatment with cross‐sex hormones. Eur J Endocrinol 159, 197–202. [DOI] [PubMed] [Google Scholar]
- Nieves JW, Formica C, Ruffing J, et al. (2005) Males have larger skeletal size and bone mass than females, despite comparable body size. J Bone Miner Res 20, 529–535. [DOI] [PubMed] [Google Scholar]
- Pan W, Quarles LD, Song LH, et al. (2005) Genistein stimulates the osteoblastic differentiation via NO/cGMP in bone marrow culture. J Cell Biochem 94, 307–316. [DOI] [PubMed] [Google Scholar]
- Pantelic J, Ajdzanovic V, Medigovic I, et al. (2013) Genistein affects parathyroid gland and NaPi 2a cotransporter in an animal model of the andropause. J Physiol Pharmacol 64, 361–368. [PubMed] [Google Scholar]
- Parfitt AM, Drezner MK, Glorieux FH, et al. (1987) Bone histomorphometry: standardization of nomenclature, symbols, and units. J Bone Miner Res 2, 595–610. [DOI] [PubMed] [Google Scholar]
- Penrod JD, Litke A, Hawkes WG, et al. (2008) The association of race, gender, and comorbidity with mortality and function after hip fracture. J Gerontol A Biol Sci Med Sci 63, 867–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picherit C, Coxam V, Bennetau‐Pelissero C, et al. (2000) Daidzein is more efficient than genistein in preventing ovariectomy‐induced bone loss in rats. J Nutr 130, 1675–1681. [DOI] [PubMed] [Google Scholar]
- Roselli CE, Abdelgadir SE, Resko JA (1997) Regulation of aromatase gene expression in the adult rat brain. Brain Res Bull 44, 351–357. [DOI] [PubMed] [Google Scholar]
- Sathyapalan T, Aye M, Rigby AS, et al. (2017) Effect of soy on bone turn‐over markers in men with type 2 diabetes and hypogonadism – a randomised controlled study. Sci Rep 7, 15366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqi A, Parsons MP, Lewis JL, et al. (2002) TR expression and function in human bone marrow stromal and osteoblast‐like cells. J Clin Endocrinol Metab 87, 906–914. [DOI] [PubMed] [Google Scholar]
- Simpson ER, Dowsett M (2002) Aromatase and its inhibitors: significance for breast cancer therapy. Recent Prog Horm Res 57, 317–338. [DOI] [PubMed] [Google Scholar]
- Sliwinski L, Folwarczna J, Janiec W, et al. (2005) Differential effects of genistein, estradiol and raloxifene on rat osteoclasts in vitro. Pharmacol Rep 57, 352–359. [PubMed] [Google Scholar]
- Smith DC, Redman BG, Flaherty LE, et al. (1998) A phase II trial of oral DES as a second‐line hormonal agent in advanced prostate cancer. Urology 52, 257–260. [DOI] [PubMed] [Google Scholar]
- Šošić‐Jurjević B, Filipović B, Ajdzanović V, et al. (2010) Suppressive effects of genistein and daidzein on pituitary‐thyroid axis in orchidectomized middle‐aged rats. Exp Biol Med 235, 590–598. [DOI] [PubMed] [Google Scholar]
- Šošić‐Jurjević B, Filipović B, Renko K, et al. (2012) Orchidectomy of middle‐aged rats decreases liver deiodinase 1 and pituitary deiodinase 2 activity. J Endocrinol 215, 247–256. [DOI] [PubMed] [Google Scholar]
- Šošić‐Jurjević B, Filipović B, Wirth EK, et al. (2014) https://www.ncbi.nlm.nih.gov/pubmed/24793811 Soy isoflavones interfere with thyroid hormone homeostasis in orchidectomized middle‐aged rats. Toxicol Appl Pharmacol 278, 124–134. [DOI] [PubMed] [Google Scholar]
- Sternberger LA, Hardy PH Jr, Cuculis JJ, et al. (1970) The unlabeled antibody enzyme method of immunohistochemistry. Preparation and properties of soluble antigen–antibody complex (horseradish peroxidase‐antihorseradish peroxidase) and its use in identification of spirochetes. J Histochem Cytochem 18, 315–333. [DOI] [PubMed] [Google Scholar]
- Tangpricha V, Duchrame SH, Barber TW, et al. (2003) Endocrinologic treatment of gender identity disorders. Endocr Pract 1, 12–21. [DOI] [PubMed] [Google Scholar]
- Tuukkanen J, Peng Z, Väänänen HK (1994) Effect of running exercise on the bone loss induced by orchidectomy in the rat. Calcif Tissue Int 55, 33–37. [DOI] [PubMed] [Google Scholar]
- Vanderschueren D, Laurent MR, Claessens F, et al. (2014) Sex steroid actions in male bone. Endocr Rev 35, 906–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga F, Spitzer S, Klaushofer K (2004) Triiodothyronine (T3) and 1,25‐dihydroxyvitamin D 3 (1,25‐D 3) inversely regulate OPG gene expression in dependence of the osteoblastic phenotype. Calcif Tissue Int 74, 382–387. [DOI] [PubMed] [Google Scholar]
- Varga F, Rumpler M, Zoehrer R, et al. (2010) T3 affects expression of collagen I and collagen cross‐linking in bone cell cultures. Biochem Biophys Res Commun 402, 180–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warshawsky H, Goltzman D, Rouleau MF, et al. (1980) Direct in vivo demonstration by radioautography of specific binding sites for calcitonin in skeletal and renal tissues of the rat. J Cell Biol 85, 682–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K, Takekoshi S, Kakudo K (1992) Effects of ipriflavone on calcitonin synthesis in C cells of the rat thyroid. Calcif Tissue Int 51, 27–29. [DOI] [PubMed] [Google Scholar]
- Waung JA, Bassett JH, Williams GR (2012) Thyroid hormone metabolism in skeletal development and adult bone maintenance. Trends Endocrinol Metab 23, 155–162. [DOI] [PubMed] [Google Scholar]
- Weibel ER (1979) Practical Methods for Biological Morphometry. Stereological Methods, Vol. 1. London: Academic Press. [Google Scholar]


