Abstract
Background
Ovarian cancer is the second most common malignant tumor of the female reproductive system and is the leading cause of death of gynecological malignancies, but at present there is no effective and safe therapy. There is no previously published report on the anti-cancer effect of prucalopride, which is a high-affinity 5-HT4 receptor. The aim of the present study was to determine whether prucalopride can inhibit proliferation of ovarian cancer cells.
Material/Methods
The cell viability was detected by use of the Cell Counting Kit-8 (CCK-8) assay. The invasion and migration of SKOV3 and OVCAR3 cells was detected by Transwell assay. The cell apoptosis was detected by apoptosis flow detection and Caspase-Glo 3/7 Assay Systems. The apoptosis-related proteins, autophagy marker proteins, and the related-factors of phosphatidylinositol 3-kinase (PI3K) were detected by Western blot.
Results
The CCK-8 proliferation test showed that prucalopride inhibited the growth of ovarian cancer cell lines SKOV3 and OVCAR3. In the Transwell assay, prucalopride inhibited cell invasion and migration. Furthermore, we found the expression of anti-apoptotic protein Bcl-2 decreased, whereas the expression of pro-apoptotic protein Caspase3 and Bax increased in the SKOV3 cell line treated with prucalopride, as well as cleaved PARP. In addition, the expression of p-AKT, p-mTOR, and p70S6K decreased in the prucalopride-treated group, and the expression of autophagy marker protein LC3-II/I and Beclin1 significantly increased, whereas the expression of p62 protein decreased.
Conclusions
The present study reveals that in ovarian cancer cells, prucalopride inhibits proliferation, migration, and invasion, and induces apoptosis and autophagy, which may be regulated by the PI3K signaling pathway. These results suggest prucalopride has potential as a new drug for clinical ovarian cancer treatment.
MeSH Keywords: Antineoplastic Agents, Autophagy, Ovarian Neoplasms
Background
Ovarian cancer is the most deadly gynecological malignancy, with more than 120 000 new deaths in the world every year, which pose a serious threat to women’s health [1–3]. Approximately 70% of patients with ovarian cancer are diagnosed in the later period, and the 5-year survival rate for ovarian cancer is only 30–40% [4,5]. Chemotherapy is a commonly used method for the treatment of ovarian cancer, but there are some problems with this approach [6]. For example, ovarian cancer patients are more likely to develop resistance to chemotherapy drugs due to the continuous use of drugs [7]. Therefore, it is urgent to develop low-toxicity and efficient new drugs for ovarian cancer treatment.
The 5-hydroxytryptamine 4 (5-HT4) receptor is a member of the G-protein-coupled family of receptors, which is widely expressed in smooth muscle cells (including ovarian smooth muscle cells) and on enteric neurons in the gastrointestinal tract [8]. Studies have shown that the activation of 5-HT4 receptor in the cholinergic nerve endings of the enteric nervous system increases the release of acetylcholine in motor neurons, thereby stimulating gastrointestinal motility [9]. Thus, a new generation of 5-HT4 receptor agonists is being used for the treatment of gastrointestinal peristalsis disorders [10]. Studies have found that 5-HT4 receptors up-regulated the expression of estrogen receptor β in hormone-naive prostates, which may affect the progression of prostate cancer [11]. It is also reported that the functional role of 5-HT4 receptors in ovarian physiology is consistent with a tumor suppressor role in ovarian carcinogenesis [12]. Prucalopride, as a high-affinity 5-HT4 receptor, has potent gastrointestinal and colonic prokinetic activity [13,14]. Prucalopride is different from other 5-HT4 receptor agonists in that its cardiac adverse effects are negligible in pharmacology [15]. Studies have shown that prucalopride can be used to treat chronic constipation without significant adverse effects [16]. Prucalopride also accelerates gastric emptying and reduces esophageal acid exposure in healthy adults, suggesting that it has potential application in the treatment of gastro-esophageal reflux disease [15]. However, there are no previously published studies on the roles of prucalopride in cancer treatments.
In the present study, we found that in ovarian cancer cells, prucalopride treatment can inhibit proliferation, migration, and invasion, and induce apoptosis and autophagy, which might be regulated by the phosphatidylinositol 3-kinase (PI3K) signaling pathway.
Material and Methods
Cell lines and cell culture
The ovarian cancer cell lines SKOV3 and OVCAR3 were obtained from the USA ATCC Company. Cells were cultured in Roswell Park Memorial Institute-1640 (RPMI-1640) medium (HYCLONE, USA) supplemented with 10% (for OVCAR3, 20%) serum (Gibco, USA), 100 U/ml penicillin (SIGMA, USA), and 0.1 mg/ml streptomycin (SIGMA, USA), at 37°C in an incubator with 5% CO2. When the cells were cultured to enter the logarithmic growth phase, they were washed 3 times with PBS and digested with trypsin (Beijing Solarbio Science & Technology, China). After the cells become round, RPMI-1640 was added to end the digestion, and repeated shaking was performed to transform the cell suspension into a single cell suspension, then the cells were seeded into 6-well plates for subsequent experiments.
Cell Counting Kit-8 (CCK8) proliferation test
The conventional ovarian cancer cultured cells were digested and counted for preparing cell suspension. We seeded 100 μl of cell suspension onto 96-well plates with 1000 cells per well; 0.1% DMSO (AMRESCO, USA) was added in the negative control (NC) group, and 0.1 μM, 1 μM, and 10 μM prucalopride (MCE, USA) were added in the experimental group. Then, the cells were cultured in a CO2 incubator, and we assessed cell viability once every 24 h. Before detection, 10 μl of CCK8 solution (CWBIO, China) was added into each well and incubated at 37°C for 1.5 h. The OD value was measured with a microplate reader in 450-nm excitation light. Finally, the multiplication curve was plotted.
Cell invasion and migration assessed by Transwell assay
We added 100 μl of Matrigel (BD, USA) dissolved overnight (serum-free RPMI-1640 medium diluted 1: 6) to the upper chamber of a 24-well Transwell device (Millipore, USA). After shaking, the Matrigel was placed into the CO2 incubator for 4–6 h of culturing at 37°C until gel formation. After drying the culture medium, 500 μl of serum-free medium was added to the bottom of wells to hydrate the basement membrane for 30 min. The cells suspension, treated with prucalopride for 24 h, was prepared using serum-free RPMI-1640 medium. We loaded 100 μl (1×105) of cell suspension into the upper Transwell chamber and 500 μl of complete culture medium was added to the bottom Transwell chamber for incubation overnight. On the next day (24 h for SKOV3 and 48 h for OVCAR3), the Transwell was removed, and cells remaining on the upper chamber were removed with a cotton swab. After washing with PBS, the cells adhering to the membrane were fixed in 4% paraformaldehyde for 30 min, and then were stained with 0.1% crystal violet for 20 min. After washing with PBS, 5 visual fields were selected random using a microscope, and we took pictures to observe the count. The migration experiment procedure was similar to that of the invasion assay, and the Matrigel was not required to be used in the Transwell chamber and the number of cells was 5000.
Cell apoptosis assay
After SKOV3 cells were treated with prucalopride for 24 h, the medium was removed and replaced with serum-free medium. After starvation under conventional conditions for 24 h, cells were harvested, digested with trypsin digestion without EDTA, collected in a centrifuge tube, centrifuged at 1000 rpm for 5 min, and resuspended in pre-cooling PBS at 4°C. Then, the cells were centrifuged once again and the supernatant was carefully aspirated. We added 1× binding buffer to resuspend the cells, and regulated the cell density to 1–5×106/ml. We transferred 100 μl of cell suspension into a 5-ml flow tube, after first staining with 5 μl Annexin V/FITC (Beijing 4A Biotech, China) for 5 min at room temperature in the dark. Then, after staining with 10 μl propidium iodide (PI) and 400 μl PBS, cells were collected and assessed using flow cytometry. Results were analyzed by use of FlowJo software.
Caspase-Glo 3/7 assay
The assay was performed with the Caspase-Glo 3/7 Assay System (Promega). SKOV3 cells were seeded onto 96-well plates. After the cells grew to 50% confluence (24 h), 10 μM prucalopride was added in the experimental group. Following the above treatments for 24 h, 100 μL reagent was added to each well and gently mixed for 1 min. The 96-well plates were then incubated at room temperature for 1 h. The luminescence of each sample was measured in a plate-reading luminometer. Data are presented as the mean ±SD from 3 replicates.
Western blot
When the cell density reached about 80%, the cells were treated with 10 μM prucalopride for 24 h, and the NC group was treated with 0.1% DMSO. Then, the protein was extracted with RIPA lysis buffer (including protease inhibitor) (CWBIO, China). The concentration was determined by BCA (CWBIO, China) method. Then, the protein was heated at 95°C for 5 min. Approximately 20 μg of protein per group was added to each well in the vertical electrophoresis tank, then it was separated in 10% SDS-PAGE electrophoresis and transferred onto a PVDF membrane. After blocking with 5% non-fat milk for 1 h, the membrane was incubated with primary antibodies at 4°C overnight. On the second day, the membrane was washed 3 times in 0.1% Tween 20-TBS (TBST) for 5 min each time, then incubated with secondary antibodies at room temperature for 1 h. After the membrane was washed, an enhanced-chemiluminescence (ECL, PTG, USA) chromogenic substrate was added to visualize the bands. The gray value was scanned by use of Quantity One software. The GAPDH was recognized as the internal control. The relative expression of each protein was calculated by Objective protein/GAPDH. Western blot analyses were performed with the following antibodies: primary antibody rabbit anti-human, PARP(1: 1000, CST, USA), Bcl-2 (1: 1000, PTG, USA), Bax (1: 1000, PTG, USA), Active-Caspase3 (1: 1000, PTG, USA), AKT (1: 1000, CST, USA), p-AKT (1: 1000, CST, USA), mTOR (1: 1000, CST, USA), p-mTOR (1: 1000, CST, USA), p70S6K (1: 1000, PTG, USA), LC3 (1: 1000, PTG, USA), Beclin-1 (1: 1000, PTG, USA), p62 (1: 1000, PTG, USA), GAPDH (1: 5000, PTG, USA); secondary antibody HRP-labeled goat anti-rabbit/goat anti-mouse (1: 5000, PTG, USA).
Statistical analysis
The experimental data were analyzed by SPSS18.0 statistical analysis software. The data are expressed as the mean ±SD. The t test was used to compare 2 groups. P < 0.05 was considered as indicating a statistically significant difference.
Results
Prucalopride inhibits ovarian cancer cell SKOV3 and OVCAR3 proliferation
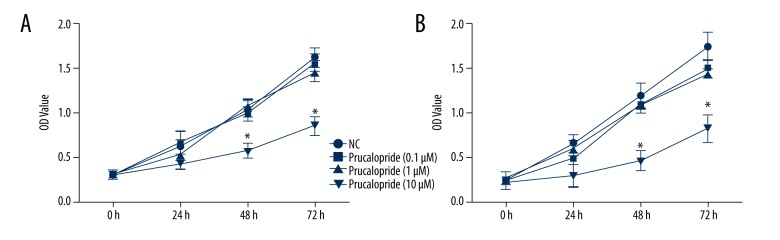
To evaluate the effect of prucalopride on ovarian cancer, we selected ovarian cancer cell lines SKOV3 and OVCAR3, and used the concentrations of 0.1 μM, 1 μM, and 10 μM prucalopride to detect cell proliferation. In this experiment, the cells were cultured for 24 h, 48 h, and 72 h. The results of the CCK8 proliferation test indicated that only the 10-μM prucalopride treatment apparently reduced the number cells at 48 h and 72 h (P<0.05, Figure 1). Both SKOV3 (Figure 1A) and OVCAR3 (Figure 1B) cell lines showed the similar results. These results suggest that prucalopride effectively inhibits ovarian cancer cell proliferation in a dose- and time-dependent manner. We then used 10 μM prucalopride in the following experiments.
Figure 1.
Effect of prucalopride on SKOV3 and OVCAR3 cell proliferation by CCK-8 assay. (A) SKOV3 cells were treated with prucalopride (0.1, 1, 10 μM) for different lengths of time (24, 48, 72 h). (B) OVCAR3 cells were treated with prucalopride (0.1, 1, 10 μM) for the same different time points. * P<0.05 compared with NC group.
Prucalopride inhibits ovarian cancer cell SKOV3 invasion and migration
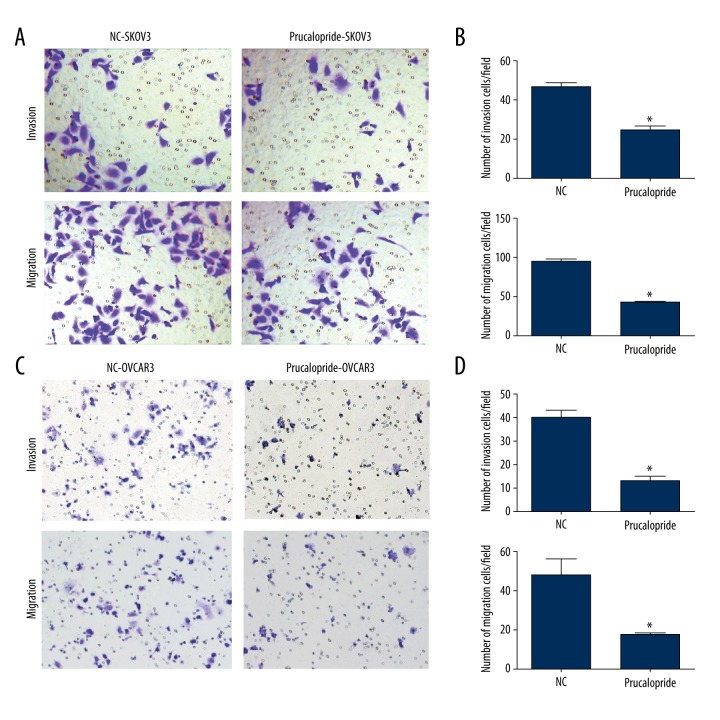
Then we used Transwell assay to analyze the effects of prucalopride on the invasion and migration ability of SKOV3 and OVCAR3. The number of SKOV3 cells with positive crystal violet staining was reduced by prucalopride treatment in the invasion experiment (P<0.05, Figure 2A), and SKOV3 cells were also reduced in the migration experiment (P<0.05, Figure 2A). The quantification results of SKOV3 cell line were showed in Figure 2B. The same results were found in OVCAR3 cell line (P<0.05, Figure 2C). The quantification results of OVCAR3 cell line were showed in Figure 2D These results suggest that prucalopride significantly inhibits invasion and migration of ovarian cancer cells.
Figure 2.
Prucalopride inhibits SKOV3 and OVCAR3 cell invasion and migration. (A, C) The images show the invasion and migration ability of SKOV3 and OVCAR3 cells stained by crystal violet. Images were captured using an inverted microscope with ×100 magnification. (B, D) The invading and migrating cells are shown by quantification. * P<0.05 compared with NC group.
Prucalopride promotes ovarian cancer cell SKOV3 apoptosis
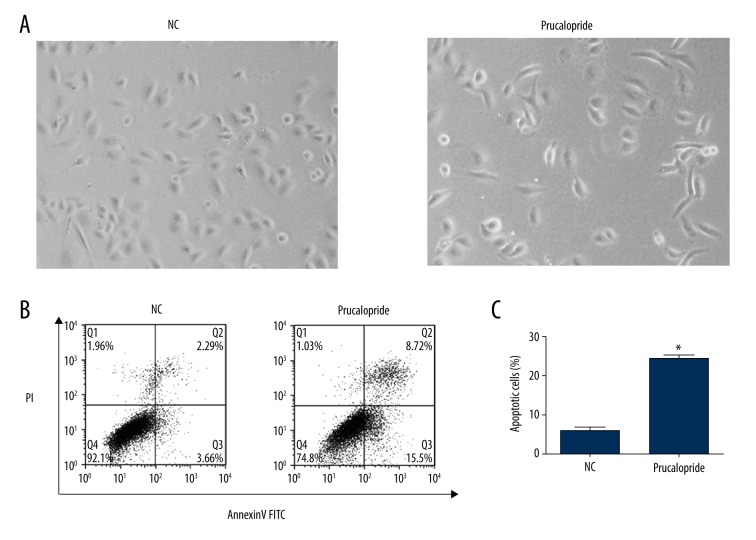
After treatment with prucalopride, the ovarian cancer cell line SKOV3 displayed typical morphological changes of apoptosis, including cell shrinkage, increased brightness, and detachment from the substratum (Figure 3A). Annexin V-FITC and PI double-staining assay were also used to determine the effect of prucalopride on SKOV3 cell apoptosis. After induction of serum-free apoptosis, the apoptosis rate in the prucalopride-treated group was significantly increased compared with the control group (P<0.05, Figure 3B). The quantification results were showed in Figure 3C.
Figure 3.
Prucalopride induces SKOV3 cell apoptosis as shown by morphological changes and Annexin V-FITC/PI staining assay. (A) Morphological changes in SKOV3 cells treated with prucalopride, including cell shrinkage, increased brightness, and detachment from the substratum. (B) The apoptosis of SKOV3 cells (treated with prucalopride for 24 h) was analyzed by Annexin V-FITC and PI, illustrated by representative flow charts and quantification. (C) The quantification results were showed. * P<0.05 compared with NC group.
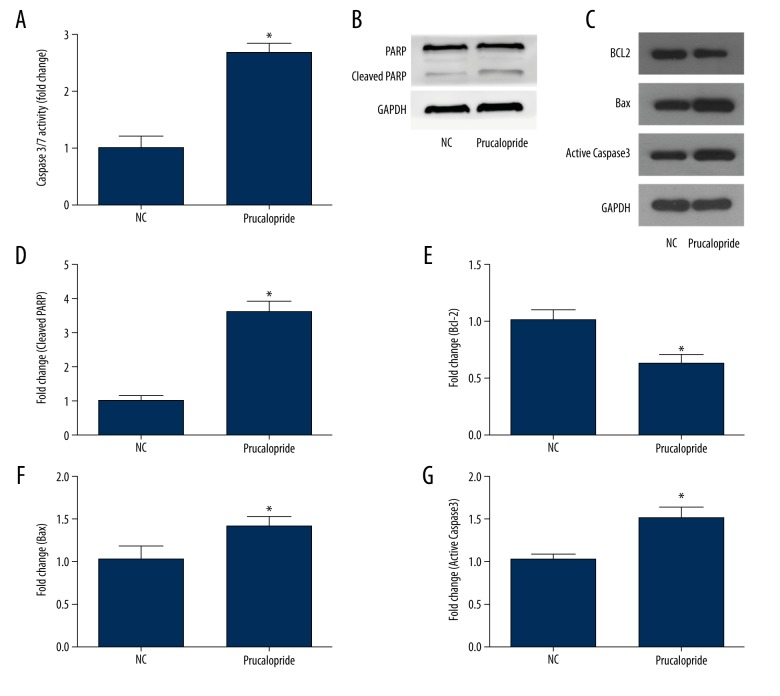
Caspase-Glo 3/7 assay was applied to detect the activity of caspase 3/7, showing that prucalopride-treated cells had a significant increase in caspase 3/7 activity (P<0.05, Figure 4A). Moreover, Western blot was used to analyze the apoptosis regulators, such as anti-apoptotic protein Bcl-2, pro-apoptotic protein Caspase3, Bax, and cleaved PARP (Figure 4B–4G). Compared with the control group, the expression of pro-apoptotic protein Caspase3, Bax, and cleaved PARP increased in the prucalopride-treated group, and the expression of anti-apoptotic protein Bcl-2 decreased (P<0.05, Figure 4C, 4E). These results indicate that prucalopride promotes SKOV3 cell apoptosis.
Figure 4.
Prucalopride induces SKOV3 cell apoptosis as shown by Caspase-Glo 3/7 assay and Western blot analysis. (A) Prucalopride-treated cells had a significant increase in caspase 3/7 activity. (B, C) Western blot analysis of SKOV3 cells treated with prucalopride for 24 h. The band intensities were quantified. The results were all normalized to the GAPDH loading control. (D–G) Relative protein levels of apoptosis-related protein. * P<0.05 compared with NC group.
Prucalopride promotes autophagy of ovarian cancer cell line SKOV3
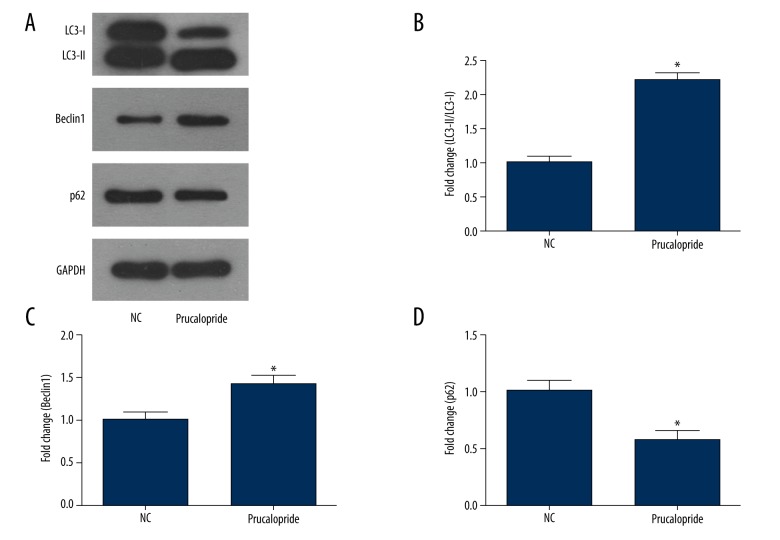
To determine if prucalopride can promote autophagy of SKOV3, we used Western blot analysis. The results showed that the autophagy of SKOV3 cells in the prucalopride-treated group was enhanced (P<0.05, Figure 6A), and the expression of autophagy marker protein LC3-II/I was significantly increased (P<0.05, Figure 6B). The expression of Beclin1 increased and the expression of P-62 protein decreased (P<0.05, Figure 6C, 6D). These results indicate that prucalopride promotes SKOV3 cell autophagy.
Figure 6.
Prucalopride promotes autophagy of ovarian cancer cell SKOV3. (A) Expression levels of LC3-II, Beclin1, and p62 were measured in the SKOV3 cells treated with prucalopride using Western blot analysis. (B–D) The relative protein levels of LC3-II, Beclin1, and p62. * P<0.05 compared with NC group.
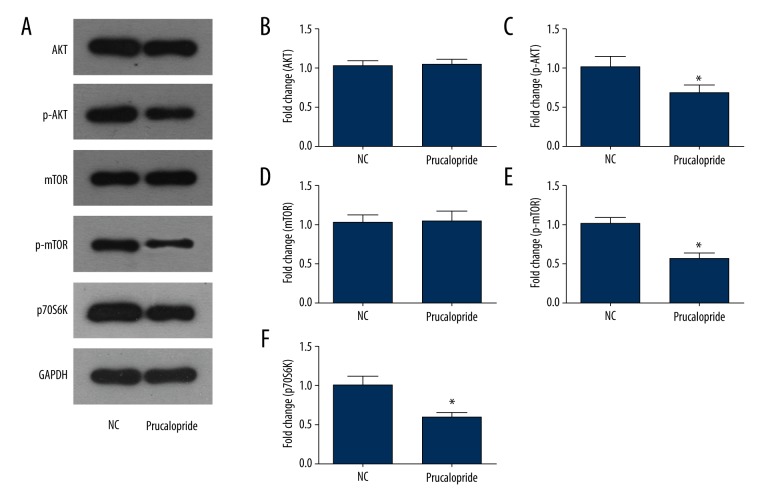
Prucalopride suppresses the PI3K pathway in SKOV3 ovarian cancer cells
PI3K is a very important signaling pathway in cancer. After prucalopride treatment, AKT, mTOR, and p70S6K proteins were selected as indicators to evaluate the activity of the PI3K signaling pathway (Figure 5A). The results of Western blot analysis indicated that the phosphorylation level of AKT and mTOR decreased significantly in the prucalopride-treated SKOV3 cells (P<0.05, P<0.05, Figure 5B–5E). Similarly, the expression level of p70S6K was reduced in the prucalopride-treated group (P<0.05, Figure 5F). These results suggest that prucalopride-induced SKOV3 cell growth is regulated by the PI3K pathway.
Figure 5.
Effects of prucalopride on the PI3K signaling pathway in SKOV3 cells. (A) Expression levels of AKT, p-AKT, mTOR, p-mTOR, and p70S6K were measured in the SKOV3 cell treatment with prucalopride using Western blot analysis. (B–F) The relative protein levels of AKT, p-AKT, mTOR, p-mTOR, and p70S6K. * P<0.05 compared with NC group.
Discussion
In this study we conducted a preliminary investigation on the anti-tumor effect of prucalopride, a high-affinity 5-HT4 receptor agonist, in ovarian cancer, and explored its potential molecular mechanism. We found that prucalopride inhibits SKOV3 and OVCAR3 cell proliferation, invasion, and migration, and showed that cell apoptosis was significantly promoted by prucalopride. Finally, we found that the effects of prucalopride on ovarian cancer cells might be related to the PI3K signal pathway and autophagy.
In the present study, ovarian cancer cells were treated with prucalopride. The results of the CCK8 proliferation test indicated that prucalopride inhibits ovarian cancer cell proliferation in a dose- and time-dependent manner. In the Transwell assay, prucalopride significantly inhibited invasion and migration of SKOV3 and OVCAR3 cells. Studies have found that 5-HT plays a role in promoting in vitro proliferation of human hepatoma cells [17]. It has been reported that 5-HT plays positive and negative roles in liver cancer. On the one hand, platelet-derived 5-HT can stimulate liver regeneration in mouse hepatectomy models, and 5-hydroxy chromatin plays an important role in human liver regeneration and contributes to the recovery of liver function in patients with liver cancer. On the other hand, 5-HT can regulate levels and activity of 5-HT receptor on endothelial cells and tumor cells to stimulate tumor growth, which is not conducive to the recovery of patients with liver cancer [18,19]. In our report, the mechanism of prucalopride inhibited ovarian cancer cell line SKOV3 proliferation, which was similar to the 5-HT positive effect.
We also found that prucalopride can affect the expression of apoptosis-related proteins. The results of Western blot analysis showed that the expression of Bcl-2 decreased and the expression of Bax, cleaved PARP, and Caspase3 increased in the prucalopride-treated group; these 4 are well-characterized regulators of apoptosis [20]. Bcl-2 is an anti-apoptotic protein, while Bax and Caspase3 are pro-apoptotic proteins [21]. Apoptosis is an important pathway for anti-tumor effects, and many antineoplastic agents play an important role in cancer through it [11].
Our results of Western blot analysis showed that p-AKT, p-mTOR, and p70S6K were decreased significantly in the prucalopride-treated group. The activation of apoptosis is regulated by many signaling pathways, among which PI3K is an important pathway [22]. PI3K, as a member of the lipid kinases family, plays a major role in regulation of apoptosis, angiogenesis, cellular metabolism, and senescence [23]. The key proteins AKT and mTOR, which are the PI3K downstream kinases, have important effects on the proliferation and metastasis of cancer cells [24]. Studies also have indicated that p70S6K is located downstream of the PI3K/AKT/mTOR pathway, which is closely associated with cell proliferation [25]. Our results are consistent with these studies, indicating that prucalopride targets key components of the PI3K pathway, which might be important in the successful treatment of ovarian cancer.
Our results of Western blot analysis also found that the autophagy of SKOV3 cells was enhanced in the prucalopride-treated group. The expression of autophagy marker proteins LC3 II and Beclin1 were significantly increased, while the expression of P-62 was decreased in the prucalopride-treated group. Autophagy (type II procedural cell death) is a highly regulated process that is generally activated in reaction to adverse environments [26]. In the field of cancer research, autophagy is defined as a lysosomal degradation of cellular components, which has recently received considerable attention [27]. Among the various proteins involved in autophagy, light chain 3 (LC3), Beclin1, and p62 markers are commonly used to assess autophagic activity [28]. LC3 participates in elongation and contributes to autophagosome formation [29]. Beclin1, a mammalian orthologue of yeast autophagy-related protein 6 encoded by the Becn1 gene, interacts with several cofactors to induce nucleation and initiation of the isolation during autophagy [30]. p62 is a scaffold protein that transfers the ubiquitinated protein to the autophagosome [31]. Our results are consistent with these studies, showing that prucalopride targets key proteins in autophagy, which might be important in the treatment of ovarian cancer.
Clinically, prucalopride is used in patients with severe chronic constipation in whom laxatives fail to provide adequate relief. Once-daily prucalopride at 4 mg for 4 weeks is effective and well tolerated in patients [32]. However, studies about the roles of prucalopride in cancer treatments have not been reported. Drug repositioning is a method used to find a new pharmacological effect of a drug for which human safety and pharmacokinetics are established and to expand the therapeutic range of the drug to other diseases [33]. For gynecologic tumors, new drugs in this field may be found by drug repositioning, such as inhibitors of the mevalonate pathway [34] and metformin [35]. These drug discoveries can be performed at quickly and at low cost, based on the results of previous clinical trials, which is promising in the field of ovarian cancer treatment.
Conclusions
We showed that prucalopride inhibits ovarian cancer cell proliferation, invasion, and migration, and promotes cell apoptosis through the PI3K signaling pathway and autophagy. Our study may provide a prospect for further clinical research on prucalopride in ovarian cancer treatment. However, our investigation of the mechanism of prucalopride in ovarian cancer was only a preliminary study, and further verification is needed, such as in vivo experimental verifications and data on the impact of other signaling pathways.
Footnotes
Source of support: This study was supported by Key research and development projects in Shandong Province, China (2016GSF201150)
Conflicts of interest
None.
References
- 1.Worzfeld T, Pogge von Strandmann E, Huber M, et al. The unique molecular and cellular microenvironment of ovarian cancer. Front Oncol. 2017;7:24. doi: 10.3389/fonc.2017.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yeung TL, Leung CS, Li F, et al. Targeting stromal-cancer cell crosstalk networks in ovarian cancer treatment. Biomolecules. 2016;6:3. doi: 10.3390/biom6010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yan B, Yin F, Wang QI, et al. Integration and bioinformatics analysis of DNA-methylated genes associated with drug resistance in ovarian cancer. Oncol Lett. 2016;12:157–66. doi: 10.3892/ol.2016.4608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Faber MT, Kjaer SK, Dehlendorff C, et al. Cigarette smoking and risk of ovarian cancer: A pooled analysis of 21 case-control studies. Cancer Causes Control. 2013;24:989–1004. doi: 10.1007/s10552-013-0174-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weidle UH, Birzele F, Kollmorgen G, Rueger R. Mechanisms and targets involved in dissemination of ovarian cancer. Cancer Genomics Proteomics. 2016;13:407–23. doi: 10.21873/cgp.20004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bax HJ, Josephs DH, Pellizzari G, et al. Therapeutic targets and new directions for antibodies developed for ovarian cancer. MAbs. 2016;8:1437–55. doi: 10.1080/19420862.2016.1219005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Correa RJ, Valdes YR, Peart TM, et al. Combination of AKT inhibition with autophagy blockade effectively reduces ascites-derived ovarian cancer cell viability. Carcinogenesis. 2014;35:1951–61. doi: 10.1093/carcin/bgu049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shin A. Patient considerations in the management of chronic constipation: Focus on prucalopride. Patient Prefer Adherence. 2016;10:1373–84. doi: 10.2147/PPA.S92550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morita H, Mochiki E, Takahashi N, et al. Effects of 5-HT2B, 5-HT3 and 5-HT4 receptor antagonists on gastrointestinal motor activity in dogs. World J Gastroenterol. 2013;19:6604–12. doi: 10.3748/wjg.v19.i39.6604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang B, Sun X, Wang S, et al. Comparative pharmacokinetics of (S)-MP3950, a novel 5-HT4 receptor agonist, in normal and atropine-induced gastrointestinal motility disorders rats. Xenobiotica. 2017 doi: 10.1080/00498254.2017.1365974. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 11.Nakamura Y, Ise K, Yamazaki Y, et al. Serotonin receptor 4 (5-hydroxytryptamine receptor Type 4) regulates expression of estrogen receptor beta and cell migration in hormone-naive prostate cancer. Indian J Pathol Microbiol. 2017;60:33–37. doi: 10.4103/0377-4929.200022. [DOI] [PubMed] [Google Scholar]
- 12.Henriksen R, Dizeyi N, Abrahamsson PA. Expression of serotonin receptors 5-HT1A, 5-HT1B, 5-HT2B and 5-HT4 in ovary and in ovarian tumours. Anticancer Res. 2012;32:1361–66. [PubMed] [Google Scholar]
- 13.Van de Velde V, Vandeplassche L, et al. Effect of prucalopride on the pharmacokinetics of oral contraceptives in healthy women. Drugs R D. 2013;13:43–51. doi: 10.1007/s40268-013-0008-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Emmanuel A, Cools M, Vandeplassche L, Kerstens R. Prucalopride improves bowel function and colonic transit time in patients with chronic constipation: An integrated analysis. Am J Gastroenterol. 2014;109:887–94. doi: 10.1038/ajg.2014.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yi CH, Lei WY, Hung JS, et al. Effects of prucalopride on esophageal secondary peristalsis in humans. Clin Transl Gastroenterol. 2016;7:e202. doi: 10.1038/ctg.2016.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Camilleri M, Piessevaux H, Yiannakou Y, et al. Efficacy and safety of prucalopride in chronic constipation: An integrated analysis of six randomized, controlled clinical trials. Dig Dis Sci. 2016;61:2357–72. doi: 10.1007/s10620-016-4147-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fatima S, Shi X, Lin Z, et al. 5-Hydroxytryptamine promotes hepatocellular carcinoma proliferation by influencing beta-catenin. Mol Oncol. 2016;10:195–212. doi: 10.1016/j.molonc.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pang Q, Liu C, Zhang JY, et al. Serotonin in liver tumor: Friend or foe? Hepatology. 2015;62:319. doi: 10.1002/hep.27568. [DOI] [PubMed] [Google Scholar]
- 19.Starlinger P, Assinger A, Haegele S, et al. Evidence for serotonin as a relevant inducer of liver regeneration after liver resection in humans. Hepatology. 2014;60:257–66. doi: 10.1002/hep.26950. [DOI] [PubMed] [Google Scholar]
- 20.Mei JM, Niu CS. Effects of CDNF on 6-OHDA-induced apoptosis in PC12 cells via modulation of Bcl-2/Bax and caspase-3 activation. Neurol Sci. 2014;35:1275–80. doi: 10.1007/s10072-014-1700-1. [DOI] [PubMed] [Google Scholar]
- 21.Zhuang RJ, Ma J, Shi X, et al. Cold-inducible protein RBM3 protects UV irradiation-induced apoptosis in neuroblastoma cells by affecting p38 and JNK pathways and Bcl2 family proteins. J Mol Neurosci. 2017;63(2):142–51. doi: 10.1007/s12031-017-0964-3. [DOI] [PubMed] [Google Scholar]
- 22.Zhou ZW, Li XX, He ZX, et al. Induction of apoptosis and autophagy via sirtuin1- and PI3K/Akt/mTOR-mediated pathways by plumbagin in human prostate cancer cells. Drug Des Devel Ther. 2015;9:1511–54. doi: 10.2147/DDDT.S75976. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Akinleye A, Avvaru P, Furqan M, et al. Phosphatidylinositol 3-kinase (PI3K) inhibitors as cancer therapeutics. J Hematol Oncol. 2013;6:88. doi: 10.1186/1756-8722-6-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang L, Huo X, Liao Y, et al. Zeylenone, a naturally occurring cyclohexene oxide, inhibits proliferation and induces apoptosis in cervical carcinoma cells via PI3K/AKT/mTOR and MAPK/ERK pathways. Sci Rep. 2017;7:1669. doi: 10.1038/s41598-017-01804-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Halacli SO, Dogan AL. FOXP1 regulation via the PI3K/Akt/p70S6K signaling pathway in breast cancer cells. Oncol Lett. 2015;9:1482–88. doi: 10.3892/ol.2015.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shim BY, Sun DS, Won HS, et al. Role of autophagy-related protein expression in patients with rectal cancer treated with neoadjuvant chemoradiotherapy. BMC Cancer. 2016;16:207. doi: 10.1186/s12885-016-2250-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim HM, Kim ES, Koo JS. Expression of autophagy-related proteins in different types of thyroid cancer. Int J Mol Sci. 2017;18(3) doi: 10.3390/ijms18030540. pii: E540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi JH, Cho YS, Ko YH, et al. Absence of autophagy-related proteins expression is associated with poor prognosis in patients with colorectal adenocarcinoma. Gastroenterol Res Pract. 2014;2014:179586. doi: 10.1155/2014/179586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alirezaei M, Flynn CT, Wood MR, et al. Coxsackievirus can exploit LC3 in both autophagy-dependent and -independent manners in vivo. Autophagy. 2015;11:1389–407. doi: 10.1080/15548627.2015.1063769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Correa RJ, Valdes YR, Shepherd TG, DiMattia GE. Beclin-1 expression is retained in high-grade serous ovarian cancer yet is not essential for autophagy induction in vitro. J Ovarian Res. 2015;8:52. doi: 10.1186/s13048-015-0182-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bortnik S, Gorski SM. Clinical applications of autophagy proteins in cancer: From potential targets to biomarkers. Int J Mol Sci. 2017;18(7) doi: 10.3390/ijms18071496. pii: E1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coremans G, Kerstens R, De Pauw M, Stevens M. Prucalopride is effective in patients with severe chronic constipation in whom laxatives fail to provide adequate relief. Results of a double-blind, placebo-controlled clinical trial. Digestion. 2003;67:82–89. doi: 10.1159/000070202. [DOI] [PubMed] [Google Scholar]
- 33.Banno K, Iida M, Yanokura M, et al. Drug repositioning for gynecologic tumors: A new therapeutic strategy for cancer. ScientificWorldJournal. 2015;2015:341362. doi: 10.1155/2015/341362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kobayashi Y, Kashima H, Rahmanto YS, et al. Drug repositioning of mevalonate pathway inhibitors as antitumor agents for ovarian cancer. Oncotarget. 2017;8:72147–56. doi: 10.18632/oncotarget.20046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Irie H, Banno K, Yanokura M, et al. Metformin: A candidate for the treatment of gynecological tumors based on drug repositioning. Oncol Lett. 2016;11:1287–93. doi: 10.3892/ol.2016.4075. [DOI] [PMC free article] [PubMed] [Google Scholar]