Abstract
Coarse ground meat was mixed with non-meat ingredients and starter culture (Pediococcus acidilactici) and then inoculated with an 8-strain cocktail of Shiga toxin-producing Escherichia coli (ca. 7.0 log CFU/g). Batter was fine ground, stuffed into fibrous casings, and fermented at 35.6°C and ca. 85% RH to a final target pH of ca. pH 4.6 or ca. pH 5.0. After fermentation, the pepperoni-like sausage were heated to target internal temperatures of 37.8°, 43.3°, 48.9°, and 54.4°C and held for 0.5 to 12.5 h. Regardless of the heating temperature, the endpoint pH in products fermented to a target pH of pH 4.6 and pH 5.0 was pH 4.56±0.13 (range of pH 4.20 to pH 4.86) and pH 4.96±0.12 (range of pH 4.70 to pH 5.21), respectively. Fermentation alone delivered ca. a 0.3- to 1.2-log CFU/g reduction in pathogen numbers. Fermentation to ca. pH 4.6 or ca. pH 5.0 followed by post-fermentation heating to 37.8° to 54.4°C and holding for 0.5 to 12.5 h generated total reductions of ca. 2.0 to 6.7 log CFU/g.
Key words: Shiga toxin-producing Escherichia coli, Fermentation, Food Safety, Thermal Inactivation, Heating
Introduction
Shiga toxin-producing cells of Escherichia coli (STEC) continue to pose a significant threat to public health as evidenced by their recovery from a variety of higher volume and higher risk foods, the observance of both large and small recalls of such foods due to the presence of regulated serotypes of these bacteria, and the frequency and documentation of severe illnesses attributed to pathogenic strains of these bacteria associated with consumption of contaminated and perhaps under-processed and/or improperly handled foods (CDC, 1995a; 1995b; 2010; USDA-FSIS, 2010). Although not the sole/primary vehicle of sporadic cases and outbreaks, raw and further processed beef was responsible for several illnesses and recalls over the past 35 years (Griffin et al., 2003; Kaspar et al., 2010; Page, 2018). Several reports have been published, particularly since the early- to mid-1990’s and likely in response to the much publicized salami outbreaks in the U.S. (CDC, 1995a) and Australia (CDC, 1995b), detailing the fate of serotype O157:H7 cells of STEC in a variety of both short- and longterm ripened cured-dried sausage and reporting on validated interventions and processes for their control (Balamurugan et al., 2017; Calicioglu et al., 2002; Faith et al., 1997, 1998; Glass et al., 2012; Heir et al., 2013; Holck et al., 2011; Hinkens et al., 1996; McLeod et al., 2016; Riordan et al., 1998; Rode et al., 2012). As detailed in our previous publication (Hinkens et al., 1996), although there are a variety of pepperoni types and sizes, three main categories dominate the market: i) small diameter (28-36 mm) for the deli case, ii) medium diameter (49-55 mm) for pizza topping, and iii) large diameter (60-80 mm) for sandwiches. As a result of the Jack-in-the-Box outbreak attributed to undercooked hamburger patties in the early 1990’s in the U.S. (CDC 1993) and to some extent the salami outbreaks attributed to survival of STEC in salami products soon thereafter (CDC 1995a, 1995b), both serotype O157:H7 and subsequently the following serotypes of STEC, namely O26:H11, O45:H2, O103:H2, O111:H-, O121:H19, and O145:NM (aka The Big Six), are considered adulterants in raw/non-intact meats (USDA-FSIS, 2011). As such, producers are required by the United States Department of Agriculture-Food Safety and Inspection Service (USDA-FSIS) to validate a 2- or 5-log reduction of these pathogens during manufacture of fermented meats (Reed, 1995a, 1995b). With the exception of a study by Glass et al. (2012) wherein strains of the six regulated non-O157:H7 serotypes of STEC were evaluated, most prior studies evaluated the fate of serotype O157:H7 strains of E. coli in dry-fermented-type sausage (Faith et al., 1997, 1998; Hinkens et al., 1996; Riordan et al., 1998). Given the current regulatory posture that The Big Six strains of non-O157:H7 serotypes and strains of O157:H7 are considered adulterants in raw/non-intact meats (USDA-FSIS, 2011), further studies are warranted to validate the comparative fate of these additional STEC strains/serotypes of E. coli in fermented meats.
Numerous studies were conducted since the early 1990’s to monitor viability of STEC in a variety of dry and semi-dry fermented sausage. In general, results to date established that fermentation alone is sufficient to deliver about a 1- to 2-log reduction of pathogen levels in products such as soudjouk, pepperoni, Genoa salami, and Norwegian dry-sausage (Calicioglu et al., 2002; Faith et al., 1997, 1998; Glass et al., 2012; Heir et al., 2013; Hinkens et al., 1996; Holck et al., 2011; Nissen and Holck, 1998; Porto-Fett et al., 2008, 2010; Riordan et al., 1998; Rode et al., 2012). In addition to the abovementioned peer-reviewed publications that quantify reductions in levels of STEC during fermentation and drying of fermented meats, in the mid-1990’s the National Cattlemen’s Beef Association (NCBA) commissioned a study to validate typical processing parameters used by industry for comparative and collective lethality during manufacture of dry and semi-dry fermented sausage. In general, the resulting NCBA Blue Ribbon Task Force Report (Nickelson et al., 1996) validated several processes that achieved either a 2- or a 5-log reduction of E. coli O157:H7 and identified processes/steps in the manufacture of dry/fermented sausage“…useful in evaluating greater (more severe) or lesser processes when evaluating a lower or higher risk….” Processes/steps shown to be of higher risk included: i) high pH, ii) beef ingredient, iii) high initial coliform count – ingredient, and iv) low fermentation temperature. It is general knowledge that pH, salt, fermentation and drying temperatures and times, in combination with relative humidity, curing salts, and presence of secondary metabolites, may also appreciably affect viability of STEC in fermented sausage. That being said, most of the data published to date suggests that post-fermentation heating is the only effective and reliable method to achieve a 5-log reduction of STEC in certain dry-fermented sausage products such as pepperoni without adversely affecting product quality (Faith et al., 1997, 1998; Glass et al., 2012; Heir et al., 2013; Hinkens et al., 1996; Holck et al., 2011; Riordan et al., 1998). Collectively, these data confirmed that traditional processes for pepperoni production were only sufficient to deliver about a 2-log reduction of E. coli O157:H7, and with the possible exception of a relatively recent paper (Glass et al., 2012), there has been little information published on the fate of non-O157:H7 cells of STEC in fermented sausage. For control of E. coli O157:H7, the two most widely accepted and practiced post-fermentation heating parameters are heating to internal temperatures of 62.8°C instantaneous or to 53.3°C and then holding for 60 min (Hinkens et al., 1996; Nickelson et al., 1996). Thus, the purpose of this study was to validate the lethality of post-fermentation heating times and temperatures for lethality towards STEC to provide manufacturers with additional processes/options for ensuring the safety of fermented sausage.
Materials and Methods
Bacterial strains
The eight rifampicin-resistant (Rifr) strains of Shiga toxin-producing Escherichia coli [STEC-8; (i) USDA-FSIS 380-94 (meat isolate, serotype O157:H7), (ii) JB1-95 (clinical isolate, serotype O111:H-), (iii) CDC 96-3285 (human stool, serotype O45:H2), (iv) CDC 90-3128 (human stool, serotype O103:H2), (v) CDC 97-3068 (human stool, serotype O121:H19), serotype O121:H19, (vi) 83-75 (human stool, serotype O145:NM), (vii) H30 (infant with diarrhea, serotype O26:H11), and (viii) ATCC BAA-2326 (human stool, serotype O104:H4)] used in this study were confirmed, cultured, and maintained as described previously (Luchansky et al., 2008).
Manufacture of a pepperoni-type sausage
The pepperoni-type sausage for this study was prepared essentially as described previously (Hinkens et al., 1996) using fresh pork and beef trimmings (75:25 pork:beef with 30% fat) obtained from a local butcher (Illg’s Meats, Chalfont, PA, USA). The dry ingredients, starter culture, and casings were donated by a cooperating commercial sausage manufacturing company (John Morrell Food Group, Lisle, IL, USA). The batter was comprised of a dry spice mix (3.69%; Saratoga Food Specialties, Bolingbrook, IL, USA), cure salt (3.60%; 6.25% sodium nitrite), and a commercial starter culture (0.0188%; Pediococcus acidilactici; Saga 200; Kerry Ingredients & Flavors, Beloit, WI, USA). For each trial, the batter (ca. 7 kg) was inoculated with 160 ml of the STEC-8 cocktail to achieve an initial level of ca. 7.0 log CFU/g. Next, the inoculated batter was fine ground through a 3/8-inch plate (Model 4346; Hobart, Troy, OH, USA), and then stuffed into a 55-mm fibrous casing using a floor-type, hydraulic-driven piston stuffer (50 lb capacity; Model SC-50, Koch Equipment, Kansas City, MO, USA). The resulting chubs (ca. 290 g; 18 cm L × 5.5 cm D) were stapled/clipped (Max HR-PS II; Salco, East Syracuse, NY, USA) and then fermented at 35.6°C and 85% relative humidity (RH) in an environmental chamber (Model ES 2000 CDC-DW; Bahnson Environmental Specialties, Winston Salem, NC, USA) until an endpoint target pH of either ca. pH 4.6 or ca. pH 5.0 was achieved. After fermentation (ca. 8 to 12 h), the environmental chamber temperature was adjusted to 37.8°C, 43.3°C, 48.9°C, or 54.4°C and the chubs were heated for up to 12.5, 8, 4, and 4 h, respectively, at a RH of 95%. Note, the resulting chubs were not dried after the post-fermentation heating component of this study. A single trial consisted of a freshly-grown cocktail comprised of each of the 8 STEC strains inoculated into a fresh meat block that was subsequently fermented to a single endpoint pH (either ca. pH 4.6 or ca. pH 5.0) and then separately subjected to one of each of the 4 post-fermentation heating regimens. At least two trials (N=2), but up to 4 trials (N=4), were conducted for each endpoint pH in combination with one of each of the associated 4 heating regimens.
Microbiological and physical-chemical analyses
At each sampling interval, a 25-gram portion from each of 3 chubs was separately analyzed (n=3) essentially as described (Hinkens et al., 1996). The samples were macerated (Stomacher 400; Seward, Cincinnati, OH, USA) and then plated, with and without prior dilution in 0.1% peptone water, onto sorbitol-MacConkey (Difco, BD, Franklin Lakes, NJ, USA) plus rifampicin (100 µg/ml; Sigma Chemical Company, St. Louis, MO, USA) agar plates. When pathogen levels decreased to below the detection limit (≤0.47 log CFU/g) by direct plating, chubs testing negative for the pathogen by direct plating were enriched as previously described (Hinkens et al., 1996). In addition to enumerating surviving STEC-8, the pH of each sample was measured as described (Porto-Fett et al., 2008) using a model 6000P pH/temperature electrode and a model 5500 pH meter (Daigger, Vernon Hills, IL, USA). Water activity was measured using an electronic water activity meter (Decagon Aqualab Model Series 3; Decagon Devices, Pullman, WA, USA).
Statistical analyses
Means and standard deviations were calculated for each of the endpoint pH and for each of the post-fermentation heating regimens using triplicate sausage samples at each sampling interval. Data were analyzed using the Microsoft Excel 2013 software (Redmond, WA).
Results
Fermentation at 35.6°C and 85% RH to a comparatively lower (i.e., pH 4.6) or a comparatively higher (i.e., pH 5.0) endpoint pH delivered a 1- to 2-log decrease in levels of STEC-8 inoculated into the pepperoni-type sausage evaluated in the present study (Figures 1-4). More specifically, the average pH of the batter was pH 5.77±0.30 (range of pH 5.28 to pH 6.60), whereas the average pH in products fermented to a target pH of ca. pH 4.6 and ca. pH 5.0 was pH 4.56±0.13 (range of pH 4.20 to pH 4.86) and pH 4.96±0.12 (range of pH 4.70 to pH 5.21), respectively. No changes in aw were observed during fermentation; the average aw of the batter was aw 0.944±0.006 (range of aw 0.934 to aw 0.959), whereas after fermentation the average aw was aw 0.944±0.011 and aw 0.940±0.008 (range of aw 0.917 to aw 0.964) in products fermented to a target pH of ca. pH 4.6 and ca. pH 5.0, respectively. Fermentation delivered reductions of ca. 0.9 to 1.1 and ca. 0.3 to 1.2 log CFU/g in products fermented to an endpoint pH of ca. pH 4.6 and pH 5.0, respectively.
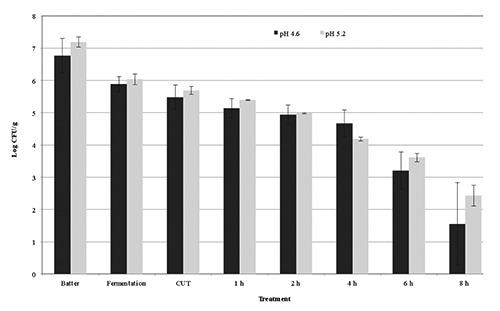
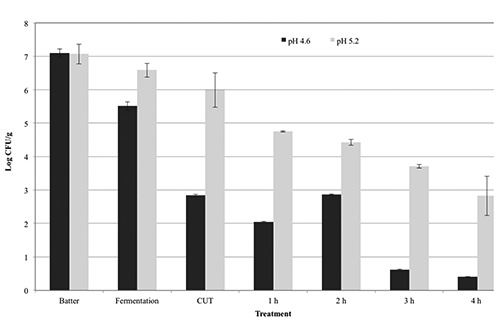
Products were also subjected to a postfermentation heating step for lethality towards STEC-8. In general, additional reductions of ca. 0.4 to 2.0 and 0.4 to 1.2 log CFU/g were observed during the come-up-time (CUT) after fermentation to pH 4.6 and pH 5.0, respectively, until achievement of post-fermentation heating temperatures of 43.3° to 54.4°C (Figures 1-4); no additional reductions were observed during the CUT for post-fermentation heating temperatures of 37.8°C. Lastly, for sausage fermented to pH 4.6, additional reductions of 0.1 to 1.5, 0.3 to 4.0, 0.2 to 2.3, and 1.9 to 3.7 log CFU/g in levels of STEC-8 were achieved during post-fermentation heating to 37.8°, 43.3°, 48.9°, and 54.4°C, respectively. Similarly, for sausage fermented to pH 5.0, additional reductions of ca. 0.2 to 1.1, 0.3 to 5.2, 1.2 to 3.2, and 2.4 to 4.4 log CFU/g were achieved during post-fermentation heating to 37.8°, 43.3°, 48.9°, and 54.4°C, respectively., After post-fermentation heating, an additional decrease in pH of the sausage was observed for all fermentation and heating conditions tested. More specifically, the pH of sausage fermented to ca. pH 4.6 and ca. pH 5.0 after post-fermentation heating decreased to pH 4.14 to pH 4.30 and pH 4.23 to pH 4.40, respectively. However, no appreciable changes in aw were observed after post-fermentation heating for sausage fermented to either ca. pH 4.6 (aw 0.944) or ca. pH 5.0 (aw 0.940). Regardless of the target endpoint pH, the average aw of sausage after fermentation and heating was aw 0.945±0.007 (range from aw 0.929 to aw 0.953). In general, longer times at higher temperatures and lower pH levels delivered greater reductions of STEC-8; however, survivors were recovered by direct plating and/or by enrichment for all pH, time, and temperature conditions tested. Although post-fermentation heating to 48.9° and 54.4°C for longer than 2 h resulted in greater reductions in STEC-8 numbers, these treatments adversely affected the texture of the product. Perhaps due to the fat content of the product (ca. 30%), as well as the above mentioned extended post-fermentation heating times and higher temperatures, liquified fat was observed throughout portions of some sausage chubs.
Discussion
Fermented sausage have been produced and consumed for centuries, and largely without untoward consequences until some 30 years ago with the emergence of acid-tolerant serotypes of Escherichia coli that produce Shiga toxins (Griffin et al., 2003). In general, STEC are somewhat more tolerant of the lower pH and aw of a traditional fermented sausage than most other food-borne pathogens; therefore, to achieve a 2-or 5-log reduction in levels of STEC as stipulated by USDA-FSIS (Reed, 1995a, 1995b) it may be necessary to develop additional ingredients or starter cultures to inhibit this pathogen or to validate post-fermentation interventions, including heat, high pressure, storage at ambient temperatures, freezing – thawing, and/or irradiation to deliver the required lethality (Heir et al., 2013; Holck et al., 2011; Rode et al., 2012). In so doing, every effort must also be made to preserve product quality and to manage costs. For these reasons, and based on information already published, the goal of the present study was to validate post-fermentation time/temperature heating regimens for lethality towards STEC.
Prior to the present study, the primary post-fermentative heating regimens for dry-fermented sausage that were validated and published in a peer-reviewed journal were: (i) holding the product at 53.3°C for up to 60 min, or (ii) heating the product to an internal instantaneous temperature of 62.8°C (Glass et al., 2012; Hinkens et al., 1996). Both of these heating regimens were selected primarily based on meeting the requirements for trichinae destruction (Hinkens et al., 1996). Additionally, the process of heating to an internal instantaneous temperature of 62.8°C approximated conditions established by USDA-FSIS for cooked and/or roast beef, and was subsequently approved as an option to control E. coli O157:H7 in fermented sausage (Hinkens et al., 1996). The process for heating to 53.3°C and holding for 60 min was selected because it was less damaging to the sensory attributes of pepperoni than heating to 62.8°C (Hinkens et al., 1996). In related studies, (Heir et al., 2013), salami and Norwegian Morr dry-fermented sausage were subjected to post-fermentation heating at 32°, 43°, 50°, 60°, or 65°C for 30 min to 6 days depending on the temperature; reductions of 3.5 to >5.5 log CFU/g were achieved with only minor untoward effects on product quality. According to these authors, the abovementioned time and temperature parameters were selected based on guidelines to achieve a 5-log reduction of STEC in ready-to-eat fermented sausage as published by the Health Protection Branch of the Health Canada Agency. In contrast, Graumann and Holley (2008) reported reductions of ca. 3.5 to 7.0 log CFU/g within 30 days of drying of a salami-type sausage via inclusion of 2 to 6% of non-deheated ground mustard to the batter with the cure ingredients.
The literature is replete with studies confirming that fermentation alone will only deliver a ≤2.0 log decrease in levels of STEC, and that post-fermentation heating is the only effective and reliable intervention to achieve a 5-log reduction of vegetative cells of most foodborne pathogens in fermented sausage while limiting untoward consequences on product quality (Faith et al., 1997, 1998; Glass et al., 2012; Hinkens et al., 1996; Holck et al., 2011; Incze, 1998; Lindqvist and Lindblad, 2009; Riordan et al., 1998). In addition to the lowering of pH by the action of the starter culture, sodium nitrite, a common ingredient in fermented sausage, can also inhibit serotype O157:H7 strains of STEC (Morita et al., 2004; Tsai and Chou, 1996). Collectively, inclusion of both intrinsic and extrinsic hurdles such as nitrite, starter cultures, or competitive flora, along with smoking and drying, can in large measure enhance product safety (Leistner, 2000; Bohnlein et al., 2016). That being said, the use of unrealistically high initial levels of the pathogen coupled with the uneven distribution in the meat of the pathogen, and the acid elaborated by the starter culture may explain, at least in part, the recovery of sporadic survivors of STEC even after post-fermentation heating of product as observed in the present study. More specifically, for sausage fermented to pH 4.6 and then heated, a ≥5-log CFU/g reduction was achieved in 8 h at 43.3°C, in >4 h at 48.9°C, and in 1 h at 54.4°C, whereas for sausage fermented to pH 5.0 and then heated, a ≥5-log CFU/g reduction was achieved in 8 h at 43.3°C, in 5 h at 48.9°C, and in 2 h at 54.4°C. Note, heating to 37.8°C delivered total reductions of 2.5 log CFU/g for chubs fermented to pH 4.6 and total reductions of 1.4 log CFU/g for chubs fermented to pH 5.0. Although a post-fermentation heating/drying step per se was not conducted in the present study, it is highly likely that further reductions in pathogen levels would be achieved following a typical drying regimen for a pepperoni-type sausage for a pepperoni-type sausage following a post-fermentation heating step.
Conclusions
Our results validated that fermentation to pH 4.6 or pH 5.0 delivered about a ≥5-log reduction in pathogen levels, but only after post-fermentation heating for 1 to 8 hours at 43.3° to 54.4°C. Further reductions likely achieved during subsequent drying may allow for lower temperatures and shorter times for post-fermentation heating and would be the primary objective of an interesting companion study. Regardless, the data herein provide manufacturers of dry-fermented sausage with several options to validate/achieve the required reduction of STEC while producing a high-quality and wholesome product. These data also confirm that processes previously validated as effective for serotype O157:H7 strains of E. coli will likely be as effective toward strains of the other six regulated serotypes of STEC and strains of serotype O104:H4 of E. coli.
Figure 1.
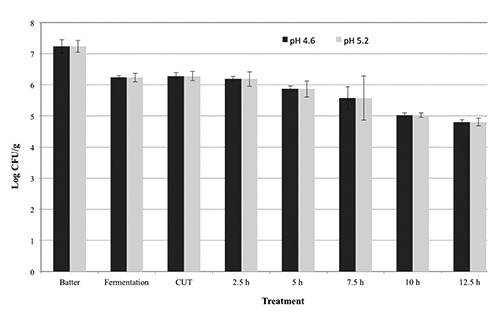
Inactivation of STEC in pepperoni-type sausage fermented to pH 4.6 or pH 5.0 with heating and holding at 37.8°C. Error bars represent the standard deviation of the mean (N=4, n=3).
Figure 3.
Inactivation of STEC in pepperoni-type sausage fermented to pH 4.6 or pH 5.2 with heating and holding at 48.9°C. Error bars represent the standard deviation of the mean (N=2, n=3).
Figure 2.
Inactivation of STEC in pepperoni-type sausage fermented to pH 4.6 or pH 5.0 with heating and holding at 43.3°C. Error bars represent the standard deviation of the mean (N=2, n=3).
Figure 4.
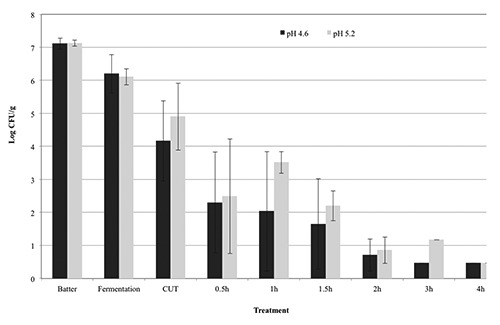
Inactivation of STEC in pepperoni-type sausage fermented to pH 4.6 or pH 5.2 with heating and holding at 54.4°C. Error bars represent the standard deviation of the mean (N=4, n=3).
Acknowledgments
The authors offer sincere appreciation to Nelly Osoria and Sarah Wadsworth (USDA-ARS, PA, USA) for their assistance on this project. Special thanks are also extended to Valentina Klein (Delaware Valley University, PA, USA), Deena Ghazzi and Kelsey Yoo (Ursinus College, PA, USA), and Maddie Munson (Drexel University, PA, USA).
Funding Statement
Funding: This material is based upon work that is supported by the National Institute of Food and Agriculture, United States Department of Agriculture, under award number #2012-68003-30155. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the United States Department of Agriculture.
References
- Balamurugan S, Ahmed R, Gao A, Strange P, 2017. Comparison of the fate of the top six non-O157 shiga-toxin producing Escherichia coli (STEC) and E. coli O157:H7 during the manufacture of dry fermented sausages. Int J Food Microbiol 259:14-21. [DOI] [PubMed] [Google Scholar]
- Böhnlein C, Kabisch J, Meske D, Franz CMAP, Pichner R, 2016. Fitness of Enterohemorrhagic Escherichia coli (EHEC)/Enteroaggregative E. coli O104:H4 in comparison to that of EHEC O157: survival studies in food and in vitro. Appl Environ Microbiol 82:6326-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calicioglu M, Faith NG, Buege DR, Luchansky JB, 2002. Viability of Escherichia coli O157:H7 during manufacturing and storage of a fermented, semidry soudjouk-style sausage. J Food Prot 65:1541-4. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, 1993. Update: Multistate outbreak of Escherichia coli O157:H7 infections from hamburgers — Western United States, 1992-1993. Morbid Mortal Weekly Rep 42:258-63. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, 1995a. Community outbreak of hemolytic uremic syndrome attributable to Escherichia coli O111:NM — South Australia, 1995. Morbid Mortal Weekly Rep 44:550-7. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, 1995b. Escherichia coli O157:H7 outbreak linked to commercially distributed dry-cured salamiWashington and California, 1994. Morbid Mortal Weekly Rep 44:157-60. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, 2010. Two multistate outbreaks of Shiga toxin-producing Escherichia coli linked to beef from a single slaughter facility. Morbid Mortal Weekly Rep 59:557-560. [PubMed] [Google Scholar]
- Faith N, Wierzba R, Ihnot A, Roering A, Lorang T, Kaspar C, Luchansky JB, 1998. Survival of Escherichia coli O157:H7 in full- and reduced-fat pepperoni after manufacture of sticks, storage of slices at 4°C or 21°C under air and vacuum, and baking of slices on frozen pizza at 135, 191 and 246°C. J Food Prot 61:383-9. [DOI] [PubMed] [Google Scholar]
- Faith N, Parniere N, Larson T, Lorang T, Luchansky JB, 1997. Viability of Escherichia coli O157:H7 in pepperoni during the manufacture of sticks and the subsequent storage of slices at 21, 4 and -20°C under air, vacuum and CO2. Int J Food Microbiol 37:47-54. [DOI] [PubMed] [Google Scholar]
- Glass KA, Kaspar CW, Sindelar JJ, Milkowski AL, Lotz BM, Kang J, Faith NG, Enache E, Katoaka A, Henry C, 2012. Validation of pepperoni process for control of Shiga toxin-producing Escherichia coli. J Food Prot 75:838-46. [DOI] [PubMed] [Google Scholar]
- Graumann GH, Holley RA, 2008. Inhibition of Escherichia coli O157:H7 in ripening dry fermented sausage by ground yellow mustard. J Food Prot 71:486-93. [DOI] [PubMed] [Google Scholar]
- Griffin PM, Mead PS, Sivapalasingam S, 2003. Escherichia coli O157:H7 and other enterohemorrhagic E. coli. Blaser MJ, Smith PD, Ravdin JI, Greenberg HB, Guerrant RL, eds. Infections of the gastrointestinal tract. Lippincott, Williams, and Wilkins, Philadelphia, PA, pp 627-642. [Google Scholar]
- Heir E, Holck AL, Omer MK, Alvseike O, Hoy M, Rode TM, Sidhu MS, Axelsson L, 2013. Effect of post-processing treatments on sensory quality and Shiga toxigenic Escherichia coli reductions in dry-fermented sausages. Meat Sci 94:47-54. [DOI] [PubMed] [Google Scholar]
- Hinkens JC, Faith NG, Lorang TD, Bailey P, Buege D, Kaspar CW, Luchansky JB, 1996. Validation of pepperoni processes for control of Escherichia coli O157:H7. J Food Prot 59:1260-6. [DOI] [PubMed] [Google Scholar]
- Holck AL, Axelsson L, Rode TM, Høy M, Mǻge I, Alvseike O, L’Abée-Lund TM, Omer MK, Granum PE, Heir E, 2011. Reduction of verotoxigenic Escherichia coli in production of fermented sausages. Meat Sci 89:286-95. [DOI] [PubMed] [Google Scholar]
- Incze K, 1998. Dry fermented sausages. Meat Sci 49:S169-77. [PubMed] [Google Scholar]
- Kaspar CW, Doyle ME, 2010. White paper on non-O157:H7 Shiga toxin-producing Escherichia coli from meat and non-meat sources. Available from: http://fri.wisc.edu/docs/pdf/FRI_Brief_NonO157STEC_4_10.pdf Accessed 19 September 2017. [Google Scholar]
- Leistner L, 2000. Basic aspects of food preservation by hurdle technology. Int J Food Microbiol 55:181-6. [DOI] [PubMed] [Google Scholar]
- Lindqvist R, Lindblad M, 2009. Inactivation of Escherichia coli, Listeria monocytogenes and Yersinia enterocolitica in fermented sausages during maturation/storage. Int J Food Microbiol 129:59-67. [DOI] [PubMed] [Google Scholar]
- Luchansky JB, Phebus RK, Thippareddi H, Call JE, 2008. Translocation of surface-inoculated Escherichia coli O157:H7 into beef subprimals following blade tenderization. J Food Prot 71:2190-7. [DOI] [PubMed] [Google Scholar]
- McLeod A, Måge I, Heir E, Axelsson L, Holck AL, 2016. Effect of relevant environmental stresses on survival of enterohemorrhagic Escherichia coli in dry-fermented sausage. Int J Food Microbiol 229:15-23. [DOI] [PubMed] [Google Scholar]
- Morita H, Yoshikawa H, Suzuki T, Hisamatsu S, Kato Y, Sakata R, Nagata Y, Yoshimura T, 2004. Anti-microbial action against verotoxigenic Escherichia coli O157:H7 of nitric oxide derived from sodium nitrite. Biosci Biotechnol Biochem 68:1027-34. [DOI] [PubMed] [Google Scholar]
- Nickelson R, Luchansky II JB, Kaspar C, Johnson E, 1996. Dry fermented sausage Escherichia coli O157:H7 validation research. An executive summary prepared by The Blue Ribbon Task Force of the National Cattlemen’s Beef Association. Research Report No. 11-316. Available from: http://www.beefresearch.org/cmdocs/beefresearch/safety_meeting_exec_summaries/1996_dry_fermented_sausage.pdf. [Google Scholar]
- Nissen H, Holck A, 1998. Survival of Escherichia coli O157:H7, Listeria monocytogenes and Salmonella Kentucky in Norwegian fermented, dry sausage. Food Microbiol 15:273-9. [Google Scholar]
- Page ET, 2018. Trends in food recalls:2004-2013. Available from: https://www.ers.usda.gov/webdocs/publications/88497/eib191-summary.pdf. [Google Scholar]
- Porto-Fett ACS, Call JE, Shoyer BE, Hill DE, Pshebniski C, Cocoma GJ, Luchansky JB, 2010. Effectiveness of fermentation, drying, and/or high pressure processing on viability of Listeria monocytogenes, Escherichia coli O157:H7, Salmonella spp., and Trichinella spiralis in raw pork and Genoa salami. Int J Food Microbiol 140:61-75. [DOI] [PubMed] [Google Scholar]
- Porto-Fett ACS, Hwang CA, Call JE, Juneja V, Ingham S, Ingham B, Luchansky JB, 2008. Viability of multi-strain mixtures of Listeria monocytogenes, Salmonella typhimurium, or Escherichia coli O157:H7 inoculated into the batter or onto the surface of a soudjouk-style fermented semi-dry sausage. Food Microbiol 25:793-801. [DOI] [PubMed] [Google Scholar]
- Reed CA, 1995a. Approaches for ensuring the safety of dry and semi-dry fermented sausage products. U.S. Department of Agriculture, Food Safety and Inspection Service, Washington, DC: Letter to Plant Managers, 21 August 1995. [Google Scholar]
- Reed CA, 1995b. Challenge study – Escherichia coli O157:H7 in fermented sausage. U.S. Department of Agriculture, Food Safety and Inspection Service, Washington, DC. Letter to Plant Managers: 29 April 1995. [Google Scholar]
- Riordan DCR, Duffy G, Sheridan JJ, Eblen BS, Whiting RC, Blair IS, Mcdowell DA, 1998. Survival of Escherichia coli O157:H7 during the manufacture of pepperoni. J Food Prot 61:146-51. [DOI] [PubMed] [Google Scholar]
- Rode TM, Holck A, Axelsson L, Hoy M, Heir E, 2012. Shiga toxigenic Escherichia coli show strain dependent reductions under dry-fermented sausage production and post-processing conditions. Int J Food Microbiol 155:227-233. [DOI] [PubMed] [Google Scholar]
- Tsai S., Chou C. 1996. Injury, inhibition and inactivation of Escherichia coli O157:H7 by potassium sorbate and sodium nitrite as affected by pH and temperature. J Sci Food Agric 71:10-2. [Google Scholar]
- U.S. Department of Agriculture Food Safety and Inspection Service, 2010. Pennsylvania firm recalls ground beef products due to possible E. coli O26 contamination. Available at: http://orig-inwww.fsis.usda.gov/News_&_Events/Recall_050_2010_Release/index.asp Accessed 18 September 2017. [Google Scholar]
- U.S. Department of Agriculture Food Safety and Inspection Service, 2011. Designation extends zero tolerance policy for E. coli O157:H7 to six additional E. coli serogroups. U.S. Department of Agriculture Office of Communications, release no. 0400.11. U.S. Department of Agriculture, Washington, DC. [Google Scholar]


