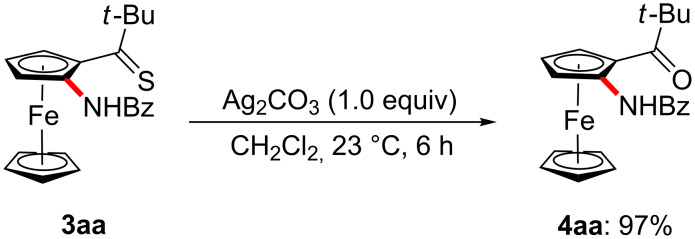Abstract
Versatile C–H amidations of synthetically useful ferrocenes were accomplished by weakly-coordinating thiocarbonyl-assisted cobalt catalysis. Thus, carboxylates enabled ferrocene C–H nitrogenations with dioxazolones, featuring ample substrate scope and robust functional group tolerance. Mechanistic studies provided strong support for a facile organometallic C–H activation manifold.
Keywords: amidation, C–H activation, cobalt, ferrocene, mechanochemistry
Introduction
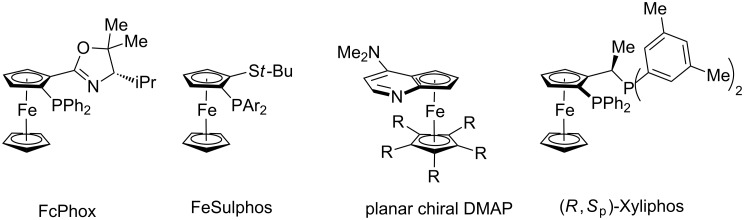
C–H activation has surfaced as a transformative tool in molecular sciences [1–9]. While major advances have been accomplished with precious 4d transition metals, recent focus has shifted towards more sustainable base metals [10–17], with considerable progress by earth-abundant cobalt catalysts [18–22]. In this context, well-defined cyclopentadienyl-derived cobalt(III) complexes have proven instrumental for enabling a wealth of C–H transformations [23–41], prominently featuring transformative C–H nitrogenations [42–43] in an atom- and step-economical fashion [44–59]. Within our program on cobalt-catalyzed C–H activation [60–68], we have now devised C–H nitrogenations assisted by weakly-coordinating [69] thiocarbonyls [70–71], allowing the direct C–H activation on substituted ferrocenes [72–93] – key structural motifs of powerful transition metal catalyst ligands and organocatalysts (Figure 1) [94–97]. During the preparation of this article, the use of strongly-coordinating, difficult to remove directing groups has been reported [70–71]. In sharp contrast, notable features of our approach include (i) cobalt-catalyzed C–H amidations of thiocarbonylferrocenes by weak coordination, (ii) thermal and mechanochemical [98–100] cobalt-catalyzed ferrocene C–H nitrogenations, (iii) versatile access to synthetically useful aminoketones, and (iv) key mechanistic insights on facile C–H cobaltation.
Figure 1.
Selected ferrocene-based ligands and organocatalysts.
Results and Discussion
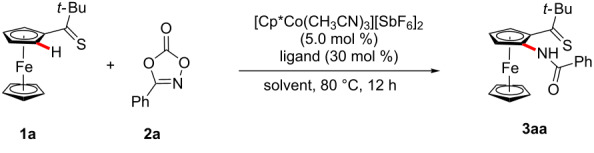
We initiated our studies by probing various reaction conditions for the envisioned C–H amidation of ferrocene 1a (Table 1). Among a variety of ligands, N-heterocyclic carbenes and phosphines provided unsatisfactory results (Table 1, entries 1–3), while the product 3aa was formed when using amino acid derivatives, albeit as of yet in a racemic fashion (Table 1, entries 4–7). Yet, optimal catalytic performance was realized with 1-AdCO2H (Table 1, entries 8 and 9) [101–104], particularly when using DCE as the solvent (Table 1, entries 9–12). A control experiment verified the essential nature of the cobalt catalyst (Table 1, entry 13). In contrast to the thiocarbonyl-assisted C–H amidation, the corresponding ketone failed thus far to deliver the desired product, under otherwise identical reaction conditions.
Table 1.
Thiocarbonyl-assisted C−H nitrogenation of ferrocene 1a.a
 | |||
| Entry | Solvent | Ligand | Yield (%) |
| 1 | DCE | – | – |
| 2 | DCE | IMes·HCl | – |
| 3 | DCE | PPh3 | – |
| 4 | DCE | Boc-Leu-OH | 40 |
| 5 | DCE | Boc-Val-OH | 55 |
| 6 | DCE | Boc-Pro-OH | 30 |
| 7 | DCE | Boc-Ala-OH | 62 |
| 8 | DCE | MesCO2H | 80 |
| 9 | DCE | 1-AdCO2H | 84 |
| 10 | 1,4-dioxane | 1-AdCO2H | 75 |
| 11 | toluene | 1-AdCO2H | 79 |
| 12 | GVL | 1-AdCO2H | 35 |
| 13 | DCE | 1-AdCO2H | –b |
aReaction conditions: 1a (0.13 mmol), 2a (0.15 mmol), ligand (30 mol %), [Co] (5.0 mol %), solvent (1.0 mL). bReaction performed in the absence of [Cp*Co(CH3CN)3][SbF6]2. Yields of isolated product.
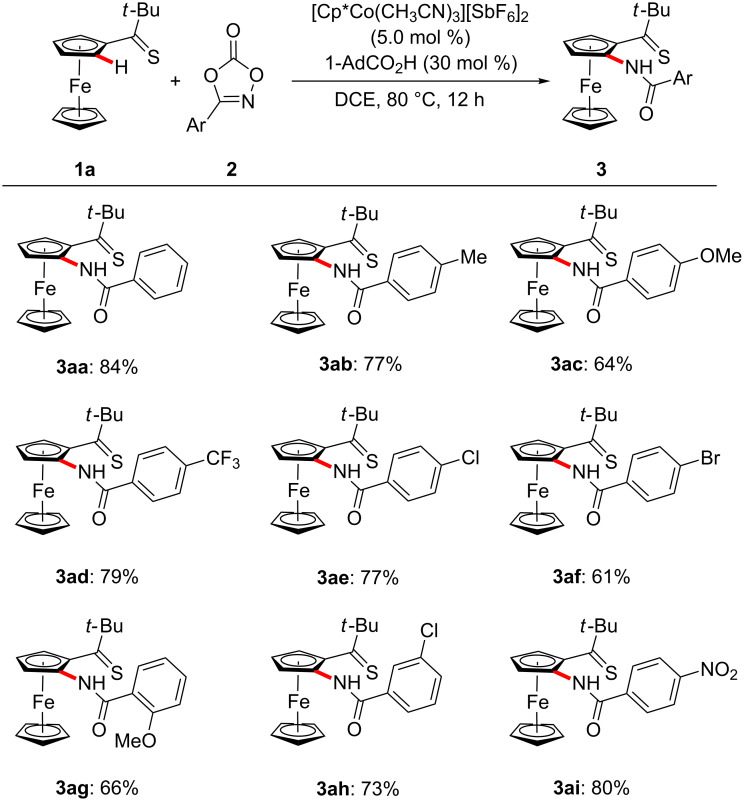
With the optimized reaction conditions in hand, we explored the robustness of the cobalt-catalyzed ferrocene C–H amidation with a variety of 1,4,2-dioxazol-5-ones 2 (Scheme 1). Hence, the chemoselectivity of the cobalt catalyst was reflected by fully tolerating sensitive electrophilic functional groups, including amido, chloro, bromo and nitro substituents in the para-, meta- and even the more congested ortho-position.
Scheme 1.
Scope of substituted dioxazolones 2.
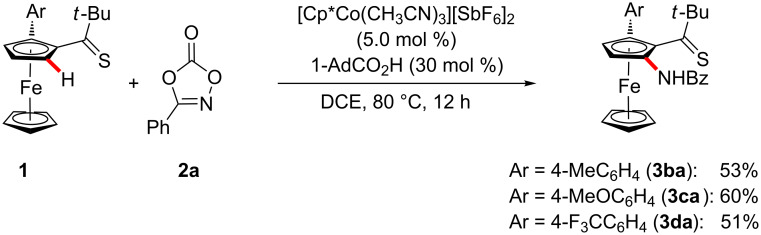
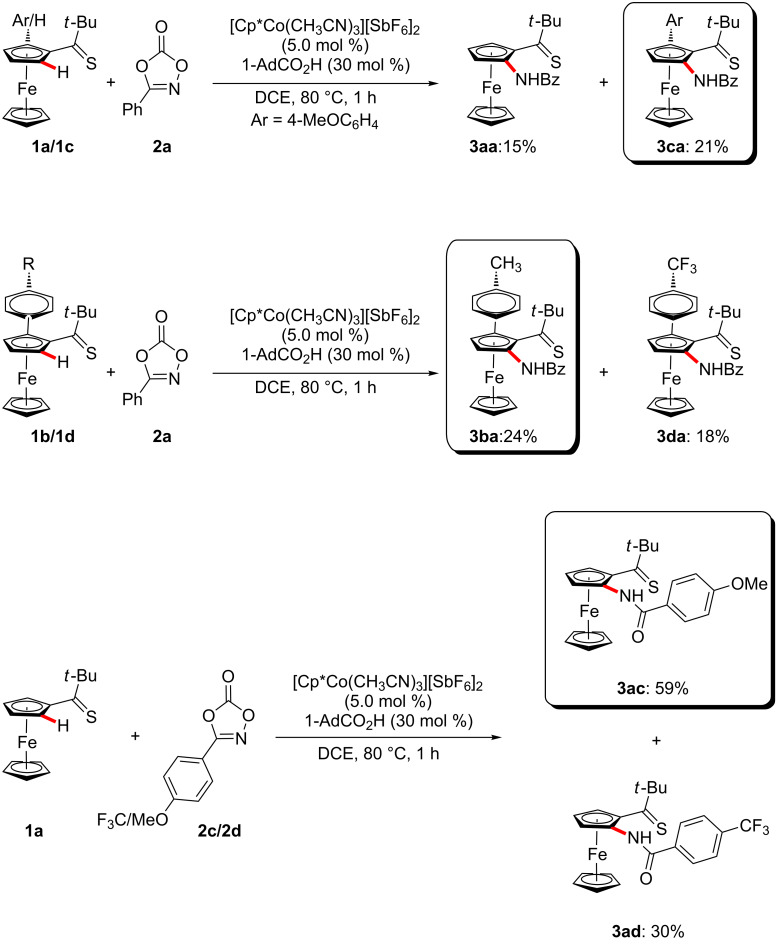
The versatile cobalt-catalyzed C–H amidation was not limited to mono-substituted ferrocenes 1 (Scheme 2). Indeed, the arylated ferrocenes 1b–d were identified as viable substrates likewise.
Scheme 2.
C–H Amidation of arylated ferrocenes 1.
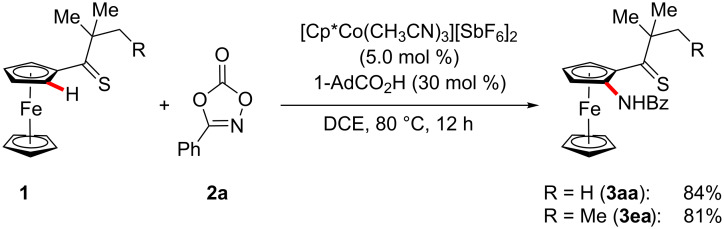
Moreover, differently substituted thiocarbonyls 1 were found to be amenable within the cobalt-catalyzed C–H amidation manifold by weak-coordination (Scheme 3).
Scheme 3.
Thiocarbonyl-assisted C–H amidation.
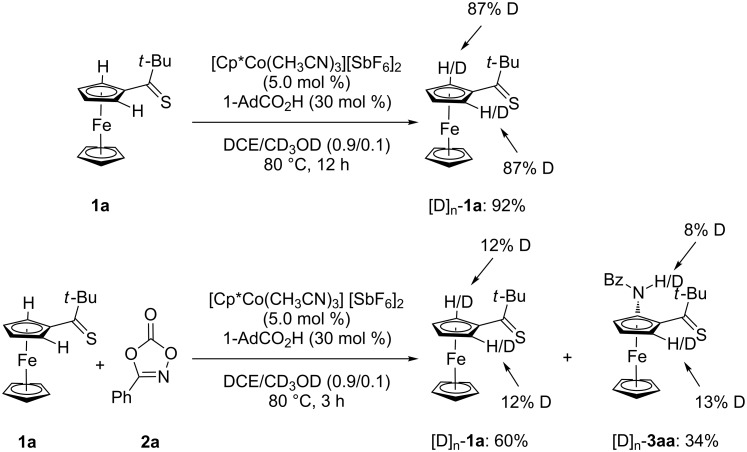
Given the versatility of the cobalt-catalyzed C–H nitrogenation, we became intrigued to delineating its mode of action. To this end, C–H amidations in the presence of isotopically labelled co-solvents led to a significant H/D scrambling in proximity to the thiocarbonyl group. These findings are indicative of a reversible, thus facile organometallic C–H cobaltation regime (Scheme 4).
Scheme 4.
H/D Exchange reactions.
Next, intermolecular competition experiments revealed that electron-rich arylated thiocarbonylferrocene 1 reacted preferentially, which can be rationalized with a base-assisted internal electrophilic substitution (BIES) [24,105] C–H cobaltation mechanism. In addition, the electron-rich amidating reagent 2c was found to be inherently more reactive (Scheme 5).
Scheme 5.
Intermolecular competition experiments.
As to further late-stage manipulation of the thus-obtained products, the amidated thiocarbonylferrocene 3aa could be easily transformed into the corresponding synthetically useful aminoketone 4aa (Scheme 6), illustrating the unique synthetic utility of our strategy.
Scheme 6.
Synthesis of aminoketone 4aa.
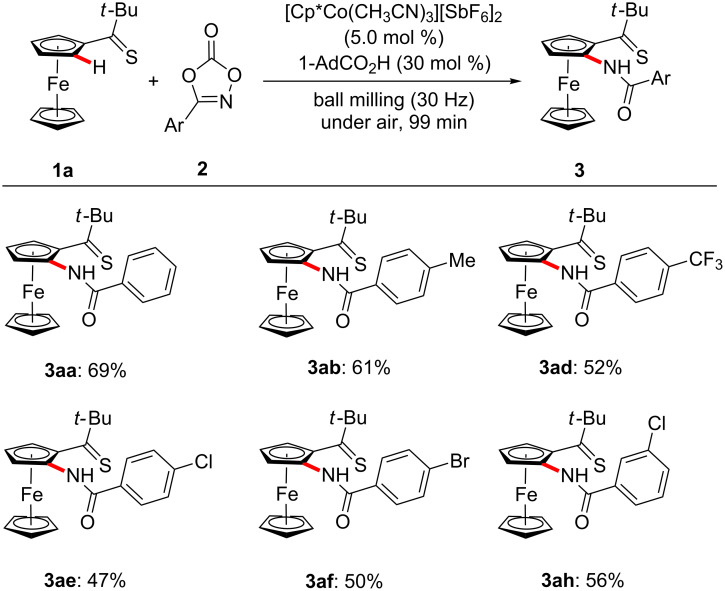
Mechanochemical molecular synthesis has attracted recent renewed attention as an attractive alternative for facilitating sustainable organic syntheses [106]. Thus, we were delighted to observe that the mechanochemical C–H nitrogenations proved likewise viable by thiocarbonyl assistance in an effective manner (Scheme 7).
Scheme 7.
Mechanochemical ferrocene C–H nitrogenation.
Conclusion
In conclusion, we have reported on the unprecedented cobalt-catalyzed C–H nitrogenation of ferrocenes by weakly-coordinating thiocarbonyls. The carboxylate-assisted cobalt catalysis was characterized by high functional group tolerance and ample substrate scope. Mechanistic studies provided evidence for a facile C–H activation. The C–H amidation was achieved in a thermal fashion as well as by means of mechanochemistry, providing access to synthetically meaningful aminoketones.
Supporting Information
Experimental procedures, characterization data, and NMR spectra for new compounds.
Acknowledgments
Generous support by the DFG (Gottfried Wilhelm Leibniz prize), and the CSC (fellowships to Z.S. and H.W.) is gratefully acknowledged.
This article is part of the thematic issue "Cobalt catalysis".
References
- 1.Gandeepan P, Ackermann L. Chem. 2018;4:199–222. doi: 10.1016/j.chempr.2017.11.002. [DOI] [Google Scholar]
- 2.Wei Y, Hu P, Zhang M, Su W. Chem Rev. 2017;117:8864–8907. doi: 10.1021/acs.chemrev.6b00516. [DOI] [PubMed] [Google Scholar]
- 3.Ma W, Gandeepan P, Li J, Ackermann L. Org Chem Front. 2017;4:1435–1467. doi: 10.1039/C7QO00134G. [DOI] [Google Scholar]
- 4.He J, Wasa M, Chan K S L, Shao Q, Yu J-Q. Chem Rev. 2017;117:8754–8786. doi: 10.1021/acs.chemrev.6b00622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng Q-Z, Jiao N. Chem Soc Rev. 2016;45:4590–4627. doi: 10.1039/C6CS00107F. [DOI] [PubMed] [Google Scholar]
- 6.Borie C, Ackermann L, Nechab M. Chem Soc Rev. 2016;45:1368–1386. doi: 10.1039/C5CS00622H. [DOI] [PubMed] [Google Scholar]
- 7.Ye B, Cramer N. Acc Chem Res. 2015;48:1308–1318. doi: 10.1021/acs.accounts.5b00092. [DOI] [PubMed] [Google Scholar]
- 8.Satoh T, Miura M. Chem – Eur J. 2010;16:11212–11222. doi: 10.1002/chem.201001363. [DOI] [PubMed] [Google Scholar]
- 9.Ackermann L, Vicente R, Kapdi A R. Angew Chem, Int Ed. 2009;121:9976–10011. doi: 10.1002/ange.200902996. [DOI] [Google Scholar]
- 10.Hu Y, Zhou B, Wang C. Acc Chem Res. 2018;51:816–827. doi: 10.1021/acs.accounts.8b00028. [DOI] [PubMed] [Google Scholar]
- 11.Liu W, Ackermann L. ACS Catal. 2016;6:3743–3752. doi: 10.1021/acscatal.6b00993. [DOI] [Google Scholar]
- 12.Cera G, Ackermann L. Top Curr Chem. 2016;374:191–224. doi: 10.1007/s41061-016-0059-6. [DOI] [PubMed] [Google Scholar]
- 13.Nakao Y. Chem Rec. 2011;11:242–251. doi: 10.1002/tcr.201100023. [DOI] [PubMed] [Google Scholar]
- 14.Castro L C M, Chatani N. Chem Lett. 2015;44:410–421. doi: 10.1246/cl.150024. [DOI] [Google Scholar]
- 15.Hirano K, Miura M. Chem Lett. 2015;44:868–873. doi: 10.1246/cl.150354. [DOI] [Google Scholar]
- 16.Yamaguchi J, Muto K, Itami K. Eur J Org Chem. 2013:19–30. doi: 10.1002/ejoc.201200914. [DOI] [Google Scholar]
- 17.Kulkarni A A, Daugulis O. Synthesis. 2009:4087–4109. doi: 10.1055/s-0029-1217131. [DOI] [Google Scholar]
- 18.Yoshino T, Matsunaga S. Adv Synth Catal. 2017;359:1245–1262. doi: 10.1002/adsc.201700042. [DOI] [Google Scholar]
- 19.Wei D, Zhu X, Niu J-L, Song M-P. ChemCatChem. 2016;8:1242–1263. doi: 10.1002/cctc.201600040. [DOI] [Google Scholar]
- 20.Moselage M, Li J, Ackermann L. ACS Catal. 2016;6:498–525. doi: 10.1021/acscatal.5b02344. [DOI] [Google Scholar]
- 21.Gao K, Yoshikai N. Acc Chem Res. 2014;47:1208–1219. doi: 10.1021/ar400270x. [DOI] [PubMed] [Google Scholar]
- 22.Ackermann L. J Org Chem. 2014;79:8948–8954. doi: 10.1021/jo501361k. [DOI] [PubMed] [Google Scholar]
- 23.Zell D, Müller V, Dhawa U, Bursch M, Presa R R, Grimme S, Ackermann L. Chem – Eur J. 2017;23:12145–12148. doi: 10.1002/chem.201702528. [DOI] [PubMed] [Google Scholar]
- 24.Zell D, Bursch M, Müller V, Grimme S, Ackermann L. Angew Chem, Int Ed. 2017;56:10378–10382. doi: 10.1002/anie.201704196. [DOI] [PubMed] [Google Scholar]
- 25.Ikemoto H, Tanaka R, Sakata K, Kanai M, Yoshino T, Matsunaga S. Angew Chem, Int Ed. 2017;56:7156–7160. doi: 10.1002/anie.201703193. [DOI] [PubMed] [Google Scholar]
- 26.Yan Q, Chen Z, Liu Z, Zhang Y. Org Chem Front. 2016;3:678–682. doi: 10.1039/C6QO00059B. [DOI] [Google Scholar]
- 27.Lu Q, Vásquez-Céspedes S, Gensch T, Glorius F. ACS Catal. 2016;6:2352–2356. doi: 10.1021/acscatal.6b00367. [DOI] [Google Scholar]
- 28.Kong L, Yu S, Zhou X, Li X. Org Lett. 2016;18:588–591. doi: 10.1021/acs.orglett.5b03629. [DOI] [PubMed] [Google Scholar]
- 29.Kalsi D, Laskar R A, Barsu N, Premkumar J R, Sundararaju B. Org Lett. 2016;18:4198–4201. doi: 10.1021/acs.orglett.6b01845. [DOI] [PubMed] [Google Scholar]
- 30.Bunno Y, Murakami N, Suzuki Y, Kanai M, Yoshino T, Matsunaga S. Org Lett. 2016;18:2216–2219. doi: 10.1021/acs.orglett.6b00846. [DOI] [PubMed] [Google Scholar]
- 31.Boerth J A, Hummel J R, Ellman J A. Angew Chem, Int Ed. 2016;55:12650–12654. doi: 10.1002/anie.201603831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hummel J R, Ellman J A. J Am Chem Soc. 2015;137:490–498. doi: 10.1021/ja5116452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang H, Koeller J, Liu W, Ackermann L. Chem – Eur J. 2015;21:15525–15528. doi: 10.1002/chem.201503624. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki Y, Sun B, Sakata K, Yoshino T, Matsunaga S, Kanai M. Angew Chem, Int Ed. 2015;54:9944–9947. doi: 10.1002/anie.201503704. [DOI] [PubMed] [Google Scholar]
- 35.Sen M, Kalsi D, Sundararaju B. Chem – Eur J. 2015;21:15529–15533. doi: 10.1002/chem.201503643. [DOI] [PubMed] [Google Scholar]
- 36.Sauermann N, González M J, Ackermann L. Org Lett. 2015;17:5316–5319. doi: 10.1021/acs.orglett.5b02678. [DOI] [PubMed] [Google Scholar]
- 37.Ma W, Ackermann L. ACS Catal. 2015;5:2822–2825. doi: 10.1021/acscatal.5b00322. [DOI] [Google Scholar]
- 38.Li J, Ackermann L. Angew Chem, Int Ed. 2015;54:3635–3638. doi: 10.1002/anie.201409247. [DOI] [PubMed] [Google Scholar]
- 39.Li J, Ackermann L. Angew Chem, Int Ed. 2015;54:8551–8554. doi: 10.1002/anie.201501926. [DOI] [PubMed] [Google Scholar]
- 40.Ikemoto H, Yoshino T, Sakata K, Matsunaga S, Kanai M. J Am Chem Soc. 2014;136:5424–5431. doi: 10.1021/ja5008432. [DOI] [PubMed] [Google Scholar]
- 41.Yoshino T, Ikemoto H, Matsunaga S, Kanai M. Angew Chem, Int Ed. 2013;52:2207–2211. doi: 10.1002/anie.201209226. [DOI] [PubMed] [Google Scholar]
- 42.Park Y, Kim Y, Chang S. Chem Rev. 2017;117:9247–9301. doi: 10.1021/acs.chemrev.6b00644. [DOI] [PubMed] [Google Scholar]
- 43.Jiao J, Murakami K, Itami K. ACS Catal. 2016;6:610–633. doi: 10.1021/acscatal.5b02417. [DOI] [Google Scholar]
- 44.Borah G, Borah P, Patel P. Org Biomol Chem. 2017;15:3854–3859. doi: 10.1039/C7OB00540G. [DOI] [PubMed] [Google Scholar]
- 45.Zhang W, Deng H, Li H. Org Chem Front. 2017;4:2202–2206. doi: 10.1039/C7QO00542C. [DOI] [Google Scholar]
- 46.Xia J, Yang X, Li Y, Li X. Org Lett. 2017;19:3243–3246. doi: 10.1021/acs.orglett.7b01356. [DOI] [PubMed] [Google Scholar]
- 47.Huang J, Huang Y, Wang T, Huang Q, Wang Z, Chen Z. Org Lett. 2017;19:1128–1131. doi: 10.1021/acs.orglett.7b00120. [DOI] [PubMed] [Google Scholar]
- 48.Barsu N, Bolli S K, Sundararaju B. Chem Sci. 2017;8:2431–2435. doi: 10.1039/C6SC05026C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang X, Lerchen A, Glorius F. Org Lett. 2016;18:2090–2093. doi: 10.1021/acs.orglett.6b00716. [DOI] [PubMed] [Google Scholar]
- 50.Wu F, Zhao Y, Chen W. Tetrahedron. 2016;72:8004–8008. doi: 10.1016/j.tet.2016.10.032. [DOI] [Google Scholar]
- 51.Wang J, Zha S, Chen K, Zhang F, Song C, Zhu J. Org Lett. 2016;18:2062–2065. doi: 10.1021/acs.orglett.6b00691. [DOI] [PubMed] [Google Scholar]
- 52.Wang H, Lorion M M, Ackermann L. Angew Chem, Int Ed. 2016;55:10386–10390. doi: 10.1002/anie.201603260. [DOI] [PubMed] [Google Scholar]
- 53.Park Y, Park K T, Kim J G, Chang S. J Am Chem Soc. 2015;137:4534–4542. doi: 10.1021/jacs.5b01324. [DOI] [PubMed] [Google Scholar]
- 54.Jeon B, Yeon U, Son J-Y, Lee P H. Org Lett. 2016;18:4610–4613. doi: 10.1021/acs.orglett.6b02250. [DOI] [PubMed] [Google Scholar]
- 55.Park Y, Jee S, Kim J G, Chang S. Org Process Res Dev. 2015;19:1024–1029. doi: 10.1021/acs.oprd.5b00164. [DOI] [Google Scholar]
- 56.Park J, Lee J, Chang S. Angew Chem, Int Ed. 2017;56:4256–4260. doi: 10.1002/anie.201701138. [DOI] [PubMed] [Google Scholar]
- 57.Park J, Chang S. Angew Chem, Int Ed. 2015;54:14103–14107. doi: 10.1002/anie.201505820. [DOI] [PubMed] [Google Scholar]
- 58.Mei R, Loup J, Ackermann L. ACS Catal. 2016;6:793–797. doi: 10.1021/acscatal.5b02661. [DOI] [Google Scholar]
- 59.Liang Y, Liang Y-F, Tang C, Yuan Y, Jiao N. Chem – Eur J. 2015;21:16395–16399. doi: 10.1002/chem.201503533. [DOI] [PubMed] [Google Scholar]
- 60.Sauermann N, Mei R, Ackermann L. Angew Chem, Int Ed. 2018;57:5090–5094. doi: 10.1002/anie.201802206. [DOI] [PubMed] [Google Scholar]
- 61.Tian C, Massignan L, Meyer T H, Ackermann L. Angew Chem, Int Ed. 2018;57:2383–2387. doi: 10.1002/anie.201712647. [DOI] [PubMed] [Google Scholar]
- 62.Sauermann N, Meyer T H, Tian C, Ackermann L. J Am Chem Soc. 2017;139:18452–18455. doi: 10.1021/jacs.7b11025. [DOI] [PubMed] [Google Scholar]
- 63.Sauermann N, Loup J, Kootz D, Yatham V R, Berkessel A, Ackermann L. Synthesis. 2017;49:3476–3484. doi: 10.1055/s-0036-1590471. [DOI] [Google Scholar]
- 64.Mei R, Ackermann L. Adv Synth Catal. 2016;358:2443–2448. doi: 10.1002/adsc.201600384. [DOI] [Google Scholar]
- 65.Moselage M, Sauermann N, Richter S C, Ackermann L. Angew Chem, Int Ed. 2015;54:6352–6355. doi: 10.1002/anie.201412319. [DOI] [PubMed] [Google Scholar]
- 66.Li J, Ackermann L. Chem – Eur J. 2015;21:5718–5722. doi: 10.1002/chem.201500552. [DOI] [PubMed] [Google Scholar]
- 67.Punji B, Song W, Shevchenko G A, Ackermann L. Chem – Eur J. 2013;19:10605–10610. doi: 10.1002/chem.201301409. [DOI] [PubMed] [Google Scholar]
- 68.Song W, Ackermann L. Angew Chem, Int Ed. 2012;51:8251–8254. doi: 10.1002/anie.201202466. [DOI] [PubMed] [Google Scholar]
- 69.Sarkar S D, Liu W, Kozhushkov S I, Ackermann L. Adv Synth Catal. 2014;356:1461–1479. doi: 10.1002/adsc.201400110. [DOI] [Google Scholar]
- 70.Cheng H, Hernández J G, Bolm C. Adv Synth Catal. 2018;360:1800–1804. doi: 10.1002/adsc.201800161. [DOI] [Google Scholar]
- 71.Wang S-B, Gu Q, You S-L. J Catal. 2018;361:393–397. doi: 10.1016/j.jcat.2018.03.007. [DOI] [Google Scholar]
- 72.Cai Z-J, Liu C-X, Gu Q, You S-L. Angew Chem, Int Ed. 2018;57:1296–1299. doi: 10.1002/anie.201711451. [DOI] [PubMed] [Google Scholar]
- 73.Xu J, Liu Y, Zhang J, Xu X, Jin Z. Chem Commun. 2018;54:689–692. doi: 10.1039/C7CC09273C. [DOI] [PubMed] [Google Scholar]
- 74.Gao D-W, Gu Q, Zheng C, You S-L. Acc Chem Res. 2017;50:351–365. doi: 10.1021/acs.accounts.6b00573. [DOI] [PubMed] [Google Scholar]
- 75.Schmiel D, Butenschön H. Eur J Org Chem. 2017:3041–3048. doi: 10.1002/ejoc.201700358. [DOI] [Google Scholar]
- 76.Schmiel D, Butenschön H. Organometallics. 2017;36:4979–4989. doi: 10.1021/acs.organomet.7b00799. [DOI] [Google Scholar]
- 77.Wang S-B, Gu Q, You S-L. J Org Chem. 2017;82:11829–11835. doi: 10.1021/acs.joc.7b00775. [DOI] [PubMed] [Google Scholar]
- 78.Wang S-B, Gu Q, You S-L. Organometallics. 2017;36:4359–4362. doi: 10.1021/acs.organomet.7b00691. [DOI] [Google Scholar]
- 79.Cera G, Haven T, Ackermann L. Angew Chem, Int Ed. 2016;55:1484–1488. doi: 10.1002/anie.201509603. [DOI] [PubMed] [Google Scholar]
- 80.Gao D-W, Gu Q, You S-L. J Am Chem Soc. 2016;138:2544–2547. doi: 10.1021/jacs.6b00127. [DOI] [PubMed] [Google Scholar]
- 81.Shibata T, Uno N, Sasaki T, Kanyiva K S. J Org Chem. 2016;81:6266–6272. doi: 10.1021/acs.joc.6b00825. [DOI] [PubMed] [Google Scholar]
- 82.Urbano A, Hernández-Torres G, del Hoyo A M, Martínez-Carrión A, Carmen Carreño M. Chem Commun. 2016;52:6419–6422. doi: 10.1039/C6CC02624A. [DOI] [PubMed] [Google Scholar]
- 83.Wang S-B, Zheng J, You S-L. Organometallics. 2016;35:1420–1425. doi: 10.1021/acs.organomet.6b00020. [DOI] [Google Scholar]
- 84.Zhu D-Y, Chen P, Xia J-B. ChemCatChem. 2016;8:68–73. doi: 10.1002/cctc.201500895. [DOI] [Google Scholar]
- 85.Arae S, Ogasawara M. Tetrahedron Lett. 2015;56:1751–1761. doi: 10.1016/j.tetlet.2015.01.130. [DOI] [Google Scholar]
- 86.López L A, López E. Dalton Trans. 2015;44:10128–10135. doi: 10.1039/C5DT01373A. [DOI] [PubMed] [Google Scholar]
- 87.Deng R, Huang Y, Ma X, Li G, Zhu R, Wang B, Kang Y-B, Gu Z. J Am Chem Soc. 2014;136:4472–4475. doi: 10.1021/ja500699x. [DOI] [PubMed] [Google Scholar]
- 88.Gao D-W, Yin Q, Gu Q, You S-L. J Am Chem Soc. 2014;136:4841–4844. doi: 10.1021/ja500444v. [DOI] [PubMed] [Google Scholar]
- 89.Liu L, Zhang A-A, Zhao R-J, Li F, Meng T-J, Ishida N, Murakami M, Zhao W-X. Org Lett. 2014;16:5336–5338. doi: 10.1021/ol502520b. [DOI] [PubMed] [Google Scholar]
- 90.Pi C, Cui X, Liu X, Guo M, Zhang H, Wu Y. Org Lett. 2014;16:5164–5167. doi: 10.1021/ol502509f. [DOI] [PubMed] [Google Scholar]
- 91.Shibata T, Shizuno T. Angew Chem, Int Ed. 2014;53:5410–5413. doi: 10.1002/anie.201402518. [DOI] [PubMed] [Google Scholar]
- 92.Xie W, Li B, Xu S, Song H, Wang B. Organometallics. 2014;33:2138–2141. doi: 10.1021/om5002606. [DOI] [Google Scholar]
- 93.Kornhaaß C, Kuper C, Ackermann L. Adv Synth Catal. 2014;356:1619–1624. doi: 10.1002/adsc.201301156. [DOI] [Google Scholar]
- 94.Arrayás R G, Adrio J, Carretero J C. Angew Chem, Int Ed. 2006;45:7674–7715. doi: 10.1002/anie.200602482. [DOI] [PubMed] [Google Scholar]
- 95.Fu G C. Acc Chem Res. 2004;37:542–547. doi: 10.1021/ar030051b. [DOI] [PubMed] [Google Scholar]
- 96.Dai L-X, Tu T, You S-L, Deng W-P, Hou X-L. Acc Chem Res. 2003;36:659–667. doi: 10.1021/ar020153m. [DOI] [PubMed] [Google Scholar]
- 97.Fu G C. Acc Chem Res. 2000;33:412–420. doi: 10.1021/ar990077w. [DOI] [PubMed] [Google Scholar]
- 98.Temnikov M N, Anisimov A A, Zhemchugov P V, Kholodkov D N, Goloveshkin A S, Naumkin A V, Chistovalov S M, Katsoulis D, Muzafarov A M. Green Chem. 2018;20:1962–1969. doi: 10.1039/C7GC03862C. [DOI] [Google Scholar]
- 99.Hermann G N, Bolm C. ACS Catal. 2017;7:4592–4596. doi: 10.1021/acscatal.7b00582. [DOI] [Google Scholar]
- 100.Cheng H, Hernández J G, Bolm C. Org Lett. 2017;19:6284–6287. doi: 10.1021/acs.orglett.7b02973. [DOI] [PubMed] [Google Scholar]
- 101.Ackermann L. Acc Chem Res. 2014;47:281–295. doi: 10.1021/ar3002798. [DOI] [PubMed] [Google Scholar]
- 102.Ackermann L. Chem Rev. 2011;111:1315–1345. doi: 10.1021/cr100412j. [DOI] [PubMed] [Google Scholar]
- 103.Lapointe D, Fagnou K. Chem Lett. 2010;39:1118–1126. doi: 10.1246/cl.2010.1118. [DOI] [Google Scholar]
- 104.Ackermann L, Novák P, Vicente R, Hofmann N. Angew Chem, Int Ed. 2009;48:6045–6048. doi: 10.1002/anie.200902458. [DOI] [PubMed] [Google Scholar]
- 105.Ma W, Mei R, Tenti G, Ackermann L. Chem – Eur J. 2014;20:15248–15251. doi: 10.1002/chem.201404604. [DOI] [PubMed] [Google Scholar]
- 106.Hernández J G. Chem – Eur J. 2017;23:17157–17165. doi: 10.1002/chem.201703605. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experimental procedures, characterization data, and NMR spectra for new compounds.