Abstract
Background
Plasma metabolites such as phosphatidylcholines and sphingomyelins (SMs) are associated with an age-related cognitive decline. However, their relations to age-related physical function decline remain largely unknown.
Methods
We examined the cross-sectional relations of 12 plasma metabolites (including four phosphatidylcholines and four SMs) with physical function in 383 older adults in the At herosclerosis Risk in Communities Study at the fifth exam (2011–2013, mean age [standard deviation (SD)]: 78.0 [5.5], 54.4% women, 28.3% African Americans). Physical function was assessed using grip strength, Short Physical Performance Battery, and 4-m walking speed. Individual metabolites were log-transformed and standardized. Multivariable linear regression was performed to account for demographics, APOE genotype, cardiovascular risk factors, comorbidities, use of antihypertensive and lipid-lowering medications, depressive symptoms, and cognition.
Results
Lower concentrations of asymmetric dimethylarginine and higher concentrations of SM (OH) C22:1, SM (OH) C22:2, and SM (OH) C24:1 were associated with physical function measures. In particular, SM (OH) C22:1 and SM (OH) C24:1 were associated with all three measures of physical function: β-coefficients (95% confidence interval) with grip strength were 0.89 kg (0.00, 1.78) and 0.86 kg (0.10, 1.61) per 1 SD higher concentration, respectively; with Short Physical Performance Battery score, were 0.61 (0.34, 0.88) and 0.41 (0.19, 0.63) per 1 SD difference, respectively; with 4-m walking speed were 0.035 m/s (0.013, 0.056) and 0.035 m/s (0.028, 0.047), respectively.
Conclusions
Plasma SM (OH)s may be independently associated with physical function in older adults.
Keywords: Phosphatidylcholines, Sphingomyelins, Asymmetric dimethylarginine
Physical function decline is prevalent in older adults and leads to many adverse health outcomes such as incident disability, institutionalization, poor quality of life, and increased mortality (1–3). Studies of biological changes underlying physical function decline have been largely restricted to inflammation and neuroendocrine dysregulation (e.g. interleukin-6, insulin growth factor-1, and sex steroid hormones) (4–6). These studies fail to consider the advancement in targeted metabolomics technology, which offers the potential to enrich molecular understanding of physical function decline. Metabolomics has been widely used to understand other aging-related symptoms; collectively, these studies have revealed that plasma metabolites such as phosphatidylcholines (PCs) and sphingomyelins (SMs) are associated with brain pathology such as neurodegeneration (7) and with cognitive decline in older adults (8,9). Shared brain pathology such as neurodegeneration could contribute to both physical and cognitive decline as supported by strong correlations between gait and cognitive abnormalities (10). Hence, plasma metabolites that are associated with cognitive decline in older adults may also be associated with physical function decline. Furthermore, physical function decline is manifested in diseases such as multiple sclerosis independent of cognition due to molecular mechanisms affecting demyelination and neurodegeneration in the central and peripheral nervous systems (11). Because SMs are major lipids in the myelin sheath that insulate nerve cell axons and conduct nerve signals, plasma SMs may be associated with poorer physical function independent of cognition.
Of 188 plasma metabolites previously examined in the Atherosclerosis Risk in Communities (ARIC), 12 were associated with cognitive status (9). Because of the well-established relationships of physical and cognitive function and the biologic plausibility outlined above, we hypothesized that these 12 metabolites would be associated with physical function as follows: (i) higher concentrations of plasma asymmetric dimethylarginine (ADMA), hydroxybutyryl-l-carnitine, lyso phosphatidylcholine acyl C16: 1 (lysoPC a C16: 1), and octadecanoyl-l-carnitine (C18) are associated with poorer physical function; and (ii) higher concentrations of plasma phosphatidylcholine diacyl C 36:5 (PC aa C36:5), PC aa C36:6, PC aa C38:1, PC aa C40:2, SM C26:0, hydroxysphingomyelin C22:1 [SM (OH) C22:1], SM (OH) C22:2, and SM (OH) C24:1 are associated with better physical function. Furthermore, we hypothesized that associations between plasma SMs (SM C26:0, SM (OH) C22:1, SM (OH) C22:2, and SM (OH) C24:1) and physical function would be independent of cognitive function.
Materials and Methods
Population
The ARIC study is a prospective cohort study investigating the etiology of atherosclerotic disease in a middle-aged, predominantly biracial (black and white) population in four U.S. communities. The ARIC study and ARIC-Neurocognitive Study were approved by the Institutional Review Board of each participating center, and written informed consent was obtained from participants at each study visit. A detailed study description has been published (12). Briefly, 15,792 participants age 45–64 years were recruited in 1987–1989 (visit 1). Participants underwent triennial exams in 1990–1992, 1993–1995, and 1996–1998. In 2011–2013, ARIC conducted the fifth examination (visit 5) of 6,538 of the 10,036 living participants (65% follow-up rate), including an initial physical function assessment in the cohort. The ARIC-Neurocognitive Study, conducted in conjunction with visit 5, assessed cognitive function and adjudicated mild cognitive impairment (MCI, n=1,402) and dementia (1,281) cases (13) of the 6,538 participants.
A previous study investigated the cross-sectional relations of 188 plasma metabolites to cognitive status among 441 ARIC visit 5 participants, sampling approximately equal numbers of dementia, MCI and cognitively normal participants and equal numbers of whites and blacks (9). Cognitively normal individuals were frequency matched with dementia/MCI participants by APOE genotype, age (above or below the median age in the dementia/MCI participants), sex, education level, and study center. In this analysis, we excluded 58 participants (13%) without any physical function measure, leaving a final sample size of 383 participants with at least one physical function measure.
Measures of Physical Function
Grip strength (kg) was assessed in participant’s preferred hand using an adjustable, hydraulic grip strength dynamometer with the better of two trials used in the analysis (14,15). Lower extremity physical function was assessed using the Short Physical Performance Battery (SPPB) (3), which is comprised of three tasks: (a) repeated chair stands; (b) balance (standing, semitandem, tandem); and (c) a 4-m usual-paced walk (meters/second) (15). Walking aids were allowed for the walking task only. Scores of 0–4 were assigned for each task based on timed performance of the three tasks using established population-based thresholds and then summed for a final score of 0–12. Higher scores represent better function. Four-meter walking speed was also examined as a separate outcome, as it is the most clinically feasible of these measures and is a sensitive predictor of mortality and frailty (16–18).
Measurement of Plasma Metabolites
Plasma metabolites were measured using the Biocrates Absolute-IDQ P180 kits (Biocrates, Life Science AG, Innsbruck, Austria), as described elsewhere (9). This targeted metabolite kit measures 188 metabolites, including 40 acylcarnitines, 21 amino acids, 21 biogenic amines, 105 phospholipids [10 SM, 5 SM (OH), 38 PC aa, 38 phosphatidylcholine acyl-akyl (PC ae), 14 lysoPC a], and hexose. Fasting, frozen, never thawed plasma samples were processed per the manufacturer instructions and analyzed on a triple-quadrupole mass spectrometer (AB Sciex QTRAP 6500). Three quality controls were included in each kit to monitor imprecisions (% coefficient of variations). Imprecisions of majority of the metabolites (i.e. > 80%) calculated based on the quality controls of seven kits used to analyze the 441 samples were less than 20%. Quantitative information of the 188 metabolites was produced by the MetIQ software (Biocrates).
Covariates
Models were adjusted for age, race, sex, ARIC study center, dementia status, and Mini–Mental State Examination (Model 1), and additionally, educational level, smoking and drinking status, APOE genotype, body mass index, physical activity, coronary artery disease, stroke, heart failure, diabetes mellitus, total cholesterol, high density lipoprotein cholesterol, triglycerides, high-sensitive C-reactive protein, systolic blood pressure, use of antihypertensive medications, use of lipid lowering medications, depressive symptoms, executive function score, bodily pain score, prevalent coronary heart disease, stroke, and heart failure in the full model (Model 2). Educational level, age, sex, and race were self-reported at the baseline visit. Smoking and drinking status was self-reported at visit 5. Participants were asked to bring medications, which were then recorded. Body mass index was defined as weight in kilograms divided by the square of height in meters measured with the participant wearing light clothing. Systolic blood pressure was measured using a random zero sphygomanometer with 5 minutes of rest before each measurement, and the mean of 2 measurements was used (19). A sports index for physical activity was calculated based on the number of times per month participants engaged in vigorous, moderate, or light physical activity, and scored 1–5 as previously described (20). Prevalent coronary heart disease, stroke and heart failure were defined according to published criteria (21,22). Prevalent diabetes was defined as a fasting blood glucose ≥126 mg/dL, non-fasting glucose >200 mg/dL, self-reported physician-diagnosis of diabetes or use of anti-diabetic medication. Total cholesterol, high density lipoprotein cholesterol, triglycerides, and high-sensitive C-reactive protein were measured using standard methods (23). Depressive symptoms were assessed with the Center for Epidemiologic Studies Depression Scale Short Form and bodily pain with the SF-12v2 questionnaire (24). Executive function/psychomotor speed was evaluated using digit symbol substitution test, trail making test parts A and B, and Wechsler Adult Intelligence Scale-Revised (WAIS-R) digit span backwards; an overall Z-score for executive function was constructed by averaging the scores of test, subtracting the mean, and dividing by the standard deviation (SD) (13).
Statistical Analysis
Metabolite concentrations were log transformed and modeled in SD units. Multivariable linear regression examined the association of each metabolite in two models (base model and full model) with the three measures of physical function. Missing values for the covariates were imputed carrying forward the last available value from a prior visit or creating a category for missing values. In the full model, we weighted observations by the inverse of the probability of being selected to the study. These probabilities were calculated with a logistic regression model using selection to the study as the dependent variable and age, sex, race, education level, study center, APOE genotype, and cognitive status as predictors. In a secondary analysis, we examined the association of total concentration of each class of phospholipids (i.e. SM, SM [OH], PC aa, PC ae, lysoPC a) with each physical function measure. Interaction terms between age, sex, or APOE genotype and total concentration of each class of phospholipids were also examined. We performed a logistic regression analysis between the total concentration of each phospholipid class and physical impairment defined in two ways: (i) SPPB score of ≤ 6, (20% of the analytic sample) (25); or (ii) walking speed ≤ 0.6 m/s (13%) (26). We also performed principal component analysis to demonstrate the correlations among these 12 plasma metabolites. We then conducted linear regression analysis between five principal component analysis–derived factors with physical function measures. We used SAS v 9.3 (SAS Inc., Cary, North Carolina) for the statistical analysis.
Results
Characteristics of the Study Population
Compared to 6,155 excluded ARIC participants, the 383 participants in this study were older, less likely to be female, less likely to drink or smoke, more likely to be diabetic, carry an APOE ε4 allele, and had lower cognitive function and physical function (Table 1). Because the study population was part of a previous study that examined the relations between plasma metabolites and cognitive status, the relation between the 12 plasma metabolites and cognitive status is included in Supplementary Table 1.
Table 1.
Characteristics of the ARIC Study Population, Atherosclerosis Risk in Communities (ARIC) Study, 2011 to 2013
| Included (n = 383) | Excluded (n = 6,155) | P-value* | |
|---|---|---|---|
| Age, years | 77.5 (5.5) | 75.7 (5.2) | <.0001 |
| African American, % | 25.3 | 23.5 | .63 |
| Female, % | 52.5 | 59.2 | .01 |
| Body mass index, kg/m2 | 28.5 (5.6) | 28.8 (5.8) | .47 |
| Current drinker, % | 42.6 | 47.8 | .02 |
| Current smoker, % | 5 | 5.6 | .0008 |
| Diabetes, % | 38.1 | 32.2 | .02 |
| High school graduate or higher, % | 81.2 | 85.1 | .11 |
| HDL cholesterol, mg/dL | 51.6 (14.3) | 52.2 (14.0) | .45 |
| Hypertension medication, % | 78.9 | 75.6 | .14 |
| Lipid lowering medication, % | 55.4 | 55.7 | .91 |
| Prevalent coronary heart disease, % | 17.8 | 14.6 | .1 |
| Prevalent heart failure, % | 3.1 | 4.5 | .21 |
| Prevalent stroke, % | 4.7 | 4.1 | .54 |
| Systolic blood pressure, mmHg | 131 (19) | 131 (19) | .42 |
| Sports index (0–4) | 2.5 (0.8) | 2.6 (0.8) | .42 |
| Total cholesterol, mg/dL | 180 (42) | 181 (42) | .46 |
| Triglycerides, mg/dL | 125 (68) | 126 (64) | .83 |
| hsCRP, mg/L | 3.9 (6.2) | 4.3 (8.9) | .25 |
| APOE, % e4/e4 | 3.9 | 2.1 | .005 |
| e2/e4 or e3/e4 | 31.1 | 25.5 | |
| Other | 61.6 | 68.3 | |
| Missing | 3.4 | 4.2 | |
| Mini–Mental State Examination (MMSE) | 25.3 (4.6) | 27.1 (3.4) | <.0001 |
| CES-Depression scale | 3.4 (3.3) | 3.2 (3.0) | .23 |
| SF-12 Bodily Pain scale | 78.7 (25.0) | 77.0 (26.0) | .22 |
| Executive function score (Z-score) | −0.52 (0.43) | −0.33 (0.41) | <.0001 |
| Grip strength, kg | 28.9 (10.4) | 29.2 (10.4) | .58 |
| SPPB score (0–12) | 8.5 (2.9) | 9.3 (2.5) | <.0001 |
| 4-m walking speed, m/s | 0.86 (0.24) | 0.94 (0.23) | <.0001 |
Notes: CES = Center for Epidemiologic Studies; HDL = high density lipoprotein; hsCRP = high-sensitive C-reactive protein; SPPB = Short Physical Performance Battery.
*p-value from chi-square test for categorical variables and t-test for continuous variables.
Association of 12 Plasma Metabolites With Physical Function Measures
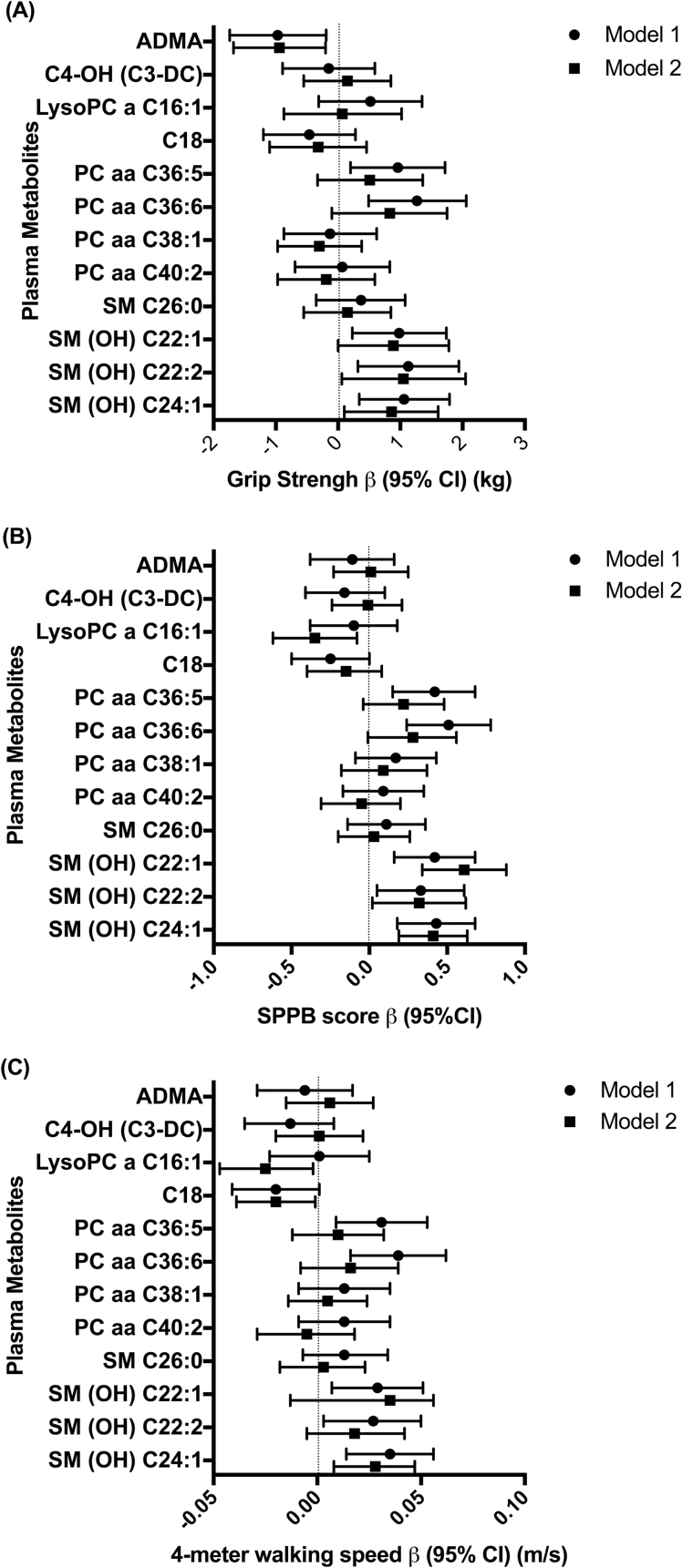
Figure 1 displays results from multivariable linear models estimating the association of the 12 metabolites with three physical function measures (also shown in Supplementary Tables 2–4). Higher ADMA was associated with worse grip strength in Model 1 and (Model 2: β-coefficients (95% CI) were −0.97 kg (−.74, −0.19) and −0.94 kg (−1.68, −0.20) per 1 SD increase in concentration, respectively. Higher PC aa C36:5 and PC aa C36:6 were associated with better physical function in all three measures in Model 1: β-coefficients were 0.96 kg (0.20, 1.72) and 1.27 kg (0.49, 2.06) per 1 SD increase in concentration, for grip strength; 0.42 (0.15, 0.68) and 0.51 (0.24, 0.78), for SPPB; and 0.031 m/s (0.009, 0.053) and 0.039 m/s (0.016, 0.062) for walking speed. Additional covariates (Model 2) attenuated the strength of the associations between PC aa C36:5 or PC aa C36:6 and all three physical function measures.
Figure 1.
Forest plots of β-coefficients (95% CI) of multivariable linear regression analyses between 12 plasma metabolites (log-transformed per 1 SD difference) and grip strength (A), SPPB (B), and 4-m walking speed (C) in the ARIC study, 2011 to 2013. Model 1: Linear regression adjusted for age, sex, race, center, dementia status, and MMSE. Model 2: Model 1 with additional adjustment for educational level, smoking and drinking status, APOE genotype, body mass index, physical activity, coronary artery disease, stroke, heart failure, diabetes mellitus, total cholesterol, high density lipoprotein (HDL) cholesterol, triglycerides, hsCRP, systolic blood pressure, use of antihypertensive medications, use of lipid lowering medications, depressive symptoms, executive function score, bodily pain score, prevalent coronary heart disease, stroke, and heart failure weighted by selection probabilities. ARIC = Atherosclerosis Risk in Communities Study; CI = confidence interval; hsCRP = high-sensitive C-reactive protein; MMSE = Mini–Mental State Examination; SPPB = Short Physical Performance Battery.
Higher SM (OH) C22:1, SM(OH) C22:2, and SM(OH) C24:1 were associated with better physical function in Model 1 and most Model 2 results. β-coefficients (95% CI) with grip strength were 0.98 kg (0.23, 1.74), 1.13 kg (0.32, 1.94), and 1.06 kg (0.34, 1.79) per 1 SD increase in concentration in Model 1 and 0.89 kg (0.00, 1.78), 1.05 kg (0.06, 2.05), 0.86 kg (0.10, 1.61) in Model 2. This was similar for SPPB β-coefficients (95% CI): 0.42 (0.16, 0.68), 0.33 (0.05, 0.61), and 0.43 (0.18, 0.68) per 1 SD increase in concentration in Model 1 and 0.61 (0.34, 0.88), 0.32 (0.02, 0.62), and 0.41 (0.19, 0.63) in Model 2. β-coefficients for 4-m walking speed were 0.029 m/s (0.007, 0.051), 0.027 m/s (0.003, 0.050), and 0.035 m/s (0.014, 0.056) per 1 SD increase in concentration in Model 1; associations with SM (OH) C22:1 and SM (OH) C24:1 were also supported in Model 2. Overall, all three SM(OH)s demonstrated moderate associations with physical function, meeting the minimal clinically meaningful differences (0.27–0.55 units) in SPPB scores and approached thresholds for small meaningful clinical differences (0.04–0.06 m/s) (27) for walking speed.
Association of Total SM (OH) With Physical Function Measures
Because of the substantial associations of all three SM (OH)s with all three physical function measures, we examined associations of total SM (OH) concentration with physical function in Models 1 and 2. Table 2 summarizes these results for five classes of total phospholipids (i.e. SM, SM[OH], PC aa, PC ae, lysoPC a). Total SM (OH) (i.e. sum of the three SM [OH]s: SM [OH] C22:1, SM [OH] C22:2, and SM [OH] C24:1, in addition to SM [OH] 14:1 and SM [OH] 16:1) was associated with each physical function measure in both models. Partial correlation coefficients (r2) between total SM (OH) concentration and physical function measures in Model 2 were 0.137 for grip strength, 0.162 for SPPB score, and 0.114 for 4-m walking speed (Supplementary Table 5). We did not find substantial interactions between sex, age, or APOE genotype and total SM(OH) concentration (Supplementary Table 6).
Table 2.
Cross-sectional Associations of Five Classes of Total Phospholipid Concentrations (Log-Transformed per 1 SD Concentration Increase) With Physical Function Measures, ARIC Study, 2011 to 2013
| Physical Function Measure | Total Phospholipid | Model | n | Difference Associated With 1 log-SD increase in the Metabolite [β] (95% CI)* |
|---|---|---|---|---|
| Grip strength (kg) | ||||
| PC aa | Model 1 | 380 | 0.77 (−0.01, 1.54) | |
| Model 2 | 380 | 0.97 (−0.45, 2.38) | ||
| PC ae | Model 1 | 380 | 0.71 (−0.04, 1.47) | |
| Model 2 | 380 | 0.75 (−0.36, 1.86) | ||
| SM | Model 1 | 380 | 0.74 (−0.06, 1.54) | |
| Model 2 | 380 | 1.19 (−0.07, 2.45) | ||
| SM (OH) | Model 1 | 380 | 1.17 (0.40, 1.94) | |
| Model 2 | 380 | 1.25 (0.26, 2.24) | ||
| LysoPC a | Model 1 | 380 | 0.55 (−0.22, 1.32) | |
| Model 2 | 380 | 0.22 (−0.62, 1.07) | ||
| SPPB score (0–12 scale) | ||||
| PC aa | Model 1 | 382 | 0.12 (−0.15, 0.39) | |
| Model 2 | 382 | 0.05 (−0.36, 0.46) | ||
| PC ae | Model 1 | 382 | 0.16 (−0.10, 0.42) | |
| Model 2 | 382 | 0.01 (−0.33, 0.35) | ||
| SM | Model 1 | 382 | 0.15 (−0.12, 0.43) | |
| Model 2 | 382 | 0.26 (−0.13, 0.66) | ||
| SM (OH) | Model 1 | 382 | 0.39 (0.13, 0.66) | |
| Model 2 | 382 | 0.51 (0.21, 0.80) | ||
| LysoPC a | Model 1 | 382 | 0.03 (−0.23, 0.30) | |
| Model 2 | 382 | −0.29 (−0.53, −0.05) | ||
| 4-m walking speed (m/s) | ||||
| PC aa | Model 1 | 377 | 0.010 (−0.012, 0.033) | |
| Model 2 | 377 | −0.003 (−0.037, 0.031) | ||
| PC ae | Model 1 | 377 | 0.020 (−0.002, 0.042) | |
| Model 2 | 377 | 0.007 (−0.021, 0.035) | ||
| SM | Model 1 | 377 | 0.017 (−0.006, 0.041) | |
| Model 2 | 377 | 0.017 (−0.014, 0.048) | ||
| SM (OH) | Model 1 | 377 | 0.030 (0.008, 0.053) | |
| Model 2 | 377 | 0.030 (0.007, 0.052) | ||
| LysoPC a | Model 1 | 377 | 0.009 (−0.013, 0.032) | |
| Model 2 | 377 | −0.021 (−0.041, 0.000) | ||
Notes: Model 1: Adjusted for age, sex, race, center, dementia status, and MMSE. Model 2: Model 1 plus educational level, smoking and drinking status, APOE genotype, body mass index, physical activity, coronary artery disease, stroke, heart failure, diabetes mellitus, total cholesterol, high density lipoprotein (HDL) cholesterol, triglycerides, hsCRP, systolic blood pressure, use of antihypertensive medications, use of lipid lowering medications, depressive symptoms, executive function, bodily pain score, coronary heart disease, stroke, and heart failure weighted by selection probabilities.
ARIC = Atherosclerosis Risk in Communities Study; CI = confidence interval; PC = phosphatidylcholine; SM = sphingomyelin; SPPB = Short Physical Performance Battery.
* β-coefficients represent the change of the predictor per 1 log-SD increase in metabolite concentration.
The principal component analysis of the 12 plasma metabolites and subsequent linear regression analysis of the association between five principal component analysis–derived factors with physical function measures showed that Factor 1, which includes the three three SM(OH)s, was substantially associated with all three physical function measures (Supplementary Tables 7 and 8), consistent with the analyses of individual SM (OH)s and total SM (OH).
Each SD higher plasma total SM (OH) concentration was associated with approximately a 30% lower odds of physical impairment defined by SPPB (odds ratio=0.66 [0.49, 0.89]) or by walking speed (odds ratio=0.70 [0.50, 1.00]) in Model 1 (Supplementary Table 9).
Associations of Total SM (OH) Concentration With Demographic Variables and Anthropometric and Behavioral Factors
Quantile analysis demonstrated that total SM (OH) concentration was not associated with age (Table 3) but was positively associated with total and high density lipoprotein cholesterol, Mini–Mental State Examination scores, SF-12 score of bodily pain (higher score means less pain), and was higher in females; SM (OH) was inversely associated with diabetes, coronary heart disease, and use of antihypertensive and lipid lowering medications.
Table 3.
Quartile Cross-sectional Associations of Total SM (OH) Concentration With Demographic Variables, Anthropometric and Behavioral Factors, ARIC Study, 2011 to 2013
| Q1 (n = 96) 9.7–12.5* |
Q2 (n = 96) 12.6–13.2 |
Q3 (n = 95) 13.3–13.7 |
Q4 (n = 96) 13.8–16.4 |
p-value** | |
|---|---|---|---|---|---|
| Age, years | 77.1 (5.3) | 77.8 (5.6) | 78.0 (5.4) | 77.3 (5.8) | .62 |
| African American, % | 32.3 | 19.8 | 26.3 | 22.9 | .23 |
| Female, % | 28.1 | 44.8 | 65.3 | 71.9 | <.0001 |
| Body mass index, kg/m2 | 29.4 (5.3) | 28.4 (6.0) | 28.6 (5.7) | 27.9 (5.3) | .34 |
| Current drinker, % | 46.9 | 39.6 | 37.9 | 45.8 | .10 |
| Current smoker, % | 7.3 | 3.1 | 7.4 | 2.1 | .22 |
| Diabetes, % | 53.1 | 42.7 | 31.6 | 25.0 | 0003 |
| High school graduate or higher, % | 76.0 | 81.3 | 84.2 | 83.3 | .44 |
| Hypertension medication, % | 91.7 | 83.3 | 80.0 | 60.4 | <.0001 |
| Lipid-lowering medication, % | 74.0 | 62.5 | 53.7 | 31.3 | <.0001 |
| Prevalent coronary heart disease, % | 24.0 | 21.9 | 22.1 | 3.1 | .0003 |
| Prevalent heart failure, % | 4.2 | 4.2 | 2.1 | 2.1 | .72 |
| Prevalent stroke, % | 8.3 | 6.3 | 3.2 | 1.0 | .08 |
| Systolic blood pressure, mmHg | 131 (20) | 130 (19) | 133 (20) | 131 (17) | .67 |
| Sports index | 2.7 (0.7) | 2.5 (0.8) | 2.5 (0.8) | 2.6 (0.8) | .27 |
| Total cholesterol, mg/dL | 149 (33) | 164 (30) | 190 (29) | 216 (40) | <.0001 |
| HDL cholesterol, mg/dL | 44.4 (13.0) | 48.5 (9.6) | 54.1 (14.1) | 59.6 (15.3) | <.0001 |
| Triglycerides, mg/dL | 133 (89) | 120 (65) | 120 (46) | 128 (64) | .50 |
| hsCRP, mg/L | 3.4 (5.7) | 3.7 (6.3) | 3.8 (4.5) | 4.8 (8.0) | .39 |
| APOE, % e4/e4 | 2.1 | 5.2 | 6.3 | 2.1 | .76 |
| e2/e4 or e3/e4 | 32.3 | 33.3 | 30.5 | 28.1 | |
| Other | 62.5 | 57.3 | 59.0 | 67.7 | |
| Missing | 3.1 | 4.2 | 4.2 | 2.1 | |
| Mini–Mental State Examination (MMSE) | 24.3 (4.4) | 25.1 (4.5) | 26.0 (4.7) | 25.8 (4.7) | .04 |
| CES-Depression scale ( | 3.5 (3.5) | 3.5 (2.9) | 3.6 (3.6) | 2.9 (3.3) | .45 |
| SF-12 Bodily Pain scale (0–100 scale) | 78.1 (26.0) | 78.7 (23.9) | 73.7 (27.4) | 84.3 (21.4) | .03 |
| Executive function z-score | -0.62 (0.45) | -0.56 (0.42) | -0.44 (0.40) | -0.44 (0.42) | .005 |
| Grip strength, kg | 31.2 (9.9) | 29.1 (10.2) | 27.9 (11.0) | 27.2 (9.9) | .04 |
| SPPB score (0–12 scale) | 8.1 (2.8) | 8.3 (3.0) | 8.6 (2.9) | 9.1 (2.8) | .10 |
| 4-m walking speed, m/s | 0.84 (0.24) | 0.83 (0.22) | 0.88 (0.25) | 0.91 (0.23) | .12 |
Notes: ARIC = Atherosclerosis Risk in Communities Study; CES = Center for Epidemiologic Studies; HDL = high density lipoprotein; hsCRP = high-sensitive C-reactive protein; SM = sphingomyelin; SPPB = Short Physical Performance Battery.
*Range of each quartile is expressed in SD units.
**p-value from chi-square test for categorical variables and t-test for continuous variables.
Discussion
The study presents novel findings on the independent association of six plasma metabolites: ADMA; PC aa C36:5; PC aa C36:6; and SM (OH) C22:1, SM (OH) C22:2, and SM (OH) C24:1 with physical function in older adults. In particular, the study findings demonstrate significant associations of SM (OH)s, a critical component of myelin surrounding peripheral nerves, with physical function measures that were robust to multiple covariate adjustments. These associations suggest that SM (OH)s may be important molecular players involved in maintaining physical function.
SM (OH)s belong to family of hydroxysphingolipids, a lack of which has been implicated in demyelination, neurodegeneration, and impaired physical function (28–34). Fatty acid 2-hydroxylase is responsible for generating hydroxysphingolipids, a subgroup of sphingolipids (28) that play important roles in long-term stability of myelin (29,34). Humans with fatty acid 2-hydroxylase gene mutations develop normally until 2–6 years of age and then develop difficulty walking and frequent falls, followed by progressive spasticity, immobility, inability to communicate, and eventually death (30–33). Because myelin sheath insulates nerve cell axons and transmits nerve signals to muscles, demyelination due to lack of SM(OH)s may also be associated with dysregulation of nerve signal transduction, leading to poorer physical function and reduced grip strength (35). This line of evidence corroborates our study findings suggesting SM (OH)s may play important roles in overall physical function in older adults.
In addition to plasma SM (OH)s, the study found associations of higher concentrations of plasma PC aa C36:5 and PC aa C36:6 with better physical function, although the strength of the associations was weakened by additional covariate adjustments. This finding builds upon a previous study that demonstrated supplementation of n-3 fatty acids improved quality of life in elderly with depression (36) by establishing associations of these n-3 fatty acids containing PCs with objective measures of functional performance in community-dwelling older adults. Finally, because plasma ADMA is associated with impaired endothelial function (37), associations of plasma ADMA with worse grip strength suggest that impaired endothelial function may be linked to muscle weakness.
This study is the first to demonstrate the independent associations between lower concentrations of plasma SM (OH)s and worse physical function in older, community-dwelling adults. A previous publication reported lower plasma SM (OH) C22:1 in centenarians compared to young adults (38). Although we did not find lower SM (OH) levels with older age, differences in SM (OH) levels may be more apparent when comparing across a broader age range than included in this study (mean age [SD] of 77.5 years [5.5]). In addition, centenarians are expected to have poorer physical function than young adults, further supporting potential relationships of SM(OH)s with physical function. Despite the primacy and strength, this study has limitations. First, the cross-sectional study design cannot assess temporality of associations; future studies should examine associations of SMs with physical function decline. Second, our study had approximately 4:4:3 ratio of participants with cognitive normal, MCI, and dementia and did not represent the distribution of cognitive impairment in the general aging population. However, analyses that incorporated weighting by selection probabilities produced similar results. Third, residual confounding could be present. Fourth, the assays used did not have a resolution to discern small mass differences (e.g. <0.5 Da), and therefore, it is possible that other SM species with similar masses as SM (OH)s could also be significantly associated with physical function. Additionally, ARIC was not designed to address the association between SM (OH)s and physical function. However, we performed secondary data analyses to address hypothesis-driven questions based on biologically plausible relationships. Lastly, adjustment for multiple comparisons was not considered and could be considered as a limitation. However, multiple comparisons present their own limitations and are not appropriate in all analyses (39). We do not believe multiple comparison tests were necessary as our study was not an exploratory but a hypothesis-driven analysis. The strength and magnitude of the associations, which are within clinically meaningful estimates of physical function outcomes, and consistency in directionality of all three SM (OH)s, suggest the associations of SM (OH)s with physical function may be important.
Conclusions
We identified several novel associations of plasma metabolites with physical function, in particular SM (OH)s which may be novel therapeutic targets for preserving physical function with aging. These findings warrant replication in other populations and use of more sensitive and specific methods to measure plasma metabolites to confirm the associations observed and/or to evaluate the associations of plasma SM (OH)s with physical function decline in older adults.
Supplementary Material
Supplementary data is available at The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences online.
Funding
The Atherosclerosis Risk in Community (ARIC) Study is carried out as a collaborative study supported by the National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN2682011000010C, HHSN2682011000011C, and HHSN2682011000012C). Neurocognitive data are collected by the support of the National Heart, Lung, and Blood Institute U01 HL096812, HL096814, HL096899, HL096902, and HL096917 with previous brain MRI examinations funded by R01-HL70825. DL is supported by a grant from the Alzheimer’s Association (NIGR-15-362392) and FY by the National Institute on Aging (1R01AG043392-01A1).
Conflict of Interest
None declared.
Supplementary Material
Acknowledgments
We thank the staff and participants of the ARIC Study for their important contributions.
References
- 1. Groessl EJ, Kaplan RM, Rejeski WJ, et al. Health-related quality of life in older adults at risk for disability. Am J Prev Med. 2007;33: 214–218. doi: 10.1016/j.amepre.2007.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55: M221–M231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49: M85–M94. [DOI] [PubMed] [Google Scholar]
- 4. Cesari M, Penninx BW, Pahor M, et al. Inflammatory markers and physical performance in older persons: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2004;59: 242–248. [DOI] [PubMed] [Google Scholar]
- 5. Papadakis MA, Grady D, Tierney MJ, Black D, Wells L, Grunfeld C. Insulin-like growth factor 1 and functional status in healthy older men. J Am Geriatr Soc. 1995;43: 1350–1355. [DOI] [PubMed] [Google Scholar]
- 6. Bonnefoy M, Patricot MC, Lacour JR, Rahmani A, Berthouze S, Kostka T. [Relation between physical activity, muscle function and IGF-1, testosterone and DHEAS concentrations in the elderly]. Rev Med Interne. 2002;23: 819–827. [DOI] [PubMed] [Google Scholar]
- 7. Savica R, Murray ME, Persson XM, et al. Plasma sphingolipid changes with autopsy-confirmed Lewy Body or Alzheimer’s pathology. Alzheimers Dement (Amst). 2016;3: 43–50. doi: 10.1016/j.dadm.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mielke MM, Bandaru VV, Haughey NJ, Rabins PV, Lyketsos CG, Carlson MC. Serum sphingomyelins and ceramides are early predictors of memory impairment. Neurobiol Aging. 2010;31: 17–24. doi: 10.1016/j.neurobiolaging.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li D, Misialek JR, Boerwinkle E, et al. Plasma phospholipids and prevalence of mild cognitive impairment and/or dementia in the ARIC Neurocognitive Study (ARIC-NCS). Alzheimers Dement (Amst). 2016;3: 73–82. doi: 10.1016/j.dadm.2016.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Buchman AS, Yu L, Wilson RS, Boyle PA, Schneider JA, Bennett DA. Brain pathology contributes to simultaneous change in physical frailty and cognition in old age. J Gerontol A Biol Sci Med Sci. 2014;69: 1536–1544. doi: 10.1093/gerona/glu117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Podbielska M, Banik NL, Kurowska E, Hogan EL. Myelin recovery in multiple sclerosis: the challenge of remyelination. Brain Sci. 2013;3: 1282–1324. doi: 10.3390/brainsci3031282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129: 687–702. [PubMed] [Google Scholar]
- 13. Knopman DS, Gottesman RF, Sharrett AR, et al. Mild cognitive impairment and dementia prevalence: the Atherosclerosis Risk in Communities Neurocognitive Study (ARIC-NCS). Alzheimers Dement (Amst). 2016;2: 1–11. doi: 10.1016/j.dadm.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kucharska-Newton AM, Palta P, Burgard S, et al. Operationalizing frailty in the Atherosclerosis Risk in Communities study cohort. J Gerontol A Biol Sci Med Sci. 2017;72: 382–388. doi: 10.1093/gerona/glw144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Burke BT, Köttgen A, Law A, et al. Physical function, hyperuricemia, and gout in older adults. Arthritis Care Res (Hoboken). 2015;67: 1730–1738. doi: 10.1002/acr.22648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cummings SR, Studenski S, Ferrucci L. A diagnosis of dismobility–giving mobility clinical visibility: a Mobility Working Group recommendation. JAMA. 2014;311: 2061–2062. doi: 10.1001/jama.2014.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fritz S, Lusardi M. White paper: “walking speed: the sixth vital sign”. J Geriatr Phys Ther. 2009;32: 46–49. [PubMed] [Google Scholar]
- 18. Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305: 50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gottesman RF, Schneider AL, Albert M, et al. Midlife hypertension and 20-year cognitive change: the atherosclerosis risk in communities neurocognitive study. JAMA Neurol. 2014;71: 1218–1227. doi: 10.1001/jamaneurol.2014.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36: 936–942. [DOI] [PubMed] [Google Scholar]
- 21. Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study). Am J Cardiol. 2008;101: 1016–1022. doi: 10.1016/j.amjcard.2007.11.061. [DOI] [PubMed] [Google Scholar]
- 22. Rosamond WD, Folsom AR, Chambless LE, et al. Stroke incidence and survival among middle-aged adults: 9-year follow-up of the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke. 1999;30: 736–743. [DOI] [PubMed] [Google Scholar]
- 23. Parrinello CM, Lutsey PL, Ballantyne CM, Folsom AR, Pankow JS, Selvin E. Six-year change in high-sensitivity C-reactive protein and risk of diabetes, cardiovascular disease, and mortality. Am Heart J. 2015;170: 380–389. doi: 10.1016/j.ahj.2015.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wu A, Coresh J, Selvin E, Tanaka H, Heiss G, Hirsch AT, et al. Lower extremity peripheral artery disease and quality of life among older individuals in the community. J Am Heart Assoc. 2017;6: e004519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cummings SR, Studenski S, Ferrucci L. A diagnosis of dismobility–giving mobility clinical visibility: a Mobility Working Group recommendation. JAMA. 2014;311: 2061–2062. doi: 10.1001/jama.2014.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332: 556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54: 743–749. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 28. Alderson NL, Maldonado EN, Kern MJ, Bhat NR, Hama H. FA2H-dependent fatty acid 2-hydroxylation in postnatal mouse brain. J Lipid Res. 2006;47: 2772–2780. doi: 10.1194/jlr.M600362-JLR200. [DOI] [PubMed] [Google Scholar]
- 29. Zöller I, Meixner M, Hartmann D, et al. Absence of 2-hydroxylated sphingolipids is compatible with normal neural development but causes late-onset axon and myelin sheath degeneration. J Neurosci. 2008;28: 9741–9754. doi: 10.1523/JNEUROSCI.0458-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cao L, Huang XJ, Chen CJ, Chen SD. A rare family with Hereditary Spastic Paraplegia Type 35 due to novel FA2H mutations: a case report with literature review. J Neurol Sci. 2013;329: 1–5. doi: 10.1016/j.jns.2013.02.026. [DOI] [PubMed] [Google Scholar]
- 31. Dick KJ, Eckhardt M, Paisán-Ruiz C, et al. Mutation of FA2H underlies a complicated form of hereditary spastic paraplegia (SPG35). Hum Mutat. 2010;31: E1251–E1260. doi: 10.1002/humu.21205. [DOI] [PubMed] [Google Scholar]
- 32. Donkervoort S, Dastgir J, Hu Y, et al. Phenotypic variability of a likely FA2H founder mutation in a family with complicated hereditary spastic paraplegia. Clin Genet. 2014;85: 393–395. doi: 10.1111/cge.12185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Garone C, Pippucci T, Cordelli DM, et al. FA2H-related disorders: a novel c.270 + 3A>T splice-site mutation leads to a complex neurodegenerative phenotype. Dev Med Child Neurol. 2011;53: 958–961. doi: 10.1111/j.1469-8749.2011.03993.x. [DOI] [PubMed] [Google Scholar]
- 34. Potter KA, Kern MJ, Fullbright G, et al. Central nervous system dysfunction in a mouse model of FA2H deficiency. Glia. 2011;59: 1009–1021. doi: 10.1002/glia.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hu X, Hicks CW, He W, et al. Bace1 modulates myelination in the central and peripheral nervous system. Nat Neurosci. 2006;9: 1520–1525. doi: 10.1038/nn1797. [DOI] [PubMed] [Google Scholar]
- 36. Rondanelli M, Giacosa A, Opizzi A, et al. Long chain omega 3 polyunsaturated fatty acids supplementation in the treatment of elderly depression: effects on depressive symptoms, on phospholipids fatty acids profile and on health-related quality of life. J Nutr Health Aging. 2011;15: 37–44. [DOI] [PubMed] [Google Scholar]
- 37. Stühlinger MC, Oka RK, Graf EE, et al. Endothelial dysfunction induced by hyperhomocyst(e)inemia: role of asymmetric dimethylarginine. Circulation. 2003;108: 933–938. doi: 10.1161/01.CIR.0000085067.55901.89. [DOI] [PubMed] [Google Scholar]
- 38. Collino S, Montoliu I, Martin FP, et al. Metabolic signatures of extreme longevity in northern Italian centenarians reveal a complex remodeling of lipids, amino acids, and gut microbiota metabolism. PLoS One. 2013;8: e56564. doi: 10.1371/journal.pone.0056564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gelman A, Hill J, Yajima M. Why we (usually) don’t have to worry about multiple comparisons. J Res Educ Eff. 2012;5: 189–211. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.