Abstract
Introduction
Sugars are major constituents and additives in traditional tobacco products, but little is known about their content or related toxins (formaldehyde, acetaldehyde, and acrolein) in electronic cigarette (e-cigarette) liquids. This study quantified levels of sugars and aldehydes in e-cigarette liquids across brands, flavors, and nicotine concentrations (n = 66).
Methods
Unheated e-cigarette liquids were analyzed using liquid chromatography mass spectrometry and enzymatic test kits. Generalized linear models, Fisher’s exact test, and Pearson’s correlation coefficient assessed sugar, aldehyde, and nicotine concentration associations.
Results
Glucose, fructose and sucrose levels exceeded the limits of quantification in 22%, 53% and 53% of the samples. Sucrose levels were significantly higher than glucose [χ2(1) = 85.9, p < .0001] and fructose [χ2(1) = 10.6, p = .001] levels. Formaldehyde, acetaldehyde, and acrolein levels exceeded the limits of quantification in 72%, 84%, and 75% of the samples. Acetaldehyde levels were significantly higher than formaldehyde [χ2(1) = 11.7, p = .0006] and acrolein [χ2(1) = 119.5, p < .0001] levels. Differences between nicotine-based and zero-nicotine labeled e-cigarette liquids were not statistically significant for sugars or aldehydes. We found significant correlations between formaldehyde and fructose (−0.22, p = .004) and sucrose (−0.25, p = .002) and acrolein and fructose (−0.26, p = .0006) and sucrose (−0.21, p = .0006). There were no significant correlations between acetaldehyde and any of the sugars or any of the aldehydes and glucose.
Conclusions
Sugars and related aldehydes were identified in unheated e-cigarette liquids and their composition may influence experimentation in naïve users and their potential toxicity.
Implications
The data can inform the regulation of specific flavor constituents in tobacco products as a strategy to protect young people from using e-cigarettes, while balancing FDA’s interest in how these emerging products could potentially benefit adult smokers who are seeking to safely quit cigarette smoking. The data can also be used to educate consumers about ingredients in products that may contain nicotine and inform future FDA regulatory policies related to product standards and accurate and comprehensible labeling of e-cigarette liquids.
Introduction
In 2016, the Food and Drug Administration (FDA) declared that electronic cigarettes (e-cigarettes) and flavored e-cigarette liquids meet the definition a tobacco product and are therefore subject to regulation that governs their manufacturing, marketing, and distribution.1 E-cigarettes are a class of battery-powered devices that can be used to aerosolize liquid when heated and inhaled by consumers. Many models of e-cigarettes exist within the product class, and some additives and constituents in the liquids may not be listed on the labels of the gallon or eye-drop size bottles in which these products are currently sold. As part of the new regulatory authority, FDA has expressed an interest in understanding constituents and toxicants in e-cigarette liquids and the aerosol.1
E-cigarette liquids come in over 400 brands and 7000 unique flavors.2 Flavored e-cigarette liquid is added to the e-cigarette component that houses the heating element (eg, cartomizer or tank) or in some cases is dripped onto the heating coils of the e-cigarette by the consumer. National data show that 63% of middle and high school student e-cigarette users reported using a flavored e-cigarette in the past 30 days.3 In 2011, 0.6% of middle school and 2% of high school students reported e-cigarette use in the past 30 days.4 By 2014, rates had dramatically increased among youth and 4% of middle school and 13% of high school students reported the use of e-cigarettes in the past 30 days.5 The growth of e-cigarette4,5 and flavored tobacco use among youth is of concern to the FDA.1 Since the majority of youth e-cigarette users report using a flavored e-cigarette, additional data are needed to understand its appeal.
Sugars are recognized as safe in food products and are important contributors to sweet taste, which contributes to flavor experiences.6 Sugars are likely to contribute to the flavor experiences of e-cigarette users, but empirical data on sugar content in e-cigarette liquids is lacking. However, there is much to learn from prior studies that have examined sugar content in other tobacco products and its effects on flavor experiences. For example, sugar in tobacco products can alter the bitter taste of nicotine, reduce the harshness and irritation of nicotine, and improve the aroma of the tobacco smoke.7 Sugar is used as a flavorant,8,9 binder, casing ingredient, formulation aid, or humectant in tobacco.7 Monosaccharides like glucose and fructose, and disaccharides like sucrose (table sugar) are naturally found in tobacco plants,8 can form during the priming and curing of tobacco,8 are added to tobacco,8,9 and are present in other tobacco additives such as fruit juices, honey, corn syrup, carob bean, or licorice.9,10
Carbohydrates, in the form of sugars, sugar esters, cellulose, pectin, and starch, may comprise 40%–50% of a tobacco product weight.8 The pyrolysis of these carbohydrates, including sugars, has been known the change sensory perceptions related to tobacco products.8 Although little is known about the types of sugars used and how they are used in e-cigarettes, it is possible that sugars in e-cigarettes are used for the same reasons that they are used in other tobacco products, which is to contribute to the flavor experience through sensory modification.
Sugar as a major constituent or additive in tobacco products can lead to exposure to harmful compounds that are produced when the sugar is heated.10–12 These harmful compounds include aldehydes such as formaldehyde, acetaldehyde, and acrolein. Prior studies have documented the quantities of aldehydes in e-cigarette aerosols following heating and suggested that these compounds result from the decomposition of propylene glycol and glycerin.13–16 In addition, harmful aldehyde constituents such as formaldehyde, acetaldehyde, and acrolein can result from the decomposition of sugars in tobacco at various temperatures depending on the type of sugar, tobacco, and presence of other constituents.7,10–12 Several studies have also identified aldehydes in unheated e-cigarette liquids.13,17–23 Such studies can help us understand how the presence of sugars and aldehydes in unheated e-cigarette liquids can inform, and potentially predict what we observe in the heated product.
Formaldehyde is a Group 1 carcinogen to humans24 and respiratory toxicant.25 Levels of formaldehyde can increase up to 73% in mainstream tobacco smoke for tobacco containing 1% w/w sugars (percent weight of solute in total weight of solution).26 Prior studies also show that “dripping” e-cigarette liquid directly onto the hot coils can result in high formaldehyde emission.27 Acetaldehyde is a Group 2B carcinogen, which may cause harm to humans,28 a cardiovascular toxicant, and has addictive properties.25 Acrolein produces eye and respiratory irritation; contributes to inflammation and cell proliferation; damages the lining of the lung;29 and contributes to cardiovascular disease.25 These aldehydes are listed on the FDA’s list of harmful and potentially harmful constituents (HPHCs) in tobacco products and smoke,25 have been identified in the aerosol of e-cigarettes under various conditions,13 and could potentially exceed levels found in cigarettes depending on the e-cigarette model.15
In addition, few studies have examined the content of vanillin in e-cigarette liquids,18,22,30,31 which is an aldehyde commonly used as a flavor additive in tobacco products32 and could potentially appeal to young people. Vanillin is the main flavor component in vanilla extract and is used widely in food products.33 In addition, when heated in cigarettes, this popular flavor additive can transfer intact into mainstream smoke at 200 degrees Celsius34 and can be toxic at the cellular level in high concentrations.33 For example, one study found that the e-cigarette liquid flavorant, ortho-vanillin, can trigger the release of interleukin-8 in human lung epithelial cells and human lung fibroblasts (Beas2B and HFL-1).35 This same study found that flavorants like vanillin can interact with nicotine to affect epithelial cell function.35 Although vanillins may produce physiological responses in human cells,35 little is known about the amount of vanillin in e-cigarette liquids and harm of vanillins in e-cigarette liquids when consumed by e-cigarette users, including young people.
To our knowledge, only one study has quantified sucrose in e-cigarette liquids.36 We are not aware of studies that have reported on multiples sugars together with the aldehydes in flavored e-cigarette liquids. This purpose of this study was to quantify the levels of sugars (glucose, sucrose, and fructose) commonly found in tobacco and aldehydes (formaldehyde, acetaldehyde, acrolein, and vanillin) in flavored unheated e-cigarette liquids. We also examined product labeling of these constituents in e-cigarette liquids. The quantification of sugars and aldehydes in unheated e-cigarette liquids can provide valuable information about the potential exposure of consumers to toxic compounds when heated. Thus, understanding the constituents in unheated e-cigarette liquids will provide valuable information to the FDA regarding its interest to protect young people from using e-cigarettes and interest in how these emerging products may influence switching from combustible tobacco to e-cigarettes among adult smokers. The data can also be used to educate consumers about ingredients in products that can contain nicotine, an addictive substance, and inform future FDA regulatory policies related to product standards and accurate and comprehensible labeling of e-cigarette liquids.
Methods
Sample
Research staff purchased a total of 66 e-cigarette liquid bottles from seven different vendors from March to April 2015 in Honolulu, Hawaii. This sample of vendor represents about 10% of vendors on the island of Oahu at the time of the study. Research staff asked each shop representative to identify the top brands sold within the store and top flavors for each top brand sold. We aimed to compare constituent and additive levels across brands, flavors, and nicotine concentrations. Thus, for each of the 8 brands, two unique flavors were selected, resulting in 16 different flavors for analysis. To examine the heterogeneity of constituents in e-cigarette liquids, four different nicotine concentrations were selected for 16 flavors. Where possible, the same nicotine concentrations were purchased across brands and flavors, but there was little consistency in nicotine concentrations across brands. For two flavors (same brand), nicotine concentrations were purchased in 0, 6, 12, 18, and 24 mg/mL because we wanted to purchase the highest concentration that would be comparable to nicotine concentrations in other brands. Rather than dropping one of the high concentrations from the analysis, we included the five different concentrations resulting in 66 samples. Nicotine concentrations that were labeled on bottles ranged from 0 mg to 24 mg. The zero nicotine concentration was selected as a baseline for each brand and flavor. The volume of the bottles ranged from 10 mL to 30 mL and in all cases, the smallest volume bottle available was purchased.
Following the purchases, each sample was assigned a unique number and was labeled and catalogued in a custom database. Research staff documented the store, brand name, stated nicotine content, stated ingredients, liquid color, and type of bottle (plastic or glass). All brand names were assigned a code (brands A–H). Samples were stored in an air-conditioned room and in a dark container to minimize the risk of constituent degradation postpurchase. When all the samples were purchased and data were documented, the 66 samples were transferred to our lab for further analysis.
Analytical methods
D-glucose, fructose, and sucrose concentrations were analyzed from e-cigarette liquids using an enzymatic kit (R-Biopharm Sucrose/D-Glucose/D-Fructose Cat#10716260035) combined with ultraviolet (UV) readings at 340 nm using a VersaMax microplate reader (Molecular Devices, Sunnyvale, CA) in a 96-well plate format. The concept of measurements is based on conversions of glucose and fructose to their 6-phosphates by hexokinase. Glucose-6-phosphate (G6P) reduces Nicotinamide adenine dinucleotide phosphate (NADP) to dihydronicotinamide adenine dinucleotide phosphate (NADPH), which can be monitored at 340 nm. Fructose-6-phosphate is converted to G6P by phosphoglucose isomerase. Sucrose is converted to glucose by ß-fructosidase. The protocol was followed exactly as directed by the manufacturer.
Formaldehyde, acetaldehyde and acrolein in e-cigarette liquids were analyzed by liquid chromatography mass spectrometry (LCMS) after dinitrophenylhydrazine (DNPH) derivatization. The e-cigarette liquids were 10-fold diluted with water and derivatized to hydrazones with DNPH. Orbitrap LCMS (model Q-Exactive, Thermo Scientific Inc., Waltham, MA) with electrospray ionization in negative ion mode was used to quantitate the hydrazones after separation on an Ascentis Express C18 column with a water/acetonitrile gradient. Mass spectrometry quantitation was performed using the exact masses 5 ppm of the deprotonated analytes using Xcalibur software (Thermo). The limits of quantification for all three compounds was based on a signal to noise ratio of 3.
Vanillin was analyzed by liquid chromatography isotope dilution electrospray ionization orbitrap mass spectrometry (model Q-Exactive, Thermo Scientific Inc., Waltham, MA) in positive ion mode. E-cigarette liquids were diluted 50000-fold with water, mixed with the isotope-labeled internal standard and injected onto a Kinetex C18 column (100 mm × 3.0 mm; 2.6 µm; Phenomenex, Torrance, CA) and separated with an aq. ammonium hydroxide/methanol gradient. Mass spectrometry quantitation was performed in positive mode using the exact mass 5 ppm of the protonated analytes using Xcalibur software (Thermo). The limit of quantification for vanillin was based on a signal to noise 3. Limits for the constituents were 6 µg/mL (glucose); 6 µg/mL (fructose); 12 µg/mL (sucrose); 5 µg/mL (vanillin); 0.01 ng/mL (formaldehyde); 0.02 ng/mL (acetaldehyde); and 0.024 ng/mL (acrolein).
The SAS 9.4 software37 was use to performed the statistical analyses. To account for limits of quantification, left-censored generalized linear model (SAS NLMIXED procedure) assessed differences in specific sugars and aldehydes across samples. Fisher’s exact test assessed if sugar and aldehyde levels (below vs. above limit of quantification) differed by nicotine level (zero-nicotine vs. nicotine-based). Pearson’s correlation coefficient assessed the associations between the sugars and aldehydes on the FDA’s list of harmful and potentially harmful constituents in tobacco.
Results
Sample Description
Brands purchased from local shops varied in their market locations, manufacturing, and distribution. Two brands were from large nationwide distributors with stores in multiple in US states. All eight brands could be purchased online. One brand advertised international distribution, and six of eight brands advertised wholesale options online. Six of the eight brands were produced locally.
Ingredient Labeling on E-cigarette Liquid Bottles
Of the 66 e-cigarette liquid bottles, 22.7% (n = 15) had no ingredient labels (Supplementary Table 1). None of the sample labels listed “sugars”, but 75.7% (n = 50) listed the natural flavors, flavoring or artificial flavors; and 12.1% (n = 8) listed natural sweeteners as ingredients. Brand E was the only brand that listed “sweeteners” on the label. None of the labels contained warning statements about aldehydes.
Sugars
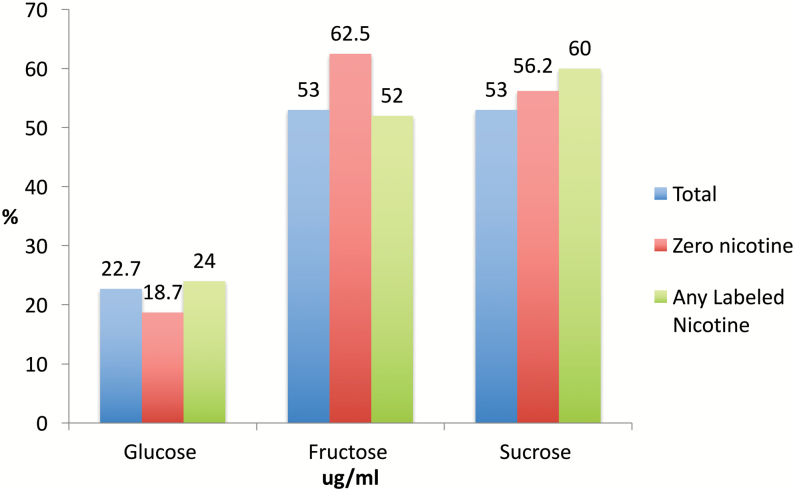
Levels of sugars varied by brand, flavor, and nicotine concentration (Supplementary Table 1). Table 1 shows the ranges for all sugars. Levels of sugars varied by brand, flavor, and nicotine concentration labeled on the samples (Supplementary Table 1). Table 1 shows the ranges for all sugars. The ranges were 6.4–88.9 µg/mL for glucose; 8.8–331.2 µg/mL for fructose; and 9.3–620.1 µg/mL for sucrose. Figure 1 shows the percentage of each sugar found in the total sample, zero nicotine concentration samples, and nicotine-based samples. Glucose was identified in 22% of the samples, and fructose and sucrose in 53% of the samples (Figure 1). Sucrose levels were significantly higher than glucose [χ2(1) = 85.9, p < .0001] and fructose [χ2(1) = 10.6, p = .001] levels in flavored e-cigarette liquids for all samples.
Table 1.
Range of Sugars and Aldehydes in E-Liquids (n = 66)
| Limit of quantification | Upper | Lower | Mean (SD)a | Medianb | |
|---|---|---|---|---|---|
| Glucose | <6 µg/mL | 88.9 | 6.4 | 20.4 (20.4) | <6.0 |
| Fructose | <6 µg/mL | 331.2 | 8.8 | 61.3 (79.9) | 9.7 |
| Sucrose | <12 µg/mL | 620.1 | 9.3 | 125.0 (153.7) | 18.9 |
| Formaldehydec | <0.01 ng/mL | 368 | 14 | 158.0 (117.5) | 66.8 |
| Acetaldehydec | <0.02 ng/mL | 4676.1 | 2.1 | 308.2 (717.1) | 73.2 |
| Acroleinc | <0.024 ng/mL | 10.1 | 0.3 | 2.2 (2.5) | 0.8 |
| Vanillin | <5 µg/mL | 11936.2 | 96.6 | 3006.3 (4174.4) | <5.0 |
aMean and standard deviation calculated for values above the limits of quantification.
bMedians calculated for values above and below the limits of quantification.
clisted on the Food and Drug Administration’s list of harmful and potentially harmful constituents in tobacco and tobacco smoke.
Flavored e-liquid samples were collected from vape” shops from March to April 2015.
Figure 1.
Percent sugars in e-liquids by labeled nicotine concentration (n = 66). Flavored e-liquid samples were collected from “vape” shops from March to April 2015 in Honolulu, Hawaii.
Of samples that exceeded the limit of quantification, Monkey Snack flavor with 18 mg/mL of nicotine list on the label had the highest level of glucose (88.9 µg/mL) and tobacco flavor with zero nicotine mg/mL listed on the label had the lowest (6.4 µg/mL). Cheesecake flavor with zero nicotine listed on the label had the highest levels of fructose (331.2 µg/mL) and Lollipop Sour Apple flavor with 24 mg/mL of nicotine (8.8 µg/mL) listed on the label had the lowest. Cheesecake flavor with zero nicotine listed on the label had the highest levels of sucrose (497.0 µg/mL) and Lemon Limed flavor with 11 mg/mL of nicotine on the label had the lowest (9.3 µg/mL) (Supplementary Table 1). We compared differences in sucrose, glucose, and fructose in nicotine-based and zero-nicotine e-cigarette liquid samples. None of the differences were statistically significant for any sugars (p > .05).
Vanillin
Levels of vanillin varied by brand, flavor, and the nicotine concentration labeled on the samples (Supplementary Table 1). Levels of vanillin range from 96.6 to 11936.2 µg/mL (Table 1). All 4 nicotine concentrations of Maui Mango flavor had the lowest detectable levels of vanillin additive and all 4 nicotine concentrations of Choco Haupia flavor had highest levels.
Aldehydes
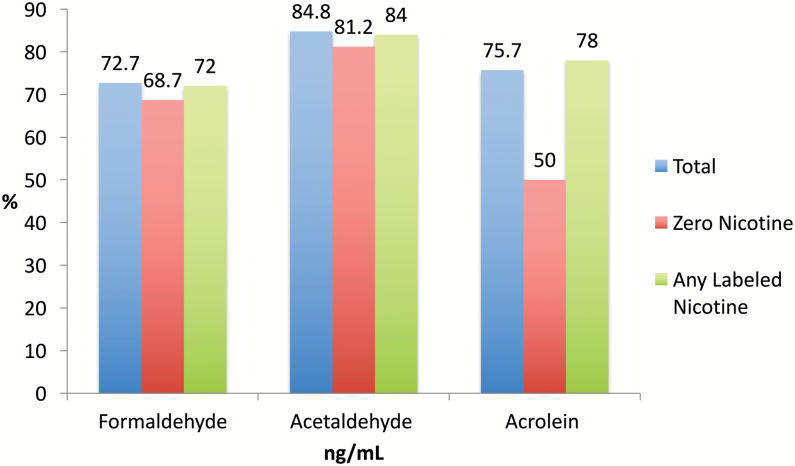
Levels of aldehydes varied by brand, flavor, and the nicotine concentration labeled on the samples (Supplementary Table 1 and Table 1). The ranges were 14–368 ng/mL for formaldehyde; 2.1–4676.1 ng/mL for acetaldehyde; 0.3–10.1 ng/mL for acrolein; and 96.6–11936.2 µg/mL for vanillin. Figure 2 shows percentage of each aldehyde found in the total sample, samples labeled as zero nicotine concentration, and sample labeled as containing nicotine. Formaldehyde was identified in 72% acetaldehyde in 84%, and acrolein in 75% of the samples (Figure 2). Acetaldehyde levels were significantly higher than formaldehyde [χ2(1) = 11.7, p = .0006] and acrolein [χ2(1) = 119.5, p < .0001] levels across all samples.
Figure 2.
Percent aldehydes in e-liquids by labeled nicotine concentration (n = 66). Flavored e-liquid samples were collected from “vape” shops from March to April 2015 in Honolulu, Hawaii.
Choco Haupia with 18mg/mL of nicotine listed on the label had the highest levels of formaldehyde (368.8 ng/mL) and Lemon Limed with 24 mg/mL of nicotine listed on the label had the lowest (1.4 ng/mL). Apple Lollipop Sour with 12 mg/mL had the highest levels of acetaldehyde (4676.1 ng/mL) and Cheesecake with zero nicotine listed on the label had the lowest (2.1 ng/mL). Pear Almond flavor with 12 mg/ml of nicotine listed on the label had the highest levels of acrolein (10.1 ng/mL) and Pina Colada flavor with zero nicotine listed on the label had the lowest (0.3 ng/mL). We compared differences in formaldehyde, acetaldehyde, and acrolein in nicotine-based and zero-nicotine e-cigarette liquid samples. Differences between nicotine-based and zero-nicotine e-cigarette liquids were not statistically significant for aldehydes (p > .05).
We examined the correlations between each sugar and formaldehyde, acetaldehyde, and acrolein (Table 2). There were no significant correlations between glucose and the aldehydes. We found significant correlations between formaldehyde and fructose (r = −0.22, p = .004) and sucrose (r = −0.25, p = .002) such that as the level of one compound increased, the level of the other decreased. Because these data are correlational, no temporal associations can be made. Significant correlations were also found between acrolein and fructose (r = −0.26, p = .0006) and sucrose (r = −0.21, p = .0006). There were no significant correlations between acetaldehyde and any of the sugars.
Table 2.
Correlations Between Aldehydes and Sugars (n = 66)
| Type of Sugar | ||||||
|---|---|---|---|---|---|---|
| Glucose | p value | Fructose | p value | Sucrose | p value | |
| Aldehydes | ||||||
| Formaldehyde | −0.10 | .17 | −0.22 | .004* | −0.25 | .001* |
| Acrolein | 0.11 | .36 | −0.26 | .0006* | −0.21 | .0006* |
| Acetaldehyde | 0.00 | .99 | −0.17 | .08 | −0.13 | .21 |
Log transformed left-censored modeling accounted for the lower limits of quantification.
Numerical values represent Pearsons correlation coefficient.
Flavored e-liquid samples were collected from “vape” shops from March to April 2015 in Honolulu, Hawaii.
*p < .001; p < .05.
Discussion
This is the first study to report levels of multiple sugars along with related aldehydes in a large sample of e-cigarette liquids and show variations across brands, flavors, and nicotine concentrations. Our study showed that fructose and sucrose levels exceeded the limit of quantification in more than 50% of the samples. Consistent with other studies,13,17–23 our data showed that aldehydes, toxins related to sugars, are present in unheated e-cigarette liquids and formaldehyde, acetaldehyde, and acrolein exceeded the limit of quantification in more than 70% of the samples. Sucrose levels were significantly higher than glucose and fructose, and acetaldehyde significantly higher than formaldehyde and acrolein levels across all samples. As formaldehyde and acrolein increased, fructose and sugars decreased. Sugars and aldehydes did not differ significantly by nicotine concentration. Furthermore, most product labels did not list sugars or provide warnings about aldehydes on the labels. The presence of FDA determined harmful tobacco constituents—formaldehyde, acetaldehyde, and acrolein—combined with high levels of sugars in unheated e-cigarette liquids, could potentially lead to higher levels of exposure to carcinogens and toxic chemicals when heated.13,15,27 This remains to be tested. Because sugar added to tobacco alters the smoke in cigarettes by modifying sensory impact of nicotine and other tobacco alkaloids,8 it is possible that sugar in e-cigarettes may make the product more appealing.
Our study found that 22.7% of the samples had no ingredients listed on the label and only 12.1% listed “sweeteners” and not specific sugars on the labels. While our study does not determine what quantity of sugar is needed to entice young people, the FDA has the authority to set product standard related to constituents and product labeling of e-cigarette liquid. Sugar reduces the irritation, bitterness, and harshness of tobacco and tobacco smoke7 and releases opioids and dopamine that stimulate the brain in ways similar to that of cocaine and morphine.38,39 Sugars, like glucose, may activate the Reward Deficiency Syndrome, a genetic and epigenetic impairment of brain reward circuitry resulting in abnormal craving behavior.38,39 However, studies on the effects of inhaled sugars via electronic cigarettes have not been conducted. How this route of administration and various levels of sugar influence reward systems in humans is unknown.
Consumers are also unaware of the existence of aldehydes in e-cigarette liquids, which were found in over 70% of our samples. Acetaldehyde, which was more prevalent in samples that other aldehydes, has addictive properties and may increase the self-administration of nicotine.40 Several prior studies that examined acetaldehyde and formaldehyde in e-cigarette liquids found higher levels of formaldehyde than our study.13,23 One study also identified formaldehyde and acetaldehyde in e-cigarette liquids, but did find acrolein above the detection limit.17 Further, formaldehyde was found in the aerosol, but not in the unheated e-cigarette liquid.19 A growing number of studies have identified vanillin as a common additive.18,22,30 Studies have examined the toxicity of vanillin,35 but the health effects associated with any of the aldehydes in e-cigarette liquids are unknown.
Product labeling related to e-cigarette parts or components is in the nascent stages. The FDA currently mandates that if e-cigarette parts or components contain nicotine, then the manufacturer must provide the information on the product label.1 Like nicotine, formaldehyde, acrolein and acetaldehyde are on the FDA’s list of HPHCs in cigarettes and are on the short-list of the FDA’s HPHCs to be reported by the manufacturers to the FDA. Manufacturers are required to report the quantity of these constituents. The literature on constituent identification in e-cigarettes is growing and several studies have identified aldehydes in heated and unheated e-cigarette liquidss.13,27 Thus, these constituents may be good candidates for product labeling in the future.
There is precedence on how to establish standards for e-cigarette product labeling and FDA’s regulations on food labeling may be useful for e-cigarette product labeling. The FDA regulates food labeling which includes guidance regarding the name of the ingredients, quantity of the contents, ingredient listing and size, nutrition labeling, label formats and graphics, and nutrition content claims.41 The FDA also has the authority to regulate food additives or food contact substances (like aldehydes), which are not intentionally added. According to food regulations, the type of sugar (eg, cane syrup, corn syrup) and amount of sugar (eg, by weight or % daily value) must be listed on food package labels under the category of carbohydrates.
We found significant correlations between formaldehyde and fructose and sucrose, such that as the level of one compound increased, the levels of the other decreased. Because these data are correlational, no temporal associations can be made. However, this is the first time that these data are presented and they provide important information for future studies that aim to compare how levels in unheated e-cigarette liquids inform what we observe in heated e-cigarette liquids. Our study did not focus on the composition of e-cigarette liquid when heated, but FDA has expressed in interest in the aerosol constituents as well.1 Determining how heated e-cigarette liquids influence exposure to toxins is still under investigation. Mods allow users to modify the battery voltage to produce more heat and studies show that higher battery voltage output is associated with higher levels of aldehydes and other constituents in the aerosol.14 High levels of sugars in e-cigarette liquids combined with the emergence of innovative high capacity heating devices can further increase consumer exposure to harmful constituents.
Strengths and Limitations of Study
Our analysis included a large number of flavored e-cigarette liquids at different nicotine concentrations. The number of e-cigarette liquids is growing rapidly and the sample may not be representative of the current market, which has not been characterized systematically. No national database of e-cigarette manufacturers and distributors existed at the time of the study. Unique to this study, we included a variety of brands, flavors, and nicotine concentrations that help us understand variations in sugars and other product constituents. Such data provide a stronger rationale for the need for product standards. Like other studies,23 we focused on commercial e-cigarette liquids sold in electronic cigarette shops and are available for purchase on the Internet. E-cigarette liquid samples were purchased from local shops, some of which are produced by major distributors in the United States. We did not measure the toxicity of these constituents or focus on risk assessments. We limited our study to the examination of glucose, fructose, and sucrose, but other sugars are found in tobacco products.12 A prior study summarized some of the analytical techniques used detect constituents in e-cigarette liquids,42 but there are no agreed upon standards for testing e-cigarette liquid products. Our study identified constituents that were identified in other studies.13–23
Conclusions
This study showed varying amounts of sugars and aldehydes commonly found in cigarettes in unheated e-cigarette liquids. Sugar has been traditionally used in tobacco products and understanding the content of sugar in e-cigarettes will help us understand how it is used to appeal to consumers. None of the constituents examined in this study were not listed on product labels, which could result in consumer misperceptions about the safety, harm, and ingredients of e-cigarette liquids unheated or heated. However, the aldehydes are on the FDA’s HPHC short-list of constituents that manufacturers have to report to FDA. Future research is needed to test flavored e-cigarette liquids for other sugars, additives, and constituents pre and postheating and at different temperatures to determine how much conversion to harmful constituents occurs. Studies are needed to examine how the levels of these constituents influence the toxicity, safety, appeal, abuse liability of the product, and delivery of sugar and toxins to consumers when heated. While this information is beneficial to FDA and the public, the data on constituents in e-cigarette liquids is also beneficial to the manufacturers. Educational efforts are also needed for e-cigarette manufacturers and distributors who may be naïve to the harmful constituents in heated and unheated e-cigarette liquids.
Supplementary Material
Supplementary data are available at Nicotine and Tobacco Research online.
Funding
This study was funded by the University of Hawaii Cancer Center. Drs. Fagan, Guy, and Eissenberg were supported in part by the National Institute on Drug Abuse of the National Institutes of Health under Award Number P50DA036105 and the Center for Tobacco Products of the U.S. Food and Drug Administration. The content is solely the responsibility of the authors and does not necessarily represent the views of the National Institutes of Health or the Food and Drug Administration.
Declaration of Interests
None declared.
Supplementary Material
Acknowledgments
We thank Alyssa Antonio and Faith Hamamura for helping to collect the data on the e-cigarette liquids and enter the data into the database.
References
- 1. United States Government, Department of Health and Human Services, Food and Drug Administration, Deeming tobacco products to be subject to the Federal Food, Drug, and Cosmetic Act, as Amended by the Family Smoking Prevention and Tobacco Control Act. Restrictions on the sale and distribution of tobacco products and required warning statements for tobacco products. Final Rule. Fed Regist. 2016;81(90):28973–9106. http://www.ncbi.nlm.nih.gov/pubmed/27192730. Accessed June 27, 2016. [PubMed] [Google Scholar]
- 2. Zhu SH, Sun JY, Bonnevie E et al. . Four hundred and sixty brands of e-cigarettes and counting: implications for product regulation. Tob Control. 2014;23(suppl 3):iii3–iii9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Corey CG, Ambrose BK, Apelberg BJ et al. . Flavored tobacco product use among middle and high school students--United States, 2014. Morb Mortal Wkly Rep. 2015;64(38):1066–1070. [DOI] [PubMed] [Google Scholar]
- 4. Centers for Disease Control and Prevention (CDC). Tobacco product use among middle and high school students--United States, 2011 and 2012. Morb Mortal Wkly Rep. 2013;62(45):893–897. Erratum in: MMWR Morb Mortal Wkly Rep. 2013;62(46):940. [PMC free article] [PubMed] [Google Scholar]
- 5. Arrazola RA, Singh T, Corey CG et al. ; Centers for Disease Control and Prevention (CDC) Tobacco use among middle and high school students - United States, 2011-2014. MMWR Morb Mortal Wkly Rep. 2015;64(14):381–385. [PMC free article] [PubMed] [Google Scholar]
- 6. Bartoshuk LM, Beauchamp GK. Chemical senses. Annu Rev Psychol. 1994;45:419–449. [DOI] [PubMed] [Google Scholar]
- 7. Talhout R, Opperhuizen A, van Amsterdam JG. Sugars as tobacco ingredient: Effects on mainstream smoke composition. Food Chem Toxicol. 2006;44(11):1789–1798. [DOI] [PubMed] [Google Scholar]
- 8. Leffingwell JC. Basic Chemical Constituents of Tobacco Leaf and Differences among Tobacco Types. In: Davis DL, Nielsen MT, eds. Tobacco: Production, Chemistry and Technology. Oxford, UK: Blackwell Sciences; 1999:265–284. [Google Scholar]
- 9. Seeman JI, Dixon M, Haussmann HJ. Acetaldehyde in mainstream tobacco smoke: formation and occurrence in smoke and bioavailability in the smoker. Chem Res Toxicol. 2002;15(11):1331–1350. [DOI] [PubMed] [Google Scholar]
- 10. Rustemeier K, Stabbert R, Haussmann HJ, Roemer E, Carmines EL. Evaluation of the potential effects of ingredients added to cigarettes. Part 2: chemical composition of mainstream smoke. Food Chem Toxicol. 2002;40(1):93–104. [DOI] [PubMed] [Google Scholar]
- 11. Baker RR, Coburn S, Liu C et al. . Pyrolysis of saccharide tobacco ingredients: a TGA-FTIR investigation. J Anal Appl Pyrolysis. 2005;74:171–180. [Google Scholar]
- 12. Baker RR, Coburn S, Liu C. The pyrolytic formation of formaldehyde from sugars and tobacco. J Anal Appl Pyrolysis. 2006;77(1):12–21. [Google Scholar]
- 13. Farsalinos KE, Kistler KA, Gillman G et al. . Evaluation of electronic cigarette liquids and aerosol for the presence of selected inhalation toxins. Nicotine Tob Res. 2015;17(2):168–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kosmider L, Sobczak A, Fik M et al. . Carbonyl compounds in electronic cigarette vapors: effects of nicotine solvent and battery output voltage. Nicotine Tob Res. 2014;16(10):1319–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jensen RP, Luo W, Pankow JF, Strongin RM, Peyton DH. Hidden formaldehyde in e-cigarette aerosols. N Engl J Med. 2015;372(4):392–394. [DOI] [PubMed] [Google Scholar]
- 16. Schripp T, Markewitz D, Uhde E, Salthammer T. Does e-cigarette consumption cause passive vaping?Indoor Air. 2013;23(1):25–31. [DOI] [PubMed] [Google Scholar]
- 17. Lim HH, Shin HS. Measurement of aldehydes in replacement liquids of electronic cigarettes by headspace gas chromatography- mass spectrometry. Bull Korean Chen Soc 2013; 34(9):2691–2696. [Google Scholar]
- 18. Hutzler C, Paschke M, Kruschinski S, Henkler F, Hahn J, Luch A. Chemical hazards present in liquids and vapors of electronic cigarettes. Arch Toxicol. 2014;88(7):1295–1308. [DOI] [PubMed] [Google Scholar]
- 19. Herrington JS, Myers C. Electronic cigarette solutions and resultant aerosol profiles. J Chromatogr A. 2015;1418:192–199. [DOI] [PubMed] [Google Scholar]
- 20. Behar RZ, Davis B, Wang Y, Bahl V, Lin S, Talbot P. Identification of toxicants in cinnamon-flavored electronic cigarette refill fluids. Toxicol In Vitro. 2014;28(2):198–208. [DOI] [PubMed] [Google Scholar]
- 21. Kucharska M, Wesołowski W, Czerczak S, Soćko R. [Testing of the composition of e-cigarette liquids - Manufacturer-declared vs. true contents in a selected series of products]. Med Pr. 2016;67(2):239–253. [DOI] [PubMed] [Google Scholar]
- 22. Tierney PA, Karpinski CD, Brown JE, Luo W, Pankow JF. Flavour chemicals in electronic cigarette fluids. Tob Control. 2016;25(e1):e10–e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Varlet V, Farsalinos K, Augsburger M, Thomas A, Etter JF. Toxicity assessment of refill liquids for electronic cigarettes. Int J Environ Res Public Health. 2015;12(5):4796–4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. International Agency for Research on Cancer (IARC). IARC monograph on the evaluation of carcinogenic risk to humans. Volume 83: Tobacco smoke and involuntary smoke. Lyon, France: World Health Organization Press; 2004. http://monographs.iarc.fr/ENG/Monographs/vol83/mono83- 1.pdf. Accessed February 19, 2016. [Google Scholar]
- 25. Harmful and Potentially Harmful Constituents in Tobacco Products and Tobacco Smoke Established List https://www.fda.gov/TobaccoProducts/GuidanceComplianceRegulatoryInformation/ucm297786.htm. Accessed November 1, 2017
- 26. Baker RR, Massey ED, Smith G. An overview of the effects of tobacco ingredients on smoke chemistry and toxicity. Food Chem Toxicol. 2004;42(suppl):S53–S83. [DOI] [PubMed] [Google Scholar]
- 27. Talih S, Balhas Z, Salman R et al. . “Direct Dripping”: A high-temperature, high-formaldehyde emission electronic cigarette use method. Nicotine Tob Res. 2015;pii:ntv080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. International Agency for Research on Cancer (IARC). IARC monograph on the evaluation of carcinogenic risks to humans. Volume 96: Alcohol consumption and ethyl carbamate. Lyon, France: World Health Organization Press; 2010. http://monographs.iarc.fr/ENG/Monographs/vol71/mono71-11.pdf. Accessed February 19, 2016. [PMC free article] [PubMed] [Google Scholar]
- 29. International Agency for Research on Cancer (IARC). IARC monograph on the evaluation of carcinogenic risks to humans. Volume 63: Dry cleaning, chlorinated solvents and other industrial chemicals. Lyon, France: World Health Organization Press; 1995. http://monographs.iarc.fr/ENG/Monographs/vol63/. Accessed February 19, 2016. [PMC free article] [PubMed] [Google Scholar]
- 30. Behar RZ, Davis B, Wang Y, Bahl V, Lin S, Talbot P. Identification of toxicants in cinnamon-flavored electronic cigarette refill fluids. Toxicol In Vitro. 2014;28(2):198–208. [DOI] [PubMed] [Google Scholar]
- 31. Hahn J, Monakhova YB, Hengen J et al. . Electronic cigarettes: overview of chemical composition and exposure estimation. Tob Induc Dis. 2014;12(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lemus R, Carmines EL, Van Miert E et al. . Toxicological comparisons of cigarettes containing different amounts of vanillin. Inhal Toxicol. 2007;19(8):683–699. [DOI] [PubMed] [Google Scholar]
- 33. Gallage NJ, Hansen EH, Kannangara R et al. . Vanillin formation from ferulic acid in Vanilla planifolia is catalysed by a single enzyme. Nat Commun. 2014;5:4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stotesbury S, Digard H, Willoughby L et al. . The pyrolysis of tobacco additives as a means of predicting their behavior in a burning cigarette. Beiträge zur Tabakforschung / Contributions to Tobacco Research. 1999;18(4):147–163. [Google Scholar]
- 35. Gerloff J, Sundar IK, Freter R et al. . Inflammatory response and barrier sysfunction by different e-cigarette flavoring chemicals identified by gas chromatography-mass spectrometry in e-liquids and e-vapors on human lung epithelial cells and fibroblasts. Appl In Vitro Toxicol. 2017;3(1):28–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kubica P, Wasik A, Kot-Wasik A, Namieśnik J. An evaluation of sucrose as a possible contaminant in e-liquids for electronic cigarettes by hydrophilic interaction liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2014;406(13):3013–3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. SAS Institute Inc. SAS software 9.4. Cary, N.C: SAS Institute Inc; 2013. [Google Scholar]
- 38. Blum K, Thanos PK, Gold MS. Dopamine and glucose, obesity, and reward deficiency syndrome. Front Psychol. 2014;5:919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Avena NM, Rada P, Hoebel BG. Evidence for sugar addiction: behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci Biobehav Rev. 2008;32(1):20–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Belluzzi JD, Wang R, Leslie FM. Acetaldehyde enhances acquisition of nicotine self-administration in adolescent rats. Neuropsychopharmacology. 2005;30(4):705–712. [DOI] [PubMed] [Google Scholar]
- 41. FDA guidance for industry: A food labeling guide (6. Ingredient list) http://www.fda.gov/Food/GuidanceRegulation/GuidanceDocuments RegulatoryInformation/LabelingNutrition/ucm064880.htm. Accessed February 19, 2016.
- 42. Famele M, Ferranti C, Abenavoli C, Palleschi L, Mancinelli R, Draisci R. The chemical components of electronic cigarette cartridges and refill fluids: review of analytical methods. Nicotine Tob Res. 2015;17(3):271–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.