Abstract
The ratio of protein and carbohydrate in an insect’s nutritional regime can significantly influence its survival, growth, and fecundity. The effects of 11 different artificial diets containing protein (p): carbohydrate (c) ratios were determined in larvae of the phytophagus ladybug, Henosepilachna vigintioctopunctata (F.) (Coleoptera: Coccinellidae). We recorded the developmental times and survival rates of the larvae and weighed their pupae. When the concentration of carbohydrates was kept constant while the concentration of proteins was increased (p29:c20, p31:c20, p33:c20, and p35:c20), H. vigintioctopunctata could successfully complete the larval and pupal stages. The highest survival rate and greatest pupal mass of H. vigintioctopunctata were 72% and 19.5 mg, respectively, when reared on the p33:c20 diet. H. vigintioctopunctata larvae, however, were unable to develop into adults when the concentration of protein remained constant while the level of carbohydrates was increased (p20:c23, p20:c25, p20:c27, and p20:c29), or when the total amount (p + c) was kept at 48% (p22:c26, p 24:c24, p26:c22). Evidently, changing the availability of quality diet, especially the total protein levels, can significantly affect the performance to H. vigintioctopunctata. Our results indicated that the maximum development and survival of H. vigintioctopunctata larvae occurred within a narrow range—when the p:c ratio was (33:20).
Keywords: Henosepilachna vigintioctopunctata, protein, carbohydrate, developmental time, survival
Insects consume specific diets to obtain the necessary mixture of nutrients that are essential to fuel their survival, growth, and fecundity (Behmer 2009). Proteins (p) and carbohydrates (c) are two significant macronutrients that provide the essential amino acids and energy, respectively, that influence their development, growth, and fecundity (Karasov and del Rio 2007; Simpson and Raubenheimer 2012). Most animals need to ingest the optimal nutritional blend of protein and carbohydrate to reduce fitness costs. Recent literature has documented that some insects such as grasshoppers, crickets, ants, and various caterpillars, among others, are capable of regulating their intake of protein and carbohydrate independently when provided the opportunity (Simpson and Raubenheimer 1993, Simpson et al. 2004, Lee et al. 2008, Dussutour and Simpson 2009, Altaye et al. 2010, Cease et al. 2012). The phytophagus ladybug beetle, Henosepilachna vigintioctopunctata (F.) (Coleoptera: Coccinellidae), typically feeds on Solanaceae plants such as black nightshade in the wild, but has adapted to many cultivated crops including tomato, potato, eggplant, etc. H. vigintioctopunctata is widespread throughout much of China (Zhou et al. 2015, Wang et al. 2017, Huang et al. 2018) and Japan (Nakamura 1976, Kawazu 2014, Sharma et al. 2012). The nutritional value of food plants varies considerably depending on the plant type, age, and growing conditions (Bernays and Chapman 1994, Awmack and Leather 2002). Because of this variability, it may be difficult for H. vigintioctopunctata beetles to continuously find and consume equally nutritious hosts. The consequences of such variation in host plant quality may be considerable to a beetle’s growth, survival, and reproductive capability.
That insects may actively regulate their intake of nutrients was only confirmed when researchers began to use defined artificial diets (Behmer 2009). Altering the nutrient composition of an artificial diet, either qualitatively and/or quantitatively, can often lead to a diet that is satisfactory to the target species, but understanding the precise interactions of the required ingredients remains a necessary to researchers. (Raubenheimer and Simpson 1997). Presently, lab populations of H. vigintioctopunctata are raised almost exclusively on natural host plants (Wang et al. 2017). This system of continually supplying host plant leaves as the diet for lab populations of H. vigintioctopunctata can be very cumbersome, both time- and labor-intensive, especially during the winter season when obtaining host plants may be difficult (Han et al. 2012). To date, however, attempts to develop artificial diets for H. vigintioctopunctata have not been successful. In 1979, Kono (1979) tried unsuccessfully to substitute sliced potato tubers as an alternative food for rearing H. vigintioctopunctata. The developmental time of the larvae was twice that of individuals reared on tomato plant leaves, and only half-sized adults were obtained. Murata et al. (1994) and Kawazu (2014) used different artificial diets to rear H. vigintioctopunctata. Both diets were successful in rearing third and fourth instar H. vigintioctopunctata larvae; the earlier instars, however, required tomato leaves to develop.
The aim at the present study was to elucidate the role of protein and carbohydrate ratios play in the development and growth of H. vigintioctopunctata. Using this information, we hope to develop a successful artificial diet for H. vigintioctopunctata larvae by altering the content and ratio of protein and carbohydrate. We used the two-sex life table method to evaluate the consequences of using different diets containing variable types of dietary protein and carbohydrates, we then assessed which protein: carbohydrate ratios were nutritionally favorable for optimal larval growth and development. Identifying the optimal p: c ratio would maximize H. vigintioctopunctata larval performance, producing a major impact on breeding H. vigintioctopunctata populations in the laboratory, and provide a basis for understanding H. vigintioctopunctata nutritional regulation.
Materials and Methods
Insects and Diets
Ten pairs of H. vigintioctopunctata adults were collected from potato plants in Jingzhou city (Hubei, China, 30.05 N, 112.14 E) and were successively reared on their wild host plant, Solanum nigrum, in the laboratory at 25 ± 1°C, 75% ± 10% RH, 12L: 12D for one generation. The eggs used in this study were laid within a 24-h period and obtained from 20 pairs of randomly chosen H. vigintioctopunctata adults. They were then placed in an environmental chamber (GZX-400BS-III, Shanghai Xin-Miao Medical Equipment Manufacturing Co., Ltd., China) at 25 ± 1°C and 75% ± 10% RH, with a photoperiod of 16L: 8D and allowed to develop.
The mixed diet consisted of commercial, chemically defined food products, including casein, cholesterol, ascorbic acid, choline, chloride, myo-insoitol, locust bean gum, Wesson’s salt, vitamin mix, cellulose, sucrose (Beijing Solarbio Science and Technology Co., Ltd., China), and yeast (Guangdong Huan Kai Microbial Technology Co., Ltd., China). The ingredients of artificial diet were weighed using an Ohaus balance (Model AX2242H/E). Table 1 gives the amount and composition of protein and carbohydrate in each ingredient. Cellulose as indigestible carbohydrate cannot be digested by H. vigintioctopunctata. Both casein and yeast provided protein, yeast and sucrose provide carbohydrate for H. vigintioctopunctata. Each of the 11 treatment combinations contained equal amounts of all ingredients, other than the three variables: Casein, sucrose, and cellulose, so that the concentration of micronutrients was constant across all diets (Raubenheimer and Simpson 1993, Yang 1994, Lee 2006).
Table 1.
The ratios and compositions of protein and carbohydrate in the ingredients used in the artificial diet
| Ratio | Total quality (g) | Yeast (g) | Casein (protein) (g) | Sucrose (carbohydrate) (g) | Cellulose (g) | Other (g) |
|---|---|---|---|---|---|---|
| p35:c20 | 12.6725 | 2.0000 | 3.7050 (3.3345) | 2.5673 (2.5160) | 0.7276 | 3.6700 |
| p33:c20 | 12.6725 | 2.0000 | 3.4234 (3.0811) | 2.5673 (2.5160) | 1.0093 | 3.6700 |
| p29:c20 | 12.6725 | 2.0000 | 3.1419 (2.8277) | 2.5673 (2.5160) | 1.2908 | 3.6700 |
| p27:c20 | 12.6725 | 2.0000 | 2.8603 (2.5743) | 2.5673 (2.5160) | 1.5724 | 3.6700 |
| p20:c23 | 12.6725 | 2.0000 | 1.5933 (1.4340) | 2.9552 (2.8961) | 2.4515 | 3.6700 |
| p20:c25 | 12.6725 | 2.0000 | 1.5933 (1.4340) | 3.2138 (3.1495) | 2.1929 | 3.6700 |
| p20:c27 | 12.6725 | 2.0000 | 1.5933 (1.4340) | 3.4723 (3.4029) | 1.9344 | 3.6700 |
| p20:c29 | 12.6725 | 2.0000 | 1.5933 (1.4340) | 3.7309 (3.6563) | 1.6758 | 3.6700 |
| p22:c26 | 12.6725 | 2.0000 | 1.8749 (1.6874) | 3.3430 (3.2762) | 1.7821 | 3.6700 |
| p24:c24 | 12.6725 | 2.0000 | 2.1564 (1.9408) | 3.0845 (3.0228) | 1.7591 | 3.6700 |
| p26:c22 | 12.6725 | 2.0000 | 2.4380 (2.1942) | 2.8259 (2.7694) | 1.7361 | 3.6700 |
The total dry mass of artificial diet is 12.67 g. The total mass of yeast, casein, sucrose, and cellulose is 9 g. The values for dietary protein and carbohydrate are based on yeast containing 55% protein and 0.9% carbohydrate, and casein containing 90% protein, and sucrose containing 98% carbohydrate.
Experimental Design
We formulated 11 treatment combinations containing different ratios of dietary protein (p) and carbohydrate (c) (Table 1). We began by maintaining the concentration of carbohydrates, and then increasing the concentration of protein (p29:c20, p31:c20, p33:c20, and p35:c20). Next, we maintained the concentration of protein, and increased the level of carbohydrates (p20:c23, p20:c25, p20:c27, and p20:c29). Finally, we kept the total protein (p) and carbohydrate (c) amount (48% by dry mass) but changed the p:c ratios (p22:c26, p 24:c24, p26:c22). Identical amounts of artificial diet were added to plastic containers (27 cm length, 15 cm width) containing 32 litter box which was disinfected prior to usage, using 75% alcohol, and then exposed to UV light for 1 h to reduce microbial contamination. The newly hatched larvae were individually transferred to containers with one of the 11 food treatments. Each diet was replaced every 3 d, and the developmental data, survival rate, and pupal weight for all individuals were recorded daily until the emergence of adults.
Statistical Analysis
The raw life table data for H. vigintioctopunctata larvae reared on different ratios of protein and carbohydrate were analyzed using the age-stage, two-sex life table theory developed by Chi and Liu (1985) and Chi (1988). The bootstrap technique (Efron and Tibshirani 1993, Huang and Chi 2012) with 100,000 resampling was used to calculate the standard errors of the larval and pupal developmental times and pupal masses. Data were analyzed using the computer program, TWOSEX-MSChart (Chi 2016) (available at: http://140.120.197.173/Ecology/Download/TWOSEX-MSChart.rar). The relationship between the age-specific survival rate (lx) which is the probability that a newly laid egg will survive to age x and the age-stage specific survival rate curves (sxj) which is the probability that a newly laid egg will survive to age x and stage j were calculated as:
| (1) |
Where k is the number of stages.
Results
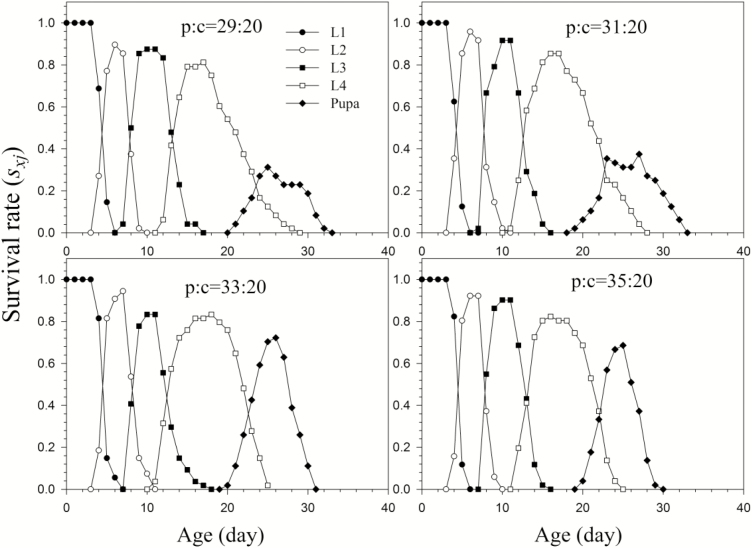
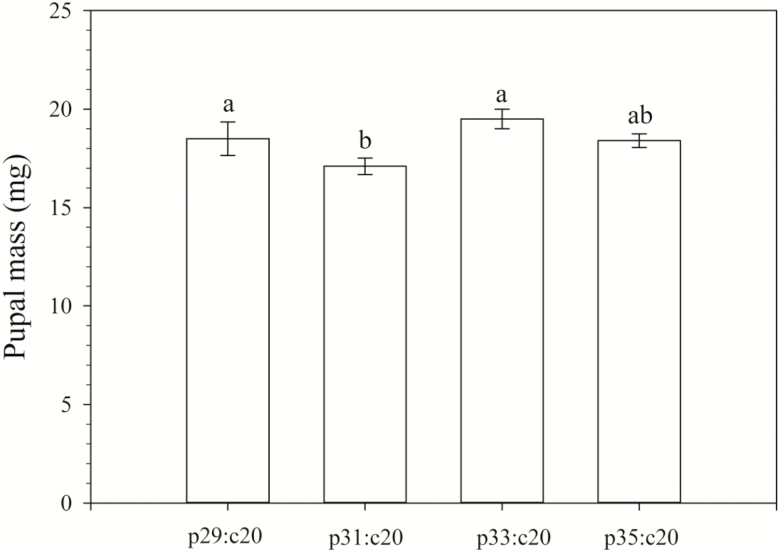
We recorded the developmental time and survival rate of H. vigintioctopunctata larvae reared on different protein: carbohydrate ratios when the carbohydrate level remained constant (Table 2 and Fig. 1). Although the lengths of the total larval stage (d) did not significantly differ in the four different protein levels, the pupal period reared on p35:c20 (5.47 d) was significantly shorter than that of the three other dietary ratios (Table 2). The pupal wet mass was the heaviest (19.5 mg) when larvae were fed on the p33:c20 diet, and the lightest (17.1 mg) when fed on the p31:c20 diet. The pupal mass when fed on diets p33:c20 (19.5 mg) and p29:c20 (18.5 mg) were both significantly higher than on diet p31:c20 (Fig. 2). The age-stage-specific survival rate curves (sxj) (Figs. 1,3,4) clearly show the stage differentiation and overlap of H. vigintioctopunctata larvae reared on the eleven diets with different p: c ratios. The highest sxj of the L1, L2, and L3 instars were not significant difference among the four different protein levels. However, the mortality of L4 larvae fed on p29:c20 and p 31:c20 was significantly higher than on the p33:c20 and p35:c20 diets (Fig. 1). The survival rates for larvae fed on diets p33:c20 (72%) and p35:c20 (68%) were significantly higher than those on the p29:c20 (31%) and p31:c20 (37%) diets (Fig. 1).
Table 2.
The developmental time of H. vigintioctopunctata larvae and pupa reared on different diet by maintaining the concentration of carbohydrates, and then increasing the concentration of protein (p29:c20, p31:c20, p33:c20, and p35:c20)
| Stage | The ratio of protein: carbohydrate | |||
|---|---|---|---|---|
| 29:20 | 31:20 | 33:20 | 35:20 | |
| L1 (d) | 4.84 ± 0.10a,b | 4.74 ± 0.09b | 5.02 ± 0.10a | 4.96 ± 0.08a,b |
| L2 (d) | 3.58 ± 0.08b | 3.82 ± 0.12a,b | 3.80 ± 0.06a | 3.55 ± 0.07b |
| L3 (d) | 5.21 ± 0.14a | 4.68 ± 0.15b | 5.06 ± 0.09a | 5.00 ± 0.13a,b |
| L4 (d) | 10.56 ± 0.41a | 10.07 ± 0.37a | 9.11 ± 0.11b | 9.10 ± 0.14b |
| Pupa (d) | 7.12 ± 0.16a | 6.28 ± 0.10b | 6.08 ± 0.10b | 5.47 ± 0.12c |
| Total larval stage (d) | 23.75 ± 0.67a | 23.19 ± 0.46a | 22.95 ± 0.22a | 22.61 ± 0.27a |
Values are means ± standard errors. Standard errors were evaluated using the bootstrap procedure with 100,000 resampling and different letters in the same row indicated significantly different (P < 0.05) between the ratio of p:c using the paired bootstrap test. (L1, the first instar; L2, the second instar, L3, the third instar, L4, the fourth instar.)
Fig. 1.
Age-stage-specific survival rate (sxj) of H. vigintioctopunctata larvae reared on diet with different protein: carbohydrate ratios by maintaining the concentration of carbohydrates, and then increasing the concentration of protein (p29:c20, p31:c20, p33:c20 and p35:c20).
Fig. 2.
Age-stage-specific survival rate (sxj) of H. vigintioctopunctata reared on diet with different protein: carbohydrate ratios by maintaining the concentration of protein and then increasing the level of carbohydrates (p20:c23, p20:c25, p20:c27 and p20:c29).
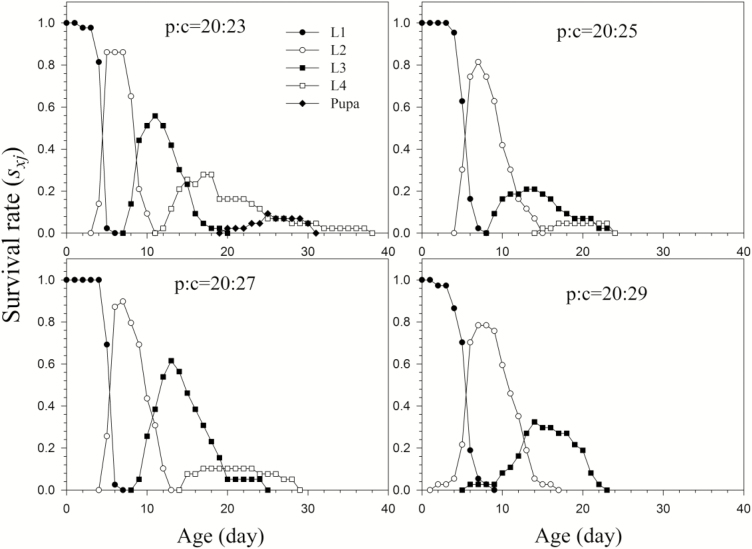
Fig. 3.
Age-stage-specific survival rate (sxj) of H. vigintioctopunctata reared on diet with different ratio of protein and carbohydrate by keeping the total protein (p) and carbohydrate (c) amount (48% by dry mass) but changed the p:c ratios (p22:c26, p 24:c24 and p26:c22).
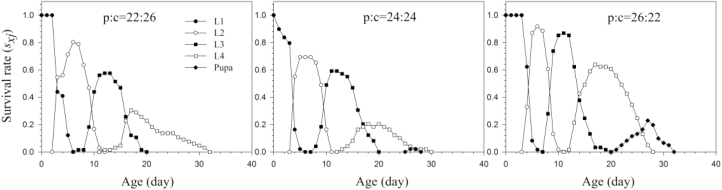
Fig. 4.
The means of pupal mass of H. vigintioctopunctata reared on different diet by maintaining the concentration of carbohydrates, and then increasing the concentration of protein (p29:c20, p31:c20, p33:c20 and p35:c20)
The number of H. vigintioctopunctata larvae surviving to the fourth instar was significantly decreased with increases in carbohydrate concentration (Fig. 3). The developmental times of L1 and L2 reared on p20:c23 were significantly faster than those on p20:c25, p20:c27, and p20:c29 (Table 3). H. vigintioctopunctata larvae could successfully develop to the pupal stage when fed on the p20:c23 diet, but the highest pupal sxj was 9.3%. The highest sxj values for L4 reared on the p20:c25 and p20:c27 diets were 4.6% and 10.3%, respectively. The larvae fed on the p20:c29 diet only survived to the third instar.
Table 3.
The developmental time of H. vigintioctopunctata larva reared on different diet by maintaining the concentration of protein and then increasing the level of carbohydrates
| Stage | The ratio of protein: carbohydrate | |||
|---|---|---|---|---|
| 20:23 | 20:25 | 20:27 | 20:29 | |
| L1 (d) | 4.83 ± 0.06b | 6.11 ± 0.12a | 5.83 ± 0.08a | 5.90 ± 0.20a |
| L2 (d) | 4.38 ± 0.14a | 5.58 ± 0.43b | 5.67 ± 0.28b | 7.17 ± 0.42c |
| L3 (d) | 5.42 ± 0.27b | 7.00 ± 0.71a | 5.60 ± 0.35a,b | - |
| L4 (d) | 9.8 ± 0.52 | - | - | - |
Values are means ± standard errors. Standard errors were evaluated using the bootstrap procedure with 100,000 resampling and different letters in the same row indicated significantly different (P < 0.05) between the ratio of p:c using the paired bootstrap test. ‘-’ means the H. vigintioctopunctata cannot finish this stage. (L1, the first instar; L2, the second instar, L3, the third instar, L4, the fourth instar.).
When the total protein (p) and carbohydrate (c) amounts (48% by dry mass) were kept the same but differed in their p:c ratios (22:c26, p 24:c24, p26:c22), the H. vigintioctopunctata larvae reared on the p26:c22 and p24: p24 diets were able to complete the entire larval stage; those fed on diet p22: c26, however, were only able to develop to L4 (Fig. 4). The developmental periods of L2 and L3 reared on the p26:c20 diet were both significantly shorter than on diet p24:c24 (Table 4).
Table 4.
The developmental time of H. vigintioctopunctata larva reared on different ratio of protein: carbohydrate levels by keeping the total protein (p) and carbohydrate (c) amount (48% by dry mass) but changed the p:c ratios
| Stage | The ratio of protein: carbohydrate | ||
|---|---|---|---|
| 26:20 | 24:24 | 22:26 | |
| L1 (d) | 4.70 ± 0.07b | 3.10 ± 0.07c | 5.20 ± 0.07a |
| L2 (d) | 4.12 ± 0.07c | 6.00 ± 0.18a | 4.39 ± 0.11b |
| L3 (d) | 5.65 ± 0.19b | 6.65 ± 0.25a | 6.00 ± 0.30a,b |
| L4 (d) | 11.94 ± 0.41 | - | - |
Values are means ± standard errors. Standard errors were evaluated using the bootstrap procedure with 100,000 resampling, and different letters in the same row indicated significantly different (P < 0.05) between the ratio of p:c using the paired bootstrap test. ‘-’ means the H. vigintioctopunctata cannot finish this stage.
Discussion
In this study, we used the life table approach to analyze the effects that two key macronutrients have on larval performance, and to obtain the optimal p:c ratio for H. vigintioctopunctata. Life tables, as one of the basic research tools for population ecology studies, are often used to determine the survival, development and reproductive capabilities of a population cohort when exposed to different treatment variables such as cultivars, temperature, humidity, etc. (Morris and Miller 1954, Jahn et al. 2005, Hu et al. 2010, Ying et al. 2012, Li et al. 2015, Yang et al. 2015).
Our results showed that the relationship between larvae performance and dietary protein/carbohydrate ratios was closely related. Clearly, altering the availability of quality diet can affect the performance of H. vigintioctopunctata larvae. Dietary carbohydrate levels, comparing with the total protein levels, seldom exerted a vital effect on survival, growth, and pupal weight. When the protein content was kept constant and the proportion of carbohydrates was gradually increased, the developmental duration of H. vigintioctopunctata larvae was significantly affected to the extent that they were unable to complete their development in the larval stage (Table 3 and Fig. 3). However, H. vigintioctopunctata larvae were not only able to complete their development, but the developmental period was shortened when the carbohydrate content was kept constant as the protein content percentage was increased (Table 2 and Fig. 1). The data demonstrated that protein deficits are more detrimental to H. vigintioctopunctata larval survival than carbohydrate deficits. This agrees with several previous reports from Simpson et al. (2004), Thompson and Redak (2000) and Wilkinson (2001) in grasshoppers, caterpillars, and aphids, respectively. However, because omnivores and herbivores regulate protein intake more intensely than they do carbohydrate, it is inevitable that they are more susceptible to insufficient protein quantities when fed on low p:c ratios (Simpson and Raubenheimer 2005).
During each animal’s developmental stage, various tissues require a specific quantity and blend of nutrients (Simpson and Raubenheimer 1993). This requirement is applicable to insects as well, where the function and effect of protein and carbohydrate will vary depending on the insect’s development, growth, and fecundity. It also can vary dramatically depending on the stage and sex where the demands made by larvae, and adult females and males for protein and carbohydrate are very different (Joern and Behmer 1997). An adult female insect cannot maximize both longevity and egg production rate on a single diet (Simpson and Raubenheimer 2009). The lifespan of both male and female insect are maximized on a high-carbohydrate low-protein diet. However, comparing the blend of nutrition necessary to maximized a female’s lifespan, with the, blend needed to achieve the optimal female reproductive performance will often produce two ratios that are considerably different (Lee et al. 2008, Maklakov et al. 2008, Fanson et al. 2009). There is a sexual difference in the trade-off between lifespan and reproduction in females and males; the females require high levels of protein for reproduction (Wheeler 1996) while males require low-protein, high-lipid diets to build energy reserves to support them in their search for females (Stockhoff 1993).
Although proteins are very important resources for promoting host resistance and producing immunological components to resist infections by pathogens (Washburn et al. 1996, Trudeau et al. 2001, Lee et al. 2006), subsisting on pure or higher than optimal level of protein, inevitably results in adversely affecting an insect’s growth and development and inducing a fitness cost (Wilkinson 2001, Cease et al. 2012). Although there was no significant difference between dietary p33:20 and p35:20 in larval survival rate and pupal mass, the survival rate (72%) and pupal mass (19.5 mg) of insects reared on dietary p33:c20 was higher than the survival rate (68%) and pupal mass (18.4 mg) for those reared on dietary p35:c20. These data suggest that the optimal p:c ratio for maximizing H. vigintioctopunctata larval performance is 33:20.
In addition, the present study found that females that were emerged from the experimental pupae were unable to produce offspring when fed on any of the artificial diets, regardless of the protein: carbohydrate ratio. This would may be that the protein and carbohydrate requirements differ considerably between the larvae and adults. Both larvae and adults of H. vigintioctopunctata feed on leaves of Solanaceae plants. H. vigintioctopunctata larvae have little, if any, opportunity to alter their diet by choosing between different host plants, so the beetle larvae are forced to feed within a relatively narrow p:c range. However, the situation is remarkably different for H. vigintioctopunctata adults, where the adults have the ability to manage imbalances by compensatory food selection. Wang et al. (2017) and Zhou et al. (2015) reported that the survival rate of H. vigintioctopunctata larvae was significantly higher on eggplant than it was on S. nigrum, while the fecundity of adults on eggplant was significantly lower than on S. nigrum. Their results indicate that, based on the reduced survival rates, the larvae were considerably less adapted on S. nigrum, than were the adults, where their fecundity was increased.
In conclusion, breeding H. vigintioctopunctata larvae using artificial diet was successful. Although the total length of the larval stage (22.95 d) when reared on the p33:c20 diet was longer than when reared on S. nigrum (14.14 d) (Wang et al. 2017), the highest sxj for H. vigintioctopunctata larvae was 72% when feed on the p33:c20 diet which was much closer to the natural host plant, S. nigrum (72.5%) (Wang et al. 2017) and 75% (Huang et al. 2018). The reason for the prolonged larval period but approximately normal survival rate when compared to the host plant may be due to insufficient micronutrients such as cholesterol in the artificial diet (Svoboda et al. 1975). Our findings are a substantial improvement over previous attempts to rear H. vigintioctopunctata on blended artificial diets (see: Kono (1979), Murata et al. (1994) and Kawazu (2014)). Although we were successfully in rearing H. vigintioctopunctata larvae, the artificial diet we used was not appropriate for rearing the adults because of their inability to produce offspring. Further refinements are needed in the breeding method, and additional studies will also be needed to determine the ideal nutrient ratio to formulate a successful artificial diet for the H. vigintioctopunctata adult.
Acknowledgments
We thank Prof. Dr. Hsin Chi for providing the Two-Sex life table program and his inspirational lectures. We are grateful to the reviewers and the editor for their valuable comments and suggestions, all of which have greatly helped us in improving this manuscript. This research was supported by the National Natural Science Foundation of China (No. 31572010).
References Cited
- Altaye S. Z., Pirk C. W., Crewe R. M., and Nicolson S. W.. 2010. Convergence of carbohydrate-biased intake targets in caged worker honeybees fed different protein sources. J. Exp. Biol. 213: 3311–3318. [DOI] [PubMed] [Google Scholar]
- Awmack C. S., and Leather S. R.. 2002. Host plant quality and fecundity in herbivorous insects. Annu. Rev. Entomol. 47: 817–844. [DOI] [PubMed] [Google Scholar]
- Behmer S. T. 2009. Insect herbivore nutrient regulation. Annu. Rev. Entomol. 54: 165–187. [DOI] [PubMed] [Google Scholar]
- Bernays E. A., and Chapman R. F.. 1994. Host-plant selection by phytophagous insects, vol. 2 pp. 7–9. Springer, New York. [Google Scholar]
- Cease A. J., Elser J. J., Ford C. F., Hao S., Kang L., and Harrison J. F.. 2012. Heavy livestock grazing promotes locust outbreaks by lowering plant nitrogen content. Science 335: 467–469. [DOI] [PubMed] [Google Scholar]
- Chi H. 1988. Life-table analysis incorporating both sexes and variable development rates among individuals. Environ. Entomol. 17: 26–34. [Google Scholar]
- Chi H. 2016. TWOSEX-MSChart: a computer program for the age-stage, two-sex life table analysis National Chung Hsing University, Taichung, Taiwan: Available from http://140.120.197.173/Ecology/Download/Twosex-MSChart.zip [Google Scholar]
- Chi H., and Liu H.. 1985. Two new methods for the study of insect population ecology. Bull. Inst. Zool. Acad. Sin. 24: 225–240. [Google Scholar]
- Dussutour A., and Simpson S. J.. 2009. Communal nutrition in ants. Curr. Biol. 19: 740–744. [DOI] [PubMed] [Google Scholar]
- Efron B., and Tibshirani R. J.. 1993. An introduction to the bootstrap. Chapman & Hall, New York, NY. [Google Scholar]
- Fanson B. G., Weldon C.W., Pérez-Staplesn D., Simpson S. J., and Taylor P. W.. 2009. Nutrients, not caloric restriction, extend lifespan in Queensland fruit flies (Bactrocera tryoni). Aging Cell 8: 514–523. [DOI] [PubMed] [Google Scholar]
- Han L., Li S., Liu P., Peng Y., and Hou M.. 2012. New artificial diet for continuous rearing of Chilo suppressalis (Lepidoptera: Crambidae). Ann. Entomol. Soc. Am. 105: 253–258. [Google Scholar]
- Hu L. X., Chi H., Zhang J., Zhou Q., and Zhang R. J.. 2010. Life-table analysis of the performance of Nilaparvata lugens (Hemiptera: Delphacidae) on two wild rice species. J. Econ. Entomol. 103: 1628–1635. [DOI] [PubMed] [Google Scholar]
- Huang Y. B., and Chi H.. 2012. Assessing the application of the jackknife and bootstrap techniques to the estimation of the variability of the net reproductive rate and gross reproductive rate: a case study in Bactrocera cucurbitae (Coquillett) (Diptera: Tephritidae). J. Agri. Fore. 61: 37–45. [Google Scholar]
- Huang H. W., Chi H., and Smith C. L.. 2018. Linking demography and consumption of Henosepilachna vigintioctopunctata (Coleoptera: Coccinellidae) fed on Solanum photeinocarpum (Solanales: Solanaceae): with a new method to project the uncertainty of population growth and consumption. J. Econ. Entomol. 111: 1–9. [DOI] [PubMed] [Google Scholar]
- Jahn G. C., Almazan L. P., and Pacia J. B.. 2005. Effect of nitrogen fertilizer on the intrinsic rate of increase of Hysteroneura setariae (Thomas) (Homoptera: Aphididae) on rice (Oryza sativa L.). Environ. Entomol. 34: 938–943. [Google Scholar]
- Joern A., and Behmer S. T.. 1997. Importance of dietary nitrogen and carbohydrates to survival, growth, and reproduction in adults of the grasshopper Ageneotettix deorum (Orthoptera: Acrididae). Oecologia 112: 201–208. [DOI] [PubMed] [Google Scholar]
- Karasov W. H., and del Rio C. M.. 2007. Physiological ecology: how animals process energy, nutrients, and toxins. Princeton University Press, Princeton. [Google Scholar]
- Kawazu K. 2014. Rearing the 28-spotted ladybird beetle, Henosepilachna vigintioctopunctata (Coleoptera: Coccinelidae), with a switchover from host plant leaves to artificial diet. Appl. Entomol. Zool. 49: 359–362. [Google Scholar]
- Kono Y. 1979. Abnormal photoperiodic and phototactic reactions of the beetle, Epilachna vigintioctopunctata, reared on sliced potatoes. Appl. Entomol. Zool. 14: 185–192. [Google Scholar]
- Lee K. P., Cory J. S., Wilson K., Raubenheimer D., and Simpson S. J.. 2006. Flexible diet choice offsets protein costs of pathogen resistance in a caterpillar. Phil. Trans. R. Soc. Lond. B. 273: 823–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. P., Simpson S. J., Clissold F. J., Brooks R., Ballard J. W. O., Taylor P. W., Soran N., and Raubenheimer D.. 2008. Lifespan and reproduction in Drosophila: new insights from nutritional geometry. Proc. Natl. Acad. Sci. USA. 105: 2498–2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Yang Y., Xie W., Wu Q., Xu B., Wang S., Zhu X., Wang S. J., and Zhang Y.. 2015. Effects of temperature on the age-stage, two-sex life table of Bradysia odoriphaga (Diptera: Sciaridae). J. Econ. Entomol. 108: 126–134. [DOI] [PubMed] [Google Scholar]
- Maklakov A. A., Simpson S. J., Zajitschek F., Hall M. D., Dessmann J., Clissold F., Raubenheimer D., Bindurianshky R., and Brooks R. C.. 2008. Sex-specific fitness effects of nutrient intake on reproduction and lifespan. Curr. Biol. 18: 1062–1066. [DOI] [PubMed] [Google Scholar]
- Morris R. F., and Miller C. A.. 1954. The development of life tables for the spruce budworm. Can. J. Zool. 32: 283–301 [Google Scholar]
- Murata M., Iwabuchi K., and Mitsuhashi J.. 1994. Partial rearing of a phytophagous lady beetle, Epilachna vigintioctopunctata (Coleoptera: Coccinelidae). Appl. Entomol. Zool. 29: 116–119. [Google Scholar]
- Nakamura K. 1976. Studies on the population dynamics of the 28-spotted lady beetle, Henosepilachna vigintioctopunctata F., 1. Analysis of life tables and mortality process in the field population. Jap. J. Ecol. 26: 49–59. [Google Scholar]
- Raubenheimer D., and Simpson S. J.. 1993. The geometry of compensatory feeding in the locust. Anim. Behav. 45: 953–964. [Google Scholar]
- Raubenheimer D., and Simpson S. J.. 1997. Integrative models of nutrient balancing: application to insects and vertebrates. Nutr. Res. Rev. 10: 151–179. [DOI] [PubMed] [Google Scholar]
- Sharma A., Thakur A., Kaur S., and Pati P. K.. 2012. Effect of Alternaria alternata on the coccinellid pest Henosepilachna vigintioctopunctata and its implications for biological pest management. J. Pest. Sci. 85: 513–518. [Google Scholar]
- Simpson S. J., and Raubenheimer D.. 1993. A multi-level analysis of feeding behaviour: the geometry of nutritional decisions. Phil. Trans. R. Soc. Lond. B. 342: 381–402. [Google Scholar]
- Simpson S. J., and Raubenheimer D.. 2005. Obesity: the protein leverage hypothesis. Obesity. Rev. 6: 133–142. [DOI] [PubMed] [Google Scholar]
- Simpson S. J., and Raubenheimer D.. 2009. Macronutrient balance and lifespan. Aging 1: 875–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson S. J., and Raubenheimer D.,. 2012. The nature of nutrition: a unifying framework from animal adaptation to human obesity. Princeton University Press, New Jersey. [Google Scholar]
- Simpson S. J., Sibly R. M., Lee K. P., Behmer S. T., and Raubenheimer D.. 2004. Optimal foraging when regulating intake of multiple nutrients. Anim. Behav. 68: 1299–1311. [Google Scholar]
- Stockhoff B.A. 1993. Ontogenetic change in dietary selection for protein and lipid by gypsy moth larvae. J. Insect. Physiol. 39: 677–686. [Google Scholar]
- Svoboda J. A., Kaplanis J. N., Robbins W. E., and Thompson M. J.. 1975. Recent developments in insect steroid metabolism. Annu. Rev. Entomol. 20: 205–220. [DOI] [PubMed] [Google Scholar]
- Thompson S. N., and Redak R. A.. 2000. Interactions of dietary protein and carbohydrate determine blood sugar level and regulate nutrient selection in the insect Manduca sexta L. Biochim. Biophys. Acta 1523: 91–102. [DOI] [PubMed] [Google Scholar]
- Trudeau D., Washburn J. O., and Volkman L. E.. 2001. Central role of hemocytes in Autographa californica M nucleopolyhedrovirus pathogenesis in Heliothis virescens and Helicoverpa zea. J. Virol. 75: 996–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z. L., Li C. R., Yuan J. J., Li S. X., Wang X. P., and Chi H.. 2017. Demographic comparison of Henosepilachna vigintioctopunctata (F.) (Coleoptera: Coccinellidae) reared on three cultivars of Solanum melongena L. and a wild hostplant Solanum nigrum L. J. Econ. Entomol. 110: 2084–2091. [DOI] [PubMed] [Google Scholar]
- Washburn J. O., Kirkpatrick B. A., and Volkman L. E.. 1996. Insect protection against viruses. Nature 383: 767. [Google Scholar]
- Wheeler D. 1996. The role of nourishment in oogenesis. Annu. Rev. Entomol. 41: 407–431. [DOI] [PubMed] [Google Scholar]
- Wilkinson T. L., Minto L. B., and Douglas A. E.. 2001. Amino acids as respiratory substrates in aphids: an analysis of Aphis fabae reared on plants and diets. Physiol. Entomol. 26: 225–228. [Google Scholar]
- Yang Y., and Joern A.. 1994. Compensatory feeding in response to variable food quality by Melanoplus differentialis. Physiol. Entomol. 19: 75–82 [Google Scholar]
- Yang Y. T., Li W. X., Xie W., Wu Q. J., Xu B. Y., Wang S. L., Li C. R., and Zhang Y. J.. 2015. Development of Bradysia odoriphaga (Diptera: Sciaridae) as affected by humidity: an age–stage, two-sex, life-table study. Appl. Entomol. Zool. 50: 3–10. [Google Scholar]
- Ying W. D., Yan W. T., Qiu G. S., Zhang H. J., and Ma C. S.. 2012. Age-stage two-sex life tables of the experimental population of Panonychus ulmi (Acari: Tetranychidae) on apples Malus sieversii subsp. kirghisorum and M. domestica golden delicious. Acta Entomol. Sinica. 55: 1230–1238. [Google Scholar]
- Zhou L., Xie B. G., and Wang X. P.. 2015. Population dynamic of Henosepilachna vigintioctopunctata in different host plants in Jianghan Plain. China J. Northern. Hort. 11: 103–105. [Google Scholar]