Abstract
Background
To what extent steroid hormones contribute to lung cancer in male and female never smokers and smokers is unclear. We examined expression of hormone receptors in lung tumors by sex and smoking.
Methods
Patients with primary non–small cell lung cancer were recruited into an Intergroup study in the United States and Canada, led by SWOG (S0424). Tumors from 813 cases (450 women and 363 men) were assayed using immunohistochemistry for estrogen receptor (ER)–α, ER-β, progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2). Linear regression was used to examine differences in expression by sex and smoking status. Cox proportional hazard models were used to estimate survival associated with the receptors. All statistical tests were two-sided.
Results
In ever smokers, postmenopause and oral contraceptive use were associated with lower nuclear ER-β (P = .02) and total (nuclear + cytoplasmic) PR expression (P = .02), respectively. Women had lower cytoplasmic ER-α (regression coefficient [β], or differences in H-scores = –15.8, P = .003) and nuclear ER-β (β = –12.8, P = .04) expression than men, adjusting for age, race, and smoking. Ever smokers had both higher cytoplasmic ER-α (β = 45.0, P < .001) and ER-β (β = 25.9, P < .001) but lower total PR (β = –42.1, P < .001) than never smokers. Higher cytoplasmic ER-α and ER-β were associated with worse survival (hazard ratio = 1.73, 95% confidence interval [CI] = 1.15 to 2.58, and HR = 1.59, 95% CI = 1.08 to 2.33, respectively; quartiles 4 vs 1).
Conclusions
Lower expression of nuclear ER-β in women supports the estrogen hypothesis in lung cancer etiology. Increasing cytoplasmic ER-α and ER-β and decreasing PR protein expression may be mechanisms whereby smoking disrupts hormone pathways.
Although lung cancer incidence in US women has plateaued and showed a trend of decline since the mid-2000s (1), incidence rates in younger women in the United States and other parts of the world are increasing (2). There is biological and epidemiological evidence that, dose for dose, women are more susceptible to tobacco smoke carcinogens than men (3,4). Among lifetime nonsmokers who are diagnosed with lung cancer, the proportion of women is consistently higher than men (3,5–7). To some extent, the lung cancer incidence in never-smoking women may be attributable to exposure to environmental tobacco smoke, but there are no facile reasons to explain the sex difference in lung cancer in never smokers (8).
The role of estrogenic steroid hormones in the etiology of lung cancer is not entirely clear. Reproductive factors (9–11) and hormone use (12–14) have been linked to lung cancer risk in women, although the evidence has not been consistent. Examining steroid hormone receptors in the lung may help clarify associations and underlying mechanisms (15). Steroid hormone-related receptors including estrogen receptor (ER)–α, ER-β, progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) can be detected in normal and tumor lung tissue (16–18) and have been linked to survival in lung cancer patients (18–20). However, it remains unclear whether receptors in the lung differ between men and women, smokers and nonsmokers, and whether they are influenced by reproductive and hormonal factors.
In a large case–case study of non–small cell lung cancer (NSCLC), we investigated protein expression of hormone receptors by sex and smoking status, hypothesizing that expression would be higher in women and in never smokers. We also posited that female reproductive and hormonal factors would influence receptor expression. In addition, we examined the relationship between expression of hormone receptors and survival in the lung cancer patients.
Methods
Study Population
Patients were prospectively enrolled in the United States and Canada from October 1, 2005, through March 15, 2011 (S0424; NCT00450281) via either SWOG or the Clinical Trials Support Unit (CTSU) of the National Cancer Institute (NCI) for the other collaborating cooperative groups. Eligible patients had newly diagnosed, histologically confirmed stage I, II, IIIA, or selected IIIB NSCLC (T4 or N3, excluding malignant pleural and pericardial effusions) (21). They may have been simultaneously enrolled in a therapeutic clinical trial. Enrollment was within 12 weeks of diagnosis, and no systemic treatment or radiation therapy was allowed until after the study blood and tissue acquisition requirements were met. A total of 981 patients (536 women and 445 men) were enrolled; 813 cases (450 women and 363 men) with protein expression data available for at least one receptor were included in statistical analyses. Expression data were unavailable because the tumor tissue was not received (n = 89), usable (n = 42), or evaluable as tissue microarray (TMA) cores or whole sections, that is, dropped cores, folded tissue, or not enough evaluable tumor cells (n = 37). The study protocol was approved by the institutional review boards at Roswell Park Cancer Institute and all participating institutions; all patients provided informed consent for participation and use of tissue.
Epidemiological Data Collection
A standardized questionnaire that included exposure to active and passive smoke as well as reproductive and hormonal factors was administered by clinical research associates at each site within seven days of registration. A never smoker was defined as someone who smoked fewer than 100 cigarettes in a lifetime; ever smokers included current (smoking within past six months) and former (quit at least six months prior to biopsy) smokers. Female reproductive history included age of menarche, parity, age at first full-term birth, breastfeeding, menopausal status, use of oral contraceptives (OCs), and hormone therapy (HT) use for menopausal symptoms. Women were considered menopausal if they had not menstruated in the past year or if they only had periods because they took hormones.
Hormone Receptor Analysis
Paraffin-embedded tissue blocks of tumors and uninvolved lung were processed per protocol within 30 days of enrollment for TMA construction at Roswell Park Cancer Institute. Whole sections or TMA sections were obtained from institutions that would not release tissue blocks, and they were stored in a desiccator until analysis. The study pathologist (WB) and laboratory staff were blinded to epidemiological and clinical variables, including sex and smoking. TMAs were sectioned at 5 µm and stained by immunohistochemistry (IHC) methods within one week, except PR, which was stained 23 days after sectioning. The antibodies and IHC conditions for each stain are provided in Supplementary Table 1 (available online). Images of staining were manually annotated to include only tumor cells. Automated digital image analysis was performed on the annotated regions using validated algorithms (Aperio Technologies, Vista, CA), with minor adjustments for cell shape and intensity thresholds. Nuclei and cytoplasm were scored for ER-α, ER-β, and PR, and membrane for HER-2, using a localized intensity score (0, 1 + [only partial or weak staining], 2 + [moderate and complete staining], and 3 + [intense and complete staining]) and percent positive cells. TMA tumor cores with 25 or more cells annotated were collapsed into case-level data using a cellularity-weighted approach (22). For each intensity score, the weighted average of percent positivity was calculated by summing the product of percent positivity and core weight across all cores per case. Core weight was calculated as the number of cells scored in a given core divided by the total number of cells scored across all cores for that case. A histological score (H-score) at the case level was calculated by the formula 1 × (% cells 1+) + 2 × (% cells 2+) + 3 × (% cells 3+) with the weighted average of percent positivity values (Supplementary Figure 1, available online) (23). For cases not on a TMA and having two or more whole sections stained, the most representative slide was selected by our study pathologist with reference to their pathology reports.
Statistical Analysis
Demographic and clinical variables were examined in relation to sex and smoking status, and, among females, reproductive history and hormonal factors were examined by smoking status. Correlations with receptor protein expression in H-score were assessed using Pearson’s correlation coefficient (Supplementary Table 2, available online). H-scores were examined according to histology and female reproductive history and hormone use. The association of smoking and sex with receptor protein expression was assessed by linear regression, as H-scores were normally distributed for several receptors including nuclear ER-β. The primary focus of analysis was the specific localization known to have high expression in the lung and previously associated with survival—cytoplasmic ER-α, nucleus and cytoplasmic ER-β, and total PR (18), and we present all localization data to explore potential differences in associations by localization. Estimates from regression models were adjusted for age, race, sex, smoking status, menopausal status, and HT use where appropriate. The regression analysis was stratified by histology, that is, adenocarcinoma and nonadenocarcinoma. The interaction between smoking and sex was tested using Wald tests for the product term between the two variables. Cox proportional hazards regressions were used to model overall survival for the quartiles of protein expression levels, adjusting for age, race, sex, smoking status, histology, and tumor stage. The proportionality assumption was verified with cumulative sums of martingale residuals over follow-up times or covariate values (24); there was no evidence of departure from the assumption (all P > .05). Tests of statistical significance were two-sided. A P value of less than .05 was considered statistically significant. All analyses were performed in SAS 9.4 (Cary, NC) at the SWOG Statistical Center.
Results
Never smokers were more likely to be Asian and diagnosed with adenocarcinoma than ever smokers (Table 1). Two-thirds of patients enrolled had stage IA and IB NSCLC, and this proportion was similar across the sex-smoking strata. In women, ever smokers with lung cancer were more likely than never smokers to be postmenopausal at diagnosis, to have a history of OC use, to have their first birth at a younger age, and to have never breastfed (Supplementary Table 3, available online). The vast majority of OC users had not used the medications for more than five years; approximately one-fifth of HT users reported current or recent (less than one year before lung cancer diagnosis) HT use.
Table 1.
Characteristics of NSCLC cases, by sex and smoking status (n = 813)
| Characteristic | Male, never smoker | Male, ever smoker | Female, never smoker | Female, ever smoker | ||
|---|---|---|---|---|---|---|
| No. (%) | No. (%) | No. (%) | No. (%) | P*, males | P*, females | |
| Total | 45 (100.0) | 318 (100.0) | 162 (100.0) | 288 (100.0) | ||
| Age, y | .006 | .05 | ||||
| <55 | 12 (28.6) | 31 (10.0) | 29 (19.0) | 47 (16.7) | ||
| 55–64 | 8 (19.0) | 85 (27.5) | 38 (24.8) | 70 (24.9) | ||
| 65–74 | 12 (28.6) | 119 (38.5) | 46 (30.1) | 116 (41.3) | ||
| 75+ | 10 (23.8) | 74 (24.0) | 40 (26.1) | 48 (17.1) | ||
| Ethnicity | .22 | .005 | ||||
| Hispanic | 0 (0.0) | 10 (3.3) | 12 (7.9) | 6 (2.2) | ||
| Non-Hispanic | 44 (100.0) | 290 (96.7) | 140 (92.1) | 268 (97.8) | ||
| Unknown | 1 | 18 | 10 | 14 | ||
| Race | <.001 | <.001 | ||||
| White | 37 (82.2) | 280 (88.1) | 119 (73.5) | 267 (92.7) | ||
| Black | 0 (0.0) | 15 (4.7) | 8 (4.9) | 11 (3.8) | ||
| Asian | 8 (17.8) | 9 (2.8) | 26 (16.0) | 3 (1.0) | ||
| Other | 0 (0.0) | 14 (4.4) | 9 (5.6) | 7 (2.4) | ||
| Family history of lung cancer | .17 | .38 | ||||
| Yes | 8 (17.8) | 84 (27.4) | 38 (24.1) | 79 (27.9) | ||
| No | 37 (82.2) | 223 (72.6) | 120 (75.9) | 204 (72.1) | ||
| Unknown | 0 | 11 | 4 | 5 | ||
| Histology | <.001 | <.001 | ||||
| Adenocarcinoma | 35 (77.8) | 134 (42.3) | 135 (83.9) | 161 (56.1) | ||
| Large cell | 1 (2.2) | 22 (6.9) | 1 (0.6) | 28 (9.8) | ||
| Other NSCLC | 0 (0.0) | 30 (9.5) | 7 (4.3) | 12 (4.2) | ||
| Squamous cell carcinoma | 3 (6.7) | 122 (38.5) | 3 (1.9) | 72 (25.1) | ||
| Bronchioloalveolar carcinoma | 6 (13.3) | 9 (2.8) | 15 (9.3) | 14 (4.9) | ||
| Unknown | 0 | 1 | 1 | 1 | ||
| Stage | .76 | .69 | ||||
| IA | 15 (34.9) | 91 (28.9) | 63 (39.4) | 120 (42.0) | ||
| IB | 14 (32.6) | 100 (31.7) | 51 (31.9) | 84 (29.4) | ||
| IIA | 4 (9.3) | 22 (7.0) | 7 (4.4) | 21 (7.3) | ||
| IIB | 3 (7.0) | 41 (13.0) | 19 (11.9) | 27 (9.4) | ||
| IIIA | 6 (14.0) | 44 (14.0) | 12 (7.5) | 24 (8.4) | ||
| IIIB | 1 (2.3) | 17 (5.4) | 8 (5.0) | 10 (3.5) | ||
| Unknown | 2 | 3 | 2 | 2 |
Ever vs never smokers, chi-square test (two-sided). NSCLC = non–small cell lung cancer.
Squamous cell carcinoma had higher expression of cytoplasmic ER-α, cytoplasmic ER-β, and total PR compared with adenocarcinoma, while adenocarcinoma had higher expression of HER2 compared with squamous cell carcinoma (Figure 1). In female ever smokers, total PR expression was lower among those who used OCs than never users (median H-score = 54 vs 90, P = .02) (Table 2). There was an indication of higher nuclear ER-β expression in postmenopausal women who had ever used HT compared with never users (median H-score = 129 vs 112), although the difference was not statistically significant (P = .09). Nuclear ER-β expression was lower in postmenopausal than premenopausal women who ever smoked (median H-score = 115 vs 181, P = .02) (Supplementary Table 4, available online).
Figure 1.
Receptor expression by non–small cell lung cancer (NSCLC) histology. NSCLC histology included adenocarcinoma (AD; symbol Ο), squamous cell carcinoma (SCC; symbol +), and other NSCLC (symbol X). P values were comparing AD and SCC (t test, two-sided). Box: interquartile range; horizontal line in the box: median; whiskers: quartile 1–1.5 × interquartile range and quartile 3 + 1.5 × interquartile range; large symbol: mean; small symbol: outlier. AD = adenocarcinoma; Cyto. = cytoplasmic; NSCLC = non–small cell lung cancer; nuc. = nuclear; SCC = squamous cell carcinoma.
Table 2.
Hormone protein expression levels in lung tumors according to use of oral contraceptives and hormone therapy
| Variable | Median H-score |
||||
|---|---|---|---|---|---|
| ER-α, cytoplasmic | ER-β, nuclear | ER-β, cytoplasmic | PR, total | HER2 | |
| Oral contraceptives | |||||
| All women | |||||
| Ever use | 60 | 120 | 80 | 74 | 42 |
| Never use | 64 | 120 | 87 | 93 | 47 |
| P* | .26 | .84 | .24 | .20 | .72 |
| Ever Smokers | |||||
| Ever use | 78 | 120 | 83 | 54 | 34 |
| Never use | 83 | 116 | 112 | 90 | 46 |
| P | .09 | .76 | .10 | .02 | .54 |
| Never smokers | |||||
| Ever use | 28 | 120 | 77 | 132 | 57 |
| Never use | 41 | 128 | 68 | 96 | 48 |
| P | .66 | .79 | .90 | .08 | .48 |
| Hormone therapy† | |||||
| All women | |||||
| Ever use | 63 | 129 | 90 | 91 | 49 |
| Never use | 63 | 112 | 70 | 77 | 32 |
| P | .93 | .09 | .18 | .20 | .10 |
| Ever Smokers | |||||
| Ever use | 80 | 128 | 97 | 70 | 45 |
| Never use | 94 | 102 | 85 | 65 | 25 |
| P | .37 | .11 | .48 | .22 | .10 |
| Never smokers | |||||
| Ever use | 30 | 137 | 87 | 124 | 57 |
| Never use | 29 | 119 | 55 | 93 | 41 |
| P | .86 | .44 | .24 | .22 | .33 |
t test (two-sided). ER = estrogen receptor; HER2 = human epidermal growth factor receptor 2; PR = progesterone receptor.
Among postmenopausal women.
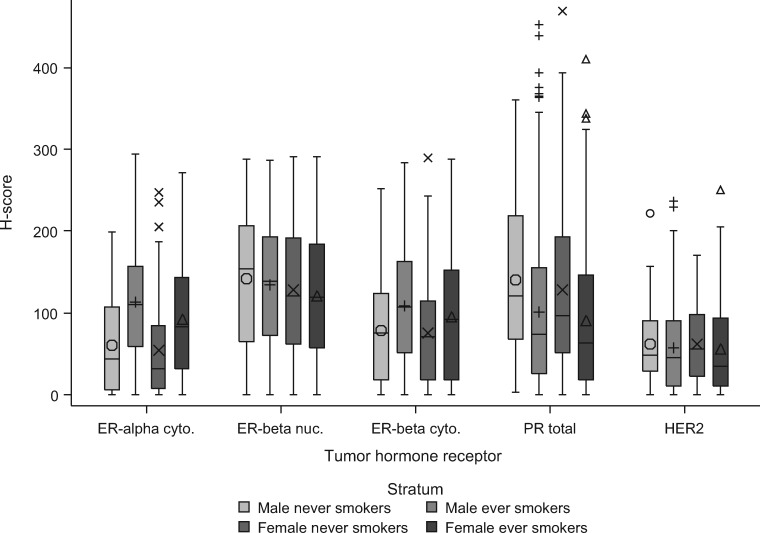
Figure 2 and Supplementary Table 5 (available online) show protein expression levels of hormone receptors by sex and smoking. Women had lower ER-α cytoplasmic (regression coefficient [β] = –15.8, 95% CI = –26.2 to –5.5, P = .003) and ER-β nuclear (β = –12.8, 95% CI = –24.7 to –0.9, P = .04) expression than men, adjusting for age, race, and smoking (Table 3). Female sex was associated with lower cytoplasmic ER-β expression in ever smokers, but not in never smokers. Smokers had higher expression of cytoplasmic ER-α (β = 45.0, 95% CI = 32.9 to 57.1, P < .001) and cytoplasmic ER-β (β = 25.9, 95% CI = 13.0 to 38.9, P < .001) but lower total PR (β = –42.1, 95% CI = –58.7 to –25.5, P < .001) than never smokers. The observed sex and smoking differences in expression were more prominent in nonadenocarcinoma, that is, squamous cell carcinoma and other NSCLCs, than adenocarcinoma. For example, the difference in ER-β nuclear expression in tumors from women compared with men was –25.8 (P = .005) for nonadenocarcinoma and –1.7 (P = .84) for adenocarcinoma (Supplementary Table 6, available online).
Figure 2.
Hormone receptor expression levels by sex and smoking status. Strata were male never smokers (symbol Ο), male ever smokers (symbol +), female never smokers (symbol X), and female ever smokers (symbol Δ). Box: interquartile range; horizontal line in the box: median; whiskers: quartile 1 – 1.5 × interquartile range and quartile 3 + 1.5 × interquartile range; large symbols: mean; small symbols: outlier. Cyto. = cytoplasmic; nuc. = nuclear.
Table 3.
Associations of sex and smoking with hormone receptor expression in lung tumors
| Hormone receptor | Difference comparing women and men (Ref.) |
Difference comparing ever and never smokers (Ref.) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All* |
Among ever smokers† |
Among never smokers† |
All‡ |
Among men† |
Among women§ |
|||||||
| β (95% CI) | P‖ | β (95% CI) | P | β (95% CI) | P | β (95% CI) | P | β (95% CI) | P | β (95% CI) | P | |
| ER-α, cytoplasmic | –15.8 | .003 | –17.5 | .004 | –7.2 | .52 | 45.0 | <.001 | 52.0 | <.001 | 44.0 | <.001 |
| (–26.2 to –5.5) | (–29.4 to –5.6) | (–28.9 to 14.6) | (32.9 to 57.1) | (28.0 to 75.9) | (29.8 to 58.1) | |||||||
| ER-β, nuclear | –12.8 | .04 | –14.5 | .03 | –14.0 | .34 | –5.6 | .43 | –5.4 | .69 | –5.2 | .55 |
| (–24.7 to –0.9) | (–27.6 to –1.5) | (–42.8 to 14.9) | (–19.6 to 8.3) | (–31.8 to 20.9) | (–22.2 to 11.8) | |||||||
| ER-β, cytoplasmic | –10.9 | .05 | –13.1 | .04 | –0.6 | .96 | 25.9 | <.001 | 34.3 | .008 | 22.6 | .004 |
| (–22.0 to 0.2) | (–25.8 to –0.3) | (–23.7 to 22.5) | (13.0 to 38.9) | (9.1 to 59.4) | (7.1 to 38.0) | |||||||
| PR, total | –11.7 | .11 | –10.4 | .19 | –14.4 | .43 | –42.1 | <.001 | –44.8 | .007 | –42.3 | <.001 |
| (–25.9 to 2.5) | (–25.9 to 5.1) | (–50.4 to 21.6) | (–58.7 to –25.5) | (–77.3 to –12.2) | (–61.9 to –22.6) | |||||||
| HER2 | –1.9 | .65 | –4.1 | .40 | 5.8 | .51 | –6.2 | .21 | 1.4 | .88 | –9.7 | .10 |
| (–10.1 to 6.3) | (–13.5 to 5.4) | (–11.5 to 23.0) | (–15.8 to 3.4) | (–17.1 to 19.9) | (–21.1 to 1.8) | |||||||
Adjusted for age, race, and smoking status. CI = confidence interval; ER = estrogen receptor; HER2 = human epidermal growth factor receptor 2; PR = progesterone receptor.
Adjusted for age and race.
Adjusted for age, race, and sex.
Adjusted for age, race, menopausal status/hormone therapy (HT) use (premenopausal, postmenopausal with never use of HT, postmenopausal with ever use of HT).
Linear regression t test (two-sided).
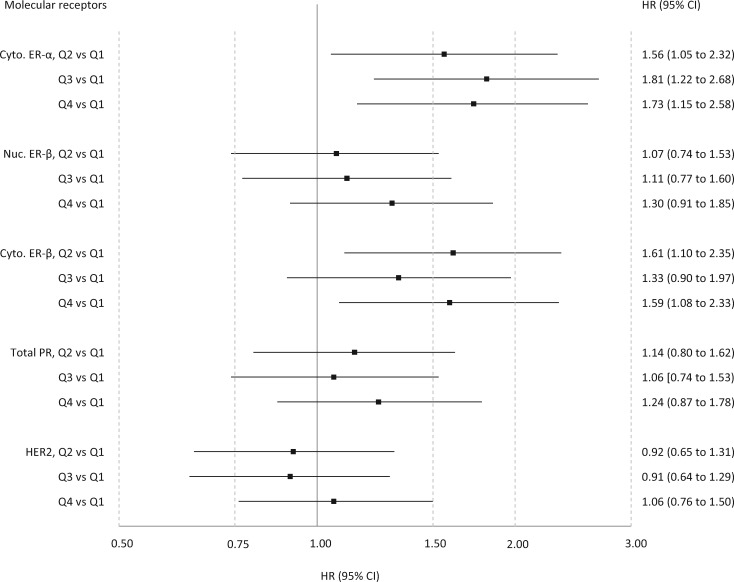
There were 297 deaths during the follow-up duration (median = 5.2 years). Patients with higher vs lower expression for cytoplasmic ER-α and ER-β had increased mortality (hazard ratio [HR] = 1.73, 95% CI = 1.15 to 2.58, and HR = 1.59, 95% CI = 1.08 to 2.33, respectively; quartiles 4 vs 1), adjusting for age, race, sex, smoking status, histology, and tumor stage (Figure 3). Nuclear ER-β, PR, and HER2 expression were not associated with survival.
Figure 3.
Associations of hormone receptor expression with overall survival. Hazard ratios were estimated for quartiles of protein expression using quartile 1 as the reference and plotted in a nature log scale. The estimates were adjusted for age, race, sex, smoking status, histology, and tumor stage. CI = confidence interval; HR = hazard ratio; Q = quartile.
Discussion
In this large case–case study, we observed distinct differences in the protein expression of ER-α, ER-β, and PR, but not HER2, in lung tumors by sex and smoking status. Lung tumors in women had lower cytoplasmic ER-α and nuclear ER-β expression compared with those in men. Smoking was associated with higher cytoplasmic ER-α and ER-β but lower total PR expression. Higher vs lower cytoplasmic ER-α and ER-β expression was associated with increased mortality.
Our findings on sex difference in ER-β nuclear expression support the estrogen hypothesis in lung cancer etiology and provide a biological mechanism for the heightened susceptibility to lung cancer in women. ER-α promotes gene transcription through binding with estrogen-responsive elements and AP-1 enhancer elements in the promoter of target genes, and the signaling process in the lung may be primarily in the cytoplasm as ER-α nuclear protein expression is very low in lung tumor tissue (18,25). Conversely, ER-β is considered the predominant subtype in the lung (26), and opposite from ER-α, it inhibits the transcription of AP-1 sites located in the cell nucleus (27,28). In our study, tumors in women had lower ER-β nuclear expression than those in men. Prior studies suggested the sex difference in nuclear ER-β expression, but had not been not confirmed, primarily because of small sample size (17,25,29). Women with higher nuclear ER-β expression in the lung may be less susceptible to hormone-related lung cancer. In a case–control study, ever vs never HT use was associated with lower risk of NSCLC (OR = 0.74, 95% CI = 0.51 to 1.06), largely in women with positive expression of nuclear ER-β in the lung (OR = 0.42, 95% CI = 0.24 to 0.74), but not in those with negative expression of nuclear ER-β (OR = 0.95, 95% CI = 0.41 to 2.22) (15). Because expression levels of ER-β were lower in postmenopausal than premenopausal women, and in HT never users than ever users, it is possible that lower circulating estrogen can be a factor for a decrease in nuclear ER-β expression; measuring circulating levels of estrogen would be warranted to establish the association. In our data, however, the sex difference in ER-β nuclear expression in never smokers was not statistically significant, in part because of the relatively small sample size in male never smokers. This association will need to be confirmed with a larger sample of never smokers. In addition, the sex difference in ER-β nuclear expression was more prominent in nonadenocarcinoma than adenocarcinoma. The study findings should be interpreted with caution when applied to adenocarcinoma.
Cigarette smoking may have a strong influence on hormone receptors. Stabile and colleagues observed no difference in cytoplasmic ER-β expression between ever and never smokers in 183 lung cancer patients (18). However, our data showed that smoking was associated with increased ER-α and ER-β in the cytoplasm, suggesting that smoking may influence ER through phosphorylation (26). NSCLC patients with higher vs lower cytoplasmic ER-β expression had a poorer prognosis (18), and we also found the same association for both cytoplasmic ER-β and ER-α. These observations are consistent with the evidence that ever-smoking lung cancer patients, who are likely to have higher cytoplasmic expression of ER-α and ER-β, have worse survival than never-smoking patients (30).
Little is known about the interplay between progesterone, PR, and smoking in lung cancer etiology. The use of HT and OC has been linked to increased lung cancer risk in some studies (10,31). However, the use of HT consisting of unopposed estrogen only did not increase lung cancer incidence (32,33). Thus, it is generally thought that progesterone in the formulation of HT may be harmful. The function of PR may be important in the lung because higher vs lower PR expression appears to be protective and associated with better survival in NSCLC patients (18,20), potentially by promoting cell differentiation. From our data on female never smokers, a pattern suggests that OC and HT use may increase PR expression in lung tumors, although the differences were not statistically significant (Table 2). Also, smoking may decrease PR expression, a finding consistent with the literature (18). Thus, a disruption of progesterone signaling pathways, by either increasing circulating progesterone levels or decreasing PR expression levels, can be an underlying hormone-related mechanism for the development of lung cancer.
HER2 expression was higher in adenocarcinoma than squamous cell carcinoma, but there were no differences in HER2 expression according to sex and smoking. A study also reported no association between HER2 expression and sex or smoking in 109 lung adenocarcinoma cases of East Asians, although the number of cases was relatively small (34).
It is unclear whether use of selective estrogen response modifiers (SERMs) and anti-estrogens can modify lung cancer incidence or risk because these medications have not been regularly used in the prevention or treatment of lung cancer. However, in vitro and in vivo evidence has shown that tamoxifen and fulvestrant can prevent lung cancer formation (35,36). To date, two observational studies have reported on the association between SERMs and lung cancer risk among breast cancer patients, who often receive SERMs for ER+ breast cancer. Data from the Geneva Cancer Registry showed no association when comparing breast cancer patients receiving tamoxifen with the general population (age-standardized incidence ratio = 0.63, 95% CI = 0.33 to 1.10); the number of observed cases was very small (n = 12) (37). More recently, a Taiwanese study using claims data showed that breast cancer patients who received SERMs (primarily tamoxifen, with a quarter of patients also receiving an aromatase inhibitor) had a lower risk of lung cancer than those who did not receive SERMs (HR = 0.77, 95% CI = 0.60 to 0.99) (38). However, a major limitation of these studies was that breast cancer patients who did not receive SERMs consisted of a large proportion of patients with ER- tumors, who may have a different risk profile of lung cancer than patients with ER+ tumors. In addition, lung cancer has not been found to increase in trials of SERMS targeting early breast cancer and women at elevated risk of breast cancer (39,40), although a longer follow-up may be needed to confirm the null association. It is also unknown whether SERMs and anti-estrogens have different effects on lung cancer risk between men and women, smokers and nonsmokers.
This study has several strengths. It was a prospective observational study conducted in the context of cooperative groups, with a large sample size of lung cancer patients, including never smokers, meeting eligibility criteria. A goal of the study was to examine associations between smoking, sex, and multiple hormone receptors in lung cancer, and detailed epidemiological data. Lung tumor was manually annotated so that influence on scoring from other components, for example, stroma, is likely minimal; the automated imaging analysis gives objective scores for each localization.
There are limitations of this study. Women's reproductive history was based on self-report, and early life events, for example, age at menarche, may be prone to misclassification. The influence of OC use on the expression of hormone receptors would need further confirmation in a larger sample of premenopausal or younger women, as 92% of our female patients were postmenopausal. We did not collect data on HT preparations, for example, estrogen alone or estrogen plus progestin, and there was no information on whether a woman had a hysterectomy. Lacking this information may have affected our assessment of the association between HT use and PR expression. In addition, HER2 status was measured by protein expression using IHC, instead of gene amplification by fluorescence in situ hybridization. Although the latter method is superior in classifying equivocal HER2 status in the clinical setting for breast cancer, results from these two methods are highly correlated (41). We did not measure protein expression of aromatase, which catalyzes androgens to estrogens, in part because research has suggested similar expression between smokers and nonsmokers, men and women (18,42). Our patient population was primarily non-Hispanic white, and whether the finding can be generalized to other populations requires further research. The influence of sex and smoking on hormone receptors may differ in other racial and ethnic groups, although a similar finding that nuclear ER-β expression was higher in never smokers than ever smokers was reported in a Japanese population (43). In addition, our study enrolled stage I–III lung cancer patients, but two-thirds were stage I patients. The generalizability of our study findings may thus be limited as the majority of lung cancers present as stages III and IV (44). Cases with data on protein expression were also more likely to be stage I lung cancer and adenocarcinoma, compared with those without the data (Supplementary Table 7, available online). However, with the increasing use of low-dose computed tomography for screening high-risk patients, it is likely that more early-stage cancers will be diagnosed, and these results would be relevant for this population.
In conclusion, there were differences in protein expression levels of ER-α, ER-β, and PR by sex and smoking status, and cytoplasmic expression of ER-α and ER-β was associated with poorer survival. The sex-related difference in nuclear ER-β expression supports the estrogen hypothesis in lung cancer etiology. Smoking may influence hormone receptor expression levels, and smoking cessation may be important to preserve the integrity of hormone receptors for both women and men.
Funding
This work was supported by the National Cancer Institute at the National Institutes of Health (grant numbers R01CA106815, U10CA180888, U10CA180819, UG1CA189974, U10CA180799, U10CA180820, U10CA180821, U10CA180868, P30CA016056, and K07CA201334) and by the National Institute of Environmental Health Sciences at the National Institutes of Health (grant number P30ES009089). Dr. Ambrosone is a recipient of funding from the Breast Cancer Research Foundation.
Note
The funders had no role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
Supplementary Material
References
- 1. Siegel RL, Miller KD, Jemal A.. Cancer statistics, 2017. CA Cancer J Clin. 2017;671:7–30. 10.3322/caac.21387 [DOI] [PubMed] [Google Scholar]
- 2. Lewis DR, Check DP, Caporaso NE et al. , . US lung cancer trends by histologic type. Cancer. 2014;12018:2883–2892. 10.1002/cncr.28749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Siegfried JM. Women and lung cancer: Does oestrogen play a role? Lancet Oncol. 2001;28:506–513. 10.1016/S1470-2045(01)00457-0 [DOI] [PubMed] [Google Scholar]
- 4. Dogan S, Shen R, Ang DC et al. , . Molecular epidemiology of EGFR and KRAS mutations in 3,026 lung adenocarcinomas: Higher susceptibility of women to smoking-related KRAS-mutant cancers. Clin Cancer Res. 2012;1822:6169–6177. 10.1158/1078-0432.CCR-11-3265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Freedman ND, Leitzmann MF, Hollenbeck AR et al. , . Cigarette smoking and subsequent risk of lung cancer in men and women: Analysis of a prospective cohort study. Lancet Oncol. 2008;97:649–656. 10.1016/S1470-2045(08)70154-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wakelee HA, Chang ET, Gomez SL et al. , . Lung cancer incidence in never smokers. J Clin Oncol. 2007;255:472–478. 10.1200/JCO.2006.07.2983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cheng TY, Cramb SM, Baade PD et al. , . The international epidemiology of lung cancer: Latest trends, disparities, and tumor characteristics. J Thorac Oncol. 2016;1110:1653–1671. 10.1016/j.jtho.2016.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Alberg AJ, Wallace K, Silvestri GA et al. , . Invited commentary: The etiology of lung cancer in men compared with women. Am J Epidemiol. 2013;1777:613–616. 10.1093/aje/kws444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Weiss JM, Lacey JV Jr, Shu XO et al. , . Menstrual and reproductive factors in association with lung cancer in female lifetime nonsmokers. Am J Epidemiol. 2008;16811:1319–1325. 10.1093/aje/kwn257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baik CS, Strauss GM, Speizer FE et al. , . Reproductive factors, hormone use, and risk for lung cancer in postmenopausal women, the Nurses' Health Study. Cancer Epidemiol Biomarkers Prev. 2010;1910:2525–2533. 10.1158/1055-9965.EPI-10-0450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schwartz AG, Ray RM, Cote ML et al. , . Hormone use, reproductive history, and risk of lung cancer: The Women's Health Initiative Studies. J Thorac Oncol. 2015;107:1004–1013. 10.1097/JTO.0000000000000558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pesatori AC, Carugno M, Consonni D et al. , . Hormone use and risk for lung cancer: A pooled analysis from the International Lung Cancer Consortium (ILCCO). Br J Cancer. 2013;1097:1954–1964. 10.1038/bjc.2013.506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hulley S, Furberg C, Barrett-Connor E et al. , . Noncardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/Progestin Replacement Study follow-up (HERS II). JAMA. 2002;2881:58–66. 10.1001/jama.288.1.58 [DOI] [PubMed] [Google Scholar]
- 14. Chlebowski RT, Schwartz AG, Wakelee H et al. , . Oestrogen plus progestin and lung cancer in postmenopausal women (Women's Health Initiative trial): A post-hoc analysis of a randomised controlled trial. Lancet. 2009;3749697:1243–1251. 10.1016/S0140-6736(09)61526-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schwartz AG, Wenzlaff AS, Prysak GM et al. , . Reproductive factors, hormone use, estrogen receptor expression and risk of non small-cell lung cancer in women. J Clin Oncol. 2007;2536:5785–5792. 10.1200/JCO.2007.13.3975 [DOI] [PubMed] [Google Scholar]
- 16. Raso MG, Behrens C, Herynk MH et al. , . Immunohistochemical expression of estrogen and progesterone receptors identifies a subset of NSCLCs and correlates with EGFR mutation. Clin Cancer Res. 2009;1517:5359–5368. 10.1158/1078-0432.CCR-09-0033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schwartz AG, Prysak GM, Murphy V et al. , . Nuclear estrogen receptor beta in lung cancer: Expression and survival differences by sex. Clin Cancer Res. 2005;1120:7280–7287. 10.1158/1078-0432.CCR-05-0498 [DOI] [PubMed] [Google Scholar]
- 18. Stabile LP, Dacic S, Land SR et al. , . Combined analysis of estrogen receptor beta-1 and progesterone receptor expression identifies lung cancer patients with poor outcome. Clin Cancer Res. 2011;171:154–164. 10.1158/1078-0432.CCR-10-0992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Meert AP, Martin B, Paesmans M et al. , . The role of HER-2/neu expression on the survival of patients with lung cancer: A systematic review of the literature. Br J Cancer. 2003;896:959–965. 10.1038/sj.bjc.6601252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ishibashi H, Suzuki T, Suzuki S et al. , . Progesterone receptor in non-small cell lung cancer—a potent prognostic factor and possible target for endocrine therapy. Cancer Res. 2005;6514:6450–6458. [DOI] [PubMed] [Google Scholar]
- 21. Greene FL, Page DL, Fleming ID et al. , . The AJCC Cancer Staging Manual. 6th ed. Chicago, IL: American Joint Committee on Cancer; 2002.
- 22. Allott EH, Cohen SM, Geradts J et al. , . Performance of three-biomarker immunohistochemistry for intrinsic breast cancer subtyping in the AMBER Consortium. Cancer Epidemiol Biomarkers Prev. 2016;253:470–478. 10.1158/1055-9965.EPI-15-0874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pirker R, Pereira JR, von Pawel J et al. , . EGFR expression as a predictor of survival for first-line chemotherapy plus cetuximab in patients with advanced non-small-cell lung cancer: Analysis of data from the phase 3 FLEX study. Lancet Oncol. 2012;131:33–42. 10.1016/S1470-2045(11)70318-7 [DOI] [PubMed] [Google Scholar]
- 24. Lin D, Wei LJ, Ying Z.. Checking the Cox model with cumulative sums of martingale-based residuals. Biometrika. 1993;80:557–572. 10.1093/biomet/80.3.557 [DOI] [Google Scholar]
- 25. Nose N, Sugio K, Oyama T et al. , . Association between estrogen receptor-beta expression and epidermal growth factor receptor mutation in the postoperative prognosis of adenocarcinoma of the lung. J Clin Oncol. 2009;273:411–417. 10.1200/JCO.2008.18.3251 [DOI] [PubMed] [Google Scholar]
- 26. Siegfried JM. Smoking out reproductive hormone actions in lung cancer. Mol Cancer Res. 2014;121:24–31. 10.1158/1541-7786.MCR-13-0580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Paech K, Webb P, Kuiper GG et al. , . Differential ligand activation of estrogen receptors ERalpha and ERbeta at AP1 sites. Science. 1997;2775331:1508–1510. 10.1126/science.277.5331.1508 [DOI] [PubMed] [Google Scholar]
- 28. Lin S, Lin CJ, Hsieh DP et al. , . ERalpha phenotype, estrogen level, and benzo[a]pyrene exposure modulate tumor growth and metabolism of lung adenocarcinoma cells. Lung Cancer. 2012;753:285–292. 10.1016/j.lungcan.2011.08.010 [DOI] [PubMed] [Google Scholar]
- 29. Kawai H, Ishii A, Washiya K et al. , . Estrogen receptor alpha and beta are prognostic factors in non-small cell lung cancer. Clin Cancer Res. 2005;1114:5084–5089. 10.1158/1078-0432.CCR-05-0200 [DOI] [PubMed] [Google Scholar]
- 30. Ferketich AK, Niland JC, Mamet R et al. , . Smoking status and survival in the national comprehensive cancer network non-small cell lung cancer cohort. Cancer. 2013;1194:847–853. 10.1002/cncr.27824 [DOI] [PubMed] [Google Scholar]
- 31. Greiser CM, Greiser EM, Doren M.. Menopausal hormone therapy and risk of lung cancer—systematic review and meta-analysis. Maturitas. 2010;653:198–204. [DOI] [PubMed] [Google Scholar]
- 32. Chlebowski RT, Anderson GL, Manson JE et al. , . Lung cancer among postmenopausal women treated with estrogen alone in the women's health initiative randomized trial. J Natl Cancer Inst. 2010;10218:1413–1421. 10.1093/jnci/djq285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Slatore CG, Chien JW, Au DH et al. , . Lung cancer and hormone replacement therapy: Association in the Vitamins and Lifestyle Study. J Clin Oncol. 2010;289:1540–1546. 10.1200/JCO.2009.25.9739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Toh CK, Ahmad B, Soong R et al. , . Correlation between epidermal growth factor receptor mutations and expression of female hormone receptors in East-Asian lung adenocarcinomas. J Thorac Oncol. 2010;51:17–22. 10.1097/JTO.0b013e3181c0a602 [DOI] [PubMed] [Google Scholar]
- 35. Stabile LP, Rothstein ME, Cunningham DE et al. , . Prevention of tobacco carcinogen-induced lung cancer in female mice using antiestrogens. Carcinogenesis. 2012;3311:2181–2189. 10.1093/carcin/bgs260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shen H, Yuan Y, Sun J et al. , . Combined tamoxifen and gefitinib in non-small cell lung cancer shows antiproliferative effects. Biomed Pharmacother. 2010;642:88–92. 10.1016/j.biopha.2009.06.010 [DOI] [PubMed] [Google Scholar]
- 37. Bouchardy C, Benhamou S, Schaffar R et al. , . Lung cancer mortality risk among breast cancer patients treated with anti-estrogens. Cancer. 2011;1176:1288–1295. 10.1002/cncr.25638 [DOI] [PubMed] [Google Scholar]
- 38. Chu SC, Hsieh CJ, Wang TF et al. , . Antiestrogen use in breast cancer patients reduces the risk of subsequent lung cancer: A population-based study. Cancer Epidemiol. 2017;48:22–28. 10.1016/j.canep.2017.02.010 [DOI] [PubMed] [Google Scholar]
- 39. Cuzick J, Sestak I, Bonanni B et al. , . Selective oestrogen receptor modulators in prevention of breast cancer: An updated meta-analysis of individual participant data. Lancet. 2013;3819880:1827–1834. 10.1016/S0140-6736(13)60140-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tamoxifen for early breast cancer: An overview of the randomised trials. Early Breast Cancer Trialists' Collaborative Group. Lancet. 1998;3519114:1451–1467. [PubMed] [Google Scholar]
- 41. Yoshizawa A, Sumiyoshi S, Sonobe M et al. , . HER2 status in lung adenocarcinoma: A comparison of immunohistochemistry, fluorescence in situ hybridization (FISH), dual-ISH, and gene mutations. Lung Cancer. 2014;853:373–378. 10.1016/j.lungcan.2014.06.007 [DOI] [PubMed] [Google Scholar]
- 42. Mah V, Seligson DB, Li A et al. , . Aromatase expression predicts survival in women with early-stage non small cell lung cancer. Cancer Res. 2007;6721:10484–10490. 10.1158/0008-5472.CAN-07-2607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kawaguchi T, Koh Y, Ando M et al. , . Prospective analysis of oncogenic driver mutations and environmental factors: Japan Molecular Epidemiology for Lung Cancer Study. J Clin Oncol. 2016;3419:2247–2257. 10.1200/JCO.2015.64.2322 [DOI] [PubMed] [Google Scholar]
- 44. Morgensztern D, Ng SH, Gao F et al. , . Trends in stage distribution for patients with non-small cell lung cancer: A National Cancer Database survey. J Thorac Oncol. 2010;51:29–33. 10.1097/JTO.0b013e3181c5920c [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.