Abstract
Small cell carcinoma of the ovary, hypercalcemic type (SCCOHT), is a highly aggressive monogenic cancer driven by SMARCA4 mutations. Here, we report responses to anti-PD1 immunotherapy in four patients and characterize the immune landscape of SCCOHT tumors using quantitative immunofluorescence and gene expression profiling. Unexpectedly for a low mutation burden cancer, the majority of the tumors (eight of 11 cases) demonstrated PD-L1 expression with strong associated T-cell infiltration (R2 = 0.60–0.95). PD-L1 expression was detected in both tumor and stromal cells, with macrophages being the most abundant PD-L1-positive cells in some tumors (three of 11 cases). Transcriptional profiling revealed increased expression of genes related to Th1 and cytotoxic cell function in PD-L1-high tumors, suggesting that PD-L1 acts as a pathway of adaptive immune resistance in SCCOHT. These findings suggest that although SCCOHT are low–mutational burden tumors, their immunogenic microenvironment resembles the landscape of tumors that respond well to treatment with PD-1/PD-L1 blockade.
Small cell carcinoma of the ovary, hypercalcemic type (SCCOHT), is a rare and very aggressive malignancy that occurs mostly in young women (1). We and others previously described SCCOHT as a SMARCA4-mutated monogenic disease (2–6). Despite recent advances in understanding SCCOHT biology, there are few effective treatments, and survival remains poor.
Immunotherapy utilizing antibodies against the immune checkpoint inhibitor programmed death 1 (PD-1) is active in many cancers (7–12). High tumor mutational load, presence of tumor-infiltrating lymphocytes (TILs), and PD-L1 expression in the microenvironment appear to enrich for response to PD-1/PD-L1 blockade (13–15). Studies in gynecologic malignancies, including microsatellite instability (MSI), demonstrate that an increase in mutational burden is associated with increased TILs and tumor PD-L1 expression (16–18).
SCCOHT is a monogenic disease and as such is not hypermutated (2–6), which may otherwise dampen enthusiasm for immunotherapy. The activity of PD-1/PD-L1 blockade in these tumors has not been reported, yet several patients with SCCOHT have received anti-PD1 immunotherapy with suggested benefit. We collected case histories from four patients diagnosed at age 18 to 29 years (details in the Supplementary Materials, available online). Each patient was found to have a large ovarian tumor and underwent surgery that resulted in complete tumor resection; all recurred after a disease-free interval of one to three years. Each patient received additional therapy and then began treatment with anti-PD1 immunotherapy. One patient has a sustained partial response for six months, and the three other patients have remained disease-free for 1.5 years or more. This study was determined to be institutional review board–exempt; however, each patient did provide written permission to be included in this report. All statistical tests were two-sided, and a P value of less than .05 was considered statistically significant.
Though we were unable to obtain biospecimens from these four patients, these anecdotal findings prompted us to study the tumor microenvironment to examine the rationale for immunotherapeutic approaches in SCCOHT. Using immunohistochemistry (IHC) and immunofluorescence (IF) analyses, we assessed tumor expression of PD-L1, CD3 (T cells), and CD68 (macrophages) in 11 SCCOHT cases. All but one patient demonstrated either a deleterious mutation in SMARCA4 or loss of SMARCA4 expression by IHC (Supplementary Table 1, available online) without any additional genetic alterations, highlighting the low tumor mutational burden of these tumors.
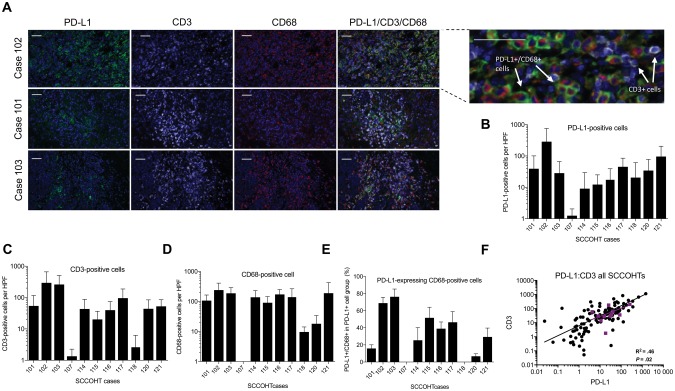
Unexpectedly for a cancer with few somatic mutations, PD-L1 expression (range = 9–287 cells per high-power field [HPF]) and prominent T-cell infiltration (range = 3–296 cells per HPF) were detected in most tumors (10 of 11 cases) (Figure 1, A–C). The majority of the tumors (10 of 11 cases) also demonstrated infiltration by CD68+ macrophages (range = 10–241 cells per HPF) (Figure 1D). Similar to reports in other solid tumors, PD-L1 expression was detected in both tumor cells and stromal cells, and in three cases tumor-associated macrophages were the most abundant PD-L1-positive cells (Figure 1, A and E; Supplementary Figure 1, available online).
Figure 1.
Small cell carcinoma of the ovary, hypercalcemic type (SCCOHT), exhibit immune-active tumor microenvironment. A) Immunohistochemistry triple-staining with the PD-L1, CD3, and CD68 antibodies of three representative SCCOHT cases. Enlarged outset demonstrates merged triple-stain, showing overlap of PD-L1- and CD68-positive cells but not CD3-positive cells. Scale bars = 50 µm. B) PD-L1-positive, C) CD3-positive, and D) CD68-positive cell counts per high-power field (HPF). The y-axis indicates number of marker-positive cells, and the x-axis indicates SCCOHT cases. Error bars represent one SD per 10 HPFs. E) Percentage of CD68+ cells out of all PD-L1-expressing cells. The y-axis indicates percentage of PD-L1-expressing macrophages, and the x-axis indicates SCCOHT cases. Error bars represent one SD per 10 HPFs. F) Correlation of PD-L1 and CD3 expression in all SCCOHT cases. Each black dot represents an individual HPF. Each purple square represents the mean of 10 HPFs for each case, and Pearson correlation is shown for these cases. All statistical tests were two-sided. HPF = high-power field; SCCOHT = small cell carcinoma of the ovary, hypercalcemic type.
There was a strong association between T-cell infiltration and PD-L1 expression across all patients (R2 = 0.46, P = .02; Pearson correlation) (Figure 1F), and on a per-field basis in each patient, apart from those with the lowest PD-L1 positivity (Supplementary Figure 2A, available online). PD-L1 expression was statistically significantly associated with T-cell infiltration in eight of 11 cases (R2 = 0.60–0.95, P < .05). There was no association between macrophages and T-cell infiltration, and there was a poor association between PD-L1 expression and tumor macrophage infiltration (Supplementary Figure 2, B and C available online). The latter finding suggests that macrophage recruitment and upregulation of PD-L1 expression are mediated by different mechanisms.
The association between PD-L1 expression and T-cell infiltration suggests that PD-L1 expression is likely not intrinsic, but rather a mechanism of adaptive immune resistance to TILs, and is likely upregulated in response to production of IFNγ in the tumor microenvironment (13,19). The differential expression of PD-L1 by the macrophages and cancer cells between the patients could imply intrinsic biologic differences between the cells or differential responses to T-cell-produced IFNγ (21).
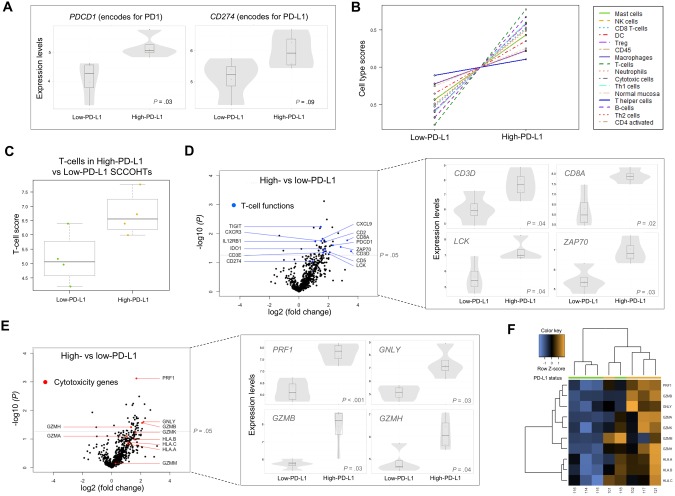
To characterize the tumor inflammatory responses and their association with PD-L1 expression in more detail, we performed gene expression profiling using NanoString’s nCounter PanCancer Immunoprofiling panel (Figure 2). Most differentially expressed genes (34 of 36, P < .05) were elevated in the high-PD-L1 group and were T-cell specific or cytotoxicity related (Supplementary Table 2, available online). The differentially expressed gene set closely corresponds to the recently reported transcriptional signature associated with response to pembrolizumab (Supplementary Table 2, available online) (22). The expression of PDCD1 (encoding for PD-1), previously associated with response to PD-1 blockade, was higher in the high-PD-L1 group (fold change = 2.3, 95% confidence interval [CI] = 1.4 to 3.8, P = .03) (Figure 2A) (13). CD274 (encoding for PD-L1) also had higher (but not statistically significant) expression in the high-PD-L1 group, possibly due to heterogeneity of the PD-L1-positive cells or post-transcriptional regulation of PD-L1 expression. Gene expression–based cell type deconvolution demonstrated that all immune cell types were more common in the high-PD-L1 group, further confirming correlation between PD-L1 positivity and enhanced immune response (Figure 2B). Among the highest cell type scores, in addition to B cells, were cell types known to be involved in antitumor activity such as T cells, Th1, CD8, and cytotoxic cells (Figure 2, B–D; Supplementary Figure 2C, available online). Two genes encoding for CD3, CD3E (fold change = 3.1, 95% CI = 1.3 to 7.2, P = .05) and CD3D (fold change = 3.5, 95% CI = 1.4 to 8.6, P = .04), were elevated in the high-PD-L1 group, supporting our observations from the IHC experiments that TILs and PD-L1-positive cells coexist in SCCOHTs (Figure 2D). Cytolytic and antigen-presenting genes were also noted to be elevated in the high-PD-L1 group (Figure 2, E and F). Among cytotoxic genes, a statistically significant increase in perforin 1 (PRF1; fold change = 3.2, 95% CI = 2.4 to 4.5, P < .001), granzyme B (GZMB; fold change = 4.3, 95% CI = 1.7 to 10.6, P = .03), granulysin (GNLY; fold change = 4.6, 95% CI = 1.8 to 11.7, P = .03), and granzyme H (GZMH; fold change = 2.5, 95% CI = 1.3 to 4.8, P = .04) (Figure 2E) highlighted the association between PD-L1 expression and cytotoxic cell activity. KEGG pathway analysis revealed increased activity in the T-cell receptor signaling pathway, supporting our observation that TILs are engaged in active immune response in SCCOHTs (Supplementary Figure 3, available online). Interestingly, two of the PD-L1-high cases exhibited macrophage-predominant PD-L1 expression (102 and 117), and the other two expressed PD-L1 predominantly in tumor cells (cases 101 and 121). Similarly, in the PD-L1-low group, PD-L1 expression was primarily seen in macrophages in cases 115 and 116 and in tumor cells in cases 114 and 118. There did not appear to be meaningful differences in T-cell-related gene expression between the subgroups in either the PD-L1-high group or the PD-L1-low group (Supplementary Figure 4, available online).
Figure 2.
PD-L1 expression is associated with T cell-related gene expression in small cell carcinoma of the ovary, hypercalcemic type (SCCOHT). A) Normalized gene expression of PDCD1 (encoding for PD1) and CD274 (encoding for PD-L1) in the high-PD-L1 vs low-PD-L1 cases. Box plots superimposed on violin plots in figures (A), (D), and (E) represent differential gene expression. Boxplot center lines represent tumor medians, box limits are the interquartile range from 25% and 75%, and whiskers represent the extent of tumors out to 1.5 times the interquartile range. The gray shading of the violin plots represents the estimated distribution of the expression values. Dots represent individual cases. B) Relative abundance of specific cell types in high-PD-L1 vs low-PD-L1 cases. Cell type scores are mean centered based on deconvolution of cell type–specific gene sets. C) T-cell scores in the high-PD-L1 vs low-PD-L1 group. D and E) Volcano plots of differentially expressed genes showing the T-cell-related genes (blue dots) and cytotoxicity genes (red dots), respectively. Expression of representative genes are shown to the right of each plot. Statistically significant genes are shown above the dashed line on the volcano plots (P = .05), and individual genes are shown adjacent. F) Unsupervised clustering of cytotoxicity-related and MHC class I genes for high-PD-L1 and low-PD-L1 cases. PD-L1 group is indicated by the color bar at the top of the figure (green: low-PD-L1; orange: high-PD-L1). All P values were calculated using the Benjamini-Yekutieli method. All statistical tests were two-sided. MHC = major histocompatibility complex.
These data suggest that SCCOHTs are immunogenic tumors and exhibit biologically significant levels of T-cell infiltration and PD-L1 expression. These findings are somewhat unexpected, given the low mutational burden in these tumors. SMARCA4 is a member of the chromatin remodeling SWI/SNF complex that is involved in many biological processes including transcriptional regulation (23). The transcriptional program regulated by SMARCA4 may influence tumor immunogenicity, leading to TIL infiltration and PD-L1 upregulation. Given that SCCOHT is a monogenic disease, this immunogenicity may derive from the loss of SMARCA4 function. Given that SCCOHT is a rare disease, the major limitation of our study is the small number of cases. Another limitation is that tissue from the patients who received anti-PD1 immunotherapy was not available.
These data describe the immune landscape and tumor microenvironment of SCCOHT tumors, which resembles other immunogenic tumors that respond well to anti-PD-1 immunotherapies, generating a strong rationale for evaluation of these agents in SCCOHT patients. The findings underscore inconsistencies between mutational burden and immunogenicity, emphasizing the need for additional biomarkers to assess tumor recognition by the immune system.
Funding
We are grateful for funding provided by the Katie Oppo Research Fund, Arnold Chavkin and Laura Chang, US Department of Defense Consortium Award W81XWH-11-2-0230, NIH P30 CA008748, NIH P30 CA016087, and support from the Small Cell Ovarian Cancer Foundation. DZ received funding from the Ovarian Cancer Research Foundation, MSKCC Cycle for Survival, and the US Department of Defense Ovarian Cancer Academy (W81XWH-16-1-0298).
Notes
We appreciate the support of all the patients who shared biologic specimens and clinical information for this report. We are thankful to Yanyun Li for help with pathology and quantification.
The funders had no role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
The authors declare no competing financial interests.
Supplementary Material
References
- 1. Young RH, Oliva E, Scully RE.. Small cell carcinoma of the ovary, hypercalcemic type. A clinicopathological analysis of 150 cases. Am J Surg Pathol. 1994;1811:1102–1116. 10.1097/00000478-199411000-00004 [DOI] [PubMed] [Google Scholar]
- 2. Jelinic P, Mueller JJ, Olvera N et al. , . Recurrent SMARCA4 mutations in small cell carcinoma of the ovary. Nat Genet. 2014;465:424–426. 10.1038/ng.2922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ramos P, Karnezis AN, Craig DW et al. , . Small cell carcinoma of the ovary, hypercalcemic type, displays frequent inactivating germline and somatic mutations in SMARCA4. Nat Genet. 2014;465:427–429. 10.1038/ng.2928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Witkowski L, Carrot-Zhang J, Albrecht S et al. , . Germline and somatic SMARCA4 mutations characterize small cell carcinoma of the ovary, hypercalcemic type. Nat Genet. 2014;465:438–443. 10.1038/ng.2931 [DOI] [PubMed] [Google Scholar]
- 5. Jelinic P, Schlappe BA, Conlon N et al. , . Concomitant loss of SMARCA2 and SMARCA4 expression in small cell carcinoma of the ovary, hypercalcemic type. Mod Pathol. 2016;291:60–66. 10.1038/modpathol.2015.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Karnezis AN, Wang Y, Ramos P et al. , . Dual loss of the SWI/SNF complex ATPases SMARCA4/BRG1 and SMARCA2/BRM is highly sensitive and specific for small cell carcinoma of the ovary, hypercalcaemic type. J Pathol. 2016;2383:389–400. 10.1002/path.4633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Topalian SL, Drake CG, Pardoll DM.. Immune checkpoint blockade: A common denominator approach to cancer therapy. Cancer Cell. 2015;274:450–461. 10.1016/j.ccell.2015.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;124:252–264. 10.1038/nrc3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hamanishi J, Mandai M, Iwasaki M et al. , . Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A. 2007;1049:3360–3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nghiem PT, Bhatia S, Lipson EJ et al. , . PD-1 blockade with pembrolizumab in advanced merkel-cell carcinoma. N Engl J Med. 2016;37426:2542–2552. 10.1056/NEJMoa1603702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brahmer J, Reckamp KL, Baas P et al. , . Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;3732:123–135. 10.1056/NEJMoa1504627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Boussiotis VA. Molecular and biochemical aspects of the PD-1 checkpoint pathway. N Engl J Med. 2016;37518:1767–1778. 10.1056/NEJMra1514296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tumeh PC, Harview CL, Yearley JH et al. , . PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;5157528:568–571. 10.1038/nature13954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Larkin J, Chiarion-Sileni V, Gonzalez R et al. , . Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;37313:1270–1271. 10.1056/NEJMc1509660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rizvi NA, Hellmann MD, Snyder A et al. , . Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;3486230:124–128. 10.1126/science.aaa1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pakish JB, Zhang Q, Chen Z et al. , . Immune microenvironment in microsatellite instable endometrial cancers: Hereditary or sporadic origin matters. Clin Cancer Res. 2017;2315:4473–4481. 10.1158/1078-0432.CCR-16-2655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Strickland KC, Howitt BE, Shukla SA et al. , . Association and prognostic significance of BRCA1/2-mutation status with neoantigen load, number of tumor-infiltrating lymphocytes and expression of PD-1/PD-L1 in high grade serous ovarian cancer. Oncotarget. 2016;712:13587–13598. 10.18632/oncotarget.7277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Howitt BE, Shukla SA, Sholl LM et al. , . Association of polymerase e-mutated and microsatellite-instable endometrial cancers with neoantigen load, number of tumor-infiltrating lymphocytes, and expression of PD-1 and PD-L1. JAMA Oncol. 2015;19:1319–1323. 10.1001/jamaoncol.2015.2151 [DOI] [PubMed] [Google Scholar]
- 19. Spranger S, Spaapen RM, Y. Z, et al. Up-regulation of PD-L1, IDO, and tregs in the melanoma tumor microenvironment is driven by CD8+ T cells. Sci Transl Med. 2013;5(200):5200:200ra116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Powles T, Eder JP, Fine GD et al. , . MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;5157528:558–562. 10.1038/nature13904 [DOI] [PubMed] [Google Scholar]
- 21. Garcia-Diaz A, Shin DS, Moreno BH et al. , . Interferon receptor signaling pathways regulating PD-L1 and PD-L2 expression. Cell Rep. 2017;196:1189–1201. 10.1016/j.celrep.2017.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ayers M, Lunceford J, Nebozhyn M et al. , . IFN-gamma-related mRNA profile predicts clinical response to PD-1 blockade. J Clin Invest. 2017;1278:2930–2940. 10.1172/JCI91190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wilson BG, Roberts CW.. SWI/SNF nucleosome remodellers and cancer. Nat Rev Cancer. 2011;117:481–492. 10.1038/nrc3068 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.