Abstract
Background
Recognition that serous tubal intraepithelial carcinoma (STIC) may represent the first manifestation of many high-grade cancers that were once considered ovarian primary tumors has led to changes in diagnostic practices that could dramatically increase the reporting of tubal carcinomas in US population-based cancer registries. Further, increased detection of early-stage tubal carcinomas through increased recognition coupled with meticulous pathology processing protocols raises important unanswered questions about the clinical behavior of such lesions, which can only be answered using large data sets. However, rates of tubal carcinomas have not been recently analyzed. Accordingly, we analyzed population-based incidence and survival data for fallopian tube carcinoma in situ (CIS; an imperfect surrogate of STIC), tubal carcinomas, and for comparison, ovarian carcinomas, in the North American Association of Central Cancer Registries (NAACCR) registries.
Methods
Total counts, standardized incidence rates, and stage-specific survival were computed using 30 NAACCR registries (1999–2012). Temporal incidence rate patterns were analyzed by joinpoint regression with estimates of annual percentage change (APC). All statistical tests were two-sided.
Results
Fallopian tube CIS incidence rates were stable from 1999 to 2002, then increased from 2002 to 2012 (APC = 16.2%, 95% confidence interval [CI] = 10.9% to 21.7%, P < .001). Rates of early- and late-stage tubal carcinomas showed similar patterns, whereas high-grade serous ovarian carcinoma rates were relatively stable. Five-year cause-specific survival was 97.9% (95% CI = 93.7% to 99.3%) for tubal CIS and 83.2% (95% CI = 77.3% to 87.7%) for early-stage high-grade serous tubal carcinoma.
Conclusions
Reporting of tubal CIS and tubal carcinoma have increased in recent years, likely reflecting changes in pathology processing of specimens and diagnosis. Developing standardized reporting for tubal neoplasms is needed to enable analysis of outcomes for these comparatively uncommon but increasingly recognized tumors.
Monitoring incidence and mortality rates of highly lethal gynecologic cancers, such as ovarian and tubal carcinomas, is an important public health measure. Recently, our understanding of the pathogenesis of these tumors has been dramatically revised, such that many cancers once supposed to arise from the ovaries are now increasingly believed to develop as a fallopian tube lesion, termed serous tubal intraepithelial carcinoma (STIC) (1–4). It is proposed that STIC may spread to the ovaries or peritoneum through exfoliation without invasion through the basement membrane of the fallopian tube (2,5), which distinguishes STIC from typical carcinoma in situ (CIS) of most organs. Given that STIC is a newly defined pathology entity, its population-based incidence and natural history are undefined. Although CIS and STIC are conceptually different and current cancer registration guidelines provide rules for coding STIC, most STIC lesions were likely coded as tubal CIS or localized/early-stage tubal carcinoma historically.
In research studies, occult STICs have been found in approximately 3% to 8% of risk-reducing salpingo-oophorectomy specimens performed among BRCA 1/2 mutation carriers (6–9) and concurrently with high-grade serous carcinoma in approximately 13% to 53% of cases (mean = 37%, 95% CI = 27% to 48%) (10–14). Among women undergoing surgery for benign indications, STIC or early tubal carcinomas are found in less than 1% of surgical pathology specimens with meticulous examination (7,15,16). Although a recent survey found that the vast majority of pathologists and gynecologists think that most high-grade serous carcinomas arise from the tubes and that defining the primary site of these cancers is clinically important (17), the population-based incidence of STIC and early-stage tubal carcinoma have not been comprehensively assessed in the United States recently (18).
We hypothesize that the reporting of tubal CIS alone and tubal CIS associated with invasive carcinoma have increased, reflecting both increased identification of early lesions by pathologists and a trend toward classifying high-grade invasive carcinomas as tubal primary tumors when STIC is identified. Accordingly, we assessed temporal patterns in the incidence and survival of tubal CIS (an imperfect surrogate of STIC) and carcinoma by stage and grade using data from the North American Association of Central Cancer Registries (NAACCR). For context, we present ovarian cancer incidence and survival patterns over the same period.
Methods
Data Source
We used the Cancer Incidence in North America (CiNA) Deluxe Analytic Files provided by NAACCR (www.naaccr.org) for this analysis. Population-based cancer incidence and mortality data were obtained from the NAACCR member registries, which are maintained by the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program or the Center for Disease Control and Prevention’s National Program of Cancer Registries (NPCR) (19). Only participating registries that meet high quality standards are included in the CiNA analytic data set. The CiNA analytic file dates to 1995, but because of missing data for some of the registries, we restricted all analyses to the year 1999 and forward. The 33 registries included in this data set are: Alabama, Alaska, California, Colorado, Connecticut, Georgia, Hawaii, Idaho, Iowa, Kentucky, Louisiana, Maine, Maryland, Detroit (Michigan), Montana, Nebraska, New Hampshire, New Jersey, New Mexico, New York, North Carolina, Pennsylvania, Rhode Island, Tennessee, Texas, Utah, Seattle, West Virginia, Wisconsin, and Wyoming. These registries cover approximately 84% of the US population. We did not include registry data from Atlanta, the Greater Bay Area, or Los Angeles to avoid double counting data already contained in Georgia and California, respectively. Thus, our analysis included fallopian tube and ovarian carcinomas with incidence data from 30 consenting population-based cancer registries (1999–2012). The study protocol received approval by the NAACCR Institutional Review Board.
Study Population
Fallopian tube and ovarian carcinomas were defined per the International Classification of Diseases for Oncology (3rd edition) topography codes (fallopian tube [C57.0] and ovarian [C56.9]). We excluded nonepithelial tumors (n = 63 fallopian tube, 18 253 ovarian) as well as those with other unclear/irrelevant histology codes (n = 9 fallopian tube, 369 ovarian). Tubal CIS was identified using topography code C57.0 and behavior in situ. Per current cancer registration guidelines, SEER cancer registries and associated hospital registries would code tubal intraepithelial carcinoma as topography code C57.0 and histology/behavior code 8010/2 and would consider STIC reportable and utilize code C57.0/8441/2 (https://seer.cancer.gov/seerinquiry/index.php?page=view&id=20170035&type=q) (20). It is unclear how coders at facilities outside of the SEER hospital registries would code STIC and if there is widespread knowledge of the use of histology/behavior code 8441/2. It is also unclear historically how STIC would have been coded. Given this uncertainty, we analyzed tubal CIS as one entity and explored the evaluation of incidence patterns for 8010/2 and 8441/2 to the extent possible. We further subdivided tumors based on histotype as follows: serous (8441, 8442, 8460, 8461, 8462, 8463, 9014), endometrioid (8380, 8381, 8382, 8383, 8560, 8570), clear cell (8310, 8313), mucinous (8470, 8471, 8472, 8480, 8481, 8482, 8490, 9015), and other epithelial (8010, 8020, 8050, 8070, 8140, 8260, 8323, 8440). Tumor grade was categorized as low grade (grade I/II) or high grade (grade III/IV). Tumor stage was categorized as early (localized: which corresponds to FIGO stage 1A, 1B, or 1, not otherwise specified) or late (regional/distant: FIGO stage >1B) (20). Regional and distant stage were combined per SEER coding instructions because the definition of regional and distant varied over the study period. We focused our comparisons on serous ovarian and fallopian tube cancers, given the prevailing view that most serous carcinomas arise from the fallopian tube (1–4).
Statistical Analysis
Age-standardized cancer incidence per 1 million women and 95% confidence intervals (CIs) were generated using SEER*Stat Software (version 8.3.2; http://www.seer.cancer.gov/seerstat) and plotted on a semilogarithmic scale by calendar year of diagnosis. Incidence rates are standardized to the 2000 US Census in five-year age groups. Estimates of the annual percentage change (APC) were calculated for the period 1999–2012 using weighted least squares regression to assess trends in age-standardized incidence from the overall population (all serous cancers) as well as by tumor stage and age. To estimate changes in data trends over time, we utilized the joinpoint regression program (version 4.1.1.3). This method describes changes in data trends by connecting several different line segments on a log scale at “joinpoints” starting with 0 (representing a straight line) and tests for model fit with a maximum of 4 joinpoints. Using Kaplan-Meier, we evaluated age-standardized cause-specific survival by stage (early, late), grade (low, high), and age (<50, ≥50 years). Cause of death was defined as death from ovarian cancer or fallopian tube cancer using the SEER cause-specific death classification variable (21). Cancer cases were excluded from survival analyses if the tumor of interest was not the first tumor, if age at diagnosis was missing, or if follow-up time was missing or 0. All statistical tests were two-sided, and P values of less than .05 were considered statistically significant. P values for APCs were calculated based on a t distribution. All other analyses were performed with SEER*Stat (version 8.3.2, Bethesda, MD) or SAS (version 9.3, Cary, NC).
Results
Population
During the period 1999 to 2012, the analytical file included 493 tubal CIS diagnoses, 7066 fallopian tube carcinomas, and 157 725 ovarian carcinomas (Table 1). Cases in which the first diagnosis was tubal CIS included 116 (24.4%) fallopian tube CIS alone, 159 (33.5%) cases in which fallopian tube CIS was diagnosed concurrently with another cancer, and 25 (5.3%) fallopian tube CIS followed by another cancer diagnosis within a year. The remaining 175 (36.8%) fallopian tube CIS were diagnosed following multiple other primary cancer diagnoses. Among the 7066 invasive tubal carcinomas, 862 (12.4%) were reported concurrently with another primary and 826 (11.9%) were followed by diagnosis of a second cancer within a year. The preceding numbers represent women with the relevant diagnoses and do not sum to the total number of tumors listed in Table 1 due to accounting for multiple diagnoses per woman. The percentage of tubal cancers staged as localized (29.6%) was twice that of ovarian cancers (14.2%), and unilateral primary site was assigned for 95.5% of tubal CIS and 87.9% of tubal cancers, whereas approximately one-third of ovarian cancers (35.3%) were considered bilateral; 47.6% of tubal CIS were coded as tubal intraepithelial carcinoma (n = 235 out of 493, 8010/2), and 22.1% were coded as serous tubal CIS (n = 109, 8441/2) based on current coding guidelines.
Table 1.
Characteristics of incident in situ carcinoma of the fallopian tube, malignant fallopian tube cancer, and malignant ovarian cancer (North American Association of Central Cancer Registries, 1999–2012)
| C57.0 Fallopian tube (in situ) | C57.0 Fallopian tube (malignant) | C56.9 Ovary (malignant) | |
|---|---|---|---|
| (n = 493) | (n = 7066) | (n = 157 725) | |
| Age, y | No. (%) | No. (%) | No. (%) |
| <50 | 121 (24.5) | 803 (11.4) | 28 823 (18.3) |
| ≥50 | 372 (75.5) | 6263 (88.6) | 128 902 (81.7) |
| Race | |||
| White | 437 (88.6) | 6216 (88.0) | 137 572 (87.2) |
| Black | 37 (7.5) | 507 (7.2) | 12 220 (7.7) |
| Asian or Pacific Islander | <16* | 277 (3.9) | 6560 (4.2) |
| American Indian/Alaska Native | <16* | 27 (0.4) | 665 (0.4) |
| Unknown | <16* | 39 (0.6) | 708 (0.4) |
| Stage | |||
| Localized (early stage) | NA | 2091 (29.6) | 22 471 (14.2) |
| Regional/distant (late stage) | NA | 4868 (68.9) | 126 021 (79.9) |
| Unknown/blank | NA | 107 (1.5) | 9233 (5.9) |
| Grade | |||
| I and II | 35 (7.1) | 1092 (15.5) | 33 107 (21.0) |
| III and IV | 28 (5.7) | 4879 (69.0) | 72 072 (45.7) |
| Unknown | 430 (87.2) | 1095 (15.5) | 52 546 (33.3) |
| Histology | |||
| Serous | 122† (24.7) | 4377 (61.9) | 71 408 (45.3) |
| Mucinous | <16* | 41 (0.6) | 10 437 (6.6) |
| Endometrioid | 26 (5.3) | 642 (9.1) | 17 210 (10.9) |
| Clear cell | <16* | 57 (0.8) | 8048 (5.1) |
| Other epithelial | 343† (69.5) | 1781 (25.2) | 47 318 (30.0) |
| Mixed | 0 (0.0) | 0 (0.0) | 3304 (2.1) |
| Laterality | |||
| Right | 213 (43.2) | 3067 (43.4) | 36 543 (23.2) |
| Left | 224 (45.4) | 3039 (43.0) | 35 878 (22.8) |
| Unilateral, side unspecified | 34 (6.9) | 105 (1.5) | 2527 (1.6) |
| Bilateral, single primary | 18 (3.7) | 684 (9.7) | 55 667 (35.3) |
| Paired, no information on laterality | <16* | 171 (2.4) | 27 105 (17.2) |
| Cancer diagnosis (n corresponds to women)‡ | |||
| First primary only | 116 (24.4) | 3346 (48.1) | 86 146 (55.1) |
| Cofirst primary (concurrent) | 159 (33.5) | 862 (12.4) | 16 702 (10.7) |
| First primary followed by 2nd cancer within 1 y | 25 (5.3) | 826 (11.9) | 18 676 (12.0) |
| Multiple primaries | 175 (36.8) | 1928 (27.7) | 34 804 (22.3) |
Cell count <16, frequencies are not reported per North American Association of Central Cancer Registries guidelines.
n = 109 were coded serous tubal CIS 8441/2; n = 235 were coded tubal intraepithelial carcinoma 8010/2.
n for cancers diagnosed does not sum to total because count is based on per woman classification rather than per tumor.
Incidence Rates and Trends
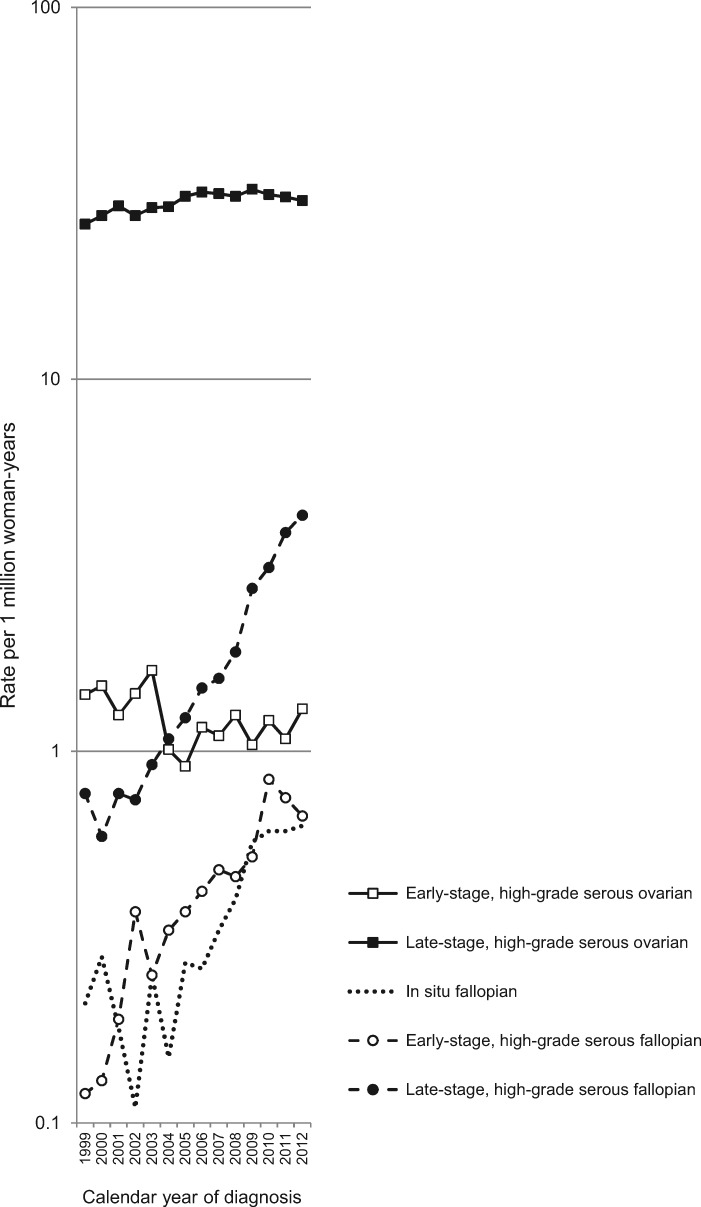
Incidence trends of tubal CIS and high-grade serous carcinomas by stage are reported in Table 2 and Figure 1. The rate of tubal CIS increased from 0.22 per 1 million women (1999–2001) to 0.62 (2011–2012). Incidence rates for tubal intraepithelial carcinoma (8010/2) and serous tubal CIS (8441/2) demonstrated increased reporting over time, with rates of less than 0.1 per 1 million women in 1999–2001 to 0.34 in 2011–2012 for intraepithelial carcinoma and 0.19 for STIC in the same period. Incidence rates of early-stage (localized) high-grade serous tubal carcinomas increased from 0.15 in 1999–2001 to 0.71 in 2011–2012; incidence rates of late-stage (regional/distant) high-grade serous fallopian tube cancers increased from 0.71 to 4.09 for the same period, respectively. In contrast to rates of less than five per 1 million women for early-stage high-grade serous tubal carcinomas and ovarian carcinomas, incidence rates hovered around 30 per 1 million women for late-stage high-grade serous ovarian carcinomas.
Table 2.
Age-standardized incidence rates (per 1 million women) and annual percent change in incidence rate for fallopian tube and ovarian cancer by behavior, stage, and grade (North American Association of Central Cancer Registries, 1999–2012)
| 1999–2001 | 2002–2004 | 2005–2007 | 2008–2010 | 2011–2012 | |||
|---|---|---|---|---|---|---|---|
| No. (Rate) | No. (Rate) | No. (Rate) | No. (Rate) | No. (Rate)* | APC (95% CI), P† | ||
| Fallopian tube | 1999–2002 | 2002–2012 | |||||
| In situ | 57 (0.22) | 48 (0.17) | 85 (0.29) | 167 (0.53) | 136 (0.62) | –13.3 (–42.3 to 30.2), .28 | 16.2 (10.9 to 21.7), <.001 |
| Intraepithelial carcinoma (8010/2) | 22 (0.09) | <16§ | 39 (0.13) | 86 (0.27) | 76 (0.34) | ||
| STIC (8441/2) | <16§ | <16§ | <16§ | 47 (0.15) | 42 (0.19) | ||
| Early-stage/low-grade serous | 28 (0.11) | 30 (0.11) | 27 (0.09) | 48 (0.15) | 30 (0.14) | 1999–2002 | 2002–2012 |
| Early-stage/high-grade serous | 38 (0.15) | 90 (0.32) | 127 (0.42) | 204 (0.61) | 167 (0.71) | 40.6 (–17.8 to 140.5), .11 | 10.4 (6.1 to 14.9), <.001 |
| Late-stage/low-grade serous | 48 (0.19) | 50 (0.18) | 74 (0.25) | 86 (0.26) | 48 (0.21) | 1999–2002 | 2002–2012 |
| Late-stage/high-grade serous | 182 (0.71) | 260 (0.92) | 428 (1.43) | 846 (2.58) | 936 (4.09) | 0.2 (–20.9 to 26.9), .86 | 20.0 (17.5 to 22.6), <.001 |
| Ovarian | |||||||
| Early-stage/low-grade serous | 507 (2.00) | 353 (1.26) | 311 (1.07) | 288 (0.91) | 145 (0.69) | 1999–2012 | |
| Early-stage/high-grade serous | 353 (1.38) | 385 (1.36) | 319 (1.06) | 377 (1.16) | 263 (1.19) | –1.8 (–3.9 to 0.3), .08 | |
| Late-stage/low-grade serous | 2599 (10.2) | 2533 (9.02) | 2339 (7.98) | 1906 (6.00) | 1034 (4.68) | 1999–2007 | 2007–2012 |
| Late-stage/high-grade serous | 7077 (27.7) | 8066 (28.5) | 9385 (31.5) | 10 267 (31.6) | 6939 (30.6) | 2.3 (1.3 to 3.4), <.001 | –0.9 (–2.7 to 1.0), .22 |
Case counts are based on two years of data compared with other strata that include three years; therefore, numbers of cases are not comparable with prior periods. Rates were age standardized to the 2000 US Population. APC = annual percentage change; CI = confidence interval; STIC = serous tubal intraepithelial carcinoma.
APC estimated from joinpoint regression for tubal carcinoma in situ and high-grade serous carcinomas by stage; time periods were selected based on regression models.
P value for a two-sided test that the true APC is zero, based on the t distribution.
Cell count <16, frequencies, and rates are not reported, per North American Association of Central Cancer Registries guidelines.
Figure 1.
Age-standardized incidence rates of fallopian tube carcinoma in situ as well as high-grade serous tumors of the fallopian tube and ovary stratified by stage (early [localized] vs late [regional/distant]; North American Association of Central Cancer Registries, 1999–2012). Rates were age standardized to the 2000 US Population.
Using joinpoint, the reporting of tubal CIS was stable from 1999 to 2002 (APC = –13.3% (95% CI = –42.3% to 30.2%, P = .28) but increased from 2002 to 2012 (APC = 16.2%, 95% CI = 10.9% to 21.7%, P < .001) (Table 2). Corresponding APCs for 1999–2012 were 16.6% (95% CI = 9.9% to 23.8%) for tubal intraepithelial carcinoma (8010/2) and 27.2% (95% CI = 15.6% to 40.0%) for serous tubal CIS (8441/2; APCs not tabled, P < .001). Similar to the trends for tubal CIS, the patterns in incidence of high-grade serous tubal carcinomas were stable, albeit imprecise between 1999 and 2002 (early stage: 40.6%, 95% CI = –17.8% to 140.5%; late stage: 0.2%, 95% CI = –20.9% to 26.9%), with increasing incidence rates between 2002 and 2012 (early stage: 10.4%, 95% CI = 6.1% to 14.9%; late stage: 20.0%, 95% CI = 17.5% to 22.6%). Rates for low-grade serous fallopian tube carcinoma were stable from 1999 to 2012 (early stage: 2.2%, 95% CI = –2.1% to 6.7%, P = .29; late stage: 2.3%, 95% CI = –0.7% to 5.4%, P = .12, data not shown).
Incidence trends for high-grade serous ovarian carcinomas were stable over the most recent period (early stage: 1999–2012 APC = –1.8%, 95% CI = –3.9% to 0.3%, P = .08; late stage: 2007–2012 APC = –0.9%, 95% CI = –2.7% to 1.0%, P = .22) (Table 2). Rates decreased for low-grade serous ovarian carcinomas over the same period (early stage: –8.4%, 95% CI = –9.8% to –7.0%, P < .001; late stage: –6.2%, 95% CI = –7.2% to –5.1%, P < .001; data not shown). Rates for the other histotypes of invasive fallopian tube and ovarian carcinomas were stable or decreasing between 1999 and 2012 (data not shown).
Survival
Five-year cause-specific survival (ie, fallopian tube or ovarian carcinoma) for women with fallopian tube CIS was 97.9% overall (95% CI = 93.7% to 99.3%), reflecting 100% survival for women younger than age 50 years and 96.8% (95% CI = 90.3% to 98.9%) for women age 50 years or older (Table 3). Five-year survival estimates for tubal intraepithelial carcinoma (8010/2) and serous tubal CIS (8441/2) were similarly high (97.1% and 96.0%, respectively; results not shown). Five-year survival estimates for early-stage serous fallopian tube carcinoma (low grade: 83.4%, 95% CI = 72.3% to 90.3%; high grade: 83.2%, 95% CI = 77.3% to 87.7%) and early-stage serous ovarian cancer (low grade: 90.7%, 95% CI = 88.7% to 92.3%; high grade: 85.2%, 95% CI = 82.7% to 87.4%) were lower than estimates for fallopian tube CIS; however, they were comparable across organ sites (fallopian tube and ovary) (Table 3). As anticipated, five-year survival estimates for late-stage serous fallopian tube carcinoma (low grade: 42.1%, 95% CI = 34.1% to 49.9%; high grade: 44.5%, 95% CI = 41.1% to 47.7%) and late-stage serous ovarian cancer (low grade: 46.1%, 95% CI = 44.9% to 47.3%; high grade: 33.8%, 95% CI = 33.2% to 34.4%) were the lowest, comparatively.
Table 3.
Age-standardized, cause-specific survival (% surviving to 60 months), stage- and histology-specific estimates overall and by age strata (North American Association of Central Cancer Registries, 1999–2012)*
| C57.0 Fallopian tube |
C56.9 Ovary |
|||
|---|---|---|---|---|
| Characteristic | No. | % (95% CI) | No. | % (95% CI) |
| In situ | 164 | 97.9 (93.7 to 99.3) | ||
| <50 y | 61 | 100 | ||
| 50+ y | 103 | 96.8 (90.3 to 98.9) | ||
| Early-stage/low-grade serous | 106 | 83.4 (72.3 to 90.3) | 1247 | 90.7 (88.7 to 92.3) |
| <50 y | <16 | —† | 394 | 96.3 (93.6 to 97.9) |
| 50+ y | 91 | 84.3 (72.5 to 91.3) | 853 | 88.1 (85.4 to 90.3) |
| Early-stage/high-grade serous | 357 | 83.2 (77.3 to 87.7) | 1198 | 85.2 (82.7 to 87.4) |
| <50 y | 45 | 80.4 (58.7 to 91.5) | 230 | 88.4 (82.6 to 92.4) |
| 50+ y | 312 | 83.8 (77.6 to 88.4) | 968 | 84.4 (81.5 to 86.9) |
| Late-stage/low-grade serous | 218 | 42.1 (34.1 to 49.9) | 8001 | 46.1 (44.9 to 47.3) |
| <50 y | 27 | 52.2 (30.1 to 70.3) | 1974 | 63.5 (61.0 to 65.8) |
| 50+ y | 191 | 40.6 (32.0 to 49.0) | 6027 | 40.5 (39.1 to 41.8) |
| Late-stage/high-grade serous | 1605 | 44.5 (41.1 to 47.7) | 29 685 | 33.8 (33.2 to 34.4) |
| <50 y | 166 | 58.7 (48.0 to 67.9) | 4358 | 44.0 (42.2 to 45.7) |
| 50+ y | 1439 | 42.7 (39.2 to 46.2) | 25 327 | 32.0 (31.3 to 32.7) |
Cause of death was defined as fallopian tube cancer or ovarian cancer, respectively, using the Surveillance, Epidemiology, and End Results cause-specific death classification variable (21). Cancer cases were excluded if not first tumor, if missing age at diagnosis, or if follow-up was missing or 0. CI = confidence interval.
Cell count <16, frequencies, and percent surviving are not reported.
Discussion
This analysis demonstrates that reporting of tubal CIS (including codes for serous intraepithelial carcinoma in recent years) and the incidence of invasive serous tubal carcinoma have increased dramatically in the last decade, likely reflecting changes in diagnostic pathology practice. Increased awareness that many high-grade carcinomas, particularly of the serous subtype, are associated with microscopic intraepithelial lesions in the fallopian tubes (ie, STIC) has led to increased identification of such lesions and a tendency to classify high-stage carcinomas as tubal carcinomas when STIC is present (17).
Previously, pathologists did not scrutinize the fallopian tube for microscopic lesions, and high-grade pelvic cancers were classified as ovarian carcinoma by default. However, implementation of the Sectioning and Extensive Examination of the Fimbria protocol (SEE-FIM) has led to dramatic increases in recognition of STIC (22). Of 231 consecutive high-grade serous carcinomas processed by SEE-FIM, 158 (68.4%) demonstrated STIC (23). Recently, the College of American Pathologists recommended histopathologic examination of all ovarian and tubal tissue from risk-reducing surgeries and examination of the entire fimbria bilaterally in cases of serous carcinoma (24), which will likely be reflected in continued increases in reporting of tubal CIS to cancer registries in coming years. Further, consensus guidelines recommend classifying a carcinoma as a fallopian tube primary when STIC is present, invasive carcinoma involves tubal mucosa, or the tube is incorporated within a tumor mass (25,26). Adoption of these rules would clarify pathology reporting and reduce the frequency with which STIC is diagnosed concurrently or sequentially with other gynecologic cancers of similar morphologic appearance. Ultimately, standardized pathology reporting of STIC and SEE-Fim processing would improve cancer registration, monitoring of incidence trends, and analysis of survival, the latter being important for clinical decision-making.
The American Congress of Obstetricians and Gynecologists recommends that physicians counsel women about salpingectomy when planning hysterectomy without oophorectomy and or laparoscopic sterilization (27). Data indicate that bilateral salpingectomy increased 77% in the United States from 2000 to 2013 and hysterectomy with bilateral salpingectomy increased fourfold from 1998 to 2001 (28). A recent analysis of US hospitalization data spanning the period of increased education about salpingectomy at the time of gynecologic surgery for ovarian cancer risk reduction (2008–2013) found that benign hysterectomy with bilateral salpingectomy (and ovarian preservation) increased from 4717 to 17 350, representing an increase of 371% (28). These changes in frequency of bilateral salpingectomy occurred at a time when the frequency of hysterectomy halved. Accordingly, practice pattern surveys suggest a growing emphasis on detecting tubal primary cancers and searching for tubal origins of high-grade serous carcinoma among women with advanced-stage disease (17). If a substantial percentage of high-grade serous carcinomas originate in the fallopian tube or from benign cells exfoliated from the tube onto the ovary (ie, endosalpingiosis) as proposed, then increased performance of salpingectomy should lower the true incidence of these cancers in the long term and lead to increased recognition of tubal CIS and early-stage cancers in the short term. Further, reported incidence rates of ovarian cancer should fall in the future as more cancers are classified as tubal, although this is not yet apparent because of the relative rarity of tubal cancers reported to date.
The term serous tubal intraepithelial carcinoma (ie, STIC) was coined to reflect the view that such lesions are full-blown carcinomas, even when invasion in the tube is not identified, and as such may spread through exfoliation onto the surface of the ovary or into the peritoneal cavity. This concept raises important questions about staging, management, and genetic testing of women who present with STIC when concurrent carcinoma is not identified. Although limited to 164 patients followed for a median of 7.2 years, our data showed that survival was 96.8% among women age 50 years and older. A literature review of STIC identified 78 cases of STIC without associated foci of cancer, including 67 found at risk-reducing surgery and 11 found incidentally. Over two to 150 months of follow-up, three (4.5%) patients developed recurrent cancer (classified as “primary peritoneal”) (29). Detection of cancer in fluids or biopsies has also been reported among women with an initial diagnosis of STIC alone, and STIC has been implicated as the potential source of 50% of tumors historically classified as primary peritoneal carcinoma (9,30). Thus, improved recording of data for women with STIC is urgently needed to generate refined US population-based estimates of incidence and survival of gynecologic cancers that complement other international efforts like the Pelvic-Ovarian Cancer INTerception (POINT) Project (30).
STIC is found with late-stage high-grade serous carcinomas in 11% to 61% of cases when the tube is extensively scrutinized; however, limited molecular data suggest that STIC is not always the source of carcinomatosis (13). A recent genomic analysis revealed STIC as a precursor of sporadic high-grade serous carcinoma in 50% (four in eight) of tumors but also suggested that 25% (two in eight) of STICs were metastases from other organ sites (eg, endometrium) (31). If corroborated, these data challenge the view that identification of STIC automatically justifies labeling a concurrent cancer as a tubal primary. Development of algorithms to enable registries to record sites and patterns of tumor distributions along with extent of histologic sampling (eg, SEE-Fim processing) could enable registries to generate useful internal data for research that could inform classification of primary site and coding. Increased understanding of the biology of STIC may enable development of evidence-based guidelines in the future, and cancer registries can contribute to this goal with optimized data collection. Our study provides preliminary evidence that survival among women with STIC alone or with concurrent cancer is excellent (>95%); the data are limited by small numbers and limited follow-up.
A strength of the current study is the use of high-quality population-based cancer registry data from 30 registries, which captured a large sample of the US population. This is the first study, to our knowledge, to examine contemporary reporting patterns for tubal CIS. The absence of increasing trends for nonserous histotypes of invasive tubal carcinomas supports the interpretation that our results are specific to serous tubal carcinomas and likely related to increased recognition of the tubal origin of serous ovarian cancers.
Our analysis of incidence and survival was limited by few tubal carcinomas; however, we analyzed the largest available database, which only included results through 2012. If reported rates continue to rise, such analyses will become increasingly tractable. In situ diagnoses of the fallopian tubes are considered reportable diagnoses by the registries, and established coding practices for cancer registration of STIC exist (20). While the increase in reporting captured in the current analysis likely reflects a true increase in reporting tubal CIS (and in recent years the increased utilization of 8441/2 for STIC), this is likely an incomplete representation of the accurate incidence of STIC. The inability to remove women with prior bilateral salpingectomy and/or bilateral oophorectomy from the at-risk population is a weakness of registry-based evaluations of fallopian tube and ovarian carcinoma incidence, which results in an underestimation of rates. Given that a reproducible and validated algorithm for diagnosing STIC was not available until 2011/2012 (32,33), future studies should evaluate whether rate patterns changed circa that period.
In conclusion, all indications are that reporting of tubal carcinomas will increase as a function of reclassification of tumors once classified as ovarian and greater emphasis on examining the fallopian tube and identifying putative early cancers. Given the anticipated changes in practices affecting both true rates and classification, it will be important to develop standardized reporting for tubal neoplasms for cancer surveillance of these highly aggressive cancers.
Funding
This work was supported by the intramural research program of the National Cancer Institute.
Notes
The funding agency did not have any role in design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication. Study results were presented at the American Association of Cancer Research Annual Meeting (April 2017).
References
- 1. Piek JM, van Diest PJ, Zweemer RP et al. , . Dysplastic changes in prophylactically removed fallopian tubes of women predisposed to developing ovarian cancer. J Pathol. 2001;1954:451–456. 10.1002/path.1000 [DOI] [PubMed] [Google Scholar]
- 2. Kurman RJ, Shih I.. Molecular pathogenesis and extraovarian origin of epithelial ovarian cancer—shifting the paradigm. Hum Pathol. 2011;427:918–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Crum CP, McKeon FD, Xian W.. BRCA, the oviduct, and the space and time continuum of pelvic serous carcinogenesis. Int J Gynecol Cancer. 2012;22:S29–S34. [DOI] [PubMed] [Google Scholar]
- 4. Dubeau L, Drapkin R.. Coming into focus: The nonovarian origins of ovarian cancer. Ann Oncol. 2013;24:28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bijron JG, Seldenrijk CA, Zweemer RP et al. , . Fallopian tube intraluminal tumor spread from noninvasive precursor lesions a novel metastatic route in early pelvic carcinogenesis. Am J Surg Pathol. 2013;378:1123–1130. 10.1097/PAS.0b013e318282da7f [DOI] [PubMed] [Google Scholar]
- 6. Sherman ME, Piedmonte M, Mai PL et al. , . Pathologic findings at risk-reducing salpingo-oophorectomy: Primary results from Gynecologic Oncology Group Trial GOG-0199. J Clin Oncol. 2014;3229:3275–3283. 10.1200/JCO.2013.54.1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shaw PA, Rouzbahman M, Pizer ES et al. , . Candidate serous cancer precursors in fallopian tube epithelium of BRCA1/2 mutation carriers. Mod Pathol. 2009;229:1133–1138. 10.1038/modpathol.2009.89 [DOI] [PubMed] [Google Scholar]
- 8. Mingels MJ, Roelofsen T, van der Laak JA et al. , . Tubal epithelial lesions in salpingo-oophorectomy specimens of BRCA-mutation carriers and controls. Gynecol Oncol. 2012;1271:88–93. 10.1016/j.ygyno.2012.06.015 [DOI] [PubMed] [Google Scholar]
- 9. Wethington SL, Park KJ, Soslow RA et al. , . Clinical outcome of isolated serous tubal intraepithelial carcinomas (STIC). Int J Gynecol Cancer. 2013;239:1603–1611. 10.1097/IGC.0b013e3182a80ac8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kindelberger DW, Lee Y, Miron A et al. , . Intraepithelial carcinoma of the fimbria and pelvic serous carcinoma: Evidence for a causal relationship. Am J Surg Pathol. 2007;312:161–169. 10.1097/01.pas.0000213335.40358.47 [DOI] [PubMed] [Google Scholar]
- 11. Roh MH, Kindelberger D, Crum CP.. Serous tubal intraepithelial carcinoma and the dominant ovarian mass: Clues to serous tumor origin? Am J Surg Pathol. 2009;333:376–383. 10.1097/PAS.0b013e3181868904 [DOI] [PubMed] [Google Scholar]
- 12. Tang SG, Onuma K, Deb P et al. , . Frequency of serous tubal intraepithelial carcinoma in various gynecologic malignancies: A study of 300 consecutive cases. Int J Gynecol Pathol. 2012;312:103–110. 10.1097/PGP.0b013e31822ea955 [DOI] [PubMed] [Google Scholar]
- 13. Chen F, Gaitskell K, Garcia MJ et al. , . Serous tubal intraepithelial carcinomas associated with high-grade serous ovarian carcinomas: A systematic review. BJOG. 2017;1246:872–878. 10.1111/1471-0528.14543 [DOI] [PubMed] [Google Scholar]
- 14. Powell CB. Risk reducing salpingo-oophorectomy for BRCA mutation carriers: Twenty years later. Gynecol Oncol. 2014;1322:261–263. 10.1016/j.ygyno.2014.01.018 [DOI] [PubMed] [Google Scholar]
- 15. Rabban JT, Garg K, Crawford B et al. , . Early detection of high-grade tubal serous carcinoma in women at low risk for hereditary breast and ovarian cancer syndrome by systematic examination of fallopian tubes incidentally removed during benign surgery. Am J Surg Pathol. 2014;386:729–742. 10.1097/PAS.0000000000000199 [DOI] [PubMed] [Google Scholar]
- 16. Seidman JD, Krishnan J, Yemelyanova A et al. , . Incidental serous tubal intraepithelial carcinoma and non-neoplastic conditions of the fallopian tubes in grossly normal adnexa: A clinicopathologic study of 388 completely embedded cases. Int J Gynecol Pathol. 2016;355:423–429. 10.1097/PGP.0000000000000267 [DOI] [PubMed] [Google Scholar]
- 17. McCluggage WG, Hirschowitz L, Gilks CB et al. , . The fallopian tube origin and primary site assignment in extrauterine high-grade serous carcinoma: Findings of a survey of pathologists and clinicians. Int J Gynecol Pathol. 2017;363:230–239. 10.1097/PGP.0000000000000336 [DOI] [PubMed] [Google Scholar]
- 18. Stewart SL, Wike JM, Foster SL et al. , . The incidence of primary fallopian tube cancer in the United States. Gynecol Oncol. 2007;1073:392–397. 10.1016/j.ygyno.2007.09.018 [DOI] [PubMed] [Google Scholar]
- 19. Jemal A, Ward EM, Johnson CJ et al. , . Annual report to the nation on the status of cancer, 1975–2014, featuring survival. J Natl Cancer Inst. 2017;1099:djx030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Adamo MB, Johnson CH, Ruhl JL, Dickie LA, eds. 2012 SEER Program Coding and Staging Manual. National Cancer Institute, NIH. Publication number 12-5581. Bethesda, MD: US Department of Health and Human Services; 2012. [Google Scholar]
- 21. Howlader N, Ries LA, Mariotto AB et al. , . Improved estimates of cancer-specific survival rates from population-based data. J Natl Cancer Inst. 2010;10220:1584–1598. 10.1093/jnci/djq366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Medeiros F, Muto MG, Lee Y et al. , . The tubal fimbria is a preferred site for early adenocarcinoma in women with familial ovarian cancer syndrome. Am J Surg Pathol. 2006;302:230–236. 10.1097/01.pas.0000180854.28831.77 [DOI] [PubMed] [Google Scholar]
- 23. Schneider S, Heikaus S, Harter P et al. , . Serous tubal intraepithelial carcinoma associated with extraovarian metastases. Int J Gynecol Cancer. 2017;273:444–451. 10.1097/IGC.0000000000000920 [DOI] [PubMed] [Google Scholar]
- 24. Gilks BC, Movahedi-Lankarani S, Baker PM et al. , . Protocol for the examination of specimens from patients with primary tumors of the ovary or fallopian tube. Northfield, IL: College of American Pathologists (CAP); 2014.
- 25. Singh N, Gilks CB, Hirschowitz L et al. , . Primary site assignment in tubo-ovarian high-grade serous carcinoma: Consensus statement on unifying practice worldwide. Gynecol Oncol. 2016;1412:195–198. 10.1016/j.ygyno.2015.10.022 [DOI] [PubMed] [Google Scholar]
- 26. Singh N, Gilks CB, Wilkinson N et al. , . The secondary Mullerian system, field effect, BRCA, and tubal fimbria: Our evolving understanding of the origin of tubo-ovarian high-grade serous carcinoma and why assignment of primary site matters. Pathology. 2015;475:423–431. 10.1097/PAT.0000000000000291 [DOI] [PubMed] [Google Scholar]
- 27. Committee on Gynecologic Practice. Committee opinion no. 620: Salpingectomy for ovarian cancer prevention. Obstet Gynecol. 2015;1251:279–281. [DOI] [PubMed] [Google Scholar]
- 28. Hicks-Courant KD. Growth in salpingectomy rates in the United States since 2000. Am J Obstet Gynecol. 2016;2155:666–667. 10.1016/j.ajog.2016.07.055 [DOI] [PubMed] [Google Scholar]
- 29. Patrono MG, Iniesta MD, Malpica A et al. , . Clinical outcomes in patients with isolated serous tubal intraepithelial carcinoma (STIC): A comprehensive review. Gynecol Oncol. 2015;1393:568–572. 10.1016/j.ygyno.2015.09.018 [DOI] [PubMed] [Google Scholar]
- 30. Chay WY, McCluggage WG, Lee CH et al. , . Outcomes of incidental fallopian tube high-grade serous carcinoma and serous tubal intraepithelial carcinoma in women at low risk of hereditary breast and ovarian cancer. Int J Gynecol Cancer. 2016;263:431–436. 10.1097/IGC.0000000000000639 [DOI] [PubMed] [Google Scholar]
- 31. Eckert MA, Pan S, Hernandez KM et al. , . Genomics of ovarian cancer progression reveals diverse metastatic trajectories including intraepithelial metastasis to the fallopian tube. Cancer Discov. 2016;612:1342–1351. 10.1158/2159-8290.CD-16-0607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Visvanathan K, Vang R, Shaw P et al. , . Diagnosis of serous tubal intraepithelial carcinoma based on morphologic and immunohistochemical features: A reproducibility study. Am J Surg Pathol. 2011;3512:1766–1775. 10.1097/PAS.0b013e31822f58bc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vang R, Visvanathan K, Gross A et al. , . Validation of an algorithm for the diagnosis of serous tubal intraepithelial carcinoma. Int J Gynecol Pathol. 2012;313:243–253. 10.1097/PGP.0b013e31823b8831 [DOI] [PMC free article] [PubMed] [Google Scholar]