Abstract
Background
Invasive lobular breast cancer (ILC) is the second most common histological subtype of breast cancer after invasive ductal cancer (IDC). Here, we aimed at evaluating the prevalence, levels, and composition of tumor-infiltrating lymphocytes (TILs) and their association with clinico-pathological and outcome variables in ILC, and to compare them with IDC.
Methods
We considered two patient series with TIL data: a multicentric retrospective series (n = 614) and the BIG 02-98 study (n = 149 ILC and 807 IDC). We compared immune subsets identified by immuno-histochemistry in the ILC (n = 159) and IDC (n = 468) patients from the Nottingham series, as well as the CIBERSORT immune profiling of the ILC (n = 98) and IDC (n = 388) METABRIC and The Cancer Genome Atlas patients. All ILC/IDC comparisons were done in estrogen receptor (ER)–positive/human epidermal growth factor receptor 2 (HER2)–negative tumors. All statistical tests were two-sided.
Results
TIL levels were statistically significantly lower in ILC compared with IDC (fold-change = 0.79, 95% confidence interval = 0.70 to 0.88, P < .001). In ILC, high TIL levels were associated with young age, lymph node involvement, and high proliferative tumors. In the univariate analysis, high TIL levels were associated with worse prognosis in the retrospective and BIG 02-98 lobular series, although they did not reach statistical significance in the latter. The Nottingham series revealed that the levels of intratumoral but not total CD8+ were statistically significantly lower in ILC compared with IDC. Comparison of the CIBERSORT profiles highlighted statistically significant differences in terms of immune composition.
Conclusions
This study shows differences between the immune infiltrates of ER-positive/HER2-negative ILC and IDC in terms of prevalence, levels, localization, composition, and clinical associations.
The presence and potential clinical value of tumor-infiltrating lymphocytes (TILs) have been assessed in many studies. Increased lymphocytic infiltration has been shown to be more frequent in triple-negative and human epidermal growth factor receptor 2 (HER2)–positive breast cancer and to be associated with an increased pathological complete response rate after neo-adjuvant chemotherapy, as well as with improved survival after adjuvant chemotherapy in these subtypes (1–3). Given that various methods have been used to quantify TILs in breast cancer, efforts to standardize their assessment were undertaken, resulting in the first guidelines for scoring TILs (4). A recent publication also showed that the assessment of stromal TILs, those present in the tumor stromal tissue, is more reproducible than intratumoral TIL evaluation (5). A complementary approach to evaluate immune infiltrates is to apply a deconvolution algorithm to gene expression data, such as CIBERSORT, which estimates the relative proportion of various immune cell types (6–8).
Invasive lobular breast cancer (ILC) represents the second most common histological subtype in breast cancer after invasive breast carcinoma of no special type (NST), previously known and further referred to as invasive ductal carcinoma (IDC), and accounts for up to 15% of cases (9). These tumors generally express estrogen receptor (ER) and lack HER2 amplification. Clinically, patients with ILC differ from those with IDC. They tend to relapse later and present a different metastatic pattern (9,10). Recently, The Cancer Genome Atlas (TCGA), the RATHER consortium, METABRIC, and our group characterized the genomic landscape of a large number of ILC tumors and consistently demonstrated that ILC presents differences regarding the frequency of alterations in several cancer genes compared with IDC (11–14). Of potential relevance, TCGA and RATHER consortia also identified an “immune-related” ILC transcriptomic subtype. However, when comparing these immune subtypes on the same data sets, it seems that they were not identifying the same tumors, suggesting that their definition should be further investigated (11,12).
While TILs in breast cancer continue to attract considerable attention, to our knowledge, there are no previous reports interrogating TILs in large cohorts of ILC. Using our multicentric retrospective cohort of ILC (13), we aimed to evaluate the distribution of TILs, correlate TIL levels with standard clinico-pathological parameters, investigate their prognostic value, and explore associations between TILs and transcriptomic features and recurrent genomic alterations in ILC. We further aimed to compare TILs between ER-positive/HER2-negative ILC and IDC tumors in the BIG 02-98 study (15). Finally, going beyond TILs to further enhance our understanding of the immune infiltrates in ER-positive/HER2-negative ILC and IDC tumors, we compared the proportion and localization of several immune subsets in the Nottingham series (16–18) and the estimates of the proportion of the different immune cell types provided by CIBERSORT in the METABRIC and TCGA cohorts (7,11,19).
Methods
Patients and Samples
The retrospective multicentric series of primary ILC has already been described (13). Supplementary Tables 1 and 2 (available online) provide overview and patient-level information, respectively, of the main clinical characteristics while Supplementary Figure 1 (available online) reports the number of patients and samples considered for each analysis. The comparison of TILs in terms of prevalence and clinico-pathological associations between ER-positive/HER2-negative ILC (n = 149) and IDC (n = 807) was assessed in the BIG 02-98 study (Supplementary Table 3, available online) (15). From this study, we only considered those patients whose tumor samples underwent central revision for ER and HER2 (20). For the Nottingham series, we only considered the ER-positive/HER2-negative ILC (n = 159) and IDC (n = 468) cases (16–18). The patient and tumor characteristics from all investigated ER-positive/HER2-negative ILC and IDC cases are reported in Supplementary Table 4 (available online). This project has been approved by the ethics committee of the Institut Jules Bordet (CE1726 and CE2560).
Assessment of Immune Infiltration
Stromal TIL, the percentage of tumor stromal area containing TILs and hereinafter referred to as “TIL,” was independently evaluated by three pathologists (GVE, GP, and RS) on hematoxylin and eosin–stained slides of the retrospective ILC cohort (n = 614) using the protocols described in Salgado et al. (4). To evaluate the reliability of TIL assessments, the two-way random intraclass correlation coefficient (ICC) was estimated (21). All cases for which the discordance between at least two pathologists reached greater than 15% were first independently rescored by all three pathologists and then reviewed together until a consensus was reached. For each pathologist, consensus values were used to recalibrate the other measurements through Passing-Bablok regression (22). The log transformation was applied to TIL values in order to stabilize variance and achieve an approximately symmetric distribution according to the measurement process (23). In the analyses, the arithmetic mean over the three pathologists of the log values was used. In the BIG 02-98 study, TILs were assessed previously by two pathologists, including RS, using the same method and the results described in Loi et al. (15). For this study, we considered the arithmetic mean of the log values from the two pathologists.
Statistical Analysis
Univariate and multiple linear regression models of log-transformed TIL percentage vs clinico-pathological and genomic variables were fitted. Point estimates and 95% confidence intervals of fold-change of TIL percentage were obtained. In the retrospective ILC cohort and the BIG2-98 series, we used the previously reported survival end points, namely breast cancer–free interval (BCFI) (13) and disease-free survival (DFS) (15), respectively. The association between the survival end points and the immune variables was evaluated using a Cox regression model by considering TILs as either continuous or categorical values, with or without adjustment for clinico-pathological variables. Statistical significance was assessed using the Wald test. For the comparison of the various immune markers in the Nottingham series, we recurred to the proportional odds logistic model extension of the Mann-Whitney test according to Harrell (24) to adjust for histology, age, grade, axillary lymph node status, and Ki67. CIBERSORT data from the METABRIC and TCGA series were obtained from Ali et al. (n = 98 ILC and n = 388 IDC) (7) and compared using the Wilcoxon rank sum test. All statistical tests were two-sided, and P values of less than .05 were considered statistically significant. Whenever appropriate, we applied correction for multiple testing using the Benjamini-Hochberg method (25). All analyses were performed using R v3.4.1 (26).
Results
Distribution of TILs
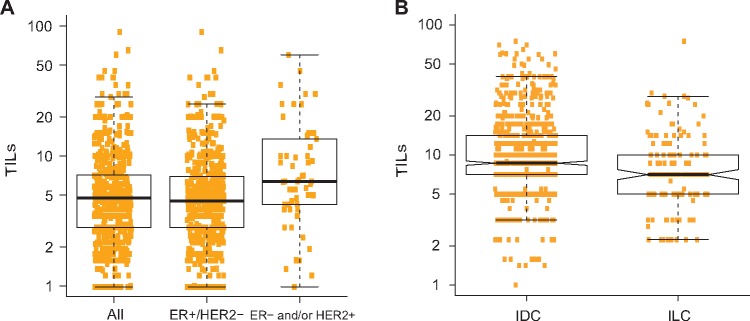
The intraclass correlation coefficient between the three pathologists for TIL evaluation on the 614 ILC cases from the multicentric retrospective series was 0.71 (95% confidence interval [CI] = 0.65 to 0.76). ILC was characterized by low lymphocytic infiltration, with a median percentage of TILs of 5% and an interquartile range of 3% to 7% (Figure 1A). Fifteen percent of the samples had more than 10% of TIL. We compared the distribution of TILs in ER-positive/HER2-negative ILC with ER-positive/HER2-negative IDC tumors from the BIG 02-98 study (15) and observed that TIL counts were slightly but statistically significantly lower in ILC compared with IDC (fold-change = 0.79, 95% CI = 0.70 to 0.88, P < .001) (Figure 1B).
Figure 1.
Distribution of tumor-infiltrating lymphocytes (TILs). Histograms displaying the distribution of the TIL counts in all invasive lobular carcinoma (ILC) cases, and only in the estrogen receptor (ER)–positive/human epidermal growth factor receptor 2 (HER2)–negative ones (A), as well as the distribution of ER-positive/HER2-negative ILC and invasive ductal carcinoma cases from the BIG 02-98 study (B). ER = estrogen receptor; IDC = invasive ductal carcinoma; ILC = invasive lobular carcinoma; TIL = tumor-infiltrating lymphocyte.
Association Between TILs and Clinico-Pathological Variables
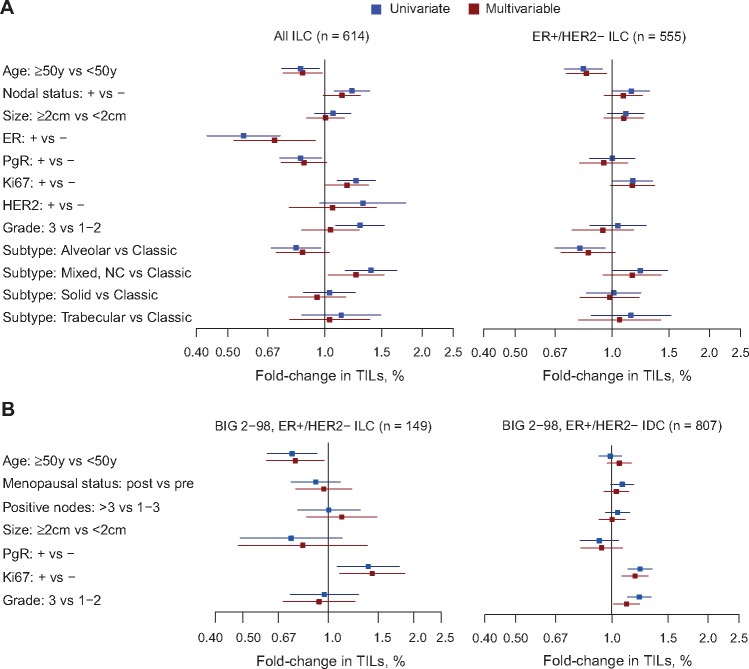
We investigated the association between TILs as a continuous variable and the standard clinico-pathological variables. In the retrospective series, we observed at the univariate level that higher TIL values were associated with ER- and PR-negative status, high proliferation status (≥20% Ki67), high grade, node-positive status, and young age at diagnosis (Figure 2A;Supplementary Table 5, available online). We further observed a statistically significant increase and decrease of TILs for the mixed nonclassic and the alveolar subtypes, respectively, compared with the more common classic subtype. In the multivariable regression, a similar trend was detected for these variables, with the exception of tumor grade, for which the association was no longer statistically significant. The associations between TILs and age, as well as Ki67 in all ILC patients from BIG 02-98 (n = 204), were consistent with our results (Supplementary Figure 2 and Supplementary Table 6, available online). The association with lymph node involvement could not be investigated as all patients included in this trial had positive lymph nodes. When restricting the analyses to the ER-positive/HER2-positive ILC in our cohort (n = 555), we made similar observations as in the global ILC population regarding the association with high Ki67, node-positive status, and young age at diagnosis (Figure 2A;Supplementary Table 7, available online). We next used the TIL data collected in the BIG 02-98 trial to compare the associations in ER-positive/HER2-negative IDC (n = 807) and ILC (n = 149). TILs were positively associated with Ki67 both in IDC and ILC. However, the associations with age and with grade were restricted to ILC and IDC, respectively (Figure 2B;Supplementary Tables 8 and 9, available online).
Figure 2.
Associations between tumor-infiltrating lymphocytes and clinico-pathological variables. Univariate and multivariable associations between tumor-infiltrating lymphocyte and clinico-pathological variables in all invasive lobular carcinoma (ILC) cases and only the estrogen receptor (ER)–positive/human epidermal growth factor receptor 2 (HER2)–negative from our series (A), as well as from the ER-positive/HER2-negative ILC and invasive ductal carcinoma (B) from the BIG 02-98 study. ER = estrogen receptor; IDC = invasive ductal carcinoma; ILC = invasive lobular carcinoma; TIL = tumor-infiltrating lymphocyte.
Association Between TILs and Genomic Alterations
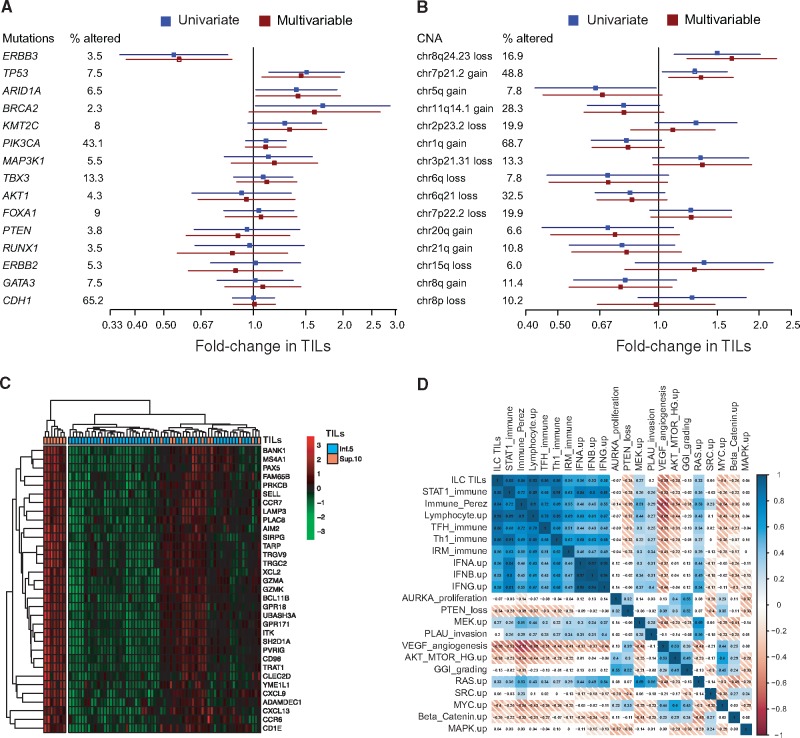
Genomic alterations with oncogenic potential (substitutions and indels as previously defined (27) were available for 399 patients of the multicentric retrospective ILC series (13), leading us to evaluate the association between TILs and the mutational status of the 15 genes that were mutated in at least 2% of the tumors. We observed statistically significantly lower TIL counts in tumors harboring ERBB3 mutations and higher TIL counts in tumors presenting somatic oncogenic TP53, ARID1A, and BRCA2 mutations compared with tumors that were not mutated in those genes. KMT2C and PIK3CA mutated tumors were associated with higher TIL, although not statistically significantly (Figure 3A;Supplementary Table 10, available online). All associations remained statistically significant in multivariable models with standard clinico-pathological variables, with the exception of BRCA2. We further investigated the association between TILs and copy number aberrations (CNAs) present in at least 5% of the patients. Genome-wide copy number data were available for 166 cases. We observed statistically significantly higher TIL values in tumors with 8q24.23 loss (which included PTK2) and 7p21.2 gains (ETV1), but statistically significantly fewer TILs in tumors with 5q gains (Figure 3B;Supplementary Table 11, available online).
Figure 3.
Associations between tumor-infiltrating lymphocytes (TILs), genomic and transcriptomic features in the retrospective invasive lobular carcinoma (ILC) cohort. Univariate and multivariable associations between TIL and recurrently mutated genes (n = 399) (A) or recurrent copy number aberrations (n = 166) (B). Heatmap of the genes statistically significantly associated with TILs at the transcriptomic level (C). Correlation matrix of the lobular immune gene expression signature (ILC TIL) with published immune signatures (D). CNA = copy number aberration; ILC = invasive lobular carcinoma; TIL = tumor-infiltrating lymphocyte.
Association Between TILs and Transcriptomic Features
In order to better understand the transcriptomic phenotype associated with infiltrated ILC tumors, we compared the gene expression profiles between ILC tumors with low (≤5%) and high TIL levels (>10%), microarray gene expression data being available for 117 tumors of the multicentric ILC cohort. Thirty-three genes were overexpressed in the high-TIL group with a fold-change of 1.2 or greater compared with the low-TIL group, and a corrected P value of .05 or less. This list included, among others, genes involved in cellular defense response (GO:0006968; ITK, CXCL9, TRAT1, SH2D1A, and CCR6) and regulation of antigen receptor–mediated signaling pathway (GO:0050854; PRKCB, TRAT1, CCR7, UBASH3A) (Figure 3C). We then computed a score based on these 33 genes and compared it with existing immune and other gene expression signature scores (28–37). We observed a strong correlation with several previously reported immune expression signatures (Figure 3D), suggesting that the immune infiltrates present in ILC might share at least some similarities with the ones found in the global breast cancer population.
Comparison of the Prevalence and Localization of Different Immune Cell Types Between ER-Positive/HER2-Negative ILC and IDC
We further aimed at extending our immune comparison between ER-positive/HER2-negative ILC and IDC beyond TIL counts by interrogating immuno-histochemical data on various immune markers according to their localization (total staining or staining restricted to the intratumoral, adjacent, or distant stromal compartments) in the Nottingham series (16–18), and the CIBERSORT immune profiles in the METABRIC and TCGA data sets (7).
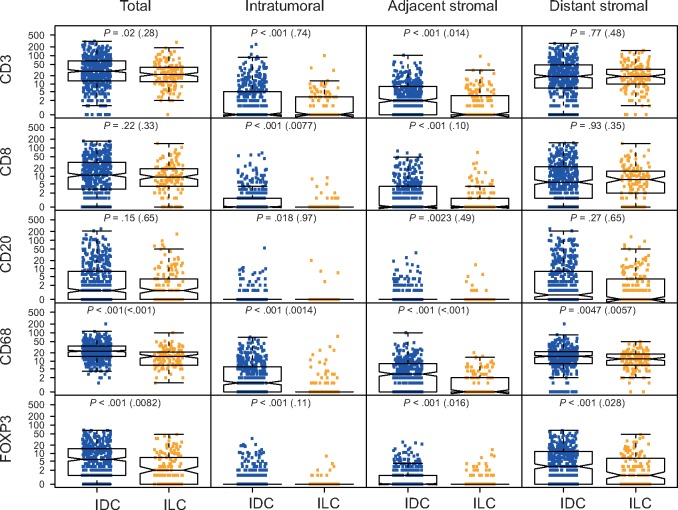
We compared the scores for CD3+, CD8+, CD20+, CD68+, and FOXP3+ cells between the 159 ILC and 468 IDC samples from the Nottingham series (Figure 4A). After adjusting for standard clinico-pathological variables, we observed statistically significantly lower levels in ILC compared with IDC regarding the adjacent stromal CD3+, the intratumoral CD8+, all measures of CD68+, and all except the intratumoral measures of FOXP3+ cells. To complement this analysis, we compared the relative proportion of the various immune cell types recognized by CIBERSORT (7) and reported the statistically significant associations in Supplementary Figure 4 (available online). Importantly, as in Ali et al. (7), we considered only samples with a CIBERSORT P value of less than .05, excluding samples without immune infiltration, leaving 388 ER-positive/HER2-negative IDC samples to the 98 ER-positive/HER2-negative ILC samples. The comparison revealed a lower frequency of follicular helper and gamma delta T cells, as well as M0, M1, and to a lesser extent M2 macrophages in ILC compared with IDC, but a higher frequency of B memory cells, monocytes, and CD8+ T cells. Unfortunately, these results do not provide information regarding the localization of these different immune cell types as CIBERSORT derives the immune subtyping from bulk transcriptomic data.
Figure 4.
Comparison of the expression of various immune cell types between estrogen receptor (ER)–positive/human epidermal growth factor receptor 2 (HER2)–negative invasive lobular carcinoma. Distribution of the expression of the various immune markers assessed by immunohistochemistry in the Nottingham cohort according to the histology and their localization. For each comparison, the value in parentheses refers to the multivariable-adjusted P value from the proportional odds logistic model extension of the Mann-Whitney test according to Harrell (24) with the following covariates: histological subtype, age, grade, axillary lymph node, and Ki67 status. ER = estrogen receptor; ILC = invasive lobular carcinoma.
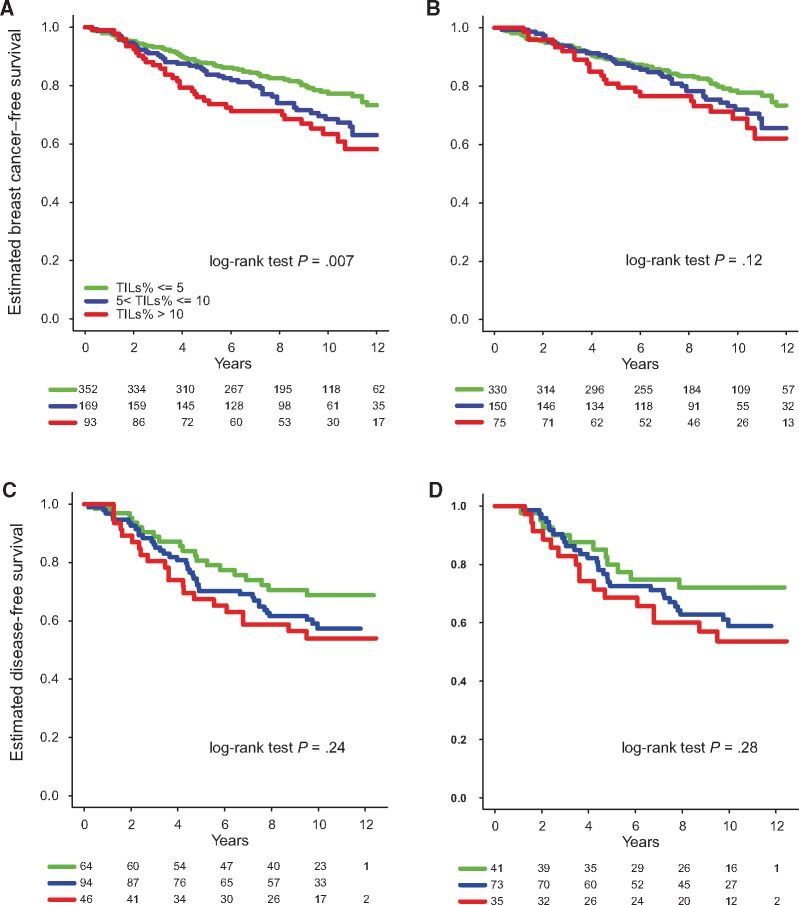
Association Between TILs and Survival
Finally, we assessed the prognostic value of TILs in ILC. TILs were initially considered a continuous variable for survival analysis, revealing that higher TILs were associated with a worse prognosis in ILC in univariate (HR = 1.22 for each 10% increment, 95% CI = 1.06 to 1.41, P = .005) but not multivariable analysis in the retrospective cohort (Supplementary Table 12, available online). TILs were next considered a categorical variable using the following groups, with the cut-points adapted from the interquartile range rounded to the nearest multiple of 5: low (≤5%), intermediate (>5 and ≤10%), and high (>10%). Both the high and intermediate groups were associated with worse breast cancer–free interval (BCFI) compared with the lowest TIL values (HRhigh vs low = 1.84, 95% CI = 1.22 to 2.78, P = .004; HRintermediate vs low = 1.46, 95% CI = 1.02 to 2.09, P = .04) (Figure 5A). In multivariable analysis adjusting for the standard clinico-pathological variables, the association between BCFI and TILs in the group with high TIL values lost statistical significance whereas the association remained statistically significant for the group with intermediate TILs (Supplementary Table 13, available online). Exploratory analyses in the ER-positive/HER2-negative subgroup revealed a biologically consistent but statistically nonsignificant difference between the high and low groups (HR = 1.58, 95% CI = 0.98 to 2.54, P = .06) (Supplementary Table 14, available online), while no statistically significant difference was observed between the TIL groups in the ER-negative and/or HER2-positive subgroup (Figure 5B;Supplementary Figure 3A and Supplementary Table 15, available online). We further assessed the prognostic value of TILs in the BIG 02-98 series, and, similar to the retrospective cohort, we observed that higher TILs were associated with worse prognosis at the univariate level, although not reaching statistical significance (Figure 5C;Supplementary Table 16, available online). While the separation of the curves was visible for the ER-positive/HER2-negative ILC subgroup (Figure 5D;Supplementary Table 17, available online), the curves were completely superimposed for the ER-positive/HER2-negative IDC (Supplementary Figure 3B and Supplementary Table 18, available online).
Figure 5.
Association between tumor-infiltrating lymphocytes (TILs) and survival. Kaplan-Meier curves displaying the estimated breast cancer–free interval probability according to the TIL group (low: ≤5%, intermediate: >5 and ≤10%, high: >10%) in all invasive lobular carcinomas (ILCs) (A) and only the estrogen receptor (ER)–positive/human epidermal growth factor receptor 2 (HER2)–negative ILC of the retrospective series (B). Kaplan-Meier curves displaying the estimated disease-free survival probability according to the TIL group in all ILCs (C) and the only the ER-positive/HER2-negative ILC (D) from the BIG 02-98 series. All statistical tests were two-sided. ER = estrogen receptor; ILC = invasive lobular carcinoma; TIL = tumor-infiltrating lymphocyte.
Discussion
This work represents to our knowledge the most detailed and comprehensive assessment of TILs using standardized, reproducible protocols and the largest series of primary ILC samples to date. We demonstrated that most ILCs are characterized by low levels of TILs. The vast majority of ILC tumors (∼90%) are ER-positive/HER2-negative, making our findings consistent with previous reports showing that TILs are more prevalent in triple-negative and HER2-positive breast cancer (15,38,39). Droeser et al. reported lower levels of lymphocytic infiltrates in ILC compared with IDC, based on the percentage of CD4+ immunohistochemistry-stained cells; however, this comparison was flawed because they included all IDC and ILC tumors irrespective of ER and HER2 status (40). Our comparison of ER-positive/HER2-negative ILC and IDC in the BIG 02-98 study, however, confirmed the slight but statistically significant increase in TILs in IDC, even after adjusting for clinico-pathological variables.
Several important findings emerged when comparing the associations between TILs, clinico-pathological variables, and survival between ER-positive/HER2-negative ILC and IDC. First, while high TIL levels were associated with high proliferation status as defined by Ki67 in both histological subtypes, the association of young age and high grade with high TIL levels was only seen in ILC and IDC, respectively. Second, the survival analyses in our retrospective ILC cohort revealed that increased TILs were associated with worse BCFI at the univariate level. This was the case when TILs were assessed either as a continuous or categorical variable. This observation was somehow contrasting to what has been reported in triple-negative and HER2-positive breast cancer, where increased TILs were associated with better prognosis (1,2). However, in the multivariable analysis, TILs lost their prognostic value at the continuous level, although in the categorical analysis, the group with intermediate TIL values remained associated with worse prognosis compared with the group with low TILs. This loss of prognostic association, once adjusted for the standard clinico-pathological variables, might be explained by the association of TILs with poor prognostic features. Of interest, in the BIG 02-98 study, we observed at the univariate level that high TILs were associated with worse prognosis in ILC, although without reaching statistical significance. Interestingly, in this study, TILs did not show any association with survival in ER-positive/HER2-negative IDC.
When we compared the immuno-histochemical scores (total, intratumoral, adjacent stromal, and distant stromal) of the CD3+, CD4+, CD8+, CD68+, and FOXP3+ cells on the Nottingham series between these two main breast cancer histologies, we noticed that while no difference was observed for total CD8+ levels, intratumoral CD8+ levels were statistically significantly lower in ER-positive/HER2-negative ILC compared with IDC, with very rare ILC cases presenting with intratumoral CD8+ cells, suggesting that ILC might present an immune-excluded phenotype where immune cells are retained in the stroma, therefore limiting cancer immunity. As tumors with intratumoral CD8+ cells have been associated with the best outcome (41), ILC tumors with high TIL levels might be associated with a worse prognosis because of the lack of intratumoral CD8+ cells. While the initial transcriptomic comparison between the less and highly infiltrated groups showed similarities with previously reported immune signatures, the CIBERSORT analyses further revealed statistically significant differences in immune composition between infiltrated ER-positive/HER2-negative IDC and ILC tumors. Whether the strong underrepresentation of M1 macrophages or follicular helper T cells in ILC compared with IDC could further explain our prognostic observations needs to be further investigated.
A unique advantage of our retrospective cohort was the ability to investigate the association between genomic alterations and TILs. Tumors presenting ARID1A, BRCA2, and TP53 mutations, as well as 8q24.23 loss and 7p21.2 gains, were associated with higher TIL counts. Although we could not find previous evidence for the association of ARID1A mutations and TILs in breast cancer, a study of hepatocellular carcinoma demonstrated that hepatocyte-specific deficiency of ARID1A leads to the infiltration of innate immune cells (42). Similarly, in colorectal cancer, ARID1A mutations are known to be more frequent in microsatellite-instable tumors, and these tumors are also associated with higher immune expression scores (43). It has to be noted, however, that ARID1A mutations were previously demonstrated to be enriched in ER-positive/HER2-negative ILC (∼6%) compared with ER-positive/HER2-negative IDC (<2%) (13). Regarding BRCA2, our observation is in line with the study of Bane et al., who reported that BRCA2-mutated tumors exhibited lymphocytic infiltration at the periphery of the tumor (44). While TP53 mutations have been shown to be associated with higher levels of TILs in ovarian cancer (45), lymphocytic infiltration in basal-like breast cancer has only recently been reported to be associated with the retention of wild-type TP53 (46). While some evidence is now beginning to accumulate pertaining to the clinical utility of mutation burden, the role of TILs and genomic alterations as potential biomarkers for immunotherapy needs to be further investigated.
We recognize that this study has limitations, which are mainly due to its retrospective nature and the clinical and pathological heterogeneity of the cohorts that were analyzed. Nevertheless, this study represents, to the best of our knowledge, the largest study investigating immune infiltration in ILC.
To conclude, we have shown, in a large cohort and using a standardized methodology for TIL assessment, that most ILCs were characterized by generally low but variable levels of lymphocytic infiltration. We further demonstrated that higher TIL levels were associated with worse prognosis in ILC, but only at the univariate level. The association of TILs with outcome in ER-positive/HER2-negative breast cancer patients should be further investigated in additional large trials to understand whether the association is limited to ILC or not, and whether high lymphocytic infiltration could drive endocrine resistance as previously suggested (47). We further highlighted several differences in terms of immune composition and localization between ILC and IDC, suggestive of an immune-exclusion phenotype. Finally, we showed that higher TIL levels were found in association with specific mutations and copy number alterations. Overall, these observations suggest that that immune infiltration could play a different role in ILC compared with IDC. Further research is needed to understand whether and how immunomodulators could reestablish and promote the antitumor immune response in ILC.
Funding
This work was supported by Susan Komen for the Cure, Fondation MEDIC, Les Amis de Bordet (CD), Fonds National de Recherche Scientifique (DB and CS), the Cancer Council Victoria John Colebatch fellowship (SL), the Breast Cancer Research Foundation, the Italian Association for Leukemia and Lymphoma, and the Italian Association for Cancer Research. We thank the Nottingham Health Science Biobank and Breast Cancer Now Tissue Bank, as well as the bio-banks from the Institut Jules Bordet, the European Institute of Oncology, the Sint Augustinus Hospital, the Cliniques Universitaires de Saint Luc, and the Institut Paoli-Calmettes for the provision of tissue samples. BIG 2-98 was conducted under the umbrella of the Breast International Group, with sponsorship and funding provided by Sanofi.
Notes
The funders had no role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
Part of this work has been presented orally at the annual San Antonio Breast Cancer Symposium in December 2015 (Desmedt et al. S1-02).
Drs Desmedt, Biganzoli, and Sotiriou had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Drs Biganzoli and Sotiriou are joint senior authors.
Author contributions: study concept and design: Desmedt, Biganzoli, Sotiriou; acquisition, analysis, or interpretation of data: all authors; drafting of the manuscript: Desmedt, Biganzoli, Sotiriou; critical revision of the manuscript for important intellectual content: all authors; statistical analysis: Fornili, Bareche, Biganzoli; obtained funding: Desmedt, Sotiriou; administrative, technical, or material support: Garaud, Rouas; study supervision: Desmedt, Biganzoli, Sotiriou.
SL received contracted research funding directly to her institute from Novartis, Pfizer, Merck, Genentech/Roche, Puma Biotechnology, and Bristol-Myers Squibb. No disclosures are reported for the other authors.
Supplementary Material
References
- 1. Savas P, Salgado R, Denkert C et al. , . Clinical relevance of host immunity in breast cancer: From TILs to the clinic. Nat Rev Clin Oncol. 2015;134:228–241. 10.1038/nrclinonc.2015.215 [DOI] [PubMed] [Google Scholar]
- 2. Pruneri G, Vingiani A, Denkert C.. Tumor infiltrating lymphocytes in early breast cancer. The Breast. 2018;37:207–214. [DOI] [PubMed] [Google Scholar]
- 3. Stanton SE, Adams S, Disis ML.. Variation in the incidence and magnitude of tumor-infiltrating lymphocytes in breast cancer subtypes. JAMA Oncol. 2016;210:1354–1360. 10.1001/jamaoncol.2016.1061 [DOI] [PubMed] [Google Scholar]
- 4. Salgado R, Denkert C, Demaria S et al. , . The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: Recommendations by an International TILs Working Group 2014. Ann Oncol. 2014;262:259–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Buisseret L, Desmedt C, Garaud S et al. , . Reliability of tumor-infiltrating lymphocyte and tertiary lymphoid structure assessment in human breast cancer. Mod Pathol. 2017; 309:1204–1212. 10.1038/modpathol.2017.43 [DOI] [PubMed] [Google Scholar]
- 6. Newman AM, Liu CL, Green MR et al. , . Robust enumeration of cell subsets from tissue expression profiles. Nat Methods. 2015;125:453–457. 10.1038/nmeth.3337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ali HR, Chlon L, Pharoah PDP, Markowetz F, Caldas C.. Patterns of immune infiltration in breast cancer and their clinical implications: A gene-expression-based retrospective study. PLoS Med. 2016:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bense RD, Sotiriou C, Piccart-Gebhart MJ et al. , . Relevance of tumor-infiltrating immune cell composition and functionality for disease outcome in breast cancer. J Natl Cancer Inst. 2017;1091:djw192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guiu S, Wolfer A, Jacot W et al. , . Invasive lobular breast cancer and its variants: How special are they for systemic therapy decisions? Crit Rev Oncol Hematol. 2014;923:235–257. 10.1016/j.critrevonc.2014.07.003 [DOI] [PubMed] [Google Scholar]
- 10. Pestalozzi BC, Zahrieh D, Mallon E et al. , . Distinct clinical and prognostic features of infiltrating lobular carcinoma of the breast: Combined results of 15 International Breast Cancer Study Group clinical trials. J Clin Oncol. 2008;2618:3006–3014. 10.1200/JCO.2007.14.9336 [DOI] [PubMed] [Google Scholar]
- 11. Ciriello G, Gatza ML, Beck AH et al. , . Comprehensive molecular portraits of invasive lobular breast cancer. Cell. 2015;1632:506–519. 10.1016/j.cell.2015.09.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Michaut M, Chin SF, Majewski I et al. , . Integration of genomic, transcriptomic and proteomic data identifies two biologically distinct subtypes of invasive lobular breast cancer. Sci Rep. 2016;6:18517. 10.1038/srep18517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Desmedt C, Zoppoli G, Gundem G et al. , . Genomic characterization of primary invasive lobular breast cancer. J Clin Oncol. 2016;3416:1872–1881. 10.1200/JCO.2015.64.0334 [DOI] [PubMed] [Google Scholar]
- 14. Pereira B, Chin SF, Rueda OM et al. , . The somatic mutation profiles of 2,433 breast cancers refines their genomic and transcriptomic landscapes. Nat Commun. 2016;7:11479. 10.1038/ncomms11479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Loi S, Sirtaine N, Piette F et al. , . Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol. 2013;317:860–867. 10.1200/JCO.2011.41.0902 [DOI] [PubMed] [Google Scholar]
- 16. Mahmoud SM, Lee AH, Paish EC, Macmillan RD, Ellis IO, Green AR.. The prognostic significance of B lymphocytes in invasive carcinoma of the breast. Breast Cancer Res Treat. 2011;1322:545–553. [DOI] [PubMed] [Google Scholar]
- 17. Mahmoud SM, Lee AH, Paish EC, Macmillan RD, Ellis IO, Green AR.. Tumour-infiltrating macrophages and clinical outcome in breast cancer. J Clin Pathol. 2011;652:159–163. [DOI] [PubMed] [Google Scholar]
- 18. Mahmoud SM, Paish EC, Powe DG et al. , . An evaluation of the clinical significance of FOXP3+ infiltrating cells in human breast cancer. Breast Cancer Res Treat. 2011;1271:99–108. [DOI] [PubMed] [Google Scholar]
- 19. Curtis C, Shah SP, Chin SF et al. , . The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature. 2012;4867403:346–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sonnenblick A, Francis PA, Azim HA Jr. et al. , . Final 10-year results of the Breast International Group 2-98 phase III trial and the role of Ki67 in predicting benefit of adjuvant docetaxel in patients with oestrogen receptor positive breast cancer. Eur J Cancer. 2015;5112:1481–1489. 10.1016/j.ejca.2015.03.018 [DOI] [PubMed] [Google Scholar]
- 21. Shrout PE FJL. Intraclass correlations: Uses in assessing rater reliability. Psychol Bull. 1979;862:420–428. 10.1037/0033-2909.86.2.420 [DOI] [PubMed] [Google Scholar]
- 22. Passing H, Bablok. A new biometrical procedure for testing the equality of measurements from two different analytical methods. Application of linear regression procedures for method comparison studies in clinical chemistry, part I. J Clin Chem Clin Biochem. 1983;2111:709–720. [DOI] [PubMed] [Google Scholar]
- 23. Box GE CD. An analysis of transformations. J R Stat Soc Ser B. 1964:211–252. [Google Scholar]
- 24. Harrell FE. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. Heidelberg, Germany: Springer; 2001. [Google Scholar]
- 25. Benjamini Yosef YH. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc Ser B. 1995;571:289–300. [Google Scholar]
- 26. R Core Team. R: A Language and Environment for Statistical Computing. R Found Stat Comput Vienna, Austria. 2017. http://www.R-project.org/.
- 27. Desmedt C, Fumagalli D, Pietri E et al. , . Uncovering the genomic heterogeneity of multifocal breast cancer. J Pathol. 2015;2364:457–466. 10.1002/path.4540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Desmedt C, Haibe-Kains B, Wirapati P et al. , . Biological processes associated with breast cancer clinical outcome depend on the molecular subtypes. Clin Cancer Res. 2008;1416:5158–5165. 10.1158/1078-0432.CCR-07-4756 [DOI] [PubMed] [Google Scholar]
- 29. Teschendorff AE, Miremadi A, Pinder SE, Ellis IO, Caldas C.. An immune response gene expression module identifies a good prognosis subtype in estrogen receptor negative breast cancer. Genome Biol. 2007;88:R157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bild AH, Yao G, Chang JT et al. , . Oncogenic pathway signatures in human cancers as a guide to targeted therapies. Nature. 2006;4397074:353–357. 10.1038/nature04296 [DOI] [PubMed] [Google Scholar]
- 31. Saal LH, Johansson P, Holm K et al. , . Poor prognosis in carcinoma is associated with a gene expression signature of aberrant PTEN tumor suppressor pathway activity. Proc Natl Acad Sci U S A. 2007;10418:7564–7569. 10.1073/pnas.0702507104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Majumder PK, Febbo PG, Bikoff R et al. , . mTOR inhibition reverses Akt-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways. Nat Med. 2004;106:594–601. 10.1038/nm1052 [DOI] [PubMed] [Google Scholar]
- 33. Creighton CJ, Hilger AM, Murthy S, Rae JM, Chinnaiyan AM, El-Ashry D.. Activation of mitogen-activated protein kinase in estrogen receptor alpha-positive breast cancer cells in vitro induces an in vivo molecular phenotype of estrogen receptor alpha-negative human breast tumors. Cancer Res. 2006;667:3903–3911. [DOI] [PubMed] [Google Scholar]
- 34. Creighton CJ. A gene transcription signature of the Akt/mTOR pathway in clinical breast tumors. Oncogene. 2007;2632:4648–4655. 10.1038/sj.onc.1210245 [DOI] [PubMed] [Google Scholar]
- 35. Der SD, Zhou A, Williams BRG, Silverman RH.. Identification of genes differentially regulated by interferon alpha, beta, or gamma using oligonucleotide arrays. Proc Natl Acad Sci U S A. 1998;9526:15623–15628. 10.1073/pnas.95.26.15623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gu-Trantien C, Loi S, Garaud S et al. , . CD4(+) follicular helper T cell infiltration predicts breast cancer survival. J Clin Invest. 2013;1237:2873–2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Alexe G, Dalgin GS, Scanfeld D et al. , . High expression of lymphocyte-associated genes in node-negative HER2+ breast cancers correlates with lower recurrence rates. Cancer Res. 2007;6722:10669–10676. [DOI] [PubMed] [Google Scholar]
- 38. Ali HR, Provenzano E, Dawson SJ et al. , . Association between CD8+ T-cell infiltration and breast cancer survival in 12,439 patients. Ann Oncol. 2014;258:1536–1543. [DOI] [PubMed] [Google Scholar]
- 39. Yamaguchi R, Tanaka M, Yano A et al. , . Tumor-infiltrating lymphocytes are important pathologic predictors for neoadjuvant chemotherapy in patients with breast cancer. Hum Pathol. 2012;4310:1688–1694. 10.1016/j.humpath.2011.12.013 [DOI] [PubMed] [Google Scholar]
- 40. Droeser R, Zlobec I, Kilic E et al. , . Differential pattern and prognostic significance of CD4+, FOXP3+ and IL-17+ tumor infiltrating lymphocytes in ductal and lobular breast cancers. BMC Cancer. 2012;12:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ali HR, Provenzano E, Dawson SJ et al. , . Association between CD8+ T-cell infiltration and breast cancer survival in 12 439 patients. Ann Oncol. 2014;258:1536–1543. [DOI] [PubMed] [Google Scholar]
- 42. Fang JZ, Li C, Liu XY, Hu TT, Fan ZS, Han ZG.. Hepatocyte-specific arid1a deficiency initiates mouse steatohepatitis and hepatocellular carcinoma. PLoS One. 2015;1011:e0143042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mlecnik B, Angell HK, Maby P et al. , . Integrative analyses of colorectal cancer show immunoscore is a stronger predictor of patient survival than microsatellite instability. Cell. 2016;443:698–711. [DOI] [PubMed] [Google Scholar]
- 44. Bane AL, Beck JC, Bleiweiss I et al. , . BRCA2 mutation-associated breast cancers exhibit a distinguishing phenotype based on morphology and molecular profiles from tissue microarrays. Am J Surg Pathol. 2007;311:121–128. 10.1097/01.pas.0000213351.49767.0f [DOI] [PubMed] [Google Scholar]
- 45. Shah CA, Allison KH, Garcia RL, Gray HJ, Goff BA, Swisher EM.. Intratumoral T cells, tumor-associated macrophages, and regulatory T cells: Association with p53 mutations, circulating tumor DNA and survival in women with ovarian cancer. Gynecol Oncol. 2008;1092:215–219. [DOI] [PubMed] [Google Scholar]
- 46. Quigley D, Silwal-Pandit L, Dannenfelser R et al. , . Lymphocyte invasion in IC10/basal-like breast tumors is associated with wild-type TP53. Mol Cancer Res. 2015;133:493–501. 10.1158/1541-7786.MCR-14-0387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dunbier AK, Ghazoui Z, Anderson H et al. , . Molecular profiling of aromatase inhibitor-treated postmenopausal breast tumors identifies immune-related correlates of resistance. Clin Cancer Res. 2013;1910:2775–2786. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.