Abstract
Age-related changes in primary lymphoid organs are well described. Less is known about age-related changes affecting peripheral lymphoid organs, although defects in old peripheral lymph nodes (pLNs) were recently described in both steady state and during viral infection. To address whether such pLN defects were intrinsic to old T cells or extrinsic (due to aging microenvironment), we employed heterochronic parabiosis. We found no age-related intrinsic or extrinsic barriers to T cell circulation and seeding of pLN, spleen, and bone marrow. However, heterochronic parabiosis failed to improve cellularity of old pLN, suggesting an environment-based limit on pLN cellularity. Furthermore, upon parabiosis, pLN of the adult partner exhibited reduced, old-like stromal and T cell cellularity, which was restored following separation of parabionts. Decay measurement of adult and old T cell subsets following separation of heterochronic parabionts delineated both T cell-intrinsic and environmental changes in T cell maintenance. Moreover, parabiotic separation revealed differences between CD4 and CD8 T cell subset maintenance with aging, the basis of which will require further investigation. Reasons for this asymmetric and subset-specific pattern of differential maintenance are discussed in light of possible age-related changes in lymph nodes as the key sites for peripheral T cell maintenance.
Keywords: Homeostasis, Lymph nodes, T cells, Parabiosis, Aging
Aging of the immune system is accompanied by a complex set of incompletely understood changes culminating in a variable degree of immune deficiency. Defects in the aged immune system begin with an early deterioration of stromal cells composing the microenvironment of the thymus and, subsequently, the bone marrow, which disrupts lymphocyte development (1–3). Subsequently, later-life deterioration of hematopoietic cell differentiation and other defects in immune cell maintenance further contribute to reduced frequencies and numbers of naïve B cells, T cells, and NK cells in aging (4).
In addition to thymic production, robust peripheral T cell pool depends upon lifelong maintenance in secondary lymphoid organs (5). Several landmark discoveries over the past decade have identified peripheral lymph nodes (pLN) as key sites for maintenance of naïve T (TN) cells and for coordination of proper primary immune responses to skin-breaching antigens, largely due to elaborate stromal networks in these organs (6,7). More recently, two reports raised the possibility that microenvironment-related defects could be occurring in secondary lymphoid organ stroma with aging (8,9). Specifically, Richner et al. demonstrated that pLNs of old mice cannot enlarge and receive additional lymphocytes in the course of a viral infection and that movement of lymphocytes within old pLN under infection is slower and more disorganized compared to adult counterparts (8). That study found evidence for defects due to both the inability of old T (and B) cells to migrate into and remain in pLN and the inability of the old pLN stromal cells to receive immigrating T cells and guide them to proper microenvironments within pLN (8). Moreover, Becklund et al. discovered disorganization of steady-state pLN stromal architecture, which prevented TN cells from accessing and proliferating in response to IL-7 in a homeostatic manner (9). Administration of exogenous IL-7 in the form of cytokine/Ab complexes (IL-7C) allowed old TN cells to proliferate normally (9).
Two questions remain that have not been addressed completely by the above studies. First, do the above problems dominantly belong to the disturbed old pLN stroma or to old T cells, with some evidence existing for both (8)? Second, are the above changes plastic and correctable? Because cell transfers result in entrapment and elimination of the majority (up to 90%) of injected cells in the liver and lung of the recipient and because the bolus of injected cells may not uniformly distribute throughout the body (10), we elected to use heterochronic parabiosis, the surgical joining of an adult and an old organism via skin suturing, to address these two questions. This technique allows one to study migration and seeding of adult and old microenvironments with old or adult cells, while assessing the impact of circulating elements that mix in a gradual manner between the old and adult animal (11). Heterochronic parabiosis has been recently used to identify and characterize potential progeronic (12–16) and antigeronic (14,15,17–19) factors that impact aging of different tissues and organs. However, the two studies addressing peripheral T cell function using heterochronic parabiosis did not examine lymph node (LN) migration and homeostasis (17,20).
Peripheral T cell homeostasis is a competitive process regulated by dynamic homeostatic mechanisms comprised of public (eg, nutrients, cytokines, and surface ligands) and clonotype-specific, private, or cognate factors (subthreshold or partial agonistic interactions between individual T Cell Receptor (TCRs) and self-peptide antigen(s) presented on MHC molecules) (21). TN cells reside in or recirculate between secondary lymphoid tissues (chiefly spleen and LNs, where they receive homeostatic signals in the form of subactivating, tonic TCR engagement with self-peptide-MHC molecules [pMHCs] that make the cell receptive to the second signal, in the form of IL-7, that maintains cellular metabolism and prevents apoptosis (5). With aging, TN cells are lost from the T cell compartment due to some combination of (i) antigenic stimulation and conversion into effector (TE) or memory (TM) cells as a consequence of lifelong encounters with microbial invaders; (ii) homeostatic conversion to memory-like cells; and/or (iii) turnover and apoptosis, presumably due to insufficient IL-7 and/or pMHC signals in the secondary lymphoid organ, mostly pLN microenvironment. The primary process countering this loss is the production of new TN cells by the thymus. It is, therefore, not surprising that aging is marked by an absolute numerical loss of TN cells and a consequent increase in representation of memory T (TM) cells (22). CD4+ and CD8+ TM cells are distributed throughout the body in both lymphoid and nonlymphoid tissues, and the bone marrow is particularly important for their maintenance (23–25). Among the TM subsets, effector memory (TEM) and tissue resident memory (TRM) cells are less abundant in lymphoid tissues and are dominantly present in nonlymphoid tissues, in order to provide immediate protection and antimicrobial responses in tissues at the site of microbial entry. Central memory T (TCM) cells, on the other hand, dominantly recirculate between secondary lymphoid tissues and bone marrow. TM cells use IL-15 as the key maintenance factor and likely need less, if any, TCR stimulation in the process (5). Still, TM, and in particular TCM cells, can also utilize IL-7 for maintenance, providing a potential point of competition with TN cells (26).
To address possible changes in lymphocyte trafficking with aging, as well as the above questions on receptiveness of pLN with aging, we employed heterochronic parabiosis. This intervention failed to reverse thymic involution, consistent with recent results (17,27). Heterochronic parabiosis also did not reverse low cellularity of old pLN; rather, pLN of the parabiotic adult partner exhibited reduced, old-like cellularity, which was restored to normal levels upon parabiont separation. Separation of parabiosed partners further showed that adult CD8+ and CD4+ T cells decayed significantly faster in the old environment compared to their old counterparts. In the adult environment, however, old CD8+, but not CD4+, T cells exhibited survival advantage, by a yet-to-be-understood extrinsic or intrinsic mechanism. This is consistent with the scenario whereby changes in peripheral factors and/or histoanatomical niches with aging limit T cell maintenance and force selection of only those T cell clones that best compete for waning maintenance resources. The results strongly suggest that the peripheral histoanatomical niches that govern T cell maintenance play a previously underappreciated role in aging of the immune system.
Materials and Methods
Animal Strains
Male C57BL/6J, Adult 45.2, and B6.SJL-PtprcaPepcb/BoyJ, Adult 45.1 mice were purchased from The Jackson Laboratory. Male old C57BL6/JNia mice were acquired from the National Institute of Aging, who rederive the strain every 6–7 years from C57BL/6J mice from The Jackson Laboratory. All animal experiments were approved by The University of Arizona’s Institutional Animal Care and Use Committee. Because these experiments were initiated at the time when the supply of old female mice from the NIA was highly restricted, the experiments remained limited to male mice.
Parabiosis Surgical Procedure
Prospective parabiotic pairs (parabionts) were temperament matched for a week prior to the surgery. On the day of surgery, mice received ketamine, xylazine, and acepromazine (45, 9, and 1.35 mg/kg, respectively) anesthetic cocktail intramuscular, followed by a subcutaneous injection of buprenorphine and meloxicam (0.1 and 5 mg/kg, respectively) for analgesia. Anesthetized mice were shaved at the corresponding lateral flanks, and matching skin incisions were made from below the base of the ear to the hip. Ipsilateral whiskers were trimmed to prevent potential agitation effects of proximal sensing among the parabionts. The subcutaneous fascia was bluntly dissected to create ~2 cm of free skin on either side of the center of the incision. Excess skin was trimmed from both sides of the incision to ensure the parabionts were comfortably restrained next to each other, while preventing them from twisting, turning, fighting, and ultimately harming themselves or each other. Laying the animals on their backs, side-by-side, the dermis of the ventral portion of the lateral incision of one parabiont was pressed against the other and was stapled together with 9 mm surgical staples. The dorsal portions of the lateral incisions were stapled together with 9 mm surgical staples. Each parabiont was then given a subcutaneous injection of 1 mL of saline and allowed to recover on a heated surgical table. Analgesia care extended for 3 days after surgery, resulting in six injections of buprenorphine, every 12 hours, and three injections of meloxicam, every 24 hours. Two weeks later, each parabiont was anesthetized with ketamine and xylazine (50 and 5 mg/kg, respectively) and the staples were removed.
Tissue Harvesting and Processing
Prior to analysis, animals were carefully checked for signs of external (skin at the site of joining, fur around the body) and internal (tumors, gross anatomical changes) abnormalities and any such animals (<2%) were excluded from the analysis. The animals were euthanized via isoflurane overdose and the tissues were harvested in the following order: First, the blood was collected via cardiac puncture and placed into vials containing heparin rings (Sarstedt). Next, the thymus, spleen, and LNs (brachial, inguinal, and popliteal separated by ipsilateral or contralateral location with respect to the surgical site) were harvested into 500 µL of RPMI/4°C, 1.5 mL of accutase (eBioscience) added and samples incubated at 37°C for 30 min. The tissues were filtered through 40 µm cell strainers (Fisher) and washed with complete RPMI (cRPMI; 10% FBS, pen/strep, L-glutamine [Gibco], and HEPES [Corning]). Both femurs were harvested and placed, together, into 5 mL of cRPMI and put on ice. The BM was flushed out with cRPMI, filtered through a 40 µm cell strainer, and washed with cRPMI. Finally, the blood was lysed via hypotonic lysis. All of the samples were counted using a Hemavet 950FS (Drew Scientific, Oxford, CT). 106 lymphocytes were plated into 96-well round-bottom plates to be stained for FCM analysis. To examine stromal contributions, LNs were cut into small pieces using a scalpel and digested using a defined collagenase blend (Liberase TL Roche) at 0.2 mg/mL and DNase I (Sigma) at 0.04 mg/mL for 1 hour in a shaking water bath at 37°C. Cells were then filtered through a 70 µm cell strainer (Fisher).
FCM Staining and Analysis
Single cell suspensions of 106 lymphocytes were washed with FACS buffer (PBS with 2% FBS) and then resuspended in 50 µL of FACS buffer containing 0.5 µg of anti-mouse CD16/CD32 (clone 93) for 15 min followed by the addition of 50 µL of antibody cocktail for each panel. Thymus panels included Annexin V, CD127 (clone SB/199), CD25 (clone PC61), CD3 (clone 17A2), CD4 (clone RM4-5), CD44 (clone IM7), CD45.1 (clone A20), CD45.2 (clone 104), CD8a (clone 53–6.7), c-Kit (clone 2B8), and intracellular Notch1 (clone mN1A) and TCRβ (clone H57). Thymus panels included a dump channel with the following antibodies: CD11b (clone M1/70), CD19 (clone 6D5), DX5, F4/80 (clone BM8), γδTCR (clone GL3), Gr1 (clone RB6-8C5), and NK1.1 (clone PK136). The T cell panel included CD3 (clone 17A2), CD4 (clone RM4-5), CD8a (clone 53–6.7), CD45.1 (clone A20), and CD45.2 (clone 104). For stromal cells, CD45 (clone 30-F11) and TER119 (clone Ter119) were used to exclude hematopoietic cells, and we gated on CD35/CD21 (clone 7E9) as a marker of stromal cells. Within that gate, gp38 (clone 8.1.1) and CD31 (clone 390) were used to determine stromal cell subsets and were classified as gp38-CD31- double-negative (DN), gp38+CD31− fibroblastic reticular cells, gp38+CD31+ lymphoid endothelial cells, and gp38-CD31+ blood endothelial cells. All antibodies and fluorescent reagents were obtained from BD Biosciences (San Diego, CA), BioLegend (San Diego, CA), Invitrogen (Carlsbad, CA), and eBioscience (San Diego, CA). Stromal cell populations were quantitated using count beads (Invitrogen). All samples were stained for viability using either Live/Dead Fixable Aqua or Live/Dead Fixable Yellow, where appropriate. Some samples were fixed with BD Cytofix/Cytoperm. All samples were collected on a custom BD Biosciences Fortessa flow cytometer. Analysis was performed on FlowJo V.10.0.8 (TreeStar, Inc., Eugene, OR).
Statistical Analysis
A repeated measures two-way analysis of variance (ANOVA) with Sidak’s post-test across tissues and ages for any given cell type was used. Adjusted p values of <.05 were considered significant. Graphs and statistics were generated using GraphPad Prism version 6 (GraphPad, La Jolla, CA).
Results
Primary and Secondary Lymphoid Organs Show Different Permissiveness to Heterochronic Engraftment
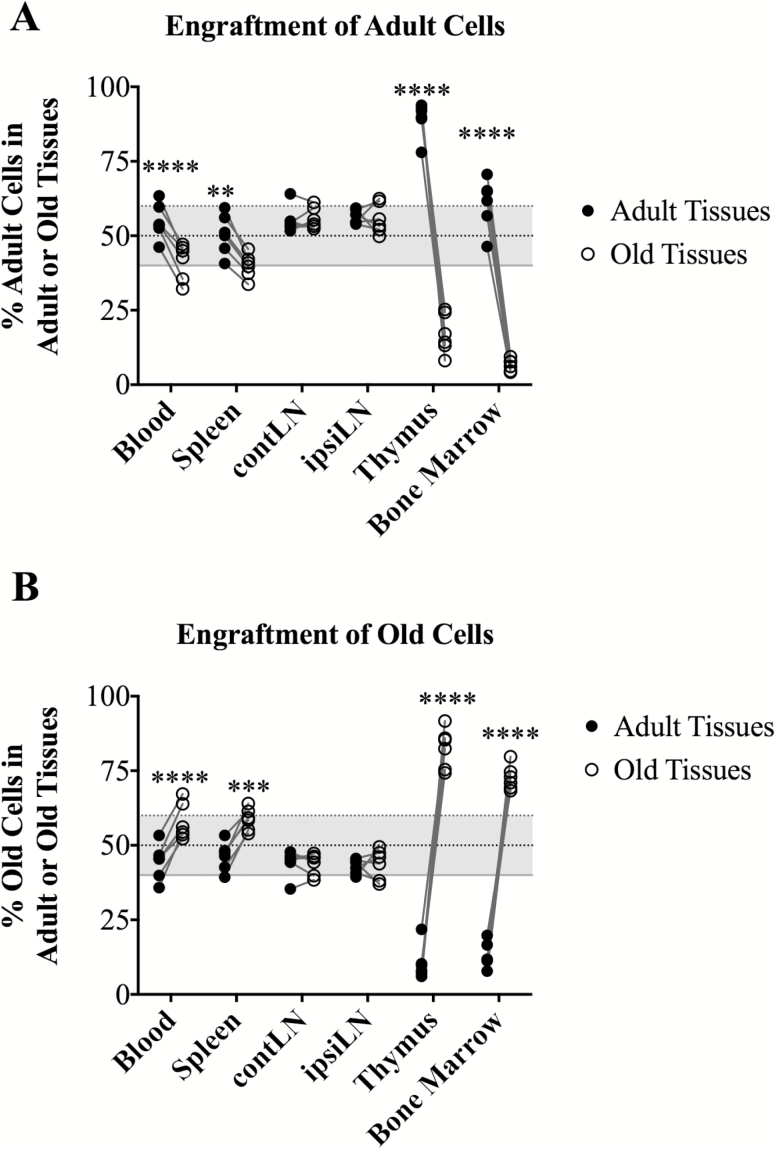
In order to assess tissue homing properties of T cell subsets as a function of age, we took advantage of CD45.1 and CD45.2 congenic markers to distinguish between old and adult hematopoietic cells, and the heterochronic parabiosis surgical technique to allow for gentle and gradual mixing of circulating cells and soluble factors between adult and old mice. Adult mice (3 months, CD45.1) and old mice (≥18 months, in the vast majority of experiments at 19 mo, CD45.2) underwent parabiosis and remained joined for 4–5 weeks prior to harvest, allowing sufficient time to establish equilibrated tissue chimerism (Figure 1), consistent with other protocols in the field (11–20). Isochronic adult, and where possible, isochronic old, mice were used as controls as indicated in figures, to control for the parabiotic procedure. In both control isochronic adult (Supplementary Figure 1) and heterochronic (Figure 1) parabionts, we observed similar extents of overall hematopoietic lineage chimerism across lymphoid tissues. That chimerism was generally equal in secondary lymphoid organs and blood, with adult and old cells being present at 50 ± 10% (Figure 1A and B). By contrast, primary lymphoid tissues, thymus and bone marrow (BM), exhibited considerable restriction, allowing only about 10% cell chimerism of the heterochronic origin (Figure 1A and B).
Figure 1.
Primary and secondary lymphoid organs show different permissiveness to parabiotic engraftment. Frequency of adult (CD45.1) or old (CD45.2) hematopoietic cells was determined by flow cytometry among cells isolated from indicated tissues in heterochronic parabiosis. (A) Engraftment of adult and old tissues by adult cells. (B) Engraftment of adult and old tissues by old cells. For all panels, data were pooled from two to three independent experiments. Significance was determined by repeated measures two-way ANOVA with Sidak’s post-test. Statistical significance: **p < .01, ***p < .001, ****p < .0001. Note that uneven engraftment of primary lymphoid organs was not a function of age, because similar differences were obtained in isochronic adult CD45.1/CD45.2 parabionts (see Supplementary Figure 1).
Because similar findings were observed in isochronic parabionts (Supplementary Figure 1A and B), these results demonstrated that neither the cellular nor the tissue age altered global leukocyte trafficking. To control for the effects of trauma and local irritation at the site of surgery and for the potential caveat that short-lived pLN cells (eg, some myeloid cells) may fail to reach equal chimerism over the 4–5 weeks of the observation period, we next separately analyzed contralateral and ipsilateral pLNs (contLNs and ipsiLNs) relative to the site of surgery. This analysis showed no significant site-specific variation among any cell type analyzed between the two sites in both adult and old animals (variation >1.2% and >0.05% for CD45+ cells, respectively, Figure 1A and B). Therefore, surgery and/or the migratory distance also did not distort leukocyte trafficking between heterochronic parabionts.
Unless otherwise stated, the remaining data presented in the manuscript will highlight the unique findings where heterochronic parabionts differ from nonsurgical (NS) and/or isochronic controls, thereby controlling for the congenic allele and surgery effects.
Old Parabiotic Environment Does Not Restrict T Cell Migration to LNs but Leads to Restricted pLN Cellularity in Both Adult and Old Parabionts
Examination of lymphoid organ cellularity revealed that, consistent with recent results (27), heterochronic parabiosis cannot rejuvenate thymic cell numbers. Specifically, old nonsurgical, old isochronic, and old heterochronic thymus cell numbers were below 20 × 106 cells per organ, contrasting with adult counterparts, that were averaging 80–100 × 106 cells per organ at 3–4 months of age (Supplementary Figure 2A). Blood (Supplementary Figure 2B) and spleen (Supplementary Figure 2C) counts of total lymphocytes did not significantly differ between adult and old mice and also did not vary between adult or old isochronic or adult:old heterochronic parabiosed mice.
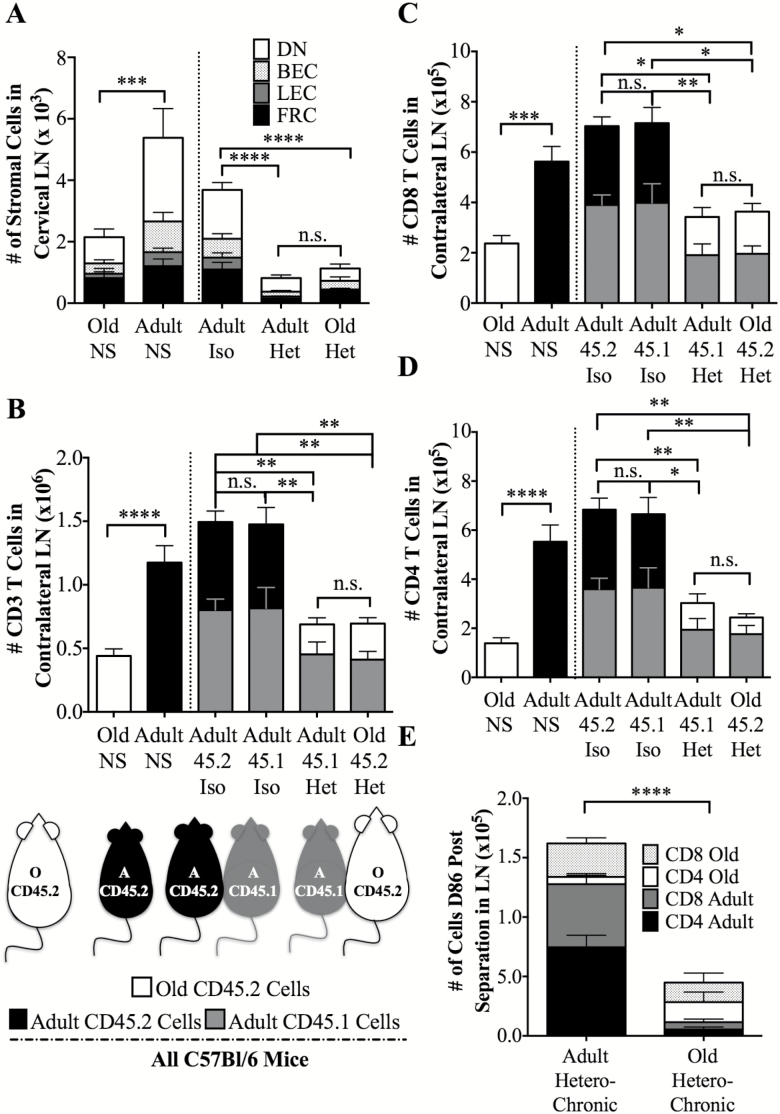
In nonparabiosed mice, however, both pLN stromal (Figure 2A) and pLN T cell cellularity (Figure 2B) decreased with age. Specifically, stromal cells from cervical LN (available from paired animals for which other LN were used for T cell analysis) decreased from 5.4 × 103 to 2.1 × 103 (Figure 2A), whereas total CD3+ T cell numbers declined from 1.17 × 106 ± 1.3 × 105 in the adult to 4.4 × 105 ± 5.6 × 104 T cells in the old, popliteal, inguinal, and brachial LNs (bilateral harvest of contralateral LNs; Figure 2B, left two bars). Both adult CD4 and CD8 T cells significantly outnumbered old CD4 and CD8 T cells in surgically unmanipulated animals (Figure 2C and D, leftmost bars). Therefore, one would expect parabiosis to lead to pervasive infiltration of adult T cells into all the permissive tissues in both the adult and old animals. Indeed, following parabiosis, we detected an increased influx and high representation of adult T cells in all old tissues. However, total T cell cellularity of old pLNs did not increase in parabiosis (Figure 2B and D), despite a strong supply of T cells from the adult partner and its functional thymus. Therefore, although old pLN were not a restricted site that would prevent immigration of circulating cells, they exhibited a ceiling effect and were not able to increase in absolute cellularity. Notably, both stromal cell and T cell cellularity decreased in parabiosed adult pLNs. Thus, both adult and old parabiont partner pLN contained T cell numbers similar to the old nonsurgical control and lower than those in adult pLN or adult isochronic parabionts (Figure 2B, p = .14 between old nonsurgical control and adult or p = .06 old heterochronic parabiont; p = .02 between adult nonsurgical control and adult heterochronic parabiont, by t test with Welch’s correction). Differences were even more impressive in the stromal cells and their subsets (Figure 2A, p = .09 between old nonsurgical control and adult or p = .21 old heterochronic parabiont; p = .01 between adult nonsurgical control and adult heterochronic parabiont, by t test with Welch’s correction).
Figure 2.
Peripheral lymph node (LN) cellularity is restricted in both adult and old heterochronic parabionts. (A) Data depict numbers and distribution of stromal (Ter119− CD45− CD35/21+) cell subsets within cervical LN in nonsurgical controls (NS) (right), and parabiosed mice (left) adult isochronic parabiosis, heterochronic parabiosis, and in old isochronic parabiosis. Shown are numbers of gp38−CD31− double-negative (DN, open parts of the bar), gp38+CD31− fibroblastic reticular cells (FRC, closed bar parts), gp38−CD31− lymphoid endothelial cells (LEC, dark gray), and gp38-CD31+ blood endothelial cells (BEC, light gray). (B) Number of adult or old CD3 T cells (derived from total cell counts and CD3 flow cytometric staining) in nonsurgical control, isochronic parabionts, and heterochronic parabionts from pooled peripheral lymph nodes (LNs; brachial, inguinal, and popliteal) contralateral to the side of surgery. Open bars indicate cell numbers originating from old mice (always CD45.2), gray bars indicate cell numbers originating from adult CD45.1 mice, and black bars indicate cell numbers from adult wildtype (CD45.2) mice. n = 8–16 mice, pooled from two to three experiments. (C) Numbers of adult or old CD8 T cells in nonsurgical control, isochronic parabionts, and heterochronic parabionts in peripheral LN, from mice analyzed in panel B. (D) Numbers of adult or old CD4 T cells in nonsurgical control, isochronic parabionts, and heterochronic parabionts in peripheral LN, from mice analyzed in panel B. (E) Adult and old LN CD4 and CD8 T cell subset numbers following heterochronic parabiosis and separation surgery (contralateral brachial and inguinal LN shown); analysis was done as in panels B-D, on n = 5 animals/age group. Significance was determined by Mann–Whitney U-test for adult and old nonsurgical controls, whereas significance for parabionts was determined by two-way ANOVA with Sidak’s post-test. Statistical significance: *p < .05, ** p < .01, *** p < .001, **** p < .0001, and ns is not significant.
There are two main explanations for this observation. One is that the adult T cell pool including T cells generated from the adult thymus now has to redistribute across two bodies rather than one (“the garden hose model,” where a garden hose would have the same throughput but would have to water twice the size of a lawn) and that this would lead to an “emptying” of adult pLN. The other is that there is/are unknown circulating progeronic factor(s), present in old mice, which adversely impact/s cellularity of adult pLNs.
To distinguish between these possibilities, we analyzed numbers of T cells, as the cell type produced by the thymus and dominantly populating, and being maintained in pLN. The “garden hose” hypothesis would predict that the sum of T cell numbers from unmanipulated adult + old animals should be approximately equal to the sum of T cells of the adult and old parabionts; the progeronic factor hypothesis would predict that the former would be larger than the latter. Figure 2A shows that total stromal cells from the cervical LN from nonsurgical controls number 7.5 × 103 cells, whereas adult + old heterochronic parabionts had 1.9 × 103 cells. Figure 2B shows that Adult + Old nonsurgical controls = 1.61 × 106 (1.17 × 106 Adult + 0.44 × 106 Old), whereas the heterochronic parabiont Adult + Old = 1.38 × 106 (0.69 × 106 Adult + 0.69 × 106 old). These results show that stromal cells in unmanipulated adult +old mice markedly outnumber the sum of stromal cells in the adult +old heterochronic parabionts and that T cells follow the same trend in a less pronounced manner. They support, although do not conclusively prove, the possibility that a progeronic factor(s) could be involved.
Another prediction of the progeronic factor(s) hypothesis is that removal and/or dilution of this factor following parabiotic separation should restore cellularity of adult pLN to the presurgery size. We tested this hypothesis and found robust recovery of pLN cellularity in adult parabiont 3 months following separation (1.6 × 106 ± 3.3 × 105 and 4.6 × 105 ± 1.3 × 105 total cells for adult and old pLNs, respectively, Figure 2E), and these cell differences were significant at p < .0001 (two-way ANOVA, Figure 2E). This result further favors the progeronic factor(s) hypothesis, that is, currently under further testing.
We found that adult and old CD4 and CD8 T cell subsets distributed across both adult and old pLN (Figure 2C and D). Among them, adult CD4 T cells outnumbered the old in both adult and old pLN (Figure 2D), whereas CD8 T cells were distributed more evenly (Figure 2C). Overall, the results unambiguously show that, at least in the context of heterochronic parabiosis, the age of pLN environment does not dictate either permissiveness or retention of T cell subset or age of the cell. Likewise, both old and adult cells were able to migrate to and populate both adult and old pLN, demonstrating that there are no cell-intrinsic age-related defects in migration to pLN. By contrast, old and heterochronic parabiotic environments severely restricted the total cellularity of these same LN (including the stromal and T cell cellularity of adult parabiont LN), despite an abundant supply of adult lymphocytes. These results are further discussed below.
T Cell Subset Homing to Bone Marrow in Heterochronic Parabiosis
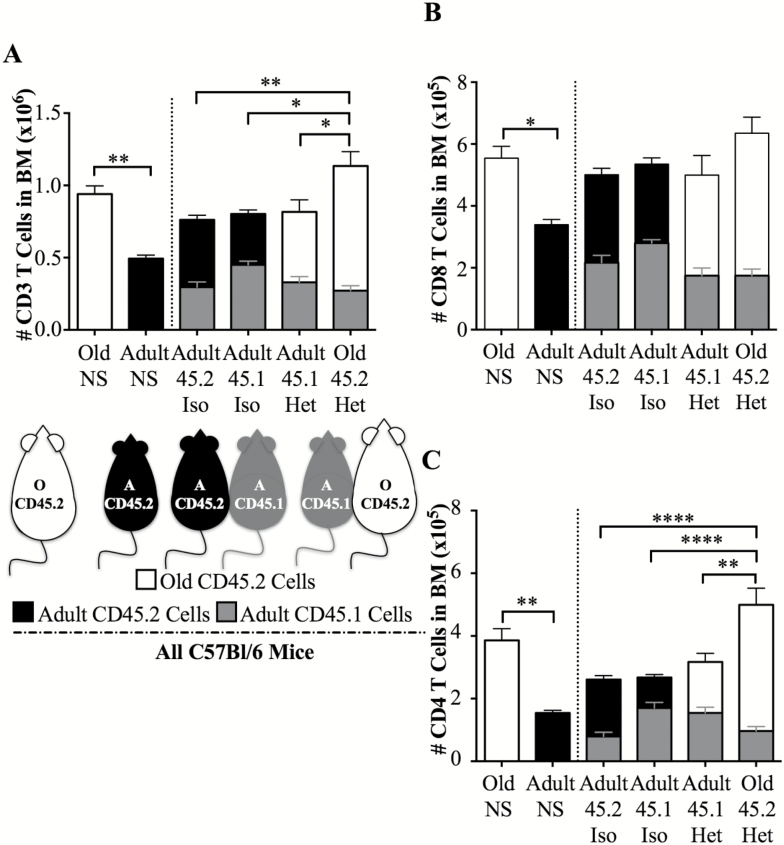
Along with the thymus, BM was another tissue exhibiting restricted (10%) hematopoietic chimerism (Figure 1). In unmanipulated control mice, old BM numerically harbored nearly twice as many T cells as adult counterparts (nonsurgical controls, Figure 3A). Upon parabiosis, even though old cells made up only 10% of the population found in adult BM, old T cells within that fraction outnumbered the resident adult T cells by nearly 2:1 (see adult heterochronic parabiont, Figure 3A). Not surprisingly, old T cells also dominated in absolute number in the BM of the old parabiont (Figure 3A). Both major T cell subsets contributed to this effect (Figure 3B and C). Unlike the ceiling effects seen with old pLN, that could not expand, or, in the case of adult pLN, that actually shrank upon parabiosis, bone marrow did not seem to exhibit space/niche constraint in either adult or old mice (Figure 3, total T cell numbers).
Figure 3.
T cell and T cell subset numbers within the bone marrow of unmanipulated adult and old animals, isochronic and heterochronic parabionts. (A) Data depict numbers of adult or old CD3 T cells in nonsurgical control, isochronic parabionts, and heterochronic parabionts in bone marrow, isolated as in Materials and Methods, and obtained by a combination of Hemavet® counts and flow cytometry. (B) Numbers of adult or old CD8 T cells in nonsurgical control, isochronic parabionts, and heterochronic parabionts in bone marrow (same animals as in A). (C) Numbers of adult or old CD4 T cells in nonsurgical control, isochronic parabionts, and heterochronic parabionts in bone marrow (same animals as in A). Each group represents five to seven mice, pooled from two to three experiments. Significance was determined by Mann–Whitney U-test for adult and old nonsurgical controls, whereas significance for parabionts was determined by two-way ANOVA with Sidak’s post-test. Statistical significance: *p < .05, **p < .01, ***p < 0.001, ****p < 0.0001, and ns (not significant) is not shown.
Aging Selects for T Cells with a Peripheral Maintenance Advantage Over Young T Cells that, in Part, Depends on the Age of the Environment
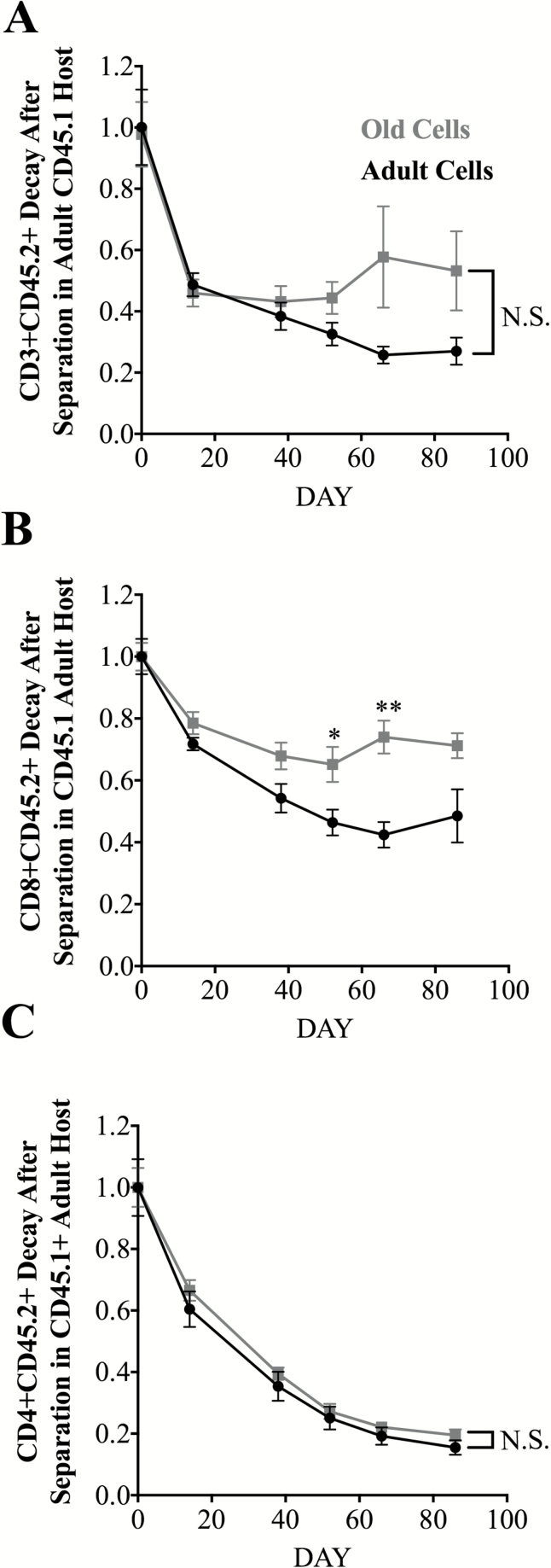
To evaluate how age-specific environmental factors contribute to T cell maintenance, we examined persistence of adult and old total CD4 and CD8 T cells in old and adult hosts following separation of parabiotic partners and re-establishment of the original (adult or old) circulating environment. In these experiments, mice underwent parabiosis surgery, remained connected for 4–5 weeks, and were then surgically separated and longitudinally bled for the following 10–12 weeks to assess T cell decay rates. To make direct comparisons, we first gated on CD3+, or CD3+CD4+ or CD3+CD8+ subsets, and then through CD45.1 or CD45.2, normalizing the initial population at the time of separation to 1.0, and comparing decay of heterochronic old CD45.2+ cells with the decay of isochronic adult CD45.2+ cells in the CD45.1+ adult host.
This comparison of persistence of old and adult cells in the adult separated parabiont revealed that total old T cells exhibited a trend to persist better than adult cells in the adult environment, but that trend did not reach significance (Figure 4A). Analysis of major subsets showed that whereas old CD8+ T cells showed significantly better survival than adult CD8+ T cells on days 50 and 64 and a trend for better survival on other days (Figure 4B), there was no difference between adult and old CD4+ T cell persistence in adult hosts (Figure 4C). Of interest, superior persistence of old CD8+ T cells in the adult circulation was evident despite the presence of a functional adult thymus in the adult animal (Supplementary Figure 2). Moreover, we found that CD4 T cells decay roughly twice as fast as CD8+ T cells, regardless of age, consistent with prior data in adult mice (28), providing further basis for their preferential disappearance with aging (Figure 4B and C).
Figure 4.
Decay kinetics of CD45.2 adult or CD45.2 old T cells or their main subsets in adult CD45.1 parabionts after separation. Data represent the frequency of adult (CD45.2) or old (CD45.2) cells among the indicated T cell subset in blood, measured longitudinally after separation in CD45.1 adult parabionts. Starting % of CD45.1 or CD45.2 within population was normalized to one so decay of population could be directly compared. (A) CD3 T cell decay kinetics in the blood. (B) CD8 kinetics in the blood. (C) CD4 kinetics in the blood. Each group represents three to six mice pooled from two independent experiments. Statistical values depict difference in individual t test of time points *p < 0.05, **p < 0.01, ***p < 0.001, ****p <0.0001, and is ns not significant.
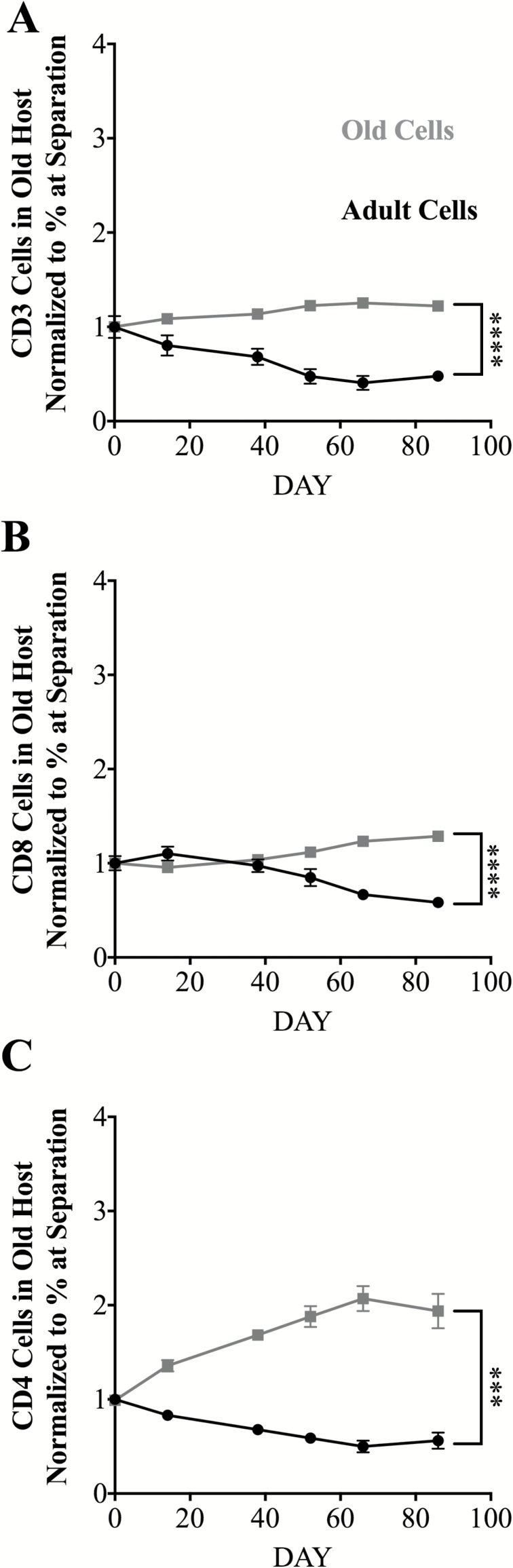
Analysis of the role of the environment in selective maintenance of T cells, from same animals, showed that, in the old separated parabiont, old total T cells, CD4+ and CD8+ cells, were maintained preferentially over their adult counterparts (Figure 5), and old CD4+ T cells even showed accumulation above the initial numbers (Figure 5C). These results demonstrate, for the first time in a direct comparison, that aging affects maintenance of peripheral CD8+ and CD4+ T cells differentially and that these differences are dictated by the age of the environment. Overall, this further supports the idea that the rules governing the maintenance of T cell subsets change significantly with aging.
Figure 5.
Adult and old T cell decay kinetics in old environments after separation. Data represent the frequency of adult (CD45.1, heterochronic parabiont) or old (CD45.1, isochronic old) cells among the indicated T cell subset in blood, measured longitudinally after separation in CD45.2 old parabionts. Starting % of CD45.1 or CD45.2 within population was normalized to one so decay of population could be directly compared. (A) CD3 T cell kinetics the blood. (B) CD8 kinetics in the blood. (C) CD4 kinetics in the blood. Each group is three to six mice in two independent experiments. Statistical values depict difference in linear regressions, where each slope is tested whether it is significantly different from the other cell type. *p < 0.05, **p < 0.01, ***p < 0.001, ****p <0.0001, and ns is not significant.
Discussion
In this report, we have used heterochronic parabiosis to investigate age-related differences in T cell homing and maintenance. While we have examined numerous other lymphoid and myeloid cell subsets, the description of which is being provided separately (29), the most striking differences were noted with different T cell subsets. The observed results have allowed us to make several novel conclusions important for our understanding of the age-related changes in T cell trafficking and maintenance. Like other recent studies using heterochronic parabiosis, we found no evidence that young circulatory factors can rejuvenate the old thymus (17,27). We extended these observations to show that heterochronic parabiosis cannot restore youthful size and cellularity to old LNs. Moreover, the adult LNs exhibited reduced size and cellularity in parabiosis. All this was occurring regardless of the normal, adult-like output from the young thymus. LN size and stromal cell cellularity suggested that this was not a simple redistribution of cells into two sets of pLN, whereas T cell cellularity showed a similar, but less pronounced, effect. The fact that stromal cells, which cannot traffic and redistribute, were markedly reduced in the adult heterochronic parabiont and further argues against simple redistribution as a cause for reduced cellularity in adult mice. Finally, experiments with separation of parabionts showed that adult, but not old, pLN returned back to the preparabiosis size. These data are suggestive of the presence of one or more unidentified peripheral circulating factor(s) in the old mice that play a dominant role in limiting the size and cellularity of pLN with aging. Though we did not directly measure IL-7 levels in our experiments, this cytokine is highly unlikely to play a role in this process for at least two reasons. First, in a recent study by Becklund et al., no differences were found between old and adult LN in levels of IL-7 mRNA or protein (9). Second, lack of IL-7 in old mice would not be consistent with a dominant reduction of cellularity in the adult parabiont upon parabiosis. Additional experiments will be necessary to address the nature of circulating factor(s) that negatively modulate LN cellularity in the adult heterochronic parabiont.
Other important conclusions from the present study are that (i) old secondary lymphoid organs (spleen and LNs) did not prevent engraftment of adult lymphocytes; (ii) old lymphocytes traffic normally to adult organs and in some cases preferentially inhabit them compared to their adult counterparts (eg, old T cells in the adult bone marrow); and (iii) following dissociation of parabionts, we found evidence of both cell-intrinsic and environment-based changes that alter longevity and maintenance of different subsets of old T cells with aging.
We found that in parabiosis, the old LNs remained hypocellular; however, they exhibited no barrier toward homing and retention of adult cells. As mentioned above, adult parabiont LNs also become hypocellular. From the standpoint of immune rejuvenation, these results bring reasons for hope as well as for concern and further research. Specifically, our data suggest that newly produced T cells originating from a potentially rejuvenated thymus would have no problem reaching the LN; however, once there, they could face serious maintenance and accumulation problems. At the present, it is unclear which specific mechanistic interactions occur in the old pLN to prevent proper TN cell maintenance. Becklund et al. recently described disorganized and disjoined networks of LN stromal cells in old pLN that could deprive TN cells of supportive, life-promoting signals (9). Moreover, in our preliminary experiments, increased fibrosis has been noted throughout the old, but not adult, pLN (H.L. Thompson et al., unpublished). Further mechanistic studies using direct genetic and cytokine manipulation of LN stromal cells will be the key to understand these changes. Regardless of the exact mechanisms, even with the intact thymic organ in the parabiosed young animal, its LN did not increase in size or T cell cellularity. Therefore, any rejuvenation strategy would have to take into account the restoration of LN function with aging.
The above results contribute to the emerging body of literature highlighting the importance of age-related LN dysfunction. Indeed, old LNs have recently been found to exhibit several functional defects over the course of an immune response, including delayed T and B cell entry into inflamed LN and disorganized and slower trafficking of T and B cells within the LN (8). Moreover, studies of steady-state old LN have shown disorganization in the LN stromal reticular networks that correlated to impaired T cell entry and homeostatic proliferation in old mice, and administration of IL-7/anti-IL-7 Ab complexes was needed to restore this defect (9). These data, together with the results of our study, stress the key role of age-related LN dysfunction in impaired T cell homeostasis and function with aging. We speculate, based on the data in this study and our other unpublished results with cell transfers (M. Jergovic et al., in preparation), that age-related defects in pLN environment may be functionally even more important than the age-related defects exhibited by lymphocytes themselves and that correction of these defects may be one of the more effective avenues to improve immunity in older individuals.
Parabiosis reversal allowed us to assess direct competition of adult and old T cell subsets in adult and old environments. Perhaps surprisingly, we found that old CD8+ T cells survived longer than their adult counterparts in the adult environment, regardless of the presence of a functional adult thymus. Further work will be necessary to dissect whether this effect holds for all of the CD8 subsets, or whether it may be selective for some, for example, the T virtual memory (TVM) cells, that with age become the main component of the old T central memory compartment (30,31). Namely, these TVM cells have been shown by us (30,32) and others (31,33) to accumulate with aging and to exhibit high CD5 levels and high TCR avidity, consistent with strong peripheral selection.
In contrast, in the old separated parabiont, both old CD8+ and old CD4+ T cells survived better than adult counterparts. This suggests that, in addition to the intrinsic changes in T cell subsets themselves (eg, accumulation of CD8+ TVM cells), there are age-related changes in the environment that affect T cell subset maintenance, perhaps in the form of different TCR ligands, that particularly strongly affected CD4+ cells. Although the nature of these changes is currently under investigation, extended half-life in old naïve CD4+ T cells was reported previously (34,35) in the old environment, albeit the use of TCR transgenic cells and of donor irradiation existed as potential confounders in one study (34). More recently, evidence for peripheral selection of old T cells, including TN cells has been accumulating, suggesting that naïve CD8+ TN cells over time may be selected for increased self-reactivity (30,32,33), whereas CD4+ TN cells may be selected with aging toward cross-reactivity (36–39). This is also consistent with results on CD4+ cell maintenance using a TCR transgenic model40 and from the decay and modeling data of Den Braber et al. in wild type, old and adult thymectomized mice (34,40), although the extent of peripheral selection in thymectomized mice has not been formally established in that study. It is also of interest to examine why old CD4+ T cells do not exhibit the same behavior in the adult separated parabiont. Novel results shown in this manuscript demonstrate that several subsets of old T cells are superior in their ability to compete for trophic factors and that this ability in part depends on the old environment. We suggest that age-related LN defects play a previously underappreciated role in this peripheral selection and immune aging.
Supplementary Material
Supplementary data are available at The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences online.
Funding
Supported by the NIAID (National Institute on Aging, National Institutes of Health) contract ADBS (N01-AI000017) and the Bowman Endowed Professorship in Medical Sciences (to J.N-Ž).
Conflict of Interest
Authors declare that there is no conflict of interest.
Supplementary Material
References
- 1. Linton PJ, Dorshkind K. Age-related changes in lymphocyte development and function. Nat Immunol. 2004;5:133–139. doi: 10.1038/ni1033. [DOI] [PubMed] [Google Scholar]
- 2. Rossi DJ, Bryder D, Zahn JM, et al. . Cell intrinsic alterations underlie hematopoietic stem cell aging. Proc Natl Acad Sci USA. 2005;102:9194–9199. doi: 10.1073/pnas.0503280102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chinn IK, Blackburn CC, Manley NR, Sempowski GD. Changes in primary lymphoid organs with aging. Semin Immunol. 2012;24:309–320. doi: 10.1016/j.smim.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Valiathan R, Ashman M, Asthana D. Effects of ageing on the immune system: infants to elderly. Scand J Immunol. 2016;83:255–266. doi: 10.1111/sji.12413. [DOI] [PubMed] [Google Scholar]
- 5. Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 6. Novkovic M, Onder L, Cupovic J, et al. . Topological small-world organization of the fibroblastic reticular cell network determines lymph node functionality. PLoS Biol. 2016;14:e1002515. doi: 10.1371/journal.pbio.1002515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Malhotra D, Fletcher AL, Turley SJ. Stromal and hematopoietic cells in secondary lymphoid organs: partners in immunity. Immunol Rev. 2013;251:160–176. doi: 10.1111/imr.12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Richner JM, Gmyrek GB, Govero J, et al. . Age-dependent cell trafficking defects in draining lymph nodes impair adaptive immunity and control of West Nile virus infection. PLoS Pathog. 2015;11:e1005027. doi: 10.1371/journal.ppat.1005027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Becklund BR, Purton JF, Ramsey C, et al. . The aged lymphoid tissue environment fails to support naïve T cell homeostasis. Sci Rep. 2016;6:30842. doi: 10.1038/srep30842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moon JJ, Chu HH, Hataye J, et al. . Tracking epitope-specific T cells. Nat Protoc. 2009;4:565–581. doi: 10.1038/nprot.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gibney BC, Chamoto K, Lee GS, et al. . Cross-circulation and cell distribution kinetics in parabiotic mice. J Cell Physiol. 2012;227:821–828. doi: 10.1002/jcp.22796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ruckh JM, Zhao JW, Shadrach JL, et al. . Rejuvenation of regeneration in the aging central nervous system. Cell Stem Cell. 2012;10:96–103. doi: 10.1016/j.stem.2011.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Loffredo FS, Steinhauser ML, Jay SM, et al. . Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell. 2013;153:828–839. doi: 10.1016/j.cell.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Salpeter SJ, Khalaileh A, Weinberg-Corem N, Ziv O, Glaser B, Dor Y. Systemic regulation of the age-related decline of pancreatic β-cell replication. Diabetes. 2013;62:2843–2848. doi: 10.2337/db13-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- 16. Miron VE, Boyd A, Zhao JW, et al. . M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat Neurosci. 2013;16:1211–1218. doi: 10.1038/nn.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pishel I, Shytikov D, Orlova T, Peregudov A, Artyuhov I, Butenko G. Accelerated aging versus rejuvenation of the immune system in heterochronic parabiosis. Rejuvenation Res. 2012;15:239–248. doi: 10.1089/rej.2012.1331. [DOI] [PubMed] [Google Scholar]
- 18. Villeda SA, Luo J, Mosher KI, et al. . The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477:90–94. doi: 10.1038/nature10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Smith LK, He Y, Park JS, et al. . β2-Microglobulin is a systemic pro-aging factor that impairs cognitive function and neurogenesis. Nat Med. 2015;21:932–937. doi: 10.1038/nm.3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shytikov DW, Shkumat MS, Yankova TM, Peregudov AG, Artyuhov IV, Pishel IM. Splenic niche cells from young heterochronic parabionts have decreased capability to amplify T-cell proliferation in vitro. Am J Biosci. 2015;3:46–54. doi:10.11648/j.ajbio.20150302.14. [Google Scholar]
- 21. Singh NJ, Bando JK, Schwartz RH. Subsets of nonclonal neighboring CD4+ T cells specifically regulate the frequency of individual antigen-reactive T cells. Immunity. 2012;37:735–746. doi: 10.1016/j.immuni.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nikolich-Žugich J. Aging of the T cell compartment in mice and humans: from no naive expectations to foggy memories. J Immunol. 2014;193:2622–2629. doi: 10.4049/jimmunol.1401174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Di Rosa F. T-lymphocyte interaction with stromal, bone and hematopoietic cells in the bone marrow. Immunol Cell Biol. 2008;87:20–29. doi: 10.1038/icb.2008.84. [DOI] [PubMed] [Google Scholar]
- 24. Herndler-Brandstetter D, Landgraf K, Tzankov A, et al. . The impact of aging on memory T cell phenotype and function in the human bone marrow. J Leukoc Biol. 2012;91:197–205. doi: 10.1189/jlb.0611299. [DOI] [PubMed] [Google Scholar]
- 25. Masopust D, Schenkel JM. The integration of T cell migration, differentiation and function. Nat Rev Immunol. 2013;13:309–320. doi: 10.1038/nri3442. [DOI] [PubMed] [Google Scholar]
- 26. Tan JT, Ernst B, Kieper WC, LeRoy E, Sprent J, Surh CD. Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J Exp Med. 2002;195:1523–1532. doi:10.1084/jem.20020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kim M-J, Miller CM, Shadrach JL, Wagers AJ, Serwold T. Young, proliferative thymic epithelial cells engraft and function in aging thymuses. J Immunol. 2015;194:4784–95. doi:10.4049/jimmunol.1403158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Polic B, Kunkel D, Scheffold A, Rajewsky K. How alpha beta T cells deal with induced TCR alpha ablation. Proc Natl Acad Sci USA. 2001;98:8744–8749. doi: 10.1073/pnas.141218898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nikolich-Žugich J, Davies JS. Homeostatic migration and distribution of innate immune cells in primary and secondary lymphoid organs with ageing. Clin Exp Immunol. 2017;187:337–344. doi: 10.1111/cei.12920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rudd BD, Venturi V, Li G, et al. . Nonrandom attrition of the naive CD8+ T-cell pool with aging governed by T-cell receptor:pMHC interactions. Proc Natl Acad Sci USA. 2011;108:13694–13699. doi: 10.1073/pnas.1107594108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chiu BC, Martin BE, Stolberg VR, Chensue SW. Cutting edge: central memory CD8 T cells in aged mice are virtual memory cells. J Immunol. 2013;191:5793–5796. doi: 10.4049/jimmunol.1302509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Renkema KR, Li G, Wu A, Smithey MJ, Nikolich-Žugich J. Two separate defects affecting true naive or virtual memory T cell precursors combine to reduce naive T cell responses with aging. J Immunol. 2014;192:151–159. doi: 10.4049/jimmunol.1301453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Decman V, Laidlaw BJ, Doering TA, et al. . Defective CD8 T cell responses in aged mice are due to quantitative and qualitative changes in virus-specific precursors. J Immunol. 2012;188:1933–1941. doi: 10.4049/jimmunol.1101098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tsukamoto H, Clise-Dwyer K, Huston GE, et al. . Age-associated increase in lifespan of naive CD4 T cells contributes to T-cell homeostasis but facilitates development of functional defects. Proc Natl Acad Sci USA. 2009;106:18333–18338. doi: 10.1073/pnas.0910139106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang B, Jia Q, Bock C, et al. . Glimpse of natural selection of long-lived T-cell clones in healthy life. Proc Natl Acad Sci USA. 2016;113:9858–9863. doi: 10.1073/pnas.1601634113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Deshpande NR, Parrish HL, Kuhns MS. Self-recognition drives the preferential accumulation of promiscuous CD4(+) T-cells in aged mice. Elife. 2015;4:e05949. doi: 10.7554/eLife.05949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rudd BD, Venturi V, Davenport MP, Nikolich-Zugich J. Evolution of the antigen-specific CD8+ TCR repertoire across the life span: evidence for clonal homogenization of the old TCR repertoire. J Immunol. 2011;186:2056–2064. doi: 10.4049/jimmunol.1003013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jiang J, Fisher EM, Murasko DM. Intrinsic defects in CD8 T cells with aging contribute to impaired primary antiviral responses. Exp Gerontol. 2013;48:579–586. doi: 10.1016/j.exger.2013.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jiang J, Fisher EM, Murasko DM. CD8 T cell responses to influenza virus infection in aged mice. Ageing Res Rev. 2011;10:422–427. doi: 10.1016/j.arr.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. den Braber I, Mugwagwa T, Vrisekoop N, et al. . Maintenance of peripheral naive T cells is sustained by thymus output in mice but not humans. Immunity. 2012;36:288–297. doi: 10.1016/j.immuni.2012.02.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.