Abstract
Immunologic graft rejection is one of the main causes of short and long-term graft failure in corneal transplantation. Steroids are the most commonly used immunosuppressive agents for postoperative management and prevention of corneal graft rejection. However, steroids delivered in eye drops are rapidly cleared from the surface of the eye, so the required frequency of dosing for corneal graft rejection management can be as high as once every 2 h. Additionally, these eye drops are often prescribed for daily use for 1 year or longer, which can result in poor patient compliance and steroid-related side effects. Here, we report a biodegradable nanoparticle system composed of Generally Regarded as Safe (GRAS) materials that can provide sustained release of corticosteroids to prevent corneal graft rejection following subconjunctival injection provided initially during transplant surgery. Poly(lactic-co-glycolic acid) (PLGA) nanoparticles containing dexamethasone sodium phosphate (DSP) exhibited a size of 200 nm, 8 wt.% drug loading, and sustained drug release over 15 days in vitro under sink conditions. DSP-loaded nanoparticles provided sustained ocular drug levels for at least 7 days after subconjunctival administration in rats, and prevented corneal allograft rejection over the entire 9-week study when administered weekly. In contrast, control treatment groups that received weekly injections of either placebo nanoparticles, saline, or DSP in solution demonstrated corneal graft rejection accompanied by severe corneal edema, neovascularization and opacity that occurred in ≤ 4 weeks. Local controlled release of corticosteroids may reduce the rate of corneal graft rejection, perhaps especially in the days immediately following surgery when risk of rejection is highest and when typical steroid eye drop administration requirements are particularly onerous.
Keywords: Dexamethasone, Corneal transplantation, Corneal rejection, PLGA, Drug delivery
1. Introduction
Corneal transplantation is the most common form of solid tissue transplantation [1,2], and is widely used to treat blindness caused by corneal diseases. Approximately 36,000 corneal transplantation surgeries are performed each year in the US alone [1]. The 2-year graft survival rate for avascular and non-inflamed “low-risk” cornea beds is up to 90%, however, the rate for “high-risk” cornea beds, which had either neovascularization, inflammation, or previous graft rejection, is as low as 50% [1,3]. Given the increased risk of future graft failure in patients who have previously rejected a corneal transplant and the limited supply of cornea tissues suitable for transplantation, corneal graft failure is a significant burden on patients, their families and the health care system.
Immunologic corneal rejection is the main cause for graft failure. Immunosuppressive therapies, such as steroids, antimetabolites, and T-cell inhibitors, have been applied to patients after cornea transplantation, either systemically or through eye drops [4–6]. Topical corticosteroids in eye drops are widely used to control rejection rates of both “low-risk” and “high-risk” corneal grafts [7–10]. Drops are generally preferred over systemic steroid administration in order to target the therapy and reduce systemic side effects. However, rapid drug clearance from the ocular surface and low drug penetration into the eye lead to a requirement for frequent administration [9,11,12]. Drop administration requirements can be as often as every 2 h during the first several days after surgery [13,14], a regimen that causes poor patient compliance that increases graft rejection rates [15,16].
In an attempt to address the need for high local steroid levels immediately after surgery, subconjunctival (SC) injection of corticosteroids at the time of surgery is often employed. SC injection of dexamethasone sodium phosphate (DSP) solution has been shown to result in increased concentrations of DSP in the aqueous humor compared to drug concentrations achieved with eye drops [17,18]. However, rapid clearance of small molecules like DSP from the ocular tissue limits the duration of therapeutic drug levels after a single SC injection, and the spike in drug concentration may cause increased ocular side effects.
Polymeric nano- and microparticles can be administered by injection and provide controlled release of drugs to target tissues at effective levels, which can increase treatment efficacy and decrease associated side effects. For this reason, polymer particle based therapies are being evaluated to deliver therapeutic agents to the eye by various routes, including intravitreal injection, topical administration and SC injection [19–23]. Here, we describe the development of a nanoparticle formulation for SC injection that can provide sustained release of DSP (DSP-NP) both in vitro and following SC injection in rats, and we demonstrate that it is effective in preventing corneal graft rejection in a corneal transplant rat model in vivo.
2. Materials and methods
2.1. Materials
Poly(D,L-lactic-co-glycolic acid; 50:50, MW ~3.2 kDa, acid terminated) (PLGA) was purchased from Lakeshore Biomaterials (Evonik, Birmingham, AL). Dexamethasone sodium phosphate salt (DSP) was purchased from MP Biomedicals (Santa Ana, CA). [3H]-labeled DSP was purchased from American Radiolabeled Chemicals (St Louis, MO). Pluronic F127 (a polyethylene oxide-polypropylene oxide-polyethylene oxide triblock copolymer, or PEO-PPO-PEO), triethanolamine (TEOA), ethylenediamine-tetraacetic acid (EDTA) solution (0.5 M), zinc acetate dihydrate and all other organic solvents were purchased from Sigma-Aldrich (St. Louis, MO). Methoxy-poly(ethylene glycol)-amine (MeO-PEG-NH2) was purchased from Creative PEGWorks (Winston Salem, NC).
2.2. Preparation of model nanoparticles
Red fluorescent carboxyl-modified PS particles of 100, 200, and 500 nm (Molecular Probes®, Life Technologies, Co., Frederick, MD) in size were covalently modified with methoxy-PEG-amine by carboxyl acid-amine reaction, as described previously [24]. PEGylated PS particles (PS-PEG) were thoroughly washed, re-suspended in water, characterized and stored at 4 °C prior to use.
2.3. Preparation of placebo nanoparticles
Placebo PLGA nanoparticles (placebo NP) were prepared by a solvent diffusion method. Briefly, 20 mg of the polymer was dissolved in 1 mL of tetrahydrofuran (THF), and added dropwise to 40 mL of 5% F127 aqueous solution under magnetic stirring at 700 rpm. After stirring for about 1 h, the solution was rotoevaporated for 30 min to remove the residual THF. PLGA nanoparticles were washed with 5% F127 by centrifugation at 10,000 g for 25 min, and re-suspended in 0.2 mL of ultrapure water.
2.4. Preparation of DSP-loaded PLGA nanoparticles
Dexamethasone sodium phosphate (DSP) was encapsulated into PLGA nanoparticles following a modified solvent diffusion method, [25,26]. Briefly, a DSP-zinc complex was formed by adding 1 mL of 0.5 M zinc acetate aqueous solution to 0.5 mL of an aqueous solution containing 10 mg of DSP. After centrifugation at 10,000 g for 5 min, the precipitated complex and 50 mg PLGA were suspended and dissolved in 2.5 mL of THF followed by the addition of 20 μL of TEOA. The mixture was added dropwise into 100 mL of 5% F127 aqueous solution with stirring to form DSP-loaded PLGA nanoparticles (DSP-NP). After complete removal of the THF by solvent evaporation, 1 mL of 0.5 M EDTA aqueous solution (pH 7.5) was added to the nanoparticle suspension to chelate excess zinc and solubilize any unencapsulated DSP-zinc complexes. The nanoparticles were collected by centrifugation at 10,000 g for 25 min, washed with 5% F127, and resuspended in 0.2 mL of ultrapure water.
2.5. Nanoparticle physiochemical characterization
Particle size and ζ-potential were determined by dynamic light scattering and laser Doppler anemometry, using a Zetasizer Nano ZS90 (Malvern Instruments, Southborough, MA). Samples were diluted in 10 mM NaCl solution at pH 7.2. Transmission electron microscope (TEM) images of DSP-NP were obtained using a Hitachi H7600 transmission electron microscope (Hitachi Co. Ltd., Tokyo, Japan).
2.6. Drug loading and in vitro drug release study
To measure the DSP content in DSP-NP, approximately 50 μL of nanoparticles was lyophilized, weighed and dissolved in 0.5 mL of acetonitrile. Subsequently, 1 mL of 50 mM EDTA was added to solubilize zinc-DSP complexes, and the DSP concentration in the solution was measured by reverse phase HPLC. Isocratic separation was performed on a Shimadzu Prominence LC system (Kyoto, Japan) equipped with a Pursuit 5 C18 column (Varian Inc, Lake Forest, CA) and mobile phase consisting of acetonitrile/water (35/65 v/v) containing 0.1% trifluoroacetic acid (flow rate = 1 mL/min). Column effluent was monitored by UV detection at 241 nm. The drug loading (DL) and encapsulation efficiency (EE) were calculated according to the following equations:
To measure the in vitro release profile of DSP, 400 μL of the nanoparticle suspension was sealed in a dialysis tubing cellulose membrane (MW cutoff: 10 kDa, Sigma Aldrich, St. Louis, MO). The sealed dialysis membrane was placed into a 50 mL conical tube containing 12 mL of release media (PBS, pH 7.4) and incubated at 37 °C on a platform shaker (140 rpm). The entire release media was collected at predetermined intervals and replaced with another 12 mL of fresh PBS. DSP concentration in the collected release media was measured by HPLC as described above.
2.7. Animals
All experimental protocols were approved by the Johns Hopkins Animal Care and Use Committee. Six to eight week old male Sprague Dawley, Lewis, and Fisher rats were purchased from Harlan (Indianapolis, IN). Sprague Dawley rats were used for in vivo safety and retention studies. For the corneal transplant studies, Lewis rats were used as the receptor animals, and Fisher rats were used as donor animals. All rats were cared for and treated in accordance with the Association for Research in Vision and Ophthalmology (ARVO) resolution concerning the use of animals in ophthalmological research. The animals were anesthetized with intramuscular injection of a mixture of Ketamine (80 mg/kg) and Xylazine (8 mg/kg) during experimental procedures.
2.8. Retention of nanoparticles following subconjunctival administration
The retention of nanoparticles after SC administration was investigated by imaging the whole eye with the Xenogen IVIS Spectrum optical imaging system (Caliper Life Sciences Inc., Hopkinton, MA). Rats were anesthetized by intramuscular injection of a mixture of Ketamine (80 mg/kg) and Xylazine (8 mg/kg). Non-degradable model particles, red fluorescent PS-PEG (hydrodynamic diameter approximately 100 nm, 260 nm and 510 nm), were injected to Sprague Dawley rats by SC administration (50 μL) using a 27-gauge needle. The injection procedure was performed under a S81 operating ophthalmic microscope (Zeiss, Germany). The eyelids were retracted during imaging with a 45G speculum (Focus Ophthalmics, LLC, Ontario, CA). The total fluorescence counts at the injection site were recorded at 550/570 nm. The images were analyzed using the Living Image 3.0 software (Caliper Lifesciences, Inc.), and the retention of nanoparticles was quantified by comparing to the fluorescence counts of the same eye immediately after injection of particles. Rat eyes without particle injection were used as the baseline.
2.9. In vivo safety profile of placebo biodegradable nanoparticles
Placebo PLGA nanoparticles (placebo NP), were administered by subconjunctival (SC) injection at a dose of 1 mg particles per eye (n = 9 for each). Control eyes received SC injection of saline (n = 9). At time points of postoperative (PO) 1 day, 7 days, and 14 days, animals were sacrificed and whole eyes together with conjunctiva tissue were harvested for histological examination after fixation and staining with hematoxylin and eosin (H&E). Enucleation was initiated by canthotomies and incision of the palpebral conjunctiva, rendering the bulbar conjunctiva intact. The SC injection site was marked with a 6-0 Nylon suture, and axial sections (5 μm thick) with antero-posterior orientation (from the cornea to the optic nerve) were cut through the SC injection site. The slides were observed and graded by a pathologist.
2.10. In vivo ocular DSP levels
In order to detect the ocular DSP level after SC administration in rats, [3H]-labeled DSP was blended with non-labeled DSP (10 μCi:1 mg DSP) and used in the preparation of DSP-NP. DSP-NP were suspended in saline at a concentration of 20 μCi/mL. Unencapsulated “free” DSP solution at 20 μCi/mL was prepared at the same blending ratio. Forty microliters (~0.8 μCi per eye) of the same formulation was administered to both eyes of the same animal (Sprague Dawley rat) through SC injection. At the indicated time intervals, PO 2 h, 1 day, 3 days, 5 days and 7 days after injection, the rats were anesthetized by intramuscular injection of Ketamine/Xylazine solution, as described above. The animal was sacrificed after collecting two drops of blood from the tail vein. The eye ball with conjunctiva tissue was carefully removed and rinsed with PBS. The aqueous, vitreous, and the remaining ocular tissues containing injection sites were carefully dissected and collected. All the samples were weighed and then dissolved with 2 mL of Solvable (Perkin Elmer, Waltham, MA) by incubation at 50 °C overnight. Blood samples were bleached with 0.2 mL H2O2 and 20 μL 0.5 M EDTA. Ten milliliters of Ultima gold scintillation medium (Perkin Elmer, Waltham, MA) was added before counting the radioactivity in a scintillation counter (Perkin Elmer, Waltham, MA). The results were expressed as a percentage of the injected dose and are the mean ± standard deviation of four eyes per data point. The level of DSP in blood was the average of two animals per time point. The total percentage of the injected dose at the injection sites and the radioactivity per mg of tissue or mL of blood were calculated.
2.11. Corneal transplantation surgery
Fisher donor rats were sacrificed and the central corneal button of both eyes were removed with a 3.5-mm trephine and kept in physiological solution ready for use. The penetrating keratoplasty (PK) surgery was performed by an experienced corneal surgeon under a S81 operating ophthalmic microscope (Zeiss, Germany). The cornea recipient Lewis rats were anesthetized with an intramuscular injection of a mixture of Ketamine (80 mg/kg) and Xylazine (8 mg/kg). Repeated instillations of 0.5% tropicamide eye drops were used on Lewis rats for total pupil dilation before surgery. A paracentesis was performed before trephinization, and the anterior chamber was filled with Healon GV™ (14 mg/mL). The corneal buttons were removed from the receptor Lewis rats with a 3.5-mm trephine. The donor corneal buttons were sutured to receptor cornea with eight interrupted sutures using 10-0 Nylon.
2.12. Postoperative treatments
Immediately after PK surgery, the animals were randomly divided into 4 groups and treated with SC injection of 50 μL of various treatments: group 1 (4 rats) saline; group 2 (5 rats) placebo NP; group 3 (5 rats) free DSP aqueous solution (DSP) (2 mg/mL); and group 4 (6 rats) DSP-NP (2 mg DSP/mL). These treatment regimens were repeated once per week in all groups until the graft was clinically deemed as failed, or until the end of the study (9 weeks following PK surgery).
2.13. Clinical evaluation
Clinical observation with a slit lamp microscope was performed every 2 days until the endpoint after PK surgery. Three parameters were evaluated for the examination of the corneal grafts: corneal transparency, edema and neovascularization. Grafts were regarded as rejected only when the total score reached 5 with opacity score of at least 3 [27]. The scoring system is presented in Table 1.
Table 1.
Evaluation of clinical parameters after transplantation (score 0–4), modified from [27].
| Clinical parameter | Score
|
||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | |
| Cornea transparency | Clear cornea | Slight opacity | Mild opacity with iris details visible | Moderate opacity, iris details not visible | Severe opacity, white cornea |
| Edema | None | Slight | Moderate stromal edema | Marked stromal edema | Severe edema |
| Neovascularization | No observable growth of new vessels | New vessels invading less than 1/3 of the recipient bed | New vessel invading less than 2/3 of the recipient bed | New vessels growing up to the limiting ring of the graft | New vessels invading the graft |
2.14. Intraocular pressure measurement
Non-invasive intraocular pressure (IOP) measurements were conducted weekly after the surgery using an Icare® Tonolab (Helsinki, Finland). The IOP recorded for each eye was the average of three consecutive measurements ± standard error of the mean (SEM).
2.15. Histological examination
All surviving animals were sacrificed by CO2 at the end of the study, and the eyes that underwent PK surgery were enucleated. Eye tissues were fixed with 10% formalin for 24 h before being embedded in paraffin. Axial sections (5 μm thick) with antero-posterior orientation (from the cornea to the optic nerve) were cut, and stained with H&E.
2.16. Statistical analysis
Statistical analysis was performed by one-way analysis of variance (ANOVA) followed by Tukey’s test. The Kaplan–Meier method was used to assess graft survival rates of corneal transplants. Differences were considered to be statistically significant at a level of P < 0.05.
3. Results
3.1. Preparation and characterization of nanoparticles
It is difficult to encapsulate dexamethasone into PLGA nanoparticles owing to incompatibility between dexamethasone and PLGA [28]. However, the water-soluble prodrug of dexamethasone, dexamethasone sodium phosphate (DSP), is converted to dexamethasone in vivo by phosphatases present in all organs, including the ocular tissues [29]. We efficiently co-encapsulated DSP with zinc into PLGA nanoparticles. The physicochemical properties of DSP-loaded PLGA nanoparticles (DSP-NP) are shown in Table 2. DSP-NP exhibited a surface charge of −8 mV, indicating the surfactant used during particle preparation, Pluronic F127, coated the particle surface. The presence of surfactant on the nanoparticle surface prevented nanoparticle aggregation and improved the yield. Without surfactant, the yield of nanoparticles was extremely low due to excessive nanoparticle aggregation during the centrifuge collection procedure (data not shown). DSP-NP possessed a hydrodynamic diameter of 200 ± 8 nm (Table 2) and were spherical (Fig. 1). DSP-NP exhibited a high drug loading of 8% w/w, corresponding to an encapsulation efficiency of 72%. The release of DSP from DSP-NP occurred in a controlled manner up to 15 days in vitro, with approximately 70% of loaded DSP released within the first 7 days (Fig. 1). The use of zinc increased the encapsulation efficiency of DSP (data not shown) and promoted its sustained release from PLGA nanoparticles, presumably via formation of an ionic bridge between the terminal carboxyl groups on PLGA and the phosphate groups on the drug molecules [25,30].
Table 2.
Physicochemical properties of nanoparticles.
| Formulation | Diameter (nm) | PDI | ζ-potential (mV) | Drug loading (wt.%) |
|---|---|---|---|---|
| DSP-NP | 200 ± 8 | 0.12 | −8 ± 1.4 | 8 |
| Placebo NP | 186 ± 13 | 0.086 | −15 ± 1 | n/a |
| PS-PEG 100 nm | 100 ± 1 | 0.01 | −5 ± 2 | n/a |
| PS-PEG 200 nm | 260 ± 12 | 0.08 | −4 ± 1 | n/a |
| PS-PEG 500 nm | 510 ± 3 | 0.05 | −6 ± 1 | n/a |
Fig. 1.
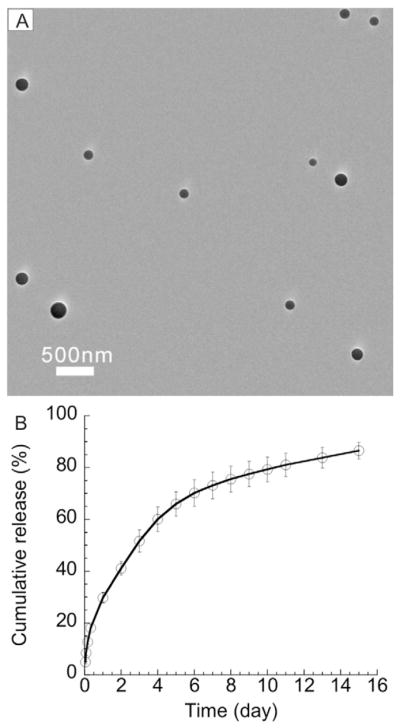
(A) TEM image and (B) in vitro DSP release profile from DSP-NP.
3.2. Ocular retention of nanoparticles
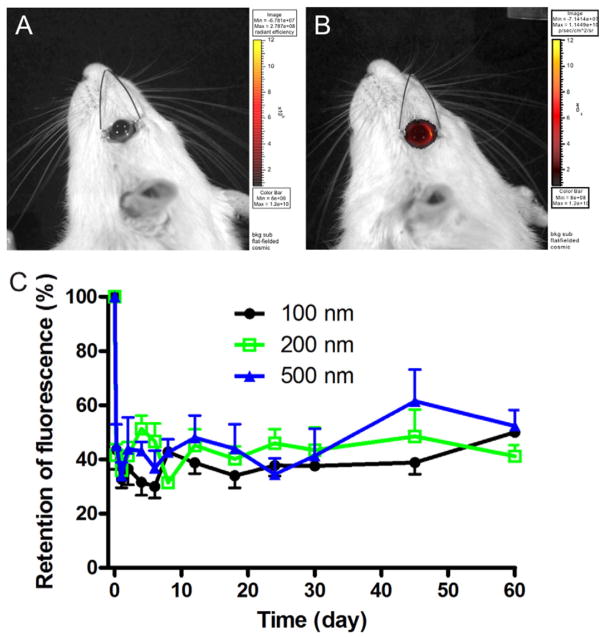
PEG-coated polystyrene (PS-PEG) particles were used to investigate the effect of size on particle retention after SC administration. PS-PEG particles with size of 100 nm, 260 nm and 510 nm, each with a near-neutral surface charge due to the PEG coating, were prepared (Table 2). Live animal imaging was used to quantify the fluorescence signal of the retained particles (Fig. 2A). PS-PEG particles (100–510 nm) exhibited a constant level of fluorescence over the 2-month retention study after an initial leakage of approximately 60% during the first 6 h after SC administration (Fig. 2B).
Fig. 2.
Fluorescence images of (A) a rat eye prior to injection and (B) a rat eye following SC administration of fluorescently-labeled nanoparticles. (C) Retention of PS-PEG particles of various diameters after SC administration to rats.
3.3. Ocular safety of placebo PLGA nanoparticles
In order to evaluate the in vivo toxicity of placebo nanoparticles (NP), we administered placebo NP to rats via SC injection. Histological examination was performed to assess the inflammatory responses in the ocular tissues (Fig. 3). Animals that received saline injection showed mild inflammation in conjunctiva tissue near the injection site at PO 2 days, and recovery at PO 7 days and PO 14 days. Similarly, conjunctiva tissue near the injection site showed mild inflammation at PO 2 days for PLGA/F127 NP, which subsided at PO 7 days and PO 14 days (grade 0). Both placebo NP and saline controls showed no inflammation in the cornea at any time point (Fig. 3).
Fig. 3.
Sample histology at PO 2 days, 7 days and 14 days showing representative images of rat conjunctiva (A, B, C, G, H, I) and cornea (D, E, F, J, K, L) after SC injection of saline or placebo NP. Conjunctival and corneal epithelia are marked with an asterisk. Foci of mildly increased inflammation were present in the underlying conjunctival substantia propria 2 days after injection but not at later time points (arrow in panels A and G). Original magnification 200×.
3.4. DSP levels in ocular tissues
DSP levels in various ocular tissues were compared following a single SC administration of either free DSP in aqueous solution or DSP-NP (both at 2 mg DSP/mL, 40μL injection). For DSP-NP, nearly 65% of the total DSP dose was retained in the conjunctiva tissue at PO 2 h, and the retained DSP levels in the conjunctiva tissue gradually decreased to 5% at PO 7 days (Fig. 4A). In comparison, only 0.4% of the total dose of free DSP was retained at the injection site at PO 2 h, and declined to just above detection limit at PO 1 day. SC injection of DSP-NP also significantly increased the concentration of DSP within the aqueous humor and vitreous up to PO 7 days (Fig. 4B and C). The concentration of DSP in both aqueous and vitreous at PO 1 day after SC administration of DSP-NP was 5160 ± 3950 ng/mL and 1290 ± 850 ng/mL, respectively. In contrast, after SC injection of free DSP, the DSP concentration within these ocular tissues rapidly dropped to undetectable levels. We also measured DSP levels in the blood at various time points to examine potential systemic exposure. SC injection of DSP-NP resulted in a low, consistent level of DSP (~50 ng/mL) from PO 2 h to PO 7 days, reflecting the sustained release; in comparison, the systemic DSP concentration after SC injection of free DSP was as high as 350 ng/mL at PO 2 h, and then diminished to baseline by PO 1 day (Fig. 4D).
Fig. 4.
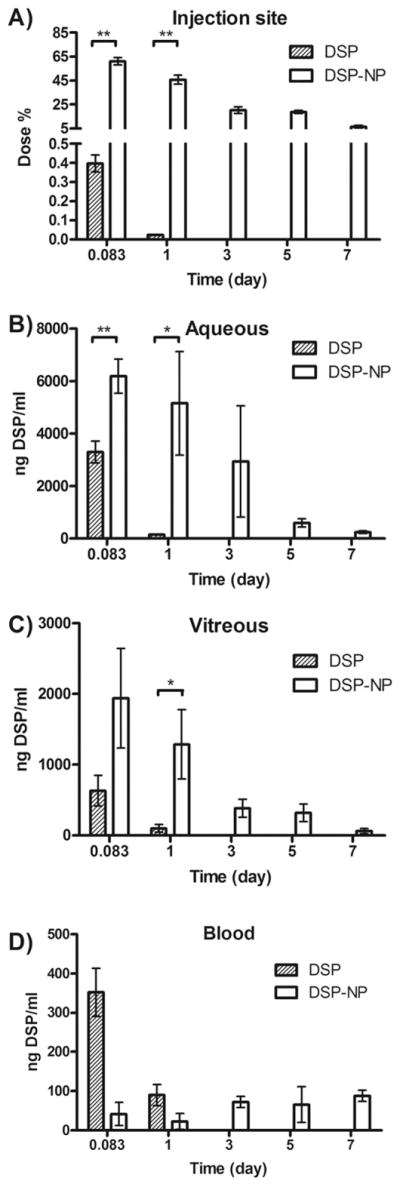
Pharmacokinetic study of free DSP solution and DSP-NP after SC administration to rats. DSP levels (A) at the injection site, (B) in the aqueous, (C) in the vitreous and (D) in blood. (*, p < 0.05; **, p < 0.01; ***, p < 0.001).
3.5. Corneal graft rejection
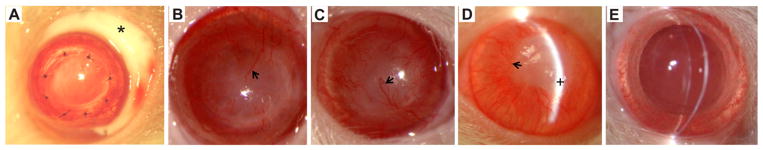
Typical PK surgery is shown in Fig. 5A. At PO 2 weeks, the corneas of animals in the saline control and placebo NP control groups exhibited severe edema, the cornea grafts were opaque, and neovascularization was evident within the corneal graft (Fig. 5B and C). By PO 4 weeks, the corneal grafts in the DSP group also developed severe edema and the cornea allografts became opaque with significant neovascularization (Fig. 5D). The DSP-NP treated group showed significantly better efficacy toward preventing corneal graft rejection, as measured by corneal transparency, edema and neovascularization (Fig. 6). There was no edema among the DSP-NP treated group, and all corneal grafts in the 6 rats were clear throughout the entire 9-week study (Fig. 5E). The neovascularization in the DSP-NP group was significantly less than all other groups (p < 0.05) (Fig. 6).
Fig. 5.
Postoperative examination of transplanted corneas. (A) A transplanted cornea immediately following SC injection of nanoparticles (site of injection marked with an asterisk). All grafts were rejected by PO 2 weeks for groups that received weekly SC injection of (B) saline and (C) placebo NP, and by PO 4 weeks for rats that received weekly SC injection of (D) free DSP. All grafts were clear until the end of study (PO 9 weeks) after weekly SC administration of (E) DSP-NP. Arrows show examples of corneal neovascularization in panels B–D. The plus sign shows site of edema in panel D.
Fig. 6.
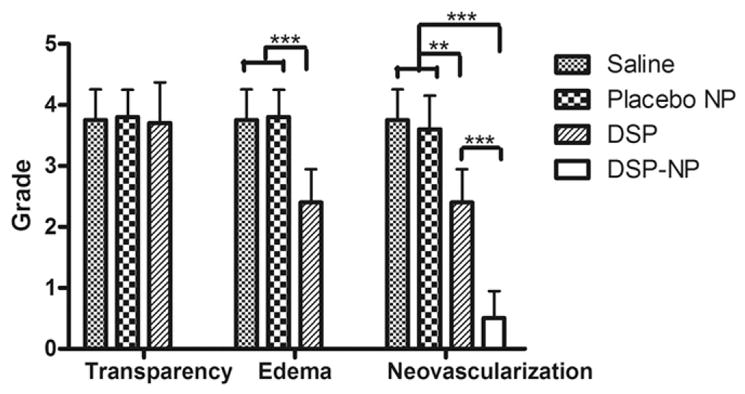
Clinical evaluation of grafts after treatment with SC injection of saline, placebo NP, free DSP or DSP-NP at the study end points (saline group at PO 2 weeks, placebo NP group at PO 2 weeks, DSP group at PO 4 weeks and DSP-NP group at PO 9 weeks). (*, p < 0.05; **, p < 0.01; ***, p < 0.001).
Animals were sacrificed when corneal graft failure occurred; the graft survival curve is shown in Fig. 7. Corneal graft rejection occurred by PO 2 weeks for saline and placebo NP control groups. A slight reduction in the rate of corneal graft rejection was achieved in animals that received weekly SC administration of free DSP, though all grafts were rejected by PO 4 weeks. In contrast, the graft survival rate for the DSP-NP treated group was 100% through to the end of the study (PO 9 weeks).
Fig. 7.
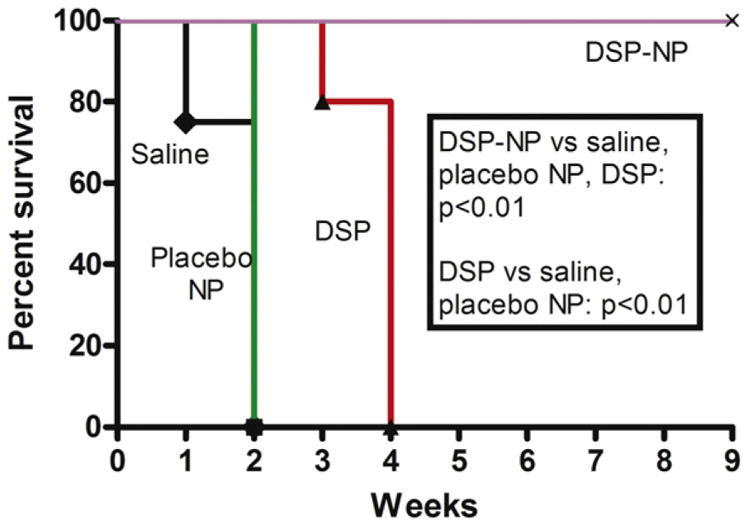
Kaplan–Meier survival curve for transplanted corneal grafts after treatment with SC injection of saline, placebo NP, free DSP or DSP-NP.
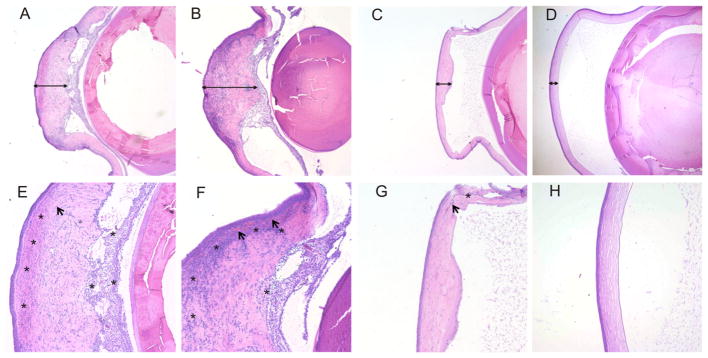
IOP was normal in all groups, except that one out of five eyes in the DSP free drug group at PO 2 weeks had an increased IOP that went back to normal levels at subsequent time points. There was no significant increase in IOP in the DSP-NP group over the course of the study (Fig. 8). Histological examination of the experimental eyes was performed upon animal sacrifice. Cornea tissues in the saline (Fig. 9A, and E), placebo NP (Fig. 9B, and F) and free DSP (Fig. 9C, and G) treated groups were all thickened, and the corneal grafts lost their structural integrity. Extensive inflammatory cell and blood vessel infiltration were observed throughout the cornea, as well as retrocorneal inflammation within the anterior chamber in some cases. In comparison, the corneas of the DSP-NP treated group had intact epithelial, stromal and endothelial layers, and no thickening or anterior chamber inflammation (Fig. 9D). In addition, neither inflammatory cells nor new blood vessel growth was observed in DSP-NP treated cornea grafts (Fig. 9H).
Fig. 8.
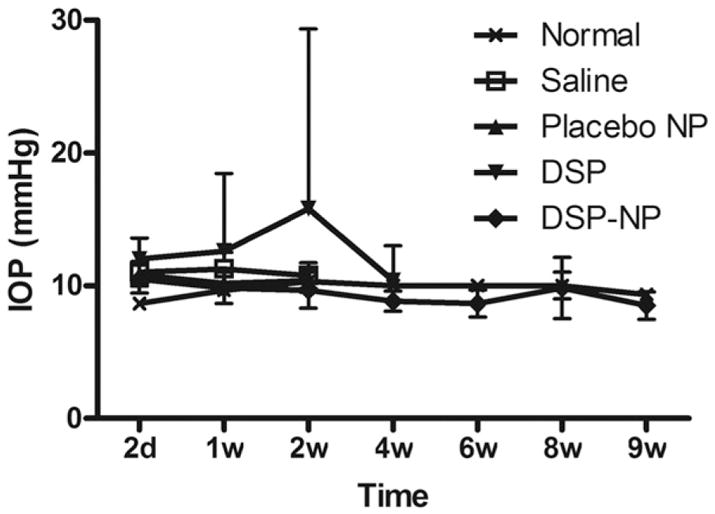
IOP measured on eyes with corneal graft transplantation and undergoing different treatments. Normal eyes without any treatment were used as the control.
Fig. 9.
Histological images of transplanted corneas after weekly SC injection with (A, E) saline at PO 2 weeks, (B, F) placebo NP at PO 2 weeks, (C, G) free DSP at PO 4 weeks and (D, H) DSP-NP at PO 9 weeks. The cornea was massively thickened in the control groups (bars, A and B), focally thickened when treated with free drug (bar, C) and normal appearing when treated with DSP-NP (bar, D). Diffuse chronic inflammation (asterisk) and associated blood vessels (arrows) were present throughout the corneal stroma in control animals. Original magnification, 40× (A, B, C, D) and 100× (E, F, G, H).
4. Discussion
Achieving effective levels of immunosuppressive agents to prevent corneal graft rejection is challenging, and requires frequent topical dosing that is difficult for patients. The most critical time is the first several days after surgery, a time when dosing requirements are also most frequent. Thus, a sustained release platform that can be administered at the time of transplantation and that provides local release of immunosuppressive agents to the eye is expected to improve patient compliance and transplant success rates [31]. Here, we demonstrated that a biodegradable nanoparticle platform with high drug loading and sustained release of DSP (DSP-NP) effectively prevented corneal allograft rejection in rats. Sustained release of DSP from DSP-NP is likely a result of both the gradual degradation of PLGA and diffusion of DSP through the PLGA matrix [25]. The DSP-NP were manufactured with components that are classified as GRAS by the FDA for various uses, and have a long history of use in pharmaceutical products, including ophthalmic formulations. These nanoparticles were found to be non-inflammatory after SC administration to healthy rats.
In addition to being difficult to comply with, frequent topical DSP drop administration also leads to high systemic drug exposure [32], making subconjunctival injection an attractive alternative route of administration. SC injection of DSP solution has been shown to more effectively deliver high levels of DSP to both the aqueous and vitreous as compared to eye drops [17,18]. It is noteworthy that we found that SC administration of free DSP resulted in more than 8-fold higher systemic DSP concentration compared to SC injection of DSP-NP at PO 2 h. Due to the sustained release of DSP, systemic exposure to DSP was minimal (50 ng/mL) over 7 days after SC administration of DSP-NP. Therefore, SC administration of DSP-NP is expected to reduce serious systemic side effects associated with corticosteroid solutions dosed as either eye drops or SC injection. Further, 65% of the original DSP dose delivered in DSP-NP was retained at the injection site on PO day 2, in contrast to only 0.4% of the original dose delivered as free DSP solution. Thus, SC injection of DSP-NP not only decreased systemic exposure, but provided sustained local drug concentrations for improved therapeutic effect.
It is well known that corticosteroids effectively inhibit the expression and action of pro-inflammatory cytokines, and have been shown to induce T-cell apoptosis [33–35]. Compared with the saline, free DSP and placebo NP control groups, the corneas of DSP-NP treated rats had no evidence of inflammatory cell infiltration. Furthermore, we observed extensive new blood vessel growth into the corneas of the rats in the control groups, but not in the DSP-NP treated group. Maintaining the avascular nature of the cornea is crucial for its immune-privileged status during cornea transplantation, and neovascularization is believed to be a driving force for corneal rejection [1,3]. SC administration of free DSP reduced neovascularization of the corneal allografts, but once weekly SC administration of free DSP was not enough to completely suppress the growth of new vessels. Even though dexamethasone shows high anti-inflammatory potency [32], the short retention of DSP solution after SC administration limits its therapeutic efficacy.
Amrite and Kompella reported that 200 nm and 2 μm non-PEGylated PS particles (carboxylate-modified) were stably retained in the subconjunctival tissue after 20–30 μL SC injection [36]. The decrease in NP retention within 6 h after SC administration observed here is likely due to leakage from the injection site caused by the higher injection volume used (50 μL). After the “leakage” during the first 6 h, non-degradable PS-PEG nanoparticles were retained in the conjunctiva tissue for the remaining 2 months of observation. Further optimization of injection conditions will likely reduce the leakage and improve retention of the DSP-NP after SC administration, thereby decreasing the required dose. DSP-NP showed better drug retention compared to free DSP after SC administration, and the DSP was released in a sustained manner from DSP-NP. Therefore, a more consistent level of DSP was achieved in the aqueous humor upon SC injection of DSP-NP. The biodegradability of PLGA will allow these nanoparticles to be gradually degraded into monomers of lactic acid and glycolic acid, which are cleared through the urine and Krebs cycle.
Long-term steroid treatment leads to increased IOP, which can result in glaucoma. Approximately 30–40% of patients given potent topical corticosteroids (dexamethasone or betamethasone) for 4–6 weeks develop increased IOP [37]. Topical ocular dexamethasone given 3 times per day significantly elevated IOP in wild-type mice after 3 weeks of treatment, and withdrawal of dexamethasone treatment reduced dexamethasone-induced elevation in IOP [38]. In our study, we did not observe increased IOP throughout the entire 9-week study for the weekly SC administration of DSP-NP in rats. Furthermore, the weekly administration regimen would allow halting the corticosteroid treatment, and a switch to other alternative immunosuppression therapy, if severe IOP elevation is observed. Finally, it is likely that this treatment will be used primarily during the time of graft transplantation only, followed by topical eye drops as needed, thereby pretreating the cornea during the critical first days after PK surgery. A 1-week treatment regimen was effective at preventing allograft rejection in the current animal model; however, formulation changes may enable the treatment regimen to be adjusted to a monthly regimen in the future.
The sustained release of corticosteroid to the anterior chamber of the eye after SC administration may be useful in treating other ocular disorders as well, such as corneal neovascularization, granulomatous iritis and uveitis.
5. Conclusions
Biodegradable nanoparticles loaded with dexamethasone sodium phosphate (DSP-NP) provided sustained release of DSP and did not cause inflammation after subconjunctival (SC) injection in rats. The nanoparticles were made of materials with a long history of safety, which should facilitate translation to human use. Treatment with DSP-NP in a rat model prevented corneal allograft rejection, whereas graft failure within 2–4 weeks occurred in the control groups, including in rats treated with weekly SC injection of free DSP. The sustained drug release provided by DSP-NP may improve patient outcomes by enhancing compliance and improving efficacy for prevention of corneal graft rejection.
Acknowledgments
The authors are grateful for the generous funding from the Raymond Kwok Family Research Fund, USA. This work was also partially funded by a grant from the King Khaled Eye Specialist Hospital of Saudi Arabia and the Eye Bank Association of America/Richard Lindstrom Research Grant 2012. The authors gratefully acknowledge Dr. Laura Ensign for helpful discussions and editing of the manuscript, as well as technical support provided by the Wilmer Microscopy and Imaging Core and the Drug Delivery and Nanotechnology Core of the Wilmer Eye Institute, each of which is supported by the NIH (P30EY001765).
References
- 1.Chong EM, Dana MR. Graft failure IV. Immunologic mechanisms of corneal transplant rejection. Int Ophthalmol. 2008;28:209–222. doi: 10.1007/s10792-007-9099-9. [DOI] [PubMed] [Google Scholar]
- 2.Tan DTH, Dart JKG, Holland EJ, Kinoshita S. Corneal transplantation. Lancet. 2012;379:1749–1761. doi: 10.1016/S0140-6736(12)60437-1. [DOI] [PubMed] [Google Scholar]
- 3.Al-Swailem SA. Graft failure: II. Ocular surface complications. Int Ophthalmol. 2008;28:175–189. doi: 10.1007/s10792-007-9127-9. [DOI] [PubMed] [Google Scholar]
- 4.Tabbara KF. Pharmacologic strategies in the prevention and treatment of corneal transplant rejection. Int Ophthalmol. 2008;28:223–232. doi: 10.1007/s10792-007-9100-7. [DOI] [PubMed] [Google Scholar]
- 5.Randleman JB, Stulting RD. Prevention and treatment of corneal graft rejection: current practice patterns (2004) Cornea. 2006;25:286–290. doi: 10.1097/01.ico.0000178731.42187.46. [DOI] [PubMed] [Google Scholar]
- 6.Hill JC. Immunosuppression in corneal transplantation. Eye. 1995;9:247–253. doi: 10.1038/eye.1995.48. [DOI] [PubMed] [Google Scholar]
- 7.Zhang EP, Schulte F, Bulfone-Paus S, Hoffmann F. The effect of corticosteroid and cyclosporin A on murine corneal allograft rejection. Graefes Arch Clin Exp Ophthalmol. 2000;238:525–530. doi: 10.1007/pl00007895. [DOI] [PubMed] [Google Scholar]
- 8.Cho YK, Uehara H, Young JR, Tyagi P, Kompella UB, Zhang X, Luo L, Singh N, Archer B, Ambati BK. Flt23k nanoparticles offer additive benefit in graft survival and anti-angiogenic effects when combined with triamcinolone. Invest Ophthalmol Vis Sci. 2012;53:2328–2336. doi: 10.1167/iovs.11-8393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shimazaki J, Iseda A, Satake Y, Shimazaki-Den S. Efficacy and safety of long-term corticosteroid eye drops after penetrating keratoplasty: a prospective, randomized, clinical trial. Ophthalmology. 2012;119:668–673. doi: 10.1016/j.ophtha.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen NX, Seitz B, Martus P, Langenbucher A, Cursiefen C. Long-term topical steroid treatment improves graft survival following normal-risk penetrating keratoplasty. Am J Ophthalmol. 2007;144:318–319. doi: 10.1016/j.ajo.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 11.Kompella UB, Kadam RS, Lee VHL. Recent advances in ophthalmic drug delivery. Ther Deliv. 2010;1:435–456. doi: 10.4155/TDE.10.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaudana R, Jwala J, Boddu SHS, Mitra AK. Recent perspectives in ocular drug delivery. Pharm Res. 2009;26:1197–1216. doi: 10.1007/s11095-008-9694-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stark WJ, Maguire MG. Design and methods of the collaborative corneal transplantation studies. Cornea. 1993;12:93–103. doi: 10.1097/00003226-199303000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Stark WJ, Maguire MG The collaborative corneal transplantation studies (CCTS) Effectiveness of histocompatibility matching in high-risk corneal transplantation. The Collaborative Corneal Transplantation Studies Research Group. Arch Ophthalmol. 1992;110:1392–1403. [PubMed] [Google Scholar]
- 15.Jones R, Rhee DJ. Corticosteroid-induced ocular hypertension and glaucoma: a brief review and update of the literature. Curr Opin Ophthalmol. 2006;17:163–167. doi: 10.1097/01.icu.0000193079.55240.18. [DOI] [PubMed] [Google Scholar]
- 16.McGhee CNJ, Dean S, Danesh-Meyer H. Locally administered ocular corticosteroids — benefits and risks. Drug Saf. 2002;25:33–55. doi: 10.2165/00002018-200225010-00004. [DOI] [PubMed] [Google Scholar]
- 17.Hosseini K, Matsushima D, Johnson J, Widera G, Nyam K, Kim L, Xu YD, Yao YJ, Cormier M. Pharmacokinetic study of dexamethasone disodium phosphate using intravitreal, subconjunctival, and intravenous delivery routes in rabbits. J Ocul Pharmacol Ther. 2008;24:301–308. doi: 10.1089/jop.2007.0117. [DOI] [PubMed] [Google Scholar]
- 18.Weijtens O, Feron EJ, Schoemaker RC, Cohen AF, Lentjes EGWM, Romijn FPHTM, van Meurs JC. High concentration of dexamethasone in aqueous and vitreous after subconjunctival injection. Am J Ophthalmol. 1999;128:192–197. doi: 10.1016/s0002-9394(99)00129-4. [DOI] [PubMed] [Google Scholar]
- 19.Xu Q, Kannan R, Kambhampati S. Nanotechnology approaches for ocular drug delivery. Middle East Afr J Ophthalmol. 2013;20:26–37. doi: 10.4103/0974-9233.106384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wadhwa S, Paliwal R, Paliwal SR, Vyas SP. Nanocarriers in ocular drug delivery: an update review. Curr Pharm Des. 2009;15:2724–2750. doi: 10.2174/138161209788923886. [DOI] [PubMed] [Google Scholar]
- 21.Jiang C, Moore MJ, Zhang X, Klassen H, Langer R, Young M. Intravitreal injections of GDNF-loaded biodegradable microspheres are neuroprotective in a rat model of glaucoma. Mol Vis. 2007;13:1783–1792. [PubMed] [Google Scholar]
- 22.Shelke N, Kadam R, Tyagi P, Rao V, Kompella U. Intravitreal poly(L-lactide) microparticles sustain retinal and choroidal delivery of TG-0054, a hydrophilic drug intended for neovascular diseases. Drug Deliv Transl Res. 2011;1:76–90. doi: 10.1007/s13346-010-0009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ayalasomayajula SP, Kompella UB. Retinal delivery of celecoxib is several-fold higher following subconjunctival administration compared to systemic administration. Pharm Res. 2004;21:1797–1804. doi: 10.1023/b:pham.0000045231.51924.e8. [DOI] [PubMed] [Google Scholar]
- 24.Nance EA, Woodworth GF, Sailor KA, Shih TY, Xu Q, Swaminathan G, Xiang D, Eberhart C, Hanes J. A dense poly(ethylene glycol) coating improves penetration of large polymeric nanoparticles within brain tissue. Sci Transl Med. 2012;4:149ra119. doi: 10.1126/scitranslmed.3003594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ishihara T, Izumo N, Higaki M, Shimada E, Hagi T, Mine L, Ogawa Y, Mizushima Y. Role of zinc in formulation of PLGA/PLA nanoparticles encapsulating betamethasone phosphate and its release profile. J Control Release. 2005;105:68–76. doi: 10.1016/j.jconrel.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 26.Ishihara T, Kubota T, Choi T, Takahashi M, Ayano E, Kanazawa H, Higaki M. Polymeric nanoparticles encapsulating betamethasone phosphate with different release profiles and stealthiness. Int J Pharm. 2009;375:148–154. doi: 10.1016/j.ijpharm.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 27.Di Tommaso C, Bourges J-L, Valamanesh F, Trubitsyn G, Torriglia A, Jeanny J-C, Behar-Cohen F, Gurny R, Möller M. Novel micelle carriers for cyclosporin A topical ocular delivery: in vivo cornea penetration, ocular distribution and efficacy studies. Eur J Pharm Biopharm. 2012;81:257–264. doi: 10.1016/j.ejpb.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 28.Gomez-Graete C, Tsapis N, Besnard M, Bochot A, Fattal E. Encapsulation of dexamethasone into biodegradable polymeric nanoparticles. Int J Pharm. 2007;331:153–159. doi: 10.1016/j.ijpharm.2006.11.028. [DOI] [PubMed] [Google Scholar]
- 29.Rautio J, Kumpulainen H, Heimbach T, Oliyai R, Oh D, Jarvinen T, Savolainen J. Prodrugs: design and clinical applications. Nat Rev Drug Discov. 2008;7:255–270. doi: 10.1038/nrd2468. [DOI] [PubMed] [Google Scholar]
- 30.Ishihara T, Takahashi M, Higaki M, Mizushima Y. Efficient encapsulation of a water-soluble corticosteroid in biodegradable nanoparticles. Int J Pharm. 2009;365:200–205. doi: 10.1016/j.ijpharm.2008.08.030. [DOI] [PubMed] [Google Scholar]
- 31.Edelhauser HF, Rowe-Rendleman CL, Robinson MR, Dawson DG, Chader GJ, Grossniklaus HE, Rittenhouse KD, Wilson CG, Weber DA, Kuppermann BD, Csaky KG, Olsen TW, Kompella UB, Holers VM, Hageman GS, Gilger BC, Campochiaro PA, Whitcup SM, Wong WT. Ophthalmic drug delivery systems for the treatment of retinal diseases: basic research to clinical applications. Invest Ophthalmol Vis Sci. 2010;51:5403–5420. doi: 10.1167/iovs.10-5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weijtens O, Schoemaker RC, Romijn FPHTM, Cohen AF, Lentjes EGWM, van Meurs JC. Intraocular penetration and systemic absorption after topical application of dexamethasone disodium phosphate. Ophthalmology. 2002;109:1887–1891. doi: 10.1016/s0161-6420(02)01176-4. [DOI] [PubMed] [Google Scholar]
- 33.Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids — new mechanisms for old drugs. N Engl J Med. 2005;353:1711–1723. doi: 10.1056/NEJMra050541. [DOI] [PubMed] [Google Scholar]
- 34.Barnes PJ. Mechanisms and resistance in glucocorticoid control of inflammation. J Steroid Biochem Mol Biol. 2010;120:76–85. doi: 10.1016/j.jsbmb.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 35.Ito K, Chung KF, Adcock IM. Update on glucocorticoid action and resistance. J Allergy Clin Immunol. 2006;117:522–543. doi: 10.1016/j.jaci.2006.01.032. [DOI] [PubMed] [Google Scholar]
- 36.Amrite AC, Kompella UB. Size-dependent disposition of nanoparticles and microparticles following subconjunctival administration. J Pharm Pharmacol. 2005;57:1555–1563. doi: 10.1211/jpp.57.12.0005. [DOI] [PubMed] [Google Scholar]
- 37.Kersey JP, Broadway DC. Corticosteroid-induced glaucoma: a review of the literature. Eye. 2006;20:407–416. doi: 10.1038/sj.eye.6701895. [DOI] [PubMed] [Google Scholar]
- 38.Zode GS, Sharma AB, Lin X, Searby CC, Bugge K, Kim GH, Clark AF, Sheffield VC. Ocular-specific ER stress reduction rescues glaucoma in murine glucocorticoid-induced glaucoma. J Clin Invest. 2014;124:1956–1965. doi: 10.1172/JCI69774. [DOI] [PMC free article] [PubMed] [Google Scholar]