A 58-year-old man from Cuba, New York was referred to our institution in July 2016 with a chief complaint of increasing confusion for one month. It was associated with irritability, decreased social interaction, memory loss, gait instability, fatigue and weight loss. He denied any fever, night sweats, depression or alcohol consumption. He worked in a warehouse and lived in a rural area. There was no recent travel history or mosquito-bite that he could recall. His only medical history was TP53 wild-type chronic lymphocytic leukemia (CLL) which was diagnosed in December 2011. He was on observation until June 2014 when he developed bulky lymphadenopathy. He was treated with bendamustine plus rituximab for six cycles and achieved complete remission. From April 2015, four weekly rituximab infusions every six months were initiated as maintenance therapy. He had completed three out of the four planned cycles. The last dose was administered three months ago.
At presentation, the vital signs were within normal limits. Neurological exam was significant for cognitive dysfunction but still communicative, extremely brisk reflexes throughout and sustained ankle clonus bilaterally. His gait was wide-based. Other physical examination findings were unremarkable.
This patient presents with rather acute cognitive impairment. Given his history of CLL, the top differential diagnoses include infectious versus paraneoplastic/autoimmune encephalitis and relapsed CLL with central nervous system (CNS) involvement. The fact that he is on rituximab therapy further increases the infection risk. Uncommon infections have also been associated with rituximab such as JC virus induced progressive multifocal leukoencephalopathy.1 CLL patients have a 5–10% risk of developing autoimmune complications. Although they primarily cause cytopaenia (i.e. autoimmune hemolytic anemia and immune thrombocytopenia),2 autoimmune limbic encephalitis has been reported as one of the non-hematological complications.3 CNS involvement by CLL occurs in <1% of the patients.4 In addition, early-onset of neurodegenerative diseases and other causes of encephalopathy should also be excluded.
Laboratory results showed profound lymphopenia of 0.65 × 109/L (1–4.5 × 109) and isolated low serum IgM of 34 (35–242 mg/dl). Total WBC, hemoglobin, platelet, lactate dehydrogenase and beta-2-microglobulin were normal. Common causes of toxic-metabolic encephalopathy were ruled out. Cerebrospinal fluid (CSF) analysis showed T-cell predominant (90%) pleocytosis of 13 WBCs/µL, elevated total protein of 189 (12–60 mg/dl) and normal glucose. An extensive workup for bacterial, viral, fungal and protozoal CNS infections including JC virus and prion disease were conducted. All test results were non-diagnostic (Tab. S1). CSF cytology, flow cytometry, paraneoplastic and autoimmune encephalitis antibody screening were negative. A contrast-enhanced brain MRI showed non-specific diffuse dural enhancement but was otherwise unremarkable. A torso CT scan was negative for lymphadenopathy and other pathology. An electroencephalogram suggested diffuse encephalopathy without seizures.
The CSF analysis is consistent with encephalitis. However, viral versus autoimmune etiology still cannot be differentiated despite extensive testing. It is important to recognize that in the majority of viral encephalitis cases, a specific pathogen is not identified.5 More than 50% cases of autoimmune encephalitis are antibody test negative.6 The absence of leukocytosis and lymphadenopathy along with normal hemoglobin and platelet count suggest the quiescence of CLL. The negative brain MRI, CSF cytology and flow cytometry results are against CNS involvement by CLL.
One week into investigation, his confusion had worsened. A five-day course of high-dose methylprednisolone followed by intravenous immunoglobulin was given for suspected antibody-negative autoimmune encephalitis. However, his mental status did not improve. A repeat CSF analysis showed decreasing WBC of 3/µL and total protein of 106 mg/dl. In order to exclude other rare infections which have not been previously associated with rituximab, the CSF was retested with an extensive PCR encephalitis panel.7 All test results returned negative except Cache Valley virus (CVV). One month after first presentation, he became non-verbal, contracted and bed-ridden. A repeat MRI showed interval development of early brain atrophy and periventricular white matter enhancement (Fig. 1). The patient died on hospice at end of August 2016.
Figure 1. Serial brain MRIs.
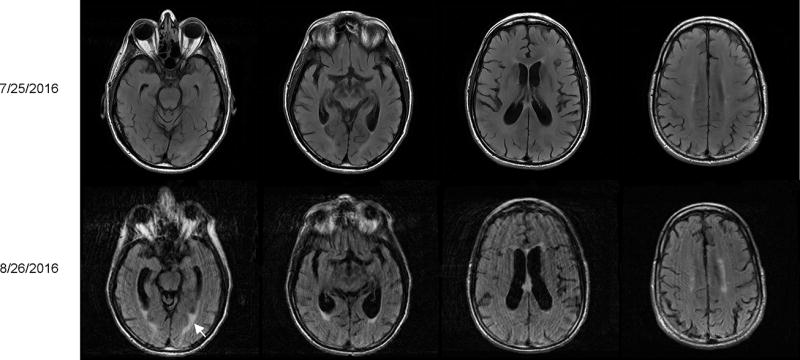
Top panels show a normal brain structure in cross section views of T2-FLAIR scans which were obtained at the initial presentation. Repeat scans five days before the patient’s death are shown in the bottom panels. There was interval development of brain atrophy with prominence of the ventricles and sulci as well as periventricular white matter enhancement (white arrow).
A brain autopsy was performed. Histopathological analysis of the parenchyma revealed focal areas of gliosis, perivascular lymphocytic cuffing and increased reactive microglia (Fig. 2A–D). No viral inclusions were observed. There was focal leptomeningeal lymphocyte aggregation. These findings are consistent with meningoencephalitis. The infiltrating cells were CD3-positive, CD20-negative suggesting a pure T-cell population (Fig. 2E–G). The masking effect of rituximab on CD20 immunostaining was excluded using additional B-cell markers PAX5 and CD79a (data not shown).
Figure 2. Histological analysis of brain autopsy.
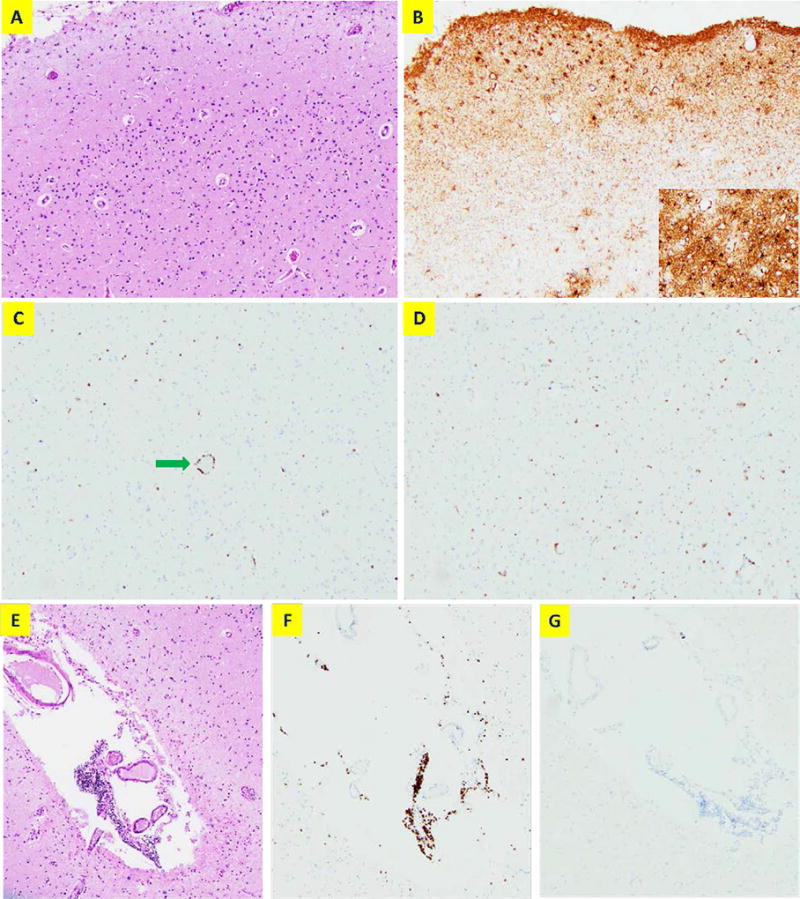
(A) A representative image from the superior temporal cortex by haematoxylin and eosin (H&E) stain (100×). No significant neuronal loss or viral inclusions are observed. (B) Immunostaining of GFAP shows mild gliosis. The insert shows higher power view (200×). (C) CD3 immunostaining shows scattered T lymphocyte infiltration with perivascular cuffing (green arrow). (D) CD68 immunostaining shows increased reactive microglia without forming nodules or clusters. (E) H&E staining showing an example of focal leptomeningeal lymphocytic aggregation (100×). These cells are CD3-positive (F) and CD20-negative (G) consistent with T lymphocytes.
CVV lineage 2 was detected and isolated from the brain tissue. Homogenates from two separate sections were tested by RT-PCR using primers and probes based on lineage 2 sequence (supplementary methods).8 The cycle thresholds were 26 and 34 respectively (positive if CT<38). CVV-infected brain cells were identified by immunohistochemistry. Sections from the left frontal cortex showed scattered CVV-positive cells with neuron morphology (Fig. 3A–B). The density of CVV-immunostained cells averaged 16.1 cells/mm2 by counting 15 one-mm2 fields from three sections. Colocalization of immunofluorescence double staining with anti-CVV and NeuN (a neuronal marker) antibodies suggested CVV selectively infecting neurons. There was no apparent overlap using anti-GFAP antibody (a glial marker) (Fig. S1). Electron microscopy was performed on the Vero cells after co-culture with the autopsy specimen for 48 hrs. Viral particles were directly visualized measuring 87nm in mean diameter (S.D. 3.2nm) which is classic for CVV (Fig. 3C).9
Figure 3. CVV specific immunostaining and electron microscopy.
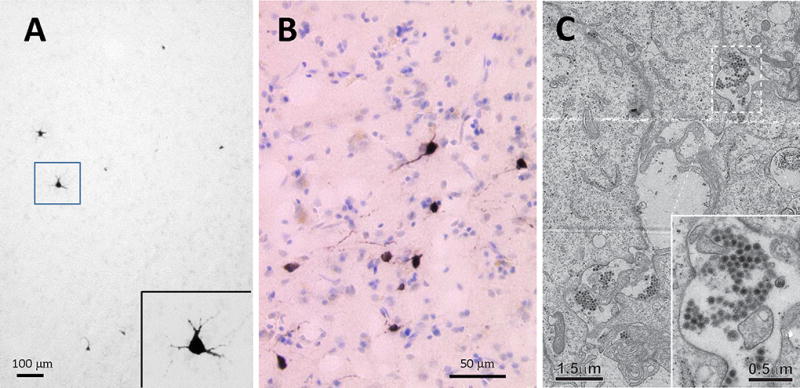
(A) A representative image of the brain showing CVV-immunoreactive cells (black) with high-magnification view in the insert; (B) Another section showing counter-stained with cresyl violet after immunostaining; (C) A representative electron micrograph of the Vero cells at 48 hrs of viral culture. Virus particles were found widely distributed in the specimen as shown. The magnified micrographs, shown in the insert, indicate that virus particles present as groups in membrane-surrounded compartments.
Commentary
Cache Valley virus is known as mosquito-borne agent causing encephalitis in livestock. Although human seroprevalence can be up to 19% in endemic areas, clinically significant infections are extremely rare.8 We describe a case of acute meningoencephalitis following lineage 2 CVV infection which appears to be emerging in Northeast United States. This report expands our knowledge of CVV infection as a fatal complication associated with rituximab maintenance (RM) therapy.
Rituximab is an engineered anti-CD20 monoclonal antibody. While it is extremely effective against B-cell lymphoma/leukemia, it also depletes normal B-cell. Four weekly doses of rituximab commonly result in undetectable peripheral B-cell for 6–12 months.1 Our patient’s symptoms started two months following last treatment which falls within the window of expected B-cell nadir. This is supported by persistent lymphopenia in serial CBCs. Physiologically B-cell controls antibody production and acts as antigen-presenting cell. Rituximab recipients exhibit decreased to absent humoral responses to new antigens. The ability to respond is correlated with the degree of B-cell recovery.10 Our patient’s decreased IgM level despite facing a life-threatening acute infection suggests ineffective antibody response as a result of B-cell deprivation. Furthermore, B-cell infiltration was strikingly obviated in the brain histopathology. It is in contrast with the findings in animal model that brain B-cell infiltration is a prerequisite for launching adaptive immune response against CVV.11 Although there was no available serology test to document the antibody deficiency, this conclusion is consistent with observations that rituximab recipients who contracted West Nile, tick-borne and eastern equine viral encephalitis failed to develop immunoglobulin responses.1,12,13 Noteworthy, our patient did not have histologically significant neuron loss in parallel to his symptoms. This mismatch along with persistently high numbers of infected neurons by immunohistochemistry suggests failure to clear the virus and transition to chronic infection accompanied by neuron dysfunction. Our finding is in agreement with a recent report that a hereditary B-cell maturation disorder predisposed a patient to chronic CVV encephalitis.14
Our patient was infected by CVV during RM therapy which has been associated with increased infection risk.1 Prolonged exposure to rituximab results in hypogammaglobulinemia, neutropenia and impaired T-cell function. Although six-month rituximab-containing chemoimmunotherapy is recommended by NCCN guidelines in certain CLL patients, the role of RM is controversial.15 A randomized clinical trial showed only 11.5-month improvement in median progression-free survival with two-year treatment compared to observation.16 Whether it will translate into overall survival benefit is questionable, but certainly there are equally effective retreat-at-relapse strategy and well-established second-line treatment options.17 There are about 45,000 patients receiving rituximab annually in the US for lymphoma at an estimated cost of two billion dollars, let alone many more rituximab recipients with autoimmune disorders and post-transplant rejections.18 The imposed infection risk and associated financial burden are hard to ignore. This case reinforces that clinicians should continue to exert extra caution before recommending such treatment.
The insidious onset of symptoms, well-known autoimmune complications of CLL and unawareness of CVV meningoencephalitis made our diagnosis challenging. In retrospect, CVV testing should have been included as part of the initial workup and glucocorticosteroids should have been avoided in this already heavily immunosuppressed patient. Our diagnosis was made through a positive PCR test from CSF. In literature review, there are four previously reported cases.9,14,19,20 While PCR is the test of choice, the specimens used were variable including blood, CSF and brain biopsy tissue. Neuroimaging findings are non-specific. Two patients had normal brain MRIs who were both immunocompetent and fully recovered after a self-limiting course of aseptic meningitis. Unlike our case, the other two patients with acute encephalitis presented with brain MRI abnormalities of focal parenchymal enhancements on T2-FLAIR. However, the affected areas were rather different ranging from leptomeninges and parietal cortex to hippocampus and amygdala. One of them had known x-linked agammaglobulinemia. There are no proven treatments. All three patients who developed encephalitis died from catastrophic neurological sequela.
Supplementary Material
Acknowledgments
The authors are grateful to the patient’s family for providing permission to share the medical information. The authors thank Dr. Kirsten St. George from New York State Department of Health for editing the manuscript.
This work was supported in part by the National Institutes of Health grants GM101026 and GM097010 (H.S.).
Footnotes
Contributions:
Y.Y. cared for the patient and wrote the manuscript with input from F.H.; J.Q. performed autopsy and pathology analysis; A.S.K. performed CVV specific immunohistochemistry; Y.W. did viral cell culture; Y.W. and S.S. prepared the sample block and EM specimens; Y.W. and H.S. performed electron microscopic analysis ; A.D. performed PCR of CSF; L.K. performed PCR of the brain tissue; F.H. provided supervision and manuscript editing.
Conflict-of-interest disclosure:
The authors declare no competing financial interests.
Contributor Information
Yuanquan Yang, Department of Medicine, Roswell Park Cancer Institute, Buffalo, New York.
Jingxin Qiu, Department of Pathology, Roswell Park Cancer Institute, Buffalo, New York.
Abigail Snyder-Keller, Wadsworth Center, New York State Department of Health.
Yongping Wu, Wadsworth Center, New York State Department of Health.
Shufeng Sun, Wadsworth Center, New York State Department of Health.
Haixin Sui, Wadsworth Center, New York State Department of Health.
Amy B. Dean, Wadsworth Center, New York State Department of Health.
Laura Kramer, Wadsworth Center, New York State Department of Health.
Francisco Hernandez-Ilizaliturri, Department of Medicine, Roswell Park Cancer Institute, Buffalo, New York.
References
- 1.Gea-Banacloche JC. Rituximab-associated infections. Seminars in hematology. 2010;47:187–98. doi: 10.1053/j.seminhematol.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Zent CS, Kay NE. Autoimmune complications in chronic lymphocytic leukaemia (CLL) Best Pract Res Clin Haematol. 2010;23:47–59. doi: 10.1016/j.beha.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nogai H, Israel-Willner H, Zschenderlein R, Pezzutto A. Improvement of paraneoplastic limbic encephalitis after systemic treatment with rituximab in a patient with B-cell chronic lymphocytic leukemia. Case Rep Hematol. 2013;2013:958704. doi: 10.1155/2013/958704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strati P, Uhm JH, Kaufmann TJ, et al. Prevalence and characteristics of central nervous system involvement by chronic lymphocytic leukemia. Haematologica. 2016;101:458–65. doi: 10.3324/haematol.2015.136556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Venkatesan A, Tunkel AR, Bloch KC, et al. Case definitions, diagnostic algorithms, and priorities in encephalitis: consensus statement of the international encephalitis consortium. Clin Infect Dis. 2013;57:1114–28. doi: 10.1093/cid/cit458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hacohen Y, Wright S, Waters P, et al. Paediatric autoimmune encephalopathies: clinical features, laboratory investigations and outcomes in patients with or without antibodies to known central nervous system autoantigens. J Neurol Neurosurg Psychiatry. 2013;84:748–55. doi: 10.1136/jnnp-2012-303807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dupuis M, Hull R, Wang H, et al. Molecular detection of viral causes of encephalitis and meningitis in New York State. J Med Virol. 2011;83:2172–81. doi: 10.1002/jmv.22169. [DOI] [PubMed] [Google Scholar]
- 8.Armstrong PM, Andreadis TG, Anderson JF. Emergence of a new lineage of Cache Valley virus (Bunyaviridae: Orthobunyavirus) in the Northeastern United States. Am J Trop Med Hyg. 2015;93:11–7. doi: 10.4269/ajtmh.15-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sexton DJ, Rollin PE, Breitschwerdt EB, et al. Life-threatening Cache Valley virus infection. N Engl J Med. 1997;336:547–9. doi: 10.1056/NEJM199702203360804. [DOI] [PubMed] [Google Scholar]
- 10.Eisenberg RA, Jawad AF, Boyer J, et al. Rituximab-treated patients have a poor response to influenza vaccination. J Clin Immunol. 2013;33:388–96. doi: 10.1007/s10875-012-9813-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodrigues Hoffmann A, Dorniak P, Filant J, et al. Ovine fetal immune response to Cache Valley virus infection. J Virol. 2013;87:5586–92. doi: 10.1128/JVI.01821-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knight A, Pauksens K, Nordmark G, Kumlien E. Fatal outcome of tick-borne encephalitis in two patients with rheumatic disease treated with rituximab. Rheumatology (Oxford) 2017;56:855–6. doi: 10.1093/rheumatology/kew495. [DOI] [PubMed] [Google Scholar]
- 13.Solomon IH, Ciarlini P, Santagata S, et al. Fatal Eastern Equine Encephalitis in a Patient on Maintenance Rituximab: A Case Report. Open Forum Infect Dis. 2017;4:ofx021. doi: 10.1093/ofid/ofx021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilson MR, Suan D, Duggins A, et al. A Novel Cause of Chronic Viral Meningoencephalitis: Cache Valley Virus. Ann Neurol. 2017 doi: 10.1002/ana.24982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wierda WG, Zelenetz AD, Gordon LI, et al. NCCN Guidelines Insights: Chronic Lymphocytic Leukemia/Small Lymphocytic Leukemia, Version 1.2017. J Natl Compr Canc Netw. 2017;15:293–311. doi: 10.6004/jnccn.2017.0030. [DOI] [PubMed] [Google Scholar]
- 16.Greil R, Obrtlikova P, Smolej L, et al. Rituximab maintenance versus observation alone in patients with chronic lymphocytic leukaemia who respond to first-line or second-line rituximab-containing chemoimmunotherapy: final results of the AGMT CLL-8a Mabtenance randomised trial. Lancet Haematol. 2016;3:e317–29. doi: 10.1016/S2352-3026(16)30045-X. [DOI] [PubMed] [Google Scholar]
- 17.Williams ME, Hong F, Gascoyne RD, et al. Rituximab extended schedule or retreatment trial for low tumour burden non-follicular indolent B-cell non-Hodgkin lymphomas: Eastern Cooperative Oncology Group Protocol E4402. Br J Haematol. 2016;173:867–75. doi: 10.1111/bjh.14007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Danese MD, Reyes CM, Gleeson ML, Halperin M, Skettino SL, Mikhael J. Estimating the Population Benefits and Costs of Rituximab Therapy in the United States from 1998 to 2013 Using Real-World Data. Med Care. 2016;54:343–9. doi: 10.1097/MLR.0000000000000486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nguyen NL, Zhao G, Hull R, et al. Cache valley virus in a patient diagnosed with aseptic meningitis. J Clin Microbiol. 2013;51:1966–9. doi: 10.1128/JCM.00252-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campbell GL, Mataczynski JD, Reisdorf ES, et al. Second human case of Cache Valley virus disease. Emerg Infect Dis. 2006;12:854–6. doi: 10.3201/eid1205.051625. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


