Abstract
The ELISpot assay prevails as one of the most sensitive and meaningful assays for the detection of antigen-specific, effector immune responses. Acquisition of cellular analyte for ELISpot analysis is typically not problematic when derived from tissues enriched in lymphocytes (e.g., lymphoid organs and blood); however, cell processing becomes more difficult when lymphocytes represent only a very minor population relative to the source tissue, especially when the source tissue is in limited supply (e.g., small mouse tumors). Traditional enzymatic-based methods for dissociating tumors often result in poor yields, inconsistent lymphocyte enrichment, and can have deleterious effects on lymphocyte phenotype and function. To address these limitations, we have developed an enzyme-free protocol for processing tumor infiltrating lymphocytes (TILs) from small mouse tumors, which enables the enumeration of antigen-specific effector lymphocytes using ELISpot analysis. This procedure is predicated on the dissociation of tumor tissue using gentle agitation with a paddle blender followed by a brief in vitro culture period to remove adherent cells, as well as to revive lymphocytes from a non-responsive state. Although this method is demonstrated with mouse intracerebral tumors, we have found that this protocol is applicable to peripheral tumors and may likely extend to alternative tissue sources wherein lymphocytes exist in low numbers.
Keywords: tumor infiltrating lymphocyte, TIL, ELISpot
1. INTRODUCTION
The detection of antigen-specific lymphocytes is crucial for immune monitoring, as well as investigating the immunological basis of cancer, autoimmunity, and pathogenic disease. Among the most widely-used assays for this purpose are the ELISpot assay, multimer analysis, and intracellular staining (ICS) (Phetsouphanh et al., 2015). Of these methods, the ELISpot assay offers several clear advantages. The ELISpot is based on the principle that soluble molecules secreted by stimulated cells are captured onto antibody coated plates, allowing effector cells to be enumerated (Czerkinsky et al., 1988). In contrast to multimer analysis, the ELISpot is a functional assay that can test lymphocyte reactivity to a large number of antigens in a high throughput manner without an underlying knowledge of exact epitopes or MHC alleles (Calarota et al., 2013). Furthermore, the ELISpot is capable of detecting low avidity T cells, which includes many tumor-reactive or autoimmune T cells and most CD4+ T cells, while the use of multimers is typically reserved for the detection of high avidity T cells (Wooldridge et al., 2009). In comparison with ICS, both methods have the ability to sensitively detect antigen-specific lymphocytes that produce a variety of soluble molecules (e.g., cytokines, chemokines, and antibodies); however, the ELISpot is superior for the detection of bona fide effector cells given that it measures only secreted, and therefore biologically-relevant, effector molecules (Calarota et al., 2013).
Tumors frequently contain an enriched population of tumor-reactive lymphocytes that are desirable for investigating tumor immunobiology. Unfortunately, isolating TILs from small mouse tumors has historically been a challenge due to their low frequencies and a limited supply of tissue (Prevost-Blondel et al., 1998). Furthermore, the oft-used method of dissociating tumors with enzymes, such as DNase and collagenase, are prone to inconsistencies and have been shown to alter surface T-cell coreceptor expression levels, which may affect T-cell function (Mulder et al., 1994). Here, we present a simple and enzyme-free protocol for processing TILs from small mouse intracerebral tumors for ELISpot analysis. Briefly, tumors are gently dissociated into a single cell suspension using a paddle blender. Cells are then plated for several hours in a tissue culture-treated flask to remove adherent cells (e.g., tumor cells, macrophages, stromal cells) that could otherwise sterically interfere with effector molecule capture onto the plate. Finally, a 2-day in vitro culture period in medium containing IL-2 allows lymphocytes to recover from a non-responsive state (Mognol et al., 2017). This methodology permits an enrichment of viable and responsive effector immune cells suitable for ELISpot analysis.
2. METHODS
2.1 Tumor cell preparation and intracerebral injection
B16F10 mouse melanoma and a variant expressing chicken ovalbumin, B16OVA, were used for this study. Upon injection of B16OVA into syngeneic C57BL/6 mice, the host immune system spontaneously activates CD8+ T cells recognizing the epitope SIINFEKL bound to the MHC I allele Kb. B16 cells were cultured in DMEM containing 10% heat inactivated FBS and 1× penicillin streptomycin to 85% confluency at 37°C/ 5% CO2. Cells were then passaged at a 1:8 dilution and harvested by trypsinization at approximately 60–80% confluency two days later. B16 melanoma cells were washed thrice in ice-cold 1× PBS, viable cells counted using trypan blue exclusion, and cells resuspended to 4×106 cells mL−1 in 1× PBS. Tumor cells were then mixed thoroughly in an equal volume of 10% methylcellulose in PBS using a sterile 1 mL syringe. 6 to 12-month-old C57BL/6 mice were premedicated with buprenorphine SR and anesthetized in an isoflurane chamber. Anesthetized mice were then placed into a stereotactic frame (Kopf), and a ~1 cm sagittal incision was made to reveal the skull. One drop of bupivacaine was added to the incision site. A 25-gauge injection needle was placed 2 mm right of the bregma and inserted to a depth of 4 mm below the surface of the skull. 1.0×104 cells in a volume of 5 µL were delivered into the caudate nucleus. Following injection, the hole was sealed using bone wax and the incision closed with a surgical staple. C57BL/6 mice inoculated intracerebrally with B16 melanoma cells become moribund in ~20–26 days. All animal experiments were performed in accordance with Duke University Institutional Animal Care and Use Committee-approved protocols.
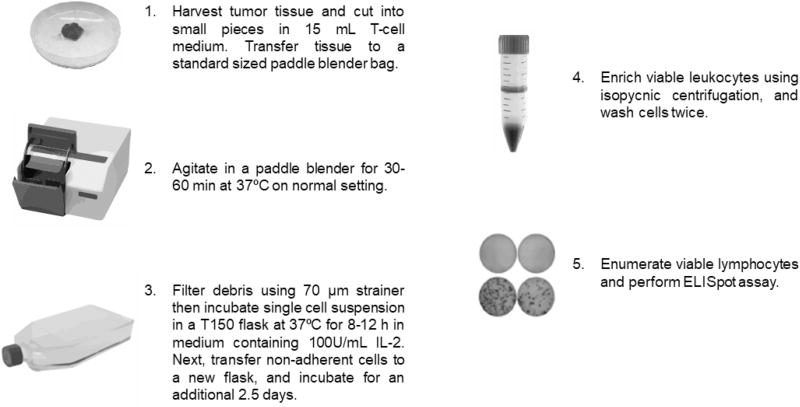
2.2 Intracerebral tumor harvesting
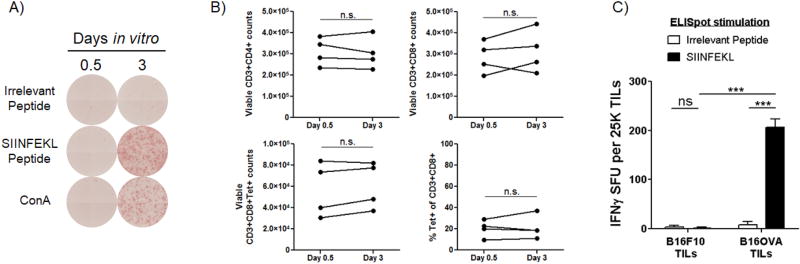
Moribund mice were sacrificed in a CO2 chamber followed by decapitation. In a laminar flow hood, using sterile forceps and scissors, the skull plate covering the tumor injection site was carefully removed. B16F10 and B16OVA tumors are distinguishable from normal brain tissue by their darker color. The tumor burden was removed using jeweler’s forceps and placed in a non -tissue culture-treated dish containing 15 mL of T-cell medium (RPMI, 10% heat inactivated FBS, 1 mM glutamine, 1× NEAA, 1× sodium pyruvate, 1× penicillin streptomycin, 55 µM β-Me, and 100U mL−1 IL-2). The tumor was minced into small pieces using scissors, transferred into a paddle blender bag (standard size, 105×155 mm), and agitated in a paddle blender (Stomacher® 80 Biomaster, Seward) for 30–60 min at 37°C on medium speed. The dissociated tumor sample was then filtered through a 70 µM strainer into a sterile 50 mL conical tube, and the single cell suspension was washed once with fresh T-cell medium. After pelleting cells at 350g for 10 min, cells were resuspended in fresh T-cell medium and transferred into a tissue culture-treated flask, maintaining a ratio of 2–3 cm2 plating area for every 1 mg of isolated tumor mass. Following an 8–12 h incubation period at 37°C/5% CO2, non-adherent cells were pelleted, resuspended in 15 mL T-cell medium, and incubated in a T75 flask at 37°C for an additional 2.5 days. This additional resting period allows TILs to become responsive to stimulation (Fig. 2A) and does not result in a significant expansion or diminished viability of TILs (Fig. 2B).
Fig. 2. Antigen-specific lymphocytes from mouse intracerebral tumors are detectable by ELISpot.
A) Day 20 intracerebral B16OVA tumor tissue was processed according to the described protocol (Fig. 1), using a 12 h in vitro incubation period to remove adherent cells. The presence of SIINFEKL-cognate T cells in 25K viable TILs was compared between samples cultured in vitro for 0.5 days vs 3 days using the ELISpot assay. B) Viable CD3+CD4+, CD3+CD8+, and CD3+CD8+SIINFEKL/Kb tetramer+ T-cell counts, determined by flow cytometry, from day 20 intracerebral B16OVA tumors at days 0.5 and 3 of in vitro resting period (n=4). Difference in viable T-cell counts not significant by Mann-Whitney U test. C) Quantification of IFNγ spot forming units (SFU) from TILs enriched from B16F10 and B16OVA intracerebral tumors (n=3 per group). ***p < 0.001 by two-way ANOVA with Bonferroni post-hoc test. Data are representative of 3 independent experiments.
2.3 Tumor infiltrating lymphocyte processing for ELISpot analysis
Non-adherent cells were washed once in R10 (RPMI, 10% heat-inactivated FBS, 1× penicillin streptomycin, and 1× NEAA) and then carefully layered onto 5 mL cell-separation gradient (Lympholyte-M, Cedarlane Labs). Isopycnic centrifugation was performed at room temperature at 1,300g for 25 min with no brake. The buffy coat layer was carefully removed and washed twice in fresh R10 medium. Viable lymphocytes were quantified using a flow cytometer (Guava® easyCyte, Millipore Sigma) ; however, we have also found that judicious counting of viable lymphocyte-sized cells using trypan blue exclusion yields very similar results. Generally, >85% of lymphocytes are viable. Following one final wash in fresh R10, cells were resuspended to the desired concentration for the ELISpot assay. Typically, 25–100K TILs per well are sufficient for analysis. While these numbers are much lower than that of traditional ELISpots (> 150K cells), we have not found evidence of insufficient antigen presentation as a result of low cell density. It is probable that the non-lymphocytic cells that are not removed via plastic adherence assist in antigen presentation.
2.4 IFNγ ELISpot
96-well PVDF plates (Multiscreen HTS filter plates, Millipore Sigma) were treated with 15 µL 70% ethanol for 30 seconds and then washed thrice with 1× PBS. 100 µL 10 µg mL−1 IFNγ antibody (clone AN18 antibody, Mabtech) was added to each treated well and ELISpot plates were incubated at 4°C overnight. The following day, ELISpot plates were washed once with 1× PBS and blocked for 2 h at 37°C with 150 µL R10 medium. Lymphocytes from the previous step were stimulated with R10 medium alone, 1 µM irrelevant peptide, 1 µM SIINFEKL peptide, or 4 µg mL−1 concanavilin A (ConA), and 25K cells were added per well, performing each sample in duplicate. ELISpot plates were incubated for 18–24 h at 37°C. Following incubation, ELISpot plates were washed 6 times with wash buffer (1× PBS + 0.05% tween). After removing wash buffer, 1 µg mL−1 biotin-conjugated IFNγ antibody (biotinylated clone R4-6A2 antibody, Mabtech) in 1× PBS plus 0.5% BSA was added to each well and incubated at 37°C for 2 h. ELISpot plates were then washed 4 times with wash buffer. 100 µL avidin-conjugated peroxidase (Vectastain ABC, Vector Laboratories) was added to each well and incubated at room temperature for 1 h. ELISpot plates were washed twice in wash buffer and then thrice in 1× PBS. ELISpot plates were developed in 100 µL AEC solution (AEC staining kit, Sigma) for 8 min and then rinsed with copious amounts of water. ELISpot plates were air dried and evaluated in blinded fashion (ZellNet Consulting, Inc., Fort Lee, NJ) using an ELISpot reader (KS ELISpot reader, Zeiss) with KS ELISpot software version 4.9.16. The plate evaluation process including the setup of optimal reading parameters followed the International guidelines on ELISpot plate evaluation (Janetzki et al., 2015).
3. RESULTS AND DISCUSSION
Using the protocol outlined above (Fig. 1), we achieve reproducible detection of antigen-specific lymphocytes from mouse intracerebral tumor tissue (Fig. 2C). We have also found that this protocol preserves the versatility of the ELISpot assay. This includes antibody-mediated MHC blockade and bead-based cell subset depletions that facilitate the determination an epitope’s MHC-restriction and effector subset, respectively (Wang et al., 2010). Additionally, titrating the dose of stimulating peptide enables an approximation of T-cell avidity (Hesse et al., 2001). This method may also be used to detect low avidity, antigen-specific CD4+ T cells that reside within the tumor environment, though for this purpose we add an additional 100K spleen-derived, non-irradiated leukocytes from an untreated mouse to provide a source of professional antigen presenting cells (APCs), as a precaution. We also advise using this approach when stimulating with elongated peptides that require processing prior to being presented on MHC molecules.
Fig. 1. An enzyme-free method for processing tumor infiltrating lymphocytes from mouse tumors for ELISpot analysis.
In conclusion, we have developed an enzyme-free protocol for processing lymphocytes from mouse tumors for subsequent analysis using the ELISpot assay. While our data here are demonstrated using an intracerebral B16 melanoma tumors, we have successfully employed this strategy for the detection of antigen-specific lymphocytes from subcutaneous tumors and non-melanoma tumors (e.g., SMA-560). Given the simplicity of this approach, this method may also be adapted to various source tissues wherein lymphocytes represent a minor population.
Acknowledgments
We wish to thank David Snyder for his assistance with animal experiments. This work was supported by funding from the National Institutes of Health: 1R01-NS099463-02 (J.H. Sampson), 5R01-NS085412-05 (J.H. Sampson), 5R01-CA177476-05 (J.H. Sampson), 5R01-NS086943-04 (J.H. Sampson), and 5P50-CA190991-04 (J.H. Sampson).
Abbreviations
- TILs
tumor infiltrating lymphocytes
- ELISpot
enzyme-linked immunosorbent spot assay
- MHC
major histocompatibility complex
- ICS
intracellular staining
- IFNγ
interferon gamma
- AEC
3-Amino-9-ethylcarbazole
- APC
antigen presenting cell
- NEAA
non-essential amino acids
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE
There are no conflicts of interests regarding the publication of this paper.
References
- Calarota SA, Baldanti F. Enumeration and characterization of human memory T cells by enzyme-linked immunospot assays. Clin Dev Immunol. 2013;2013:637649. doi: 10.1155/2013/637649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czerkinsky C, Andersson G, Ekre HP, Nilsson LA, Klareskog L, Ouchterlony O. Reverse ELISPOT assay for clonal analysis of cytokine production. I. Enumeration of gamma-interferon-secreting cells. J Immunol Methods. 1988;110(1):29–36. doi: 10.1016/0022-1759(88)90079-8. [DOI] [PubMed] [Google Scholar]
- Hesse MD, Karulin AY, Boehm BO, Lehmann PV, Tary-Lehmann M. A T cell clone's avidity is a function of its activation state. J Immunol. 2001;167(3):1353–1361. doi: 10.4049/jimmunol.167.3.1353. [DOI] [PubMed] [Google Scholar]
- Janetzki S, Price L, Schroeder H, Britten CM, Welters MJ, Hoos A. Guidelines for the automated evaluation of Elispot assays. Nat Protoc. 2015;10(7):1098–1115. doi: 10.1038/nprot.2015.068. [DOI] [PubMed] [Google Scholar]
- Mognol GP, Spreafico R, Wong V, Scott-Browne JP, Togher S, Hoffmann A, Trifari S. Exhaustion-associated regulatory regions in CD8(+) tumor-infiltrating T cells. Proc Natl Acad Sci U S A. 2017;114(13):E2776–E2785. doi: 10.1073/pnas.1620498114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder WM, Koenen H, van de Muysenberg AJ, Bloemena E, Wagstaff J, Scheper RJ. Reduced expression of distinct T-cell CD molecules by collagenase/DNase treatment. Cancer Immunol Immunother. 1994;38(4):253–258. doi: 10.1007/BF01533516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phetsouphanh C, Zaunders JJ, Kelleher AD. Detecting Antigen-Specific T Cell Responses: From Bulk Populations to Single Cells. Int J Mol Sci. 2015;16(8):18878–18893. doi: 10.3390/ijms160818878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevost-Blondel A, Zimmermann C, Stemmer C, Kulmburg P, Rosenthal FM, Pircher H. Tumor-infiltrating lymphocytes exhibiting high ex vivo cytolytic activity fail to prevent murine melanoma tumor growth in vivo. J Immunol. 1998;161(5):2187–2194. [PubMed] [Google Scholar]
- Wang M, Larsen MV, Nielsen M, Harndahl M, Justesen S, Dziegiel MH, Claesson MH. HLA class I binding 9mer peptides from influenza A virus induce CD4 T cell responses. PLoS One. 2010;5(5):e10533. doi: 10.1371/journal.pone.0010533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooldridge L, Lissina A, Cole DK, van den Berg HA, Price DA, Sewell AK. Tricks with tetramers: how to get the most from multimeric peptide-MHC. Immunology. 2009;126(2):147–164. doi: 10.1111/j.1365-2567.2008.02848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]