Abstract
Background
There is a paucity of data comparing the oncologic properties of breast cancer among patients previously having undergone breast augmentation in either the subglandular or subpectoral planes. The objective of the present systematic review was to evaluate whether implant location influenced the characteristics of breast tumors in previously augmented women.
Methods
A systematic literature search was performed to identify relevant articles reporting tumor characteristics in augmented patients. The search included published articles in three electronic databases; Ovid MEDLINE, EMBASE, and PubMed. Comparative studies (subglandular vs. subpectoral) were included.
Results
Analysis of data pooled from the included studies showed that subglandular implants had a higher frequency of tumors between 2 to 5 cm (26.5% vs. 9.9%, P = 0.0130). Subglandular implants also had a higher frequency of stage 2 tumors (42.9% vs. 23.7%, P = 0.0308). There was no significant difference in lymphovascular invasion between the 2 groups. These results of this systematic review suggest that the prognosis of patients undergoing augmentation is unaffected by implant location (subpectoral vs. subglandular).
Conclusions
With the absence of large randomized controlled trials, our study provides surgeons with an evidence-based reference to improve informed consent with regards to implant placement.
Keywords: Breast Implants, Breast Neoplasms, Mammography
INTRODUCTION
The demand for cosmetic breast augmentation continues to increase in the United States. According to the American Society of Aesthetic Plastic Surgeons, there were 310,444 cases of breast augmentation in 2016, representing a 206.8% annual increase in the number of procedures performed as compared to 20 years ago.1 With an approximate 12.4% lifetime risk of developing breast cancer, a significant proportion of augmented women will go on to develop breast cancer and delayed detection remains an ongoing concern.2 Numerous studies have been conducted to evaluate whether an etiologic link exists between implants and breast cancer. It has been concluded that women are not at an increased risk of developing breast carcinoma following augmentation.3–9 It is however well documented that the radiopaque nature of implants impairs the sensitivity of mammography, even when employing the implant displacement technique.10,11 This appears to be more pronounced when the implant is located in the subglandular plane as opposed to subpectoral.10,11 Conversely, implants have been speculated to facilitate the detection of palpable breast tumors by providing a background on which to palpate, and have also been associated with more invasive cancers.11–13 The clinical significance of these findings remains unclear. While certain studies claim that women with implants have breast cancer diagnosed at a more advanced stage, other publications presented conflicting results.14–19 To date, there does not exist a systematic review of the literature comparing the oncologic behavior of breast cancer between previously augmented patients in the subglandular and subpectoral planes. The objective of the present systematic review is to evaluate whether implant location (subglandular vs. subpectoral) influences the characteristics of breast tumors in previously augmented women.
MATERIALS AND METHODS
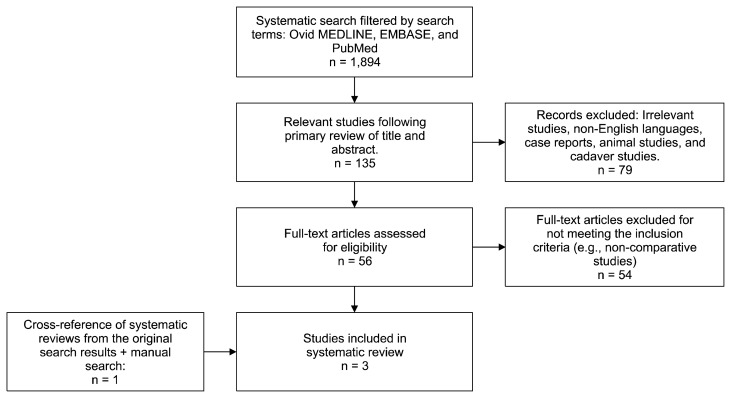
A search of the Ovid MEDLINE, EMBASE, and PubMed databases was performed, starting from the establishment of each database to August 1st 2017. An initial search was performed using different spellings and versions of the following terms: ([“breast neoplasm”] and [“implant” or “prosthesis” or “augmentation mammoplasty” or “mammoplasty” or “alloplastic”] and [“detection” or “screening”]). A separate search was also performed using different spellings and versions of the following terms: ([“prepectoral” or “subglandular” or “retropectoral” or “subpectoral”] and [“breast neoplasms”]). Citations were limited to human studies published in the English language. Studies were included if subpectoral and subglandular implant subgroups were separated and analyzed independently with regards to cancer characteristics at presentation. The endpoints of interest were tumor size, tumor grade and lymph node involvement at diagnosis. Only comparative studies were included to reduce the risk of confounding factors. Non-human studies, case reports (< 8 patients), and review articles were excluded. Two independent reviewers assessed the eligibility of the studies using the same systematic inclusion/exclusion criteria. A total of 1,894 studies were identified and further narrowed to 135 potentially eligible studies after primary review. Studies were selected based on the relevance of the title and/or abstract of retrieved records (Fig. 1). The initial screen excluded studies with evidently irrelevant titles or abstracts. If content was unclear in the initial screen based on abstract review, a formal article review was undertaken. Potentially eligible studies were further reviewed, leading to a total of 2 eligible studies. Studies were also collected from an extensive manual Internet search and from the reference list of relevant articles, yielding one additional study. The systematic review followed the guidelines provided by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.20 Subglandular and subpectoral implants were compared with respect to tumor size, tumor stage, and presence of lymphovascular invasion at initial presentation for breast cancer screening. Variables were displayed as descriptive data and compared by Fisher’s exact test and chi-square analysis using IBM SPSS ver. 22 (IBM Co., Armonk, NY, USA). P-values < 0.05 were considered to be statistically significant.
Figure 1.
Flowchart of the search criteria and strategy used for the literature review.
RESULTS
A total of three studies met the inclusion criteria (Cho et al.,21 Douglas et al.,22 and Spear et al.23) (Table 1). Tumor size, stage and the presence of lymphovascular involvement were the only variables with enough data for comparison (Table 2). Exact carcinoma subtype (lobular vs. ductal), tumor grade, and presence of metastasis could not be compared due to incomplete or insufficient data. The following analyses were performed:
Table 1.
Overview of included studies characteristics and level of evidence
Table 2.
Overall study results of included studies
| Variable | Subglandular | Subpectoral | P-value |
|---|---|---|---|
| Tumor size (cm) | 0.052a | ||
| < 2 | 30 (61.2) | 62 (76.5) | 0.0642 |
| 2–5 | 13 (26.5) | 8 (9.9) | 0.0130* |
| > 5 | 2 (4.1) | 7 (8.6) | 0.3286 |
| Unknown | 4 (8.2) | 4 (4.9) | 0.4494 |
| Tumor stage | 0.09b | ||
| 0 | 7 (16.7) | 24 (31.6) | 0.0796 |
| 1 | 15 (35.7) | 26 (34.2) | 0.8704 |
| 2 | 18 (42.9) | 18 (23.7) | 0.0308* |
| 3 | 2 (4.8) | 8 (10.5) | 0.2891 |
| 4 | 0 (0) | 0 (0) | - |
| Lymphovascular invasion | 0.60a | ||
| Present | 18 (36.7) | 23 (28.4) | 0.3255 |
| Absent | 30 (61.2) | 55 (67.9) | 0.4383 |
| Unknown | 1 (2.0) | 3 (3.7) | 0.5869 |
Values are presented as number (%).
Fisher’s exact test was used.
Fisher’s exact test was used except for tumor stage 4.
P < 0.05.
1. Tumor size
Tumor size, measured by the pathologist, was categorized into the following three categories: < 2 cm, 2 to 5 cm, and > 5 cm, based on tumor size classification in the TNM staging system.24 All 3 studies were included in the analysis with an effective sample size of 122 (Table 3). A difference in tumor sizes, trending towards statistical significance (P = 0.052) was observed between patients receiving subglandular and subpectoral implants. Subgroup analysis of data pooled from the 3 studies showed that subglandular implants were associated with a higher frequency of tumors between 2 to 5 cm (P = 0.0130). There was also a higher frequency of tumors < 2 cm in the subglandular group, with borderline statistical significance (P-value = 0.0642). There were no difference in the frequency of tumors > 5 cm.
Table 3.
Tumor size characteristics
| Variable | Tumor size (cm) (subglandular) | Tumor size (cm) (subpectoral) | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| < 2 | 2–5 | > 5 | Unknown | < 2 | 2–5 | > 5 | Unknown | |
| Cho et al. (2017)21 | 15 | 8 | 0 | 4 | 45 | 7 | 7 | 4 |
| Douglas et al. (1991)22 | 5 | 1 | 0 | 0 | 2 | 0 | 0 | 0 |
| Spear et al. (2008)23 | 10 | 4 | 2 | 0 | 15 | 1 | 0 | 0 |
Values are presented as number only.
2. Tumor stage
Tumor stage was categorized from 0 to 4, based on the TNM staging system.24 Two studies (Cho et al.21 and Spear et al.23) were included with an effective sample size of 118 (Table 4). Overall, the difference in tumor stages between both groups was not considered statistically significant (P = 0.09). Subgroup analysis of the pooled data demonstrated a higher frequency in stage 2 tumors (P = 0.0308) in the subglandular group. The remainder of the comparison demonstrated no difference in frequency between the subpectoral and subglandular groups.
Table 4.
Tumor stage characteristics
| Variable | Tumor stage (subglandular) | Tumor stage (subpectoral) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| 0 | 1 | 2 | 3 | 4 | 0 | 1 | 2 | 3 | 4 | |
| Cho et al. (2017)21 | 2 | 13 | 11 | 0 | 0 | 20 | 19 | 13 | 8 | 0 |
| Spear et al. (2008)23 | 5 | 2 | 7 | 2 | 0 | 4 | 7 | 5 | 0 | 0 |
Values are oresented as number only.
3. Lymphovascular invasion
Presence of lymphovascular invasion was a binary outcome (yes or no). All 3 studies were included with an effective sample size of 126 (Table 5). Analysis of data pooled from the 3 studies demonstrated no significant difference between lymphovascular invasion and implant position (P = 0.60).
Table 5.
LV invasion characteristics
| Variable | LV invasion (subglandular) | LV invasion (subpectoral) | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Present | Absent | Unknown | Present | Absent | Unknown | |
| Cho et al. (2017)21 | 10 | 16 | 1 | 17 | 43 | 3 |
| Douglas et al. (1991)22 | 2 | 4 | - | 1 | 1 | - |
| Spear et al. (2008)23 | 6 | 10 | - | 5 | 11 | - |
Values are presented as number only. LV, lymphovascular.
DISCUSSION
In the current review, implant location (subpectoral vs. subglandular) did not have a significant overall impact on cancer screening. Subgroup analyses demonstrated that subglandular implants had a higher frequency of tumors between 2 to 5 cm and stage 2 tumors, although no statistical significance was observed overall. There was also a difference in tumors < 2 cm, albeit not statistically significant (P = 0.0642). This finding potentially suggests that the increased distortion caused by subglandular implants may lead to delayed radiological detection of early tumors. This result however did not translate to more aggressive cancers, as there was no significant difference when comparing tumors > 5 cm, stage 3 tumors, and lymphovascular invasion between groups. These results serve to suggest that the prognosis of patients undergoing augmentation is unaffected by implant location. It is important however that patients are made aware of the inherent risk of false-negatives on mammography following augmentation,9 and counseled to undergo regular screening using both imaging and clinical examinations.
Delayed detection of occult breast cancers by means of mammography in previously augmented patients remains an area of ongoing concern. It has been reported in the literature that the radio-opaque nature of implants may obscure the visualization of breast tissue on mammography.9,25 The amount of glandular tissue obscured by implants varies from 22% to 83%.25 Silverstein et al.10 demonstrated that subglandular implants resulted in in reduced measurable tissue area as compared to subpectoral implants. These results were paralleled by Handel,9 who demonstrated that patients with subglandular implants had a 37% reduction in the visualized tissue area as compared to 17% in the subpectoral group. Despite utilization of Eklund’s displacement technique, minimal improvement was observed. Furthermore, in addition to implant placement, the presence of capsular contracture further obscures the image, with a reported range of 30% to 50% in Baker grades 1 to 2 and 3 to 4, respectively.9 Capsular contracture in the context of subglandular implant placement can potentially limit the sensitivity of mammography.
As a result of these factors, the sensitivity of mammography in previously augmented women with palpable tumors has been reported as reduced when compared to non-augmented women.26–28 This association however is mainly based on older studies and updated prospective studies are needed due to evolution in mammography quality and implants in the past decades. Tumors in women following augmentation have been shown to be primarily detected by palpation as opposed to mammography.9,13,17 This has been explained by the smaller native breast tissue of these women as well as the implant serving as a background upon which to palpate the breast tissue. Furthermore, women undergoing cosmetic procedures may be more body conscious and therefore more likely to identify changes to their breasts.12
Whether these aforementioned factors resulting in delayed detection translate to more aggressive tumors remains inconclusive in the literature. Previous studies have shown there is no increased risk of breast cancer associated with implants and even suggested that the incidence of breast carcinoma is lower than in non-augmented women.7 Conversely, in a meta-analysis performed by Lavigne et al.,8 breast augmentation was shown to adversely affect the survival of women subsequently diagnosed with breast cancer. Patients having undergone augmentation had an overall OR of 1.26 (95% CI, 0.99–1.60) for an invasive type of breast cancer at diagnosis. The studies included in that analysis did not account for potential confounding factors and should therefore be interpreted with caution. To our knowledge, implant plane (subglandular vs. subpectoral) has not been previously compared in systematic review with regards to oncologic characteristics in breast cancer screening.
This systematic review is not without limitations. Several confounding factors may have affected the overall results. These include surgical approach, implant material, texture, size etc. Due to paucity of comparative studies, we were unable to account for these factors. Heterogeneity in mammogram machines and radiologist experience could have also affected our results. Follow-up period, mean age, and patient population also varied greatly amongst the included studies. We also acknowledge that larger randomized controlled studies are required to confirm causality.
Implant location (subpectoral vs. subglandular) does not appear to have an impact on cancer screening. Implant placement should be decided according to both patient and surgeon preference in order to achieve the desired aesthetic outcome. With the absence of meta-analyses or large randomized controlled trials, our study provides surgeons with an evidence-based reference to improve informed consent with regards to implant placement.
ACKNOWLEDGMENTS
We would like to thank Dr. Xianming Tan, Ph.D., from the Department of Biostatistics and the Lineberger Comprehensive Cancer Center in North Carolina at Chapel Hill, USA. Dr. Tan’s expertise in statistics, particularly in systematic reviews and meta-analyses, was crucial to this manuscript.
Footnotes
CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
REFERENCES
- 1.Cosmetic Surgery National Data Bank statistics. Aesthet Surg J 2017;37(suppl_2):1–29. 10.1093/asj/sjx076 [DOI] [PubMed] [Google Scholar]
- 2.Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Kosary CL, et al. SEER Cancer Statistics Review, 1975–2014, National Cancer Institute; http://seer.cancer.gov/csr/1975_2014/ Accessed September 1, 2017. [Google Scholar]
- 3.Berkel H, Birdsell DC, Jenkins H. Breast augmentation: a risk factor for breast cancer? N Engl J Med 1992;326:1649–1653. 10.1056/NEJM199206183262501 [DOI] [PubMed] [Google Scholar]
- 4.Brinton LA, Lubin JH, Burich MC, Colton T, Brown SL, Hoover RN. Breast cancer following augmentation mammoplasty (United States). Cancer Causes Control 2000;11:819–27. 10.1023/A:1008941110816 [DOI] [PubMed] [Google Scholar]
- 5.Clark CP, 3rd, Peters GN, O’Brien KM. Cancer in the augmented breast. Diagnosis and prognosis. Cancer 1993;72:2170–4. [DOI] [PubMed] [Google Scholar]
- 6.Deapen DM, Pike MC, Casagrande JT, Brody GS. The relationship between breast cancer and augmentation mammaplasty: an epidemiologic study. Plast Reconstr Surg 1986;77:361–8. 10.1097/00006534-198603000-00001 [DOI] [PubMed] [Google Scholar]
- 7.Deapen D. Breast implants and breast cancer: a review of incidence, detection, mortality, and survival. Plast Reconstr Surg 2007;120:70S–80S. 10.1097/01.prs.0000286577.70026.5d [DOI] [PubMed] [Google Scholar]
- 8.Lavigne E, Holowaty EJ, Pan SY, Villeneuve PJ, Johnson KC, Fergusson DA, et al. Breast cancer detection and survival among women with cosmetic breast implants: systematic review and meta-analysis of observational studies. BMJ 2013;346:f2399. 10.1136/bmj.f2399 [DOI] [PubMed] [Google Scholar]
- 9.Handel N. The effect of silicone implants on the diagnosis, prognosis, and treatment of breast cancer. Plast Reconstr Surg 2007;120:81S–93S. 10.1097/01.prs.0000286578.94102.2b [DOI] [PubMed] [Google Scholar]
- 10.Silverstein MJ, Handel N, Gamagami P. The effect of silicone-gel-filled implants on mammography. Cancer 1991;68(5 Suppl):1159–63. [DOI] [PubMed] [Google Scholar]
- 11.Silverstein MJ, Handel N, Gamagami P, Waisman E, Gierson ED. Mammographic measurements before and after augmentation mammaplasty. Plast Reconstr Surg 1990;86:1126–30. 10.1097/00006534-199012000-00014 [DOI] [PubMed] [Google Scholar]
- 12.Miglioretti DL, Rutter CM, Geller BM, Cutter G, Barlow WE, Rosenberg R, et al. Effect of breast augmentation on the accuracy of mammography and cancer characteristics. JAMA 2004;291:442–50. 10.1001/jama.291.4.442 [DOI] [PubMed] [Google Scholar]
- 13.Skinner KA, Silberman H, Dougherty W, Gamagami P, Waisman J, Sposto R, et al. Breast cancer after augmentation mammoplasty. Ann Surg Oncol 2001;8:138–44. 10.1007/s10434-001-0138-x [DOI] [PubMed] [Google Scholar]
- 14.Silverstein MJ, Gierson ED, Gamagami P, Handel N, Waisman JR. Breast cancer diagnosis and prognosis in women augmented with silicone gel-filled implants. Cancer 1990;66:97–101. [DOI] [PubMed] [Google Scholar]
- 15.Silverstein MJ, Handel N, Gamagami P, Waisman JR, Gierson ED, Rosser RJ, et al. Breast cancer in women after augmentation mammoplasty. Arch Surg 1988;123:681–5. 10.1001/archsurg.1988.01400300023001 [DOI] [PubMed] [Google Scholar]
- 16.Xie L, Brisson J, Holowaty EJ, Villeneuve PJ, Mao Y. The influence of cosmetic breast augmentation on the stage distribution and prognosis of women subsequently diagnosed with breast cancer. Int J Cancer 2010;126:2182–90. [DOI] [PubMed] [Google Scholar]
- 17.Birdsell DC, Jenkins H, Berkel H. Breast cancer diagnosis and survival in women with and without breast implants. Plast Reconstr Surg 1993;92:795–800. 10.1097/00006534-199392050-00003 [DOI] [PubMed] [Google Scholar]
- 18.Deapen D, Hamilton A, Bernstein L, Brody GS. Breast cancer stage at diagnosis and survival among patients with prior breast implants. Plast Reconstr Surg 2000;105:535–40. 10.1097/00006534-200002000-00009 [DOI] [PubMed] [Google Scholar]
- 19.Friis S, Hölmich LR, McLaughlin JK, Kjøller K, Fryzek JP, Henriksen TF, et al. Cancer risk among Danish women with cosmetic breast implants. Int J Cancer 2006;118:998–1003. 10.1002/ijc.21433 [DOI] [PubMed] [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, Altman DGPRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010;8:336–41. 10.1016/j.ijsu.2010.02.007 [DOI] [PubMed] [Google Scholar]
- 21.Cho EH, Shammas RL, Phillips BT, Greenup RA, Hwang ES, Hollenbeck ST. Breast cancer after augmentation: oncologic and reconstructive considerations among women undergoing mastectomy. Plast Reconstr Surg 2017;139:1240e–9e. 10.1097/PRS.0000000000003342 [DOI] [PubMed] [Google Scholar]
- 22.Douglas KP, Bluth EI, Sauter ER, McKinnon WM, Bergeron RB, Merritt CR, et al. Roentgenographic evaluation of the augmented breast. South Med J 1991;84:49–54. 10.1097/00007611-199101000-00013 [DOI] [PubMed] [Google Scholar]
- 23.Spear SL, Clemens MW, Dayan JH. Considerations of previous augmentation in subsequent breast reconstruction. Aesthet Surg J 2008;28:285–93. 10.1016/j.asj.2008.02.008 [DOI] [PubMed] [Google Scholar]
- 24.American Joint Committee on Cancer (AJCC). Breast. In: AJCC, eds. AJCC Cancer Staging Manual. 7th ed New York, Springer, pp 347–69, 2010. [Google Scholar]
- 25.Hayes H, Jr, Vandergrift J, Diner WC. Mammography and breast implants. Plast Reconstr Surg 1988;82:1–8. 10.1097/00006534-198882010-00001 [DOI] [PubMed] [Google Scholar]
- 26.Silverstein MJ, Handel N, Gamagami P, Gierson ED, Furmanski M, Collins AR, et al. Breast cancer diagnosis and prognosis in women following augmentation with silicone gel-filled prostheses. Eur J Cancer 1992;28:635–40. 10.1016/S0959-8049(05)80115-7 [DOI] [PubMed] [Google Scholar]
- 27.Leibman AJ, Kruse BD. Imaging of breast cancer after augmentation mammoplasty. Ann Plast Surg 1993;30:111–5. 10.1097/00000637-199302000-00003 [DOI] [PubMed] [Google Scholar]
- 28.Fajardo LL, Harvey JA, McAleese KA, Roberts CC, Granstrom P. Breast cancer diagnosis in women with subglandular silicone gel-filled augmentation implants. Radiology 1995;194:859–62. 10.1148/radiology.194.3.7862991 [DOI] [PubMed] [Google Scholar]