Abstract
Introduction
The purpose of present study was to investigate the effect of limonin on tumor glycolysis and the underlying mechanisms in hepatocellular carcinoma (HCC).
Methods
Cell proliferation and colony formation assays were performed to evaluate the potency of limonin against HCC cells in vitro. The glucose consumption and lactate production after limonin treatment was determined. The effect of limonin on hexokinase-2 (HK-2) activity was assessed and the mitochondrial location of HK-2 was studied by immunoprecipitation. Cell apoptosis and protein expression were detected by flow cytometry and western blotting respectively. Protein overexpression by plasmid transfection was adopted to investigate the molecular mechanisms.
Results
HCC proliferation and colony formation were inhibited by limonin in vitro. With the suppression of HK-2 activity, the glycolytic level in HCC cells was substantially reduced, which was evidenced by the decrease of glucose consumption and lactate production. The phosphorylation of HK-2 was substantially inhibited by limonin, which resulted in the disassociation of HK-2 from mitochondria. Due to the reduction of HK-2 in mitochondria, increasing Bax were shifted to the mitochondria and gave rise to the release of cytochrome C, which induced HCC cells to subject to mitochondria-mediated apoptosis. Mechanism investigations revealed that the decrease of HK-2 phosphorylation was mainly due to the inhibition of Akt activity. In Akt exogenously overexpressed HCC cells, limonin-mediated cell proliferation inhibition, glycolysis suppression and apoptosis induction were significantly impaired.
Conclusion
Limonin inhibited the tumor glycolysis in hepatocellular carcinoma by suppressing HK-2 activity, and the suppression of HK-2 was closely related to the decrease of Akt activity.
Keywords: apoptosis, limonin, tumor glycolysis, hexokinase-2
Introduction
In normal cells, when oxygen is available, glucose is completely metabolized into CO2 and H2O via the tricarboxylic acid (TCA) cycle to acquire sufficient energy. However, in tumor cells, even in aerobic conditions, glucose is converted into lactic acid instead of CO2 and H2O, which is named as tumor glycolysis or Warburg effect.1 Although the amount of ATP produced by tumor glycolysis is relatively small (about 18 times less), the rate of ATP production is much higher than that of TCA cycle (about 100 times faster) and can meet the energy demand of the rapidly proliferating tumor cells.2 In the process of tumor glycolysis, the conversion of glucose to glucose-6-phosphate (G-6P) is the first irreversible step and is mainly catalyzed by hexokinases (HKs).3 Because G-6P is used as precursor in various metabolism pathways, HKs therefore have important roles in the regulation of cell metabolism. In mammalian cells, four different isoforms of HK, HK-1, HK-2, HK-3 and HK-4, have been characterized.4 In contrast with HK-4, the other three isoforms (1–3) have higher affinity for glucose. Different from HK-1 and HK-3, which are widely expressed, HK-2 is mainly expressed in insulin-sensitive tissues including skeletal and cardiac muscle cells. With the in-depth study of HK-2 biological function, increasing evidences demonstrated that HK-2 upregulation was an important contributor the metabolic re-programming in cancer cells and was closely related to the elevated tumor glycolysis and survival.5–7 By comparing the expression of HKs in liver preneoplasia and neoplasia, the results demonstrated HK-2 expression was promoted, whereas HK-4 was decreased. This shift to high-affinity HK subtype helps hepatocellular carcinoma (HCC) cells to increase glucose metabolism rate in order to meet the enormous energy needs.8 In HCC patients, high HK-2 expression was closely related to tumor grade and poor prognosis.9,10 In addition to the roles in glucose metabolism, the HK-2 located on the outer mitochondrial membrane is proved to be engaged in the protection of mitochondria integrity and the prevention of cell apoptosis.11
Limonin is a kind of limonoids that are mainly found in citrus fruits, such as lemons, oranges, pumellos, grapefruits, bergamots and mandarins.12 Just like the members of limonoids family, limonin has been reported to exhibit various biological activities such as analgesic, anti-inflammatory, antioxidant and antiviral.13–15 Recently, increasing studies reported that limonin demonstrated antitumor activities in colon cancer, pancreatic cancer, breast cancer and HCC.16–20 Several investigations revealed that induction of cell apoptosis, blockade of Wnt signaling pathway and inhibition of breast cancer resistance protein (BCRP/ABCG-2)-mediated transport were the potential mechanisms of action.21,22
In this study, we investigated the effects of limonin on tumor glycolysis in HCC cells and elucidated the relevant mechanisms. The glycolytic levels in HCC cells were significantly decreased after limonin treatment. Further studies showed that the glycolysis inhibition was mainly attributed to the decrease of HK-2 in mitochondria, and the HK-2 decrease was caused by the suppression of Akt-mediated HK-2 phosphorylation. In addition to the effect on tumor glycolysis, we also validated that the decrease of HK-2 in mitochondria played a critical role in cell apoptosis induction by limonin.
Materials and methods
Cell line and reagents
Hep3B and HepG2 cells were obtained from the Cell bank of Chinese Academy of Sciences (Shanghai, People’s Republic of China). By following the instructions of supplier, Hep3B and HepG2 cells were cultured with RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS) in a 37°C incubator with 5% CO2. Limonin was purchased from Selleck (Shanghai, People’s Republic of China). The primary antibodies used in Western blotting including anti-Glut1, anti-voltage-dependent anion channel 1 (VDAC-1), anti-LDHA, anti-PKM2, anti-cleaved caspase-3, anti-phospho-Akt substrate, anti-cleaved PARP, anti-Bax, anti-Bak, anti-cyto C, anti-Bcl-2, anti-Bcl-xl, anti-Akt, anti-phospho-Akt, anti-GSK3β, anti-phospho-GSK3β and the secondary HRP-conjugated goat anti-rabbit IgG were products of Cell Signaling Technology (Beverly, MA, USA). The anti-hexokinase-2 antibody, Glucose Uptake Assay and l-Lactate Assay Kits were products of Abcam (Cambridge, UK). The Annexin V-FITC was product of BioLegend (San Diego, CA, USA). For cell transfection, Myr-Akt1 was obtained from Addgene (Cambridge, MA, USA), and the Lipofectamine 3000 was a product of Thermo Fisher Scientific (Waltham, MA, USA).
Cell proliferation assay
After the digestion with trypsin, 100 μL of cell suspension (3×105 cell) was seeded into a 96-well plate and cultured in incubator overnight, and then different concentrations of limonin were added into the plate. After incubation with limonin for different time points, 20 μL/well CellTiter 96® AQueous One Solution (Promega, Madison, WI, USA) was added into the plate and the absorbance value was examined at 490 nM according to the instructions of manufacturer.
Colony formation assay
The anchorage-independent growth assay was conducted as described previously.23 Briefly, 3 mL of basal medium Eagle/10% FBS/0.5% bottom agar mixture that contains different concentrations of limonin was prepared and seeded into a 6-well plate. In total, 8,000 cells were suspended in 1 mL basal medium Eagle/10% FBS/0.3% top agar with the same concentration of limonin. The cells were cultured in an incubator at 37°C and 5% CO2 atmosphere for 2 weeks and the colonies were scored under a microscope using the Image-Pro Plus software (version 6.2) program (Media Cybernetics, Rockville, MD, USA).
Glucose and lactate detection
According to the protocols of manufacturer, glucose consumption and lactate production were examined by Glucose Uptake Assay Kit and l-Lactate Assay Kit (Abcam), respectively. The amount of protein in tested sample was determined by BCA kit and used to normalize glucose consumption and lactate production. The glucose consumption and lactate production in control group were taken as 100%.
Intracellular ATP determination
After incubation with different concentrations of limonin for 24 h, HCC cells were digested with trypsin, and 100 μL of cell suspension (1×104 cells) was plated in triplicates in a 96-well plate and then 100 μL ATPlite reagent (cat 6016736; Perkin-Elmer) was added and mixed uniformly by microplate shaker. The luminescence was measured using SpectraMax i3 (Molecular Devices). The luminescence value in control group was taken as 100%.
Hexokinase activity examination
HepG2 cells were seeded into a 6-well plate and grown to 70%–80% confluence, and then the medium was replaced with fresh culture medium and incubated with different concentrations of limonin for 24 h. Cell numbers were counted, and cell pellets were obtained by centrifugation and then lysed with RIPA lysis buffer (cat 89900; Thermo Fisher Scientific). Protein concentration in cell lysate was determined by BCA Kit, and the HK activity in cell lysate was examined with the Hexokinase Assay Kit (cat ab136957; Abcam) by following the instructions of manufacturer. The HK activity in the group without limonin was calculated as 100%.
Isolation of mitochondrial protein
After limonin treatment, ~2×107 cells were harvested by centrifuging at 500× g for 5 min. The mitochondrial fraction was extracted using the Mitochondria Isolation Kit (cat 89874; Thermo Fisher Scientific) according to the instructions provided. For protein immunoprecipitation experiment, the mitochondrial pellets were dissolved in the lysis buffer (20 mmol/L Tris-HCl pH 7.5, 2 mmol/L EGTA, 2 mmol/L EDTA, 1% Triton X-100) containing protease inhibitors.
Immunoprecipitation
Proteins from whole-cell extract or mitochondrial fraction were extracted with RIPA lysis buffer or the Mitochondria Isolation Kit by following the instructions of manufacturers. The protein lysates were precleared with 35 μL agarose A/G beads for 2 h at 4°C. Precleared lysates were incubated with appropriate antibodies and 35 μL of fresh agarose A/G beads overnight at 4°C. The beads were collected and washed with lysis buffer (cat 87787; Thermo Fisher Scientific) for three times. After boiling with 30 μL 2× loading buffer for 5 min, the supernatant was collected by centrifugation at 12,000× g for 5 min and then subjected to Western blotting analysis.
Western blotting
The protein samples (10 ug/lane) were loaded into 10% gel and resolved by SDS-PAGE. After separation, proteins were transferred onto PVDF membrane with Wet/Tank Blotting Systems (Bio-Rad). The membrane was blocked with 5% BSA solution at room temperature for 1–2 h and then incubated with given primary antibodies (1:1,000 diluted in 5% BSA in PBS solution) at 4°C overnight. After washing with PBST (PBS solution plus 0.1% Tween20), the membrane was incubated with the secondary antibody at room temperature for 1 h and the signals on the membrane were developed with ECL Plus Western Blotting Substrate (#32132; Pierce, Rockford, IL, USA).
Cell apoptosis assay
HepG2 cells treated with limonin were digested with trypsin and the cell suspension was collected. After centrifugation at 500× g for 5 min, the supernatant was discarded, and cell pellets were washed with PBS for three times. The binding buffer (cat 422201; BioLegend) was used to resuspend HepG2 cell pellets and the cell concentration was adjusted to 1×106/mL. Then, 5 μL Annexin V-FITC (cat 640914; BioLegend) and 10 μL propidium iodide solution (20 μg/mL) were added into 100 μL cell suspension and incubated at room temperature for 15 min avoiding light, and then 400 μL binding buffer was added, and the stained cells were analyzed by flow cytometry (FACS).
Plasmid transfection
For Myr-Akt1 transfection, Lipofectamine 3000 (Thermo Fisher Scientific) reagent was used by following the manufacturer’s instructions. Briefly, before transfection, the medium was replaced with fresh medium without FBS. Appropriate Myr-Akt1 plasmid and Lipofectamine 3000 were diluted with Opti-MEM, respectively, and then mixed together and incubated at room temperature for 10 min avoiding light; then the mixture was added into HepG2 cells. After 4–6 h, the medium was replaced with fresh culture medium. Then, 24 h after transfection, the transfected cells were used for studies.
Statistical analysis
The experiments were performed at least in triplicates, and the quantitative results are presented as mean ± SD. The statistical analysis was done by the Student’s t-test or one-way ANOVA (Graphpad prism 6.0). A p-value<0.05 indicated a statistical difference.
Results
Limonin suppressed HCC proliferation and colony formation
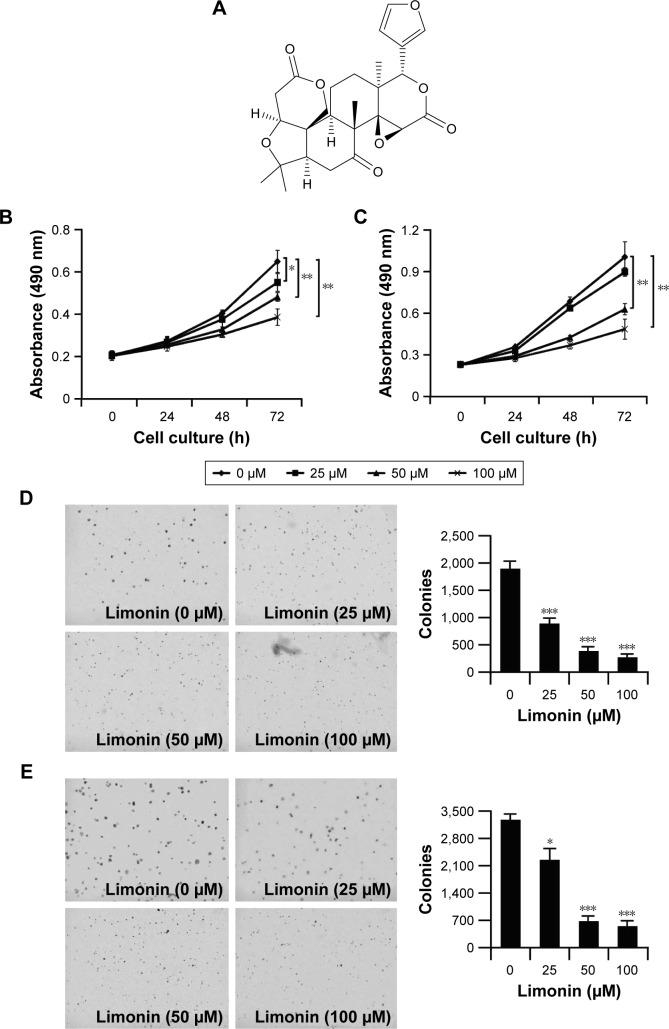
Firstly, we used cell proliferation assay and anchorage-independent assay to evaluate the antitumor activities of limonin (structure is shown in Figure 1A). The proliferation of HCC cells was significantly inhibited by limonin in a concentration- and time-dependent manner (Figure 1B and C). At 100 μM, the inhibition rate reached about 47.8% and 56.3% in Hep3B and HepG2 cells, respectively, after 72 h of treatment. In addition, limonin also demonstrated significant inhibitory effects on the colony formation. After the limonin treatment, the size and number of clones were significantly reduced (Figure 1D and E), and the number of clones formed in the soft agar was decreased to about 86.7% and 83.3%, respectively, in Hep3B and HepG2 cells at 100 μM.
Figure 1.
Limonin inhibited HCC cell proliferation and colony formation.
Notes: (A) Chemical structure of limonin, (B and C) limonin inhibited HCC cell proliferation. Hep3B (B) or HepG2 (C) cells were incubated with various concentrations of limonin for different times, and the cell viability was determined. (D and E) Limonin suppressed HCC cell anchorage-independent growth in soft agar. Hep3B (D) or HepG2 (E) cell suspensions were plated into a 6-well plate and exposed to different limonin concentrations; the anchorage-independent assay was performed. The asterisks (*p<0.05, **p<0.01, ***p<0.001, Student’s t-test) indicate significant difference versus the control.
Abbreviation: HCC, hepatocellular carcinoma.
Limonin inhibited glycolysis by decreasing HK-2 activity
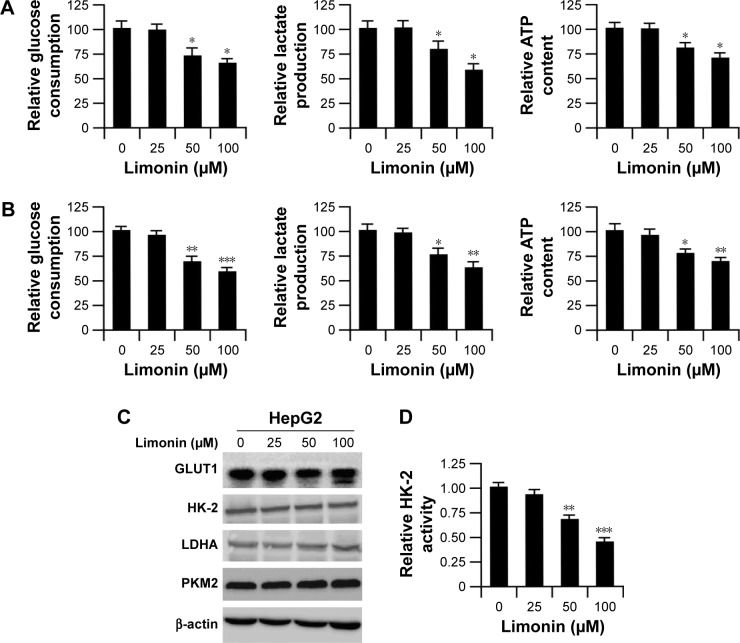
We examined the effects of limonin on tumor glycolysis by determining glucose consumption and lactate production levels. As per the results shown in Figure 2A and B, after exposure to limonin, the glucose consumption in HepG2 and Hep3B was dose dependently decreased, and the levels of lactate production was also reduced accordingly. Meanwhile, after limonin treatment, the intracellular ATP level was also significantly declined. By examining the important enzymes in glucose metabolism such as GLUT1, LDHA, HK-2 and PKM2, we found that limonin had no effect on the expression of these enzymes (Figure 2C). We further evaluated the effects of limonin on HK-2 activity and the results demonstrated that the activity of HK-2 was dose dependently suppressed after limonin treatment (Figure 2D).
Figure 2.
Limonin suppressed tumor glycolysis by decreasing HK-2 activity.
Notes: (A and B) Limonin inhibited tumor glycolysis in HepG2 and Hep3B cells. HepG2 (A) or Hep3B (B) cells were treated with different concentrations of limonin for 24 h; glucose consumption (left), lactate production (middle) and intracellular ATP levels (right) were examined as described. The asterisks (*p<0.05, **p<0.01, ***p<0.001, Student’s t-test) indicate statistical difference versus the control. (C) Limonin had no effects on the expressions of important glycolytic enzymes. After incubation of limonin for 24 h, the expression of given protein was determined by Western blotting. (D) Limonin reduced HK-2 activity dose dependently. After treatment with limonin, the activities of HK-2 were detected as described in “Materials and methods” section. The asterisks (**p<0.01, ***p<0.001, Student’s t-test) indicate significant difference in contrast with the control.
Abbreviation: HK-2, hexokinase-2.
Limonin induced the detachment of HK-2 from the mitochondria
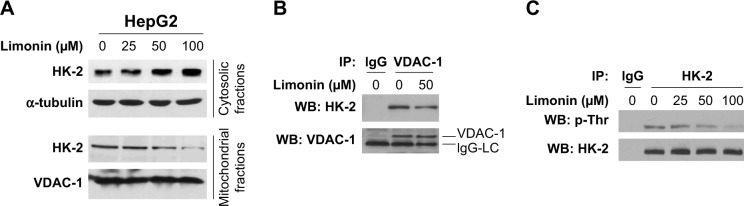
Since HK-2 is generally located on the mitochondria to perform its biological functions, we examined HK-2 expression in the cytoplasm and mitochondria, respectively. As shown in Figure 3A, upon limonin treatment, the amount of HK-2 in cytoplasm was increased, while the HK-2 in mitochondria was decreased, suggesting a translocation of HK-2 from mitochondria to cytoplasm. The immunoprecipitation results also demonstrated that the amount of HK-2 combined with VDAC-1 was significantly decreased, further confirming the detachment of HK-2 from mitochondria (Figure 3B). HK-2 phosphorylation is very important for its mitochondrial location, and so we tested the effects of limonin on HK-2 phosphorylation. Because the commercial phosphor-HK-2 antibody was not available, as previously described,24 we used HK-2 antibody to immunoprecipitate HK-2 and then examined its phosphorylation with anti-phospho-Akt substrate antibody. As demonstrated in Figure 3C, after limonin treatment, the phosphorylation of HK-2 was dose dependently decreased.
Figure 3.
Limonin decreased HK-2 expression in mitochondria.
Notes: (A) HK-2 expression in mitochondria was reduced. After limonin treatment, the cytosolic and mitochondrial fractions were separated, and HK-2 expression was assessed by Western blotting. (B) The interaction between HK-2 and VDAC-1 was decreased. The cell lysates were immunoprecipitated with VDAC-1 antibody and then probed with HK-2 antibody by Western blotting. (C) HK-2 phosphorylation was decreased after limonin treatment. Cell lysates were immunoprecipitated with HK-2 antibody and then detected with PAS antibody to examine HK-2 phosphorylation.
Abbreviations: HK-2, hexokinase-2; VDAC-1, voltage-dependent anion channel 1; PAS, phospho-Akt substrate.
Limonin induced cell apoptosis in HCC cells
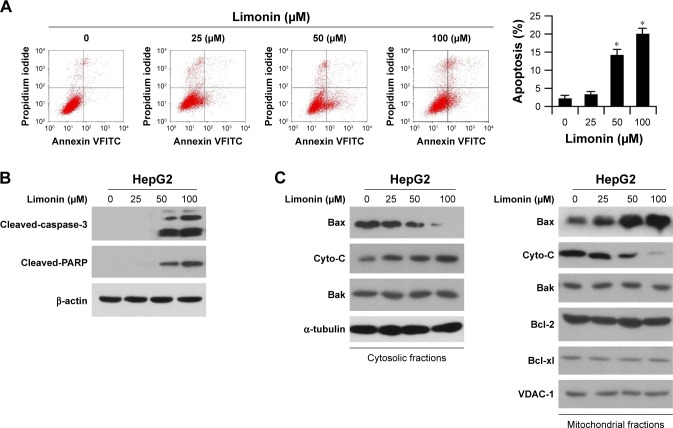
Since the HK-2 located on mitochondria has an important role in preventing cell apoptosis, we examined the effect of limonin on apoptosis induction. As shown in the FACS results, limonin significantly induced apoptosis in HepG2 cells, and about 20% of cells were subjected to apoptosis after 100 uM limonin treatment (Figure 4A). Moreover, in HepG2 cells treated with limonin, the expressions of cleaved caspase-3 and PARP, which are important indicative markers of apoptosis, were dose dependently increased (Figure 4B). Furthermore, we investigated the expressions of pro-apoptotic and anti-apoptotic proteins in cytoplasm and mitochondria, respectively. In Figure 4C, after limonin treatment, the expression of Bax in cytoplasm was decreased, while its expression in mitochondria was increased, suggesting a translocation of Bax from cytoplasm to mitochondria. By contrast, cytochrome C was increased in cytosolic fractions and decreased in mitochondria, demonstrating that cytochrome C was released from the mitochondria after limonin treatment. The expression of other pro-apoptotic/anti-apoptotic proteins showed no obvious change.
Figure 4.
Limonin induced cell apoptosis in HepG2 cells.
Notes: After limonin treatment for 24 h, HepG2 cells were subjected to Annexin V/propidium iodide double staining and FACS analysis (A) or Western blotting with indicated antibodies (B). The asterisks (*p<0.05, Student’s t-test) indicated significant difference versus the control. (C) Limonin increased Bax binding to mitochondria and induced cytochrome C release. After limonin treatment, the cytosolic and mitochondrial fractions were isolated, and the expressions of given proteins were examined with indicated antibodies.
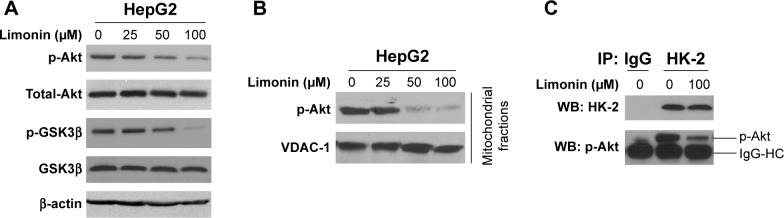
Limonin inhibited Akt kinase activity in HepG2 cells
Previous studies reported that Akt kinase was involved in HK-2 phosphorylation, and so we evaluated the effects of limonin on Akt. As shown in Figure 5A, the phosphorylation of Akt was inhibited by limonin in a dose-dependent fashion. With the suppression of Akt activation, its downstream pathway such as GSK3β was also inactivated. Furthermore, we also examined the activated Akt in mitochondria. As demonstrated, the activated Akt in mitochondria was substantially decreased (Figure 5B). The immunoprecipitation results further validated that, after exposure to limonin, the amount of activated Akt interacting with HK-2 was decreased (Figure 5C).
Figure 5.
Limonin suppressed Akt activity in HepG2 cells.
Notes: (A) Limonin inhibited Akt activity and its downstream signaling pathway. The cell lysates of HepG2 cells treated with limonin were probed with indicated antibodies. (B) Limonin blocked Akt activity in mitochondria. The mitochondrial fractions were isolated, and Akt phosphorylation was determined. (C) Limonin inhibited Akt-mediated HK-2 phosphorylation. The lysates of the mitochondria fractions were immunoprecipitated with HK-2 antibody and phosphor-Akt was examined.
Abbreviation: HK-2, hexokinase-2.
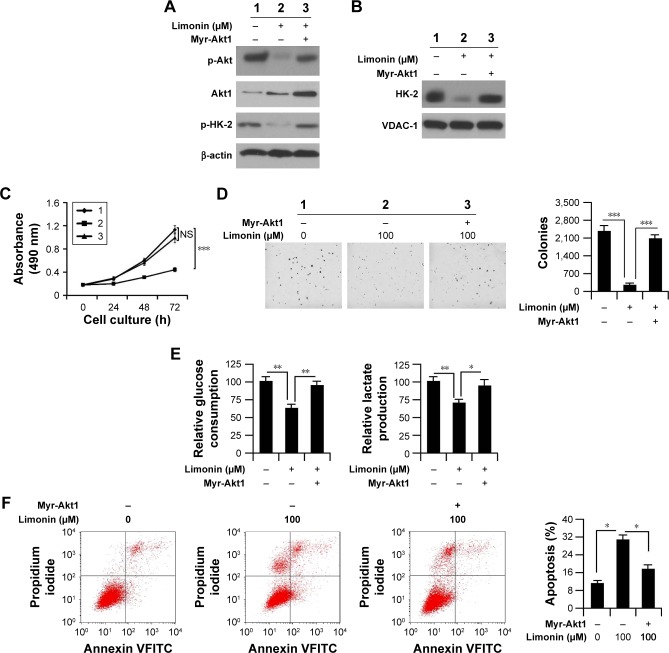
Hyperactivation of Akt attenuated limonin-mediated glycolysis inhibition and apoptosis induction
To clarify the importance of Akt inhibition, we introduced Myr-Akt1 into HepG2 cells and then examined the effects of limonin. In Myr-Akt1-transfected cells, the activity of Akt was significantly increased in contrast with the mock group. With the elevation of Akt activity, the phosphorylation of HK-2 was also substantially recovered (Figure 6A). The protein immunoprecipitation results demonstrated that the amount of HK-2 combined with VDAC-1 was increased significantly after Myr-Akt1 transfection (Figure 6B). As expected, with the promotion of HK-2 activity, the cell proliferation inhibition, tumor glycolysis suppression and cell apoptosis induced by limonin were significantly impaired. As demonstrated in Figure 6C and D, in contrast with the Mock group, the inhibitory effects of limonin on HepG2 cell proliferation and anchorage-independent growth were significantly decreased. Beyond that, the glucose consumption and lactate production were markedly recovered (Figure 6E). Moreover, Myr-Akt1 transfection resulted in a significant decrease of cell apoptosis in HepG2 cells after the exposure to limonin (Figure 6F).
Figure 6.
Exogenous hyperactivation of Akt impaired limonin-induced proliferation inhibition, glycolysis suppression and cell apoptosis.
Notes: (A) Phosphor-Akt and HK-2 expression in Myr-Akt-transfected HepG2 cells. HepG2 cells were transfected with Myr-Akt1 and then treated with 100 μM limonin; the expression of given proteins was examined by Western blotting. (B) Myr-Akt1 transfection promoted HK-2 mitochondrial location. After Myr-Akt1 transfection, the lysates of mitochondrial fractions were immunoprecipitated with VDAC-1 antibody and then probed with HK-2. (C and D) The inhibitory effects of limonin in Myr-Akt1-transfected HepG2 cells. After Myr-Akt1 transfection, the effects of limonin on cell proliferation (C) and anchorage-independent growth (D) were determined. 1: untreated; 2: limonin treated; 3: Myr-Akt1 transfected and limonin treated. The asterisks (***p<0.001, Student’s t-test) indicate significant difference between different groups. (E and F) The activities of limonin in glycolysis and apoptosis induction in Akt-hyperactivated HepG2 cells. In Myr-Akt1-transfected HepG2 cells, the effects of limonin on tumor glycolysis (E) and apoptosis induction (F) were evaluated. The asterisks (*p<0.05, **p<0.01, Student’s t-test) indicate significant difference between different groups.
Abbreviations: HK-2, hexokinase-2; VDAC-1, voltage-dependent anion channel 1; NS, no significance.
Discussion
In addition to the compounds generated by organic synthesis, the natural products, especially those that are abundant in food or herbs, are also important sources for anticancer drug discovery. Compared with the organic compounds, the natural products, especially those existing in our daily diet, may have more advantages in terms of drug safety. Previous studies have demonstrated that the suppression of proliferation-related signaling, promotion of cell death and downregulation of angiogenesis were involved in natural product-mediated antitumor activities.25–28 In this study, we evaluated the antitumor activity of limonin in HCC cells, its effects on tumor glycolysis as well as the underlying mechanisms.
As an important feature of cancer cell, aerobic glycolysis not only offers the necessary energy to support the rapid proliferation, but also provides the precursors for biomolecular synthesis. In addition, the acidic microenvironment created by lactate also provides a comfortable site for tumor cell proliferation.29 Therefore, it is an effective strategy to inhibit cancer cell proliferation by blocking tumor glycolysis.30 Inhibitors such as 2-deoxy-D-glucose and 3-bromopyruvate, which target to block glucose metabolism, are undergoing clinical trials and have achieved promising results.31,32 After limonin treatment, the glucose consumption and the lactate production were significantly decreased (Figure 2A and B), proving that glycolysis in HCC cells was inhibited by limonin. Furthermore, we also demonstrated the glycolysis inhibition was attributed to the decrease of HK-2 activity, and the expression of the important glycolytic enzymes including PKM2, LDH and GLUT1 had no obvious change after limonin treatment. Generally, HK-2 is located on the outer membrane of mitochondria, which provides the facilitation to access the ATP produced in mitochondria and promotes glucose phosphorylation. Although total HK-2 was not changed, exposure to limonin led to the translocation of HK-2 from mitochondria to cytoplasm (Figure 3) and the decrease of HK-2 activity. Previous studies demonstrated that the phosphorylation at Ser473 of HK-2 induced its conformation changes to enhance its affinity with the mitochondrial partner and increased its mitochondrial localization.33 Beyond that, the phosphorylation of HK-2 also impaired the sensitivity to G-6P, which is the product of glucose phosphorylation, and induced HK-2 to dissociate from mitochondria.34 After limonin treatment, the phosphorylation of HK-2 was decreased in a dose-dependent manner (Figure 3C), and we thought it was responsible for the decrease of HK-2 activity. In addition to participating in glycolysis regulation, the mitochondrial HK-2 also provides protection against cell apoptosis initiated by mitochondrial dysfunction.35 On the outer membrane of mitochondria, HK-2 is interacted with VDAC-1 to maintain the integrity and regulate the permeability. By coupling with ATP generation and glucose phosphorylation, HK-2 is able to increase the electron flow and eliminate the membrane potential and reduce ROS generation.36 Moreover, several studies uncovered that HK-2 was a competitor of Bax, which can bind to the VDAC-1 on mitochondria and induce cell apoptosis.37 After limonin treatment, owing to the dissociation of HK-2, Bax gained more opportunities to bind with VDAC-1 and gave rise to the translocation of Bax from cytoplasm to mitochondria, which induced the activation of Bax and the release of pro-apoptotic factor such as cytochrome C from the mitochondria (Figure 4C).
Akt is an important serine/threonine kinase and is often hyperactivated in many tumor tissues. Upon receptor stimulation or gene mutation, Akt is activated to mediate different functions such as cell proliferation, metabolism and survival by activating various downstream targets. Previous experiments have shown that Akt enhanced HK-2 expression by regulating hypoxia-inducible factor-1 (HIF-1), which is a transcriptional factor and is increased under hypoxic conditions. In HK-2 promoter region, there is consensus motif for HIF-1 binding and HK-2 expression is promoted.38 Other studies also demonstrated that Akt/mTOR pathway was involved in the regulation of HK-2 expression by targeting miRNA, such as miR-143.39 In addition to regulating HK-2 expression, Akt also enhances mitochondrial HK-2 by phosphorylating HK-2 itself.24,33 In this study, our data demonstrated that Akt activities were significantly inhibited by limonin, and the activated Akt located in mitochondria was also substantially decreased (Figure 5A and B). Co-immunoprecipitation results further clarified that the amount of phosphorylated Akt combined with HK-2 was substantially reduced, suggesting that Akt was involved in the regulation of HK-2 activity by direct phosphorylation, not through mediating its expression. Due to the decrease of HK-2 phosphorylation, HK-2 was detached from the mitochondria, which resulted in glycolysis inhibition and apoptosis induction.
Conclusion
In present study, we demonstrated that limonin had a profound antitumor activity against HCC cells by suppressing tumor glycolysis and inducing cell apoptosis. Through inhibiting Akt activity, limonin lowered the phosphorylation level of HK-2, leading to its detachment from mitochondria and the blockade of activity. Our studies provided preclinical data for further development of limonin or its analogs as effective therapeutics for HCC management.
Acknowledgments
This study was supported by the internal research fund of Jinshan Hospital.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.San-Millán I, Brooks GA. Reexamining cancer metabolism: lactate production for carcinogenesis could be the purpose and explanation of the Warburg Effect. Carcinogenesis. 2017;38(2):119–133. doi: 10.1093/carcin/bgw127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pfeiffer T, Schuster S, Bonhoeffer S. Cooperation and competition in the evolution of ATP-producing pathways. Science. 2001;292(5516):504–507. doi: 10.1126/science.1058079. [DOI] [PubMed] [Google Scholar]
- 3.Zhong JT, Zhou SH. Warburg effect, hexokinase-II, and radioresistance of laryngeal carcinoma. Oncotarget. 2017;8(8):14133–14146. doi: 10.18632/oncotarget.13044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilson JE. Isozymes of mammalian hexokinase: structure, subcellular localization and metabolic function. J Exp Biol. 2003;206(Pt 12):2049–2057. doi: 10.1242/jeb.00241. [DOI] [PubMed] [Google Scholar]
- 5.Li W, Gao F, Ma X, Wang R, Dong X, Wang W. Deguelin inhibits non-small cell lung cancer via down-regulating Hexokinases II-mediated glycolysis. Oncotarget. 2017;8(20):32586–32599. doi: 10.18632/oncotarget.15937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu D, Jin J, Yu H, et al. Chrysin inhibited tumor glycolysis and induced apoptosis in hepatocellular carcinoma by targeting hexokinase-2. J Exp Clin Cancer Res. 2017;36(1):44. doi: 10.1186/s13046-017-0514-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li W, Ma X, Li N, et al. Resveratrol inhibits hexokinases II mediated glycolysis in non-small cell lung cancer via targeting Akt signaling pathway. Exp Cell Res. 2016;349(2):320–327. doi: 10.1016/j.yexcr.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Roberts DJ, Miyamoto S. Hexokinase II integrates energy metabolism and cellular protection: Akting on mitochondria and TORCing to autophagy. Cell Death Differ. 2015;22(2):364. doi: 10.1038/cdd.2014.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwee SA, Hernandez B, Chan O, Wong L. Choline kinase alpha and hexokinase-2 protein expression in hepatocellular carcinoma: association with survival. PLoS One. 2012;7(10):e46591. doi: 10.1371/journal.pone.0046591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu J, Hu L, Wu F, Zou L, He T. Poor prognosis of hexokinase 2 overexpression in solid tumors of digestive system: a meta-analysis. Oncotarget. 2017;8(19):32332–32344. doi: 10.18632/oncotarget.15974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Camara AKS, Zhou Y, Wen PC, Tajkhorshid E, Kwok WM. Mitochondrial VDAC1: a key gatekeeper as potential therapeutic target. Front Physiol. 2017;8:460. doi: 10.3389/fphys.2017.00460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gualdani R, Cavalluzzi MM, Lentini G, Habtemariam S. The chemistry and pharmacology of citrus limonoids. Molecules. 2016;21(11):E1530. doi: 10.3390/molecules21111530. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang Y, Wang X, Zhu Q, et al. Synthesis and pharmacological evaluation of novel limonin derivatives as anti-inflammatory and analgesic agents with high water solubility. Bioorg Med Chem Lett. 2014;24(7):1851–1855. doi: 10.1016/j.bmcl.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Fujii A, Okuyama T, Wakame K, Okumura T, Ikeya Y, Nishizawa M. Identification of anti-inflammatory constituents in Phellodendri cortex and Coptidis rhizoma by monitoring the suppression of nitric oxide production. J Nat Med. 2017;71(4):745–756. doi: 10.1007/s11418-017-1107-4. [DOI] [PubMed] [Google Scholar]
- 15.Balestrieri E, Pizzimenti F, Ferlazzo A, et al. Antiviral activity of seed extract from Citrus bergamia towards human retroviruses. Bioorg Med Chem. 2011;19(6):2084–2089. doi: 10.1016/j.bmc.2011.01.024. [DOI] [PubMed] [Google Scholar]
- 16.Chidambara Murthy KN, Jayaprakasha GK, Patil BS. Citrus limonoids and curcumin additively inhibit human colon cancer cells. Food Funct. 2013;4(5):803–810. doi: 10.1039/c3fo30325j. [DOI] [PubMed] [Google Scholar]
- 17.Chidambara Murthy KN, Jayaprakasha GK, Kumar V, Rathore KS, Patil BS. Citrus limonin and its glucoside inhibit colon adenocarcinoma cell proliferation through apoptosis. J Agric Food Chem. 2011;59(6):2314–2323. doi: 10.1021/jf104498p. [DOI] [PubMed] [Google Scholar]
- 18.Patil JR, Chidambara Murthy KN, Jayaprakasha GK, Chetti MB, Patil BS. Bioactive compounds from Mexican lime (Citrus aurantifolia) juice induce apoptosis in human pancreatic cells. J Agric Food Chem. 2009;57(22):10933–10942. doi: 10.1021/jf901718u. [DOI] [PubMed] [Google Scholar]
- 19.Kim J, Jayaprakasha GK, Patil BS. Limonoids and their anti-proliferative and anti-aromatase properties in human breast cancer cells. Food Funct. 2013;4(2):258–265. doi: 10.1039/c2fo30209h. [DOI] [PubMed] [Google Scholar]
- 20.Rahman A, Siddiqui SA, Jakhar R, Kang SC. Growth inhibition of various human cancer cell lines by imperatorin and limonin from poncirus trifoliata rafin. Seeds. Anticancer Agents Med Chem. 2015;15(2):236–241. doi: 10.2174/1871520614666140922122358. [DOI] [PubMed] [Google Scholar]
- 21.Langeswaran K, Gowthamkumar S, Vijayaprakash S, Revathy R, Balasubramanian MP. Influence of limonin on Wnt signalling molecule in HepG2 cell lines. J Nat Sci Biol Med. 2013;4(1):126–133. doi: 10.4103/0976-9668.107276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tan KW, Li Y, Paxton JW, Birch NP, Scheepens A. Identification of novel dietary phytochemicals inhibiting the efflux transporter breast cancer resistance protein (BCRP/ABCG2) Food Chem. 2013;138(4):2267–2274. doi: 10.1016/j.foodchem.2012.12.021. [DOI] [PubMed] [Google Scholar]
- 23.Yu X, Liang Q, Liu W, Zhou L, Li W, Liu H. Deguelin, an aurora B kinase inhibitor, exhibits potent anti-tumor effect in human esophageal squamous cell carcinoma. EBioMedicine. 2017;26:100–111. doi: 10.1016/j.ebiom.2017.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyamoto S, Murphy AN, Brown JH. Akt mediates mitochondrial protection in cardiomyocytes through phosphorylation of mitochondrial hexokinase-II. Cell Death Differ. 2008;15(3):521–529. doi: 10.1038/sj.cdd.4402285. [DOI] [PubMed] [Google Scholar]
- 25.Gao F, Deng G, Liu W, Zhou K, Li M. Resveratrol suppresses human hepatocellular carcinoma via targeting HGF-c-Met signaling pathway. Oncol Rep. 2017;37(2):1203–1211. doi: 10.3892/or.2017.5347. [DOI] [PubMed] [Google Scholar]
- 26.Yu X, Li W, Deng Q, et al. Neoalbaconol inhibits angiogenesis and tumor growth by suppressing EGFR-mediated VEGF production. Mol Carcinog. 2017;56(5):1414–1426. doi: 10.1002/mc.22602. [DOI] [PubMed] [Google Scholar]
- 27.Fan HC, Chi CS, Chang YK, Tung MC, Lin SZ, Harn HJ. The molecular mechanisms of plant-derived compounds targeting brain cancer. Int J Mol Sci. 2018;19(2):E395. doi: 10.3390/ijms19020395. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li W, Yu X, Tan S, Liu W, Zhou L, Liu H. Oxymatrine inhibits non-small cell lung cancer via suppression of EGFR signaling pathway. Cancer Med. 2018;7(1):208–218. doi: 10.1002/cam4.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kato Y, Ozawa S, Miyamoto C, et al. Acidic extracellular microenvironment and cancer. Cancer Cell Int. 2013;13(1):89. doi: 10.1186/1475-2867-13-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ganapathy-Kanniappan S. Taming tumor glycolysis and potential implications for immunotherapy. Front Oncol. 2017;7:36. doi: 10.3389/fonc.2017.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raez LE, Papadopoulos K, Ricart AD, et al. A phase I dose-escalation trial of 2-deoxy-D-glucose alone or combined with docetaxel in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2013;71(2):523–530. doi: 10.1007/s00280-012-2045-1. [DOI] [PubMed] [Google Scholar]
- 32.Ko YH, Verhoeven HA, Lee MJ, Corbin DJ, Vogl TJ, Pedersen PL. A translational study “case report” on the small molecule “energy blocker” 3-bromopyruvate (3BP) as a potent anticancer agent: from bench side to bedside. J Bioenerg Biomembr. 2012;44(1):163–170. doi: 10.1007/s10863-012-9417-4. [DOI] [PubMed] [Google Scholar]
- 33.Roberts DJ, Tan-Sah VP, Smith JM, Miyamoto S. Akt phosphorylates HK-II at Thr-473 and increases mitochondrial HK-II association to protect cardiomyocytes. J Biol Chem. 2013;288(33):23798–23806. doi: 10.1074/jbc.M113.482026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.John S, Weiss JN, Ribalet B. Subcellular localization of hexokinases I and II directs the metabolic fate of glucose. PLoS One. 2011;6(3):e17674. doi: 10.1371/journal.pone.0017674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ben-Hail D, Begas-Shvartz R, Shalev M, et al. Novel compounds targeting the mitochondrial protein VDAC1 inhibit apoptosis and protect against mitochondrial dysfunction. J Biol Chem. 2016;291(48):24986–25003. doi: 10.1074/jbc.M116.744284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim JS, Ahn KJ, Kim JA, et al. Role of reactive oxygen species-mediated mitochondrial dysregulation in 3-bromopyruvate induced cell death in hepatoma cells: ROS-mediated cell death by 3-BrPA. J Bioenerg Biomembr. 2008;40(6):607–618. doi: 10.1007/s10863-008-9188-0. [DOI] [PubMed] [Google Scholar]
- 37.Pastorino JG, Shulga N, Hoek JB. Mitochondrial binding of hexokinase II inhibits Bax-induced cytochrome c release and apoptosis. J Biol Chem. 2002;277(9):7610–7618. doi: 10.1074/jbc.M109950200. [DOI] [PubMed] [Google Scholar]
- 38.Düvel K, Yecies JL, Menon S, et al. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol Cell. 2010;39(2):171–183. doi: 10.1016/j.molcel.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fang R, Xiao T, Fang Z, et al. MicroRNA-143 (miR-143) regulates cancer glycolysis via targeting hexokinase 2 gene. J Biol Chem. 2012;287(27):23227–23235. doi: 10.1074/jbc.M112.373084. [DOI] [PMC free article] [PubMed] [Google Scholar]