Abstract
Objectives:
HIV-positive adolescents have low-ART adherence, with consequent increased risks of mortality, morbidity, and viral resistance. Despite high rates of violence against children in the Africa region, no known studies have tested impacts on HIV-positive adolescents. We examine associations of ART adherence with adolescent violence victimization by caregivers, teachers, peers, community members, and healthcare providers.
Design and methods:
HIV-positive adolescents were interviewed (n = 1060), and clinic biomarker data collected. We sampled all 10–19-year olds ever ART-initiated within 53 clinics in 180 South African communities (90.1% reached). Analyses examined associations between nonadherence and nine violence types using sequential multivariate logistic regressions. Interactive and additive effects were tested with regression and marginal effects.
Results:
Past-week self-reported ART nonadherence was 36%. Nonadherence correlated strongly with virologic failure (OR 2.3, CI 1.4–3.8) and symptomatic pulmonary tuberculosis (OR 1.49, CI 1.18–2.05). Four violence types were independently associated with nonadherence: physical abuse by caregivers (OR 1.5, CI 1.1–2.1); witnessing domestic violence (OR 1.8, CI 1.22–2.66); teacher violence (OR 1.51, CI 1.16–1.96,) and verbal victimization by healthcare staff (OR 2.15, CI 1.59–2.93). Past-week nonadherence rose from 25% with no violence to 73.5% with four types of violence exposure.
Conclusion:
Violence exposures at home, school, and clinic are major and cumulating risks for adolescent antiretroviral nonadherence. Prevention, mitigation, and protection services may be essential for the health and survival of HIV-positive adolescents.
Keywords: adherence, adolescence, child abuse, HIV/AIDS, violence
Introduction
Adolescents are the age group with the highest rates of antiretroviral nonadherence, bringing elevated risks of AIDS-related morbidity and mortality [1,2]. AIDS is the most common cause of death amongst adolescents in Africa [3]. Nonadherence can also lead to viral resistance and greater risk of onwards HIV-transmission through increased viral load [4].
There is an emerging evidence base on factors associated with adolescent nonadherence to ART. Studies have identified lower adherence amongst older adolescents and those who are vertically infected [5]. Qualitative and quantitative reviews suggest risks related to ART formulation and higher pill burden, depression and behavioral problems, low family support, and parental death [6–14]. Studies also suggest potential impacts of economic factors such as poverty and food insecurity [7,15].
The World Health Organization (WHO) Africa region, home to 80% of HIV-positive adolescents also has the world's highest rates of violence against children and adolescents [16]. Violence exposure takes place within a range of settings and includes physical, psychological/verbal, and sexual violence, [17]. Most adolescents live at home, whereas some are exposed to abusive caregivers or relatives and witness domestic violence between adults. In addition, adolescence brings new independence and exploration, with associated increased violence exposure in community settings [18]. Studies also report physical and verbal violence from teachers and clinic verbal violence by healthcare providers, perhaps reflecting the challenges faced by overburdened and resource-constrained systems [19,20].
Two important recent studies show that exposure to violence more broadly is associated with adolescent ART nonadherence. In Malawi, Kim et al.[21] found that a dichotomous variable of any exposure to sexual, physical or household violence was strongly associated with reduced adherence. In a US study (amongst 8–15-year olds) a sum score of violence exposure (physical, sexual or crime) was associated with nonadherence, unsuppressed viral load and CD4+% less than 25% [22]. Evidence from other health behaviors shows that any form of violence victimization during adolescence is associated with negative health impacts, and that witnessing domestic violence – especially within the home – may be equally harmful [23,24]. In other non-HIV medical contexts, abuse in the home has been shown to be associated with medication nonadherence in children [25]. However, no known studies have tested potential associations of different types of violence with adolescent antiretroviral nonadherence. In addition, increased severity, chronicity, and exposure to multiple types of violence (polyvictimization) show greater impacts on other health outcomes such as increased mental health problems, substance use, sexual risk behavior, HIV-infection and educational delay [26–31]. There is a clear need to examine impacts of different types and cumulative effects of violence on adolescents living with HIV (ALHIV) in a high-prevalence setting.
The current study tests potential associations of violence exposure and antiretroviral nonadherence amongst HIV-positive adolescents in South Africa. It examines victimization and witnessing violence within home, school, community, and clinic settings. First, we test whether different types of violence are associated with nonadherence, and if so which are most salient. Second, we identify whether there are interactive or additive effects of multiple victimization on adolescent antiretroviral nonadherence.
Methods
Procedures
We conducted a cross-sectional survey of ALHIV and community controls in South Africa. The study took place in the Eastern Cape, a province with the lowest per-capita GDP nationally, and very limited social service access [32]. Within a health sub-district constituting urban, rural, and peri-urban settlements, all 53 facilities providing adolescent antiretroviral therapy (ART; hospitals, primary care clinics, and community health centres) were visited and included in the study. In each clinic, paper and computerized clinical files were reviewed to identify every patient aged 10–19 who had ever initiated ART, regardless of current healthcare attendance. To prevent sampling bias towards those attending healthcare, adolescents were not interviewed in clinics, but were traced to 180 communities and interviewed in their homes and schools. It was important to avoid risk of stigma or identification of HIV-positive status from participation in the research. Consequently, the study was presented as focusing on general adolescent use of health and social services, and as an additional stigma avoidance strategy, we also interviewed 467 adolescents who were co-resident or living in neighbouring households.
Ethical approval was given by IRBs at the Universities of Cape Town (CSSR 2013/4) and Oxford (SSD/CUREC2/12-21), the Provincial Departments of Health and Education and participating health facilities. All participants and their primary caregivers provided written informed consent for interviews and accessing clinical records. Consent procedures were also read aloud in case of low literacy. No financial incentives were used, but participants received a small gift pack, snacks, and a certificate of participation. Confidentiality was maintained except in cases of disclosure of risk of harm. Where participants reported on-going or prior abuse or violence, referrals were made to relevant child protection, health services, or police (n = 112 referrals). A registered child protection social worker oversaw referrals and subsequent follow-up.
Adolescents completed confidential 90-min tablet-based questionnaires, designed in collaboration with a Teen Advisory Group to be enjoyable and nonstigmatizing. Measures were translated and back-translated into Xhosa and English and completed in the language of participants’ choice. Audio-CASI was used for sensitive items. Researchers trained in working with vulnerable adolescents provided support for questionnaire completion, depending on adolescents’ literacy, and cognitive needs. Research tools were prepiloted with 25 HIV-positive adolescents in the Eastern Cape. In order to mitigate risk of social desirability bias, self-reported nonadherence was validated against two clinical outcomes that may be associated with nonadherence: clinic-based records of virologic failure [33] and a combination of clinic records and self-reported symptomatic tuberculosis (TB) [34]. The study was developed in collaboration with the South African National Departments of Health, Social Development and Basic Education and National AIDS Council, UNICEF, PEPFAR-USAID, and NGOs including Pediatric Adolescent Treatment for Africa.
Measures
ART nonadherence was measured using the standardized self-report Patient Medication Adherence Questionnaire, combined with measures developed in Botswana by Lowenthal et al.[35,36]. After piloting, vignettes were added to reduce social desirability bias, for example, ‘Even if Lindiwe tries her best sometimes unexpected things get in the way and prevent her from taking her pills… this is not her fault.’ Past-week nonadherence was defined as ART medication adherence below 95% over the preceding 7 days (always including both a weekend and weekdays) [37]. Two validation measures of self-reported nonadherence were applied. Virologic failure was measured using clinical records and defined as viral load greater than 1000 copies/ml [38]. All viral load measures taken in the year of interview and the prior year were recorded. Concurrent symptomatic pulmonary TB was measured as clinic report of TB diagnosis without subsequent treatment in the past year or self-reported WHO diagnostic criteria for symptomatic TB, validated against sputum specimens [39,40]. To maximize precision for case identification, we combined criteria for highest positive predictive value (chronic cough and weight loss with sensitivity 72.9%, specificity 85%, PPV 11.4) and highest negative predictive value (NPV; any cough, drenching night sweats, and weight loss with sensitivity 75%, specificity 82.4%, NPV 99.2) amongst HIV-positive participants.
Violence exposure
Ten potential violence factors were measured and coded as dichotomies of exposure/no exposure. Past-year physical abuse victimization by caregivers at home past-year verbal abuse victimization by caregivers at home and past-week witnessing domestic violence between adults in the home were measured using UNICEF Measures for National-level Monitoring of Orphans and Vulnerable Children [41]. Contact sexual violence by any perpetrator was measured using three items from the Juvenile Victimization Questionnaire and defined as any lifetime contact sexual abuse or forced sex [42]. Past-year physical violence from teachers in school was measured as being hit by a teacher ‘sometimes’/‘always.’ Past-year physical violence from peers and past-year verbal violence from peers were measured using the Social and Health Assessment peer victimization scale [43]. Past-year physical violence victimization in community settings was measured as being physically attacked in the community or being robbed and past-year witnessing of violence in community settings was measured as any of past-year witnessing of shootings or stabbings, using the Child Exposure to Community Violence checklist [44]. Past-year verbal violence in the clinic setting was measured as adolescent self-report of being shouted at angrily by clinic staff ‘sometimes’/‘always’.
Confounding variables
Nine potential confounders were identified using quantitative and qualitative literature review of factors associated with adherence amongst adult, pediatric, and adolescent populations included socio-economic factors of age, sex, urban/rural location, and living in formal or informal housing, using items based on the 2011 Census [45]. They also included family factors of maternal orphanhood and paternal orphanhood, and HIV-related factors of mode of infection (vertical/ horizontal), time on ART treatment (in years), and travel time to clinical care (dichotomized as >1 h) [46].
Analyses
Analyses were conducted in six stages in SPSS 21.0 and STATA 14. First, associations of self-reported nonadherence were tested in multivariate logistic regressions, against validation measures of virologic failure and symptomatic TB, controlling for potential confounders. Second, known characteristics (sex, age, and location) of excluded and included participants were compared to check for potential differences. Violence variables were excluded if numbers were too small for analysis. Third, sociodemographic characteristics were reported and potential associations between violence and ART nonadherence were assessed following a sequential regression approach recommended by Hosmer and Lemeshow [47]. Three logistic regression models were run: with all violence victimization factors and potential confounders entered simultaneously, with all variables significant at 0.1 or below, and with only variables significant at 0.05 or below. Fourth, we tested all potential two-way interactive effects between violence factors significant in Stage 3, using logistic regression applying Hochberg's multiple hypothesis step-up method to reduce false discovery rate. Fifth, we tested potential moderation effects of sex and age on associations between violence factors significant in Stage 3 and nonadherence, using interaction terms in the final regression model. Sixth, to test potential cumulative effects of multiple types of violence exposure on ART nonadherence, marginal effects models were run with all potential combinations of significant violence factors and summarized with a marginal effects model using a multiple-violence score of 0–4 types of violence.
Results
Of 1176 eligible ALHIV, 90.1% (n = 1060) were interviewed together with community controls (n = 467). 4.1% of ALHIV or their caregivers refused participation, 0.9% were unable to participate because of severe cognitive delay, 3.7% were untraceable and 1.2% no longer lived in the study area.
Validating self-reported adherence
Self-reported nonadherence was 36% in the past week. Only n = 412 (38.9%) of patient medical records included a viral load measure taken within the past 2 years (Table 1). In those, self-reported nonadherence was significantly associated with virologic failure [odds ratio (OR) = 2.32; CI 1.41–3.84, P = 0.001) independent of age, sex, urban/rural location, formal/informal housing, maternal orphanhood, paternal orphanhood, mode of infection, time on ART treatment, and travel time to clinic. In the full sample, self-reported nonadherence was associated with increased rates of concurrent symptomatic pulmonary TB (OR = 1.54, CI 1.07–2.22, P = 0.021), independent of potential confounders.
Table 1.
Multivariate logistic regression analyses testing associations between self-reported ART nonadherence, virologic failure, and symptomatic pulmonary tuberculosis.
| Virologic failure (N = 412) | Symptomatic pulmonary tuberculosis (N = 1060) | |||
| OR, P-value | 95% CI | OR, P-value | 95% CI | |
| Age (years) | 1.22*, 0.001 | 1.08–1.37 | 1.11*, 0.014 | 1.02–1.21 |
| Female sex | 0.71, 0.181 | 0.43–1.18 | 0.82, 0.277 | 0.56–1.18 |
| Rural location | 2.19*, 0.007 | 1.24–3.86 | 0.98, 0.919 | 0.63–1.53 |
| Informal housing | 0.96, 0.897 | 0.51–1.80 | 0.79, 0.361 | 0.48–1.30 |
| Absent mother | 1.11, 0.699 | 0.66–1.86 | 1.43, 0.068 | 0.97–2.10 |
| Absent father | 1.01, 0.976 | 0.60–1.70 | 0.87, 0.476 | 0.59–1.28 |
| Vertical infection | 2.72*, 0.026 | 1.13–6.57 | 1.65, 0.138 | 0.85–3.19 |
| Time on ART (years) | 0.92*, 0.026 | 0.86–0.99 | 0.95*, 0.047 | 0.90–1.00 |
| Travel time to clinic more than 1 h | 1.06, 0.874 | 0.49–2.29 | 1.22, 0.477 | 0.71–2.09 |
| Past week ART nonadherence | 2.32*, 0.001 | 1.41–3.84 | 1.54*, 0.021 | 1.07–2.22 |
*Statistically significant at P < 0.05.
Comparing included and excluded participants
Known factors of excluded participants (those who were not able to be traced or interviewed) were identified from clinic files as age, sex, and urban/rural location and compared using z scores and chi-square tests. They showed no significant differences from included participants on any variable.
Sociodemographic characteristics
HIV-positive participants had a mean age of 13.8, 55% were girls and 22% lived in rural areas (Table 2). Nineteen percent lived in informal housing and the remainder in formal or traditional homes, 59% were paternal orphans, 48% were maternal orphans, and 67% were vertically infected. Primary caregivers were 46% biological mothers, 28% grandmothers, 15% aunts, 4% biological fathers, 2% sisters, 1% uncles, 1% grandfathers, and 3% other caregivers such as foster parents or carers in children's homes. Mean years on ART was 5.9 (median 5.0, range 0–19 years, IQR 8.0), and mean travel time to clinical care was 45 min (median 30 min, range 0–3 h. IQR 26 min).
Table 2.
Socio-demographic characteristics of HIV+ adolescents in this sample (n = 1060).
| Age (mean, SD, SE) | 13.8, SD 2.84, SE 0.09 |
| Female | 55.9% |
| Rural location | 21.6% |
| Informal housing | 18.7% |
| Maternal orphanhood | 48.4% |
| Paternal orphanhood | 58.8% |
| Vertical infection | 74.9% |
| Time on ART in years | 6.29, SD 4.71, SE 0.15 |
| Travel time to clinic more than 1 h | 11.3% |
| Physical abuse | 19.6% |
| Verbal abuse | 18.6% |
| Domestic violence or conflict | 11.8% |
| Teacher violence | 41.2% |
| Peer physical violence | 45.7% |
| Peer verbal violence | 46.6% |
| Community violence victimization | 45.3% |
| Witnessing community violence | 22.0% |
| Clinic verbal violence | 21.7% |
Levels of violence exposure were high (Table 2). Past-year physical abuse from caregivers was 19.6%, verbal abuse from caregivers was 18.6%, witnessing domestic violence between adults at home was 11.8%, and sexual abuse was 5.2% (removed from subsequent analyses because of low n). Physical violence by teachers was 41.2% and physical violence by peers 45.7%, with verbal violence by peers at 46.6%. Past-year community violence victimization was 45.3% and witnessing of community violence 22.0%. Verbal victimization by clinic healthcare staff was experienced by 21.7% of adolescents.
Associations between violence exposure and nonadherence
In the first model, five violence factors were excluded with significance P > 0.1: verbal abuse by caregivers, peer physical violence, peer verbal violence, community violence victimization, and witnessing community violence (Table 3). Of the five remaining violence factors retained in the second model, only four were significant in the third and final model: physical abuse by caregivers, witnessing domestic violence at home, violence from a teacher at school, and verbal victimization from a clinic staff member. All four types of violence were associated with higher ART nonadherence, independent of each other and of all covariates: physical abuse by caregivers (OR 1.49, CI 1.18–2.05, P = 0.015); witnessing domestic violence at home (OR 1.80, CI 1.22–2.66, P = 0.003); violence from a teacher at school (OR 1.51, CI 1.16–1.96, P = 0.002) and clinic verbal victimization from a staff member (OR 2.15, CI 1.59–2.93, P = 0.001).
Table 3.
Multivariate logistic regression analyses testing associations between violence exposure and antiretroviral therapy nonadherence among South African HIV-positive adolescents (n = 1056a).
| Model 1: all factors | Model 2: all factors <0.1 | Model 3: all factors <0.05 | ||||
| OR , P value | 95% CI | OR, P value | 95% CI | OR, P value | 95% CI | |
| Age | 1.05, 0.199 | 0.98–1.12 | ||||
| Sex | 1.12, 0.223 | 0.90–1.57 | ||||
| Vertical infection | 1.09, 0.741 | 0.65–1.82 | ||||
| Time on treatment | 0.99, 0.555 | 0.95–1.03 | ||||
| Rural location | 139$, 0.054 | 0.99–1.03 | 1.40*, 0.033 | 1.03–1.91 | 1.44*, 0.020 | 1.06–1.97 |
| Informal housing | 0.81, 0.243 | 0.57–1.16 | ||||
| Travel to clinic more than 1 h | 1.24, 0.313 | 0.82–1.89 | ||||
| Absent father | 1.20, 0.224 | 0.90–1.60 | ||||
| Absent mother | 0.79, 0.113 | 0.59–1.06 | ||||
| Physical abuse | 1.55*, 0.012 | 1.10–2.18 | 1.42*, 0.034 | 1.03–1.99 | 1.49*, 0.015 | 1.18–2.05 |
| Verbal abuse | 1.32, 0.136 | 0.92–1.91 | 1.39$, 0.060 | 0.99–1.96 | ||
| Domestic violence | 1.61*, 0.024 | 1.07–2.44 | 1.74*, 0.007 | 1.17–2.59 | 1.80*, 0.003 | 1.22–2.66 |
| Teacher violence | 1.60*, 0.001 | 1.22–2.10 | 1.52*, 0.002 | 1.17–1.98 | 1.51*, 0.002 | 1.16, 1.96 |
| Peer physical violence | 1.14,0.452 | 0.82–1.58 | ||||
| Peer verbal violence | 1.05, 0.748 | 0.77–1.44 | ||||
| Community violence victimization | 0.77$, 0.083 | 0.57–1.04 | 0.85, 0.226 | 0.64–1.11 | ||
| Witnessing community | 0.97, 0.874 | 0.69–1.37 | ||||
| Violence | ||||||
| Clinic verbal violence | 2.06*, 0.001 | 1.49–2.86 | 2.11*, 0.001 | 1.55–2.88 | 2.15*, 0.001 | 1.59–2.93 |
| LR chi-square | 87.09 | 70.11 | 65.67 | |||
CI, confidence interval; OR, odds ratio.
aThere were four missing cases.
*Statistically significant at P < 0.05.
$Statistically significant at P < 0.1.
No potential two-way and three-way interactions were significant when P values were adjusted for multiple hypothesis testing using Hochberg's step-up method. There were no significant moderation effects of sex or age. Marginal effects models controlling for all factors significantly associated with ART nonadherence, show additive increases in nonadherence with each different combination of violence exposures (Table 4). This showed a clear pattern of increasing risk of nonadherence by additional violence exposures. Categorical Principal Components Analysis (CATPCA) established that all four violence variables loaded onto a single factor (Eigenvalue 1.5) accounting for 38.3% of the total variance. Loading factors for all four violence predictors loaded between 0.3 and 0.65. A summative index of individual violence variables (polyvictimization score) was computed (Fig. 1). This showed a strongly graded relationship of significant increases in nonadherence with each additional victimization. Nonadherence rose from 25.6% amongst adolescents with no violence, to 36.5% with any one violence victimization, 49.3% with any two victimization types, 62.2% with any three types, and 74.9% with all four violence victimization types.
Table 4.
Cumulative effects of different types of violence in combination with each other on antiretroviral therapy adherence amongst South African HIV+ adolescents (n = 1056).
| ART nonadherence | |||
| Violence types and combinations | Predicted % probability | Lower CI | Upper CI |
| No violence | 25.61% | 21.84 | 29.39 |
| Physical abuse | 33.85% | 26.36 | 41.34 |
| Teacher violence | 34.17% | 29.12 | 39.23 |
| Domestic violence | 38.24% | 28.72 | 47.77 |
| Clinic verbal violence | 42.58% | 35.11 | 50.05 |
| Teacher violence and physical abuse | 43.56% | 35.55 | 51.57 |
| Domestic violence and physical abuse | 47.93% | 36.16 | 59.71 |
| Domestic violence and teacher violence | 48.29% | 37.95 | 58.63 |
| Clinic verbal violence and physical abuse | 52.43% | 42.84 | 62.03 |
| Clinic verbal violence and teacher violence | 52.79% | 44.58 | 61.00 |
| Domestic violence and clinic verbal violence | 57.15% | 46.37 | 67.94 |
| Domestic violence, teacher violence, and physical abuse | 58.13% | 46.86 | 69.40 |
| Clinic verbal violence, teacher violence, and physical abuse | 62.44% | 53.48 | 71.40 |
| Domestic, clinic verbal violence, caregiver physical abuse | 66.48% | 55.65 | 77.30 |
| Domestic, clinic verbal violence, and teacher violence | 66.79% | 56.71 | 76.88 |
| All types | 74.94% | 65.90 | 83.98 |
ART, antiretroviral therapy; CI, confidence interval.
Fig. 1.
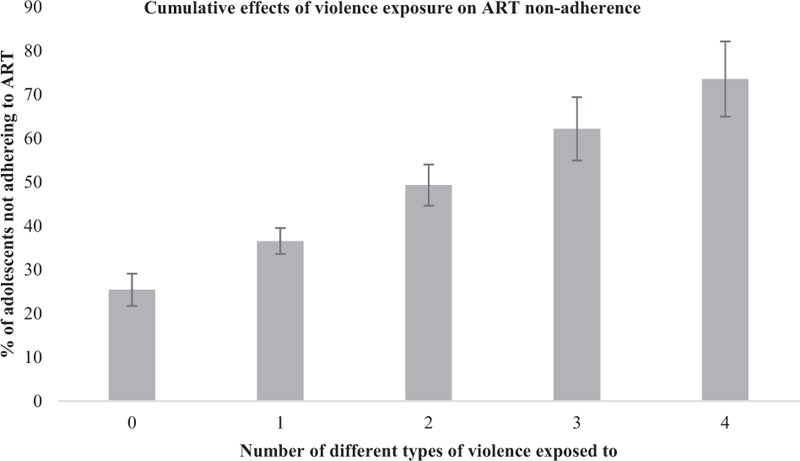
Cumulative effects of violence exposure on predicted probability of ART nonadherence among South African HIV+ adolescents.
Discussion
Despite enormous progress in ART roll-out, nonadherence remains a major impediment to the health and survival of HIV-positive adolescents [48,49]. To date, responses have focused on healthcare services, including adolescent-friendly clinics, counseling, treatment literacy, extended clinic opening hours, and peer support [50,51]. These have the potential to address important barriers to retention in care.
But this study identifies an additional, structural-level risk. Exposure to four types of violence was associated with lower ART adherence, independent of each other, and of socioeconomic, family, and HIV-related factors. They included physical abuse from caregivers, witnessing domestic violence between adults at home, physical violence from teachers, and clinic verbal victimization by healthcare providers.
Findings indicate that violence in community settings and between peers, and verbal abuse from caregivers were not associated with ART adherence. It may be that these are perceived as part of a generally high-violence environment, and whilst they likely have other negative impacts on adolescent development, they did not lead to inability to take a lifesaving medication [16,52]. It is possible that physical violence from caregivers and teachers, and clinic verbal violence from healthcare providers evoke a sense of betrayal amongst adolescents for whom they are hoped for sources of care and support. Just as positive relationships with adult role models may be protective for adolescent health behavior, abusive relationships may be exceptionally detrimental.
The violence type with the strongest individual association with nonadherence was clinic victimization. Patient care experiences may be a particularly important focus for interventions, and qualitative studies of adult ART patients in sub-Saharan Africa report negative provider–patient interactions as a major barrier to adherence [53,54]. Adolescents also report experiences of being shouted at or treated harshly, whilst healthcare workers report frustration and worry regarding their adolescent patients [8,55]. A recent situational analysis of 218 healthcare facilities across sub-Saharan Africa found that healthcare workers lacked training or protocols for supporting HIV-positive adolescents, suggesting that more targeted support may be essential [56].
This was primarily a young sample, with a mean age of 13 years and only 18% sexually active. As these adolescents become older, it will be essential to also measure impacts of intimate partner violence on ART adherence. A recent meta-analysis of intimate partner violence amongst HIV-positive adult women (primarily in US-based studies) found associations with lower ART use [25,57], supported by quantitative and qualitative studies of adult women in sub-Saharan Africa [58,59]. A study this year of HIV-positive adolescents and young women in South Africa found associations between intimate partner violence and reduced medication adherence [60]. We also need to understand if and how intimate partner violence interacts with other types of violence and whether this increases other risks, particularly for young women.
It will be important to further examine the pathways by which violence from different sources may impact adolescent adherence. There is substantive evidence of mental health problems associated with violence victimization, and this may lead to reduced self-esteem, future orientation, and lowered motivation for adherence [24]. It is possible that high-violence home and school environments are also less conducive to safe storage and medication-taking, and may also contribute to increased stigma and secrecy. Qualitative research suggests that negative healthcare experiences can reduce trust in medical treatment, and encourage care-seeking outside of clinical settings [20]. Clinic verbal violence from healthcare providers may alienate adolescents from attending clinics, may trigger defiant behavior in terms of nonadherence, and may inhibit them from disclosing mental health distress or other risks for nonadherence.
This study has a number of limitations. First, all associations are cross-sectional, thus no certainty regarding causality can be held. In particular, shouting and scolding by clinic staff may be both a cause of and a response to nonadherence by adolescents. However, measurement crossover for this association was reduced by reporting periods – we measured past-week adherence, whilst only a handful of adolescents had received clinical care in the past week. The possibility of reverse causality is also unlikely across all four types of violence that were found to be associated with nonadherence. Second, we did not measure intimate partner violence experienced by adolescents, and rates of reported sexual abuse within the HIV-positive sample were too small for inclusion in analyses: these are important potential risk factors that require further investigation.
Third, a strength of this study was that it was a real-world sample of adolescents initiated on ART in government healthcare services in a low-resource South African province. We had overall high inclusion rates, and community-tracing from all clinical files allowed inclusion of adolescents regardless of healthcare retention. However, 9% of ever-initiated adolescents were untraceable, had severe cognitive delay, or did not consent. These may be important subgroups for risk of violence victimization and nonadherence – in particular, there is specific evidence that children with any form of disability may be particularly vulnerable to violence [61–63]. Finally, this study uses self-reported nonadherence, which has the risk of under-reporting because of recall and social desirability biases. However, reviews have identified some unreliability in all methods of measuring ART adherence [64,65]. This study found high correlation between self-reported nonadherence, virological suppression failure, and symptomatic TB, as found in other multisite studies [66,67].
Despite limitations, this study has major implications for adolescent HIV care. It is the first to examine associations of multiple types of violence victimization to nonadherence. It finds impacts of violence – both witnessing and victimization – and strong and graded effects of polyvictimization with increased nonadherence. Amongst adolescents exposed to four types of violence, only 25% were adherent in the past week, compared with 75% of nonvictimized adolescents.
Findings suggest that violence prevention and response may be an essential, and insufficiently addressed, component of adolescent HIV care. Fortunately, there is an increasing evidence-base for effective violence prevention programs for adolescents in high HIV-prevalence countries. The Good Schools program has been shown to reduce teacher and peer violence against learners in Uganda, and the Parenting for Lifelong Health programs show reductions in caregiver violence in South Africa [68–71]. Also in Uganda, the SASA! program reduced intimate partner violence between adults, and in South Africa the PREPARE trial showed reduced IPV victimization amongst school-going adolescents [72] with further trials are underway [31,73,74]. There are no known effective programs shown to reduce verbal violence towards adolescents within clinical settings, although reviews suggest that reducing workload and providing patient-centred care skills may be helpful [75].
Integrating violence prevention and HIV treatment is a complex challenge in resource-constrained settings. It will be important to ensure that HIV-positive adolescents access services for violence prevention and response, without exposing families to dual stigma. However, new initiatives such as kNOw Violence and the Global Partnership to End Violence against Children's INSPIRE package provide important opportunities for advocacy, evidence-building and multiagency collaboration [17,76]. Sustainable Development Goal 17 promotes integration between goals, and these findings demonstrate the importance of ending violence against children (SDG 16) for the promotion of HIV healthcare (SDG 3). If we do not rise to the challenge of addressing violence, the goal of ending AIDS will remain out of reach.
Acknowledgements
L.C., E.T., F.M., L.S., and R.H. contributed to conceptualization of the study. L.C., F.M., and E.T. oversaw research fieldwork. L.C., M.O., F.M., E.T., and L.S. conducted analyses and interpreted findings. All authors provided comments towards drafts of the article and approved the final version for publication.
We thank all the adolescents, parents, and their communities who participated in this study. We thank Eda He, McKenzie Berezin, and Marija Pantelic. The study was supported by the Nuffield Foundation under Grant CPF/41513, the Johnson and Johnson Global Health Programme, Evidence for HIV Prevention in Southern Africa, a UKAID programme managed by Mott MacDonald (MM/EHPSA/UCT/05150014), the John Fell Fund (103/757), the International AIDS Society through the CIPHER grant (155-Hod), and the Clarendon-Green Templeton College Scholarship (MP/ET). Additional support for L.C. was provided by the European Research Council (ERC) under the European Union's Seventh Framework Programme (FP7/2007–2013)/ERC grant agreement n°313421 and the Philip Leverhulme Trust (PLP-2014-095). F.M. was funded by the Economic and Social Research Council (ESRC, ES/N017447/1) and the project received ESRC Impact Acceleration Account Support.
Funding: This research was supported by Nuffield Foundation, DFID, Johnson & Johnson, Leverhulme Trust, IAS, ERC, ESRC, John Fell Fund.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Nachega JB, Hislop M, Nguyen H, Dowdy DW, Chaisson RE, Regensberg L, et al. Antiretroviral therapy adherence, virologic and immunologic outcomes in adolescents compared with adults in southern Africa. J Acquir Immune Defic Syndr 2009; 51:65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.UNICEF. Children and AIDS 2015 Statistical Update. New York: UNICEF; 2015. [Google Scholar]
- 3.World Health Organisation. WHO, Health for the world's adolescents: a second chance in the second decade. Geneva: WHO; 2014. [Google Scholar]
- 4.Kim SH, Gerver SM, Fidler S, Ward H. Adherence to antiretroviral therapy in adolescents living with HIV: systematic review and meta-analysis. AIDS 2014; 28:1945–1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dachew BA, Tesfahunegn TB, Birhanu AM. Adherence to highly active antiretroviral therapy and associated factors among children at the University of Gondar Hospital and Gondar Poly Clinic, Northwest Ethiopia: a cross-sectional institutional based study. BMC Public Health 2014; 14:875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vreeman RC, Wiehe SE, Pearce EC, Nyandiko WM. A systematic review of pediatric adherence to antiretroviral therapy in low- and middle-income countries. Pediatr Infect Dis J 2008; 27:686–691. [DOI] [PubMed] [Google Scholar]
- 7.Hudelson C, Cluver L. Factors associated with adherence to antiretroviral therapy among adolescents living with HIV/AIDS in low- and middle-income countries: a systematic review. AIDS Care 2015; 27:805–816. [DOI] [PubMed] [Google Scholar]
- 8.Adejumo OA, Malee KM, Ryscavage P, Hunter SJ, Taiwo BO. Contemporary issues on the epidemiology and antiretroviral adherence of HIV-infected adolescents in sub-Saharan Africa: a narrative review. J Int AIDS Soc 2015; 18:20049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biressaw S, Abegaz WE, Abebe M, Taye WA, Belay M. Adherence to antiretroviral therapy and associated factors among HIV infected children in Ethiopia: unannounced home-based pill count versus caregivers’ report. BMC Pediatr 2013; 13:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lowenthal E, Lawler K, Harari N, Moamogwe L, Masunge J, Masedi M, et al. Rapid psychosocial function screening test identified treatment failure in HIV+ African youth. AIDS care 2012; 24:722–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mellins CA, Malee KM. Understanding the mental health of youth living with perinatal HIV infection: lessons learned and current challenges. J Int AIDS Soc 2013; 16:18593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malee K, Williams P, Montepiedra G, McCabe M, Nichols S, Sirois PA, et al. Medication adherence in children and adolescents with HIV infection: associations with behavioral impairment. AIDS Patient Care STDs 2011; 25:191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gross R, Bandason T, Langhaug L, Mujuru H, Lowenthal E, Ferrand R. Factors associated with self-reported adherence among adolescents on antiretroviral therapy in Zimbabwe. AIDS Care 2015; 27:322–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haberer J, Mellins C. Pediatric adherence to HIV antiretroviral therapy. Curr HIV/AIDS Rep 2009; 6:194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ssewamala FM. Evaluating a youth-focused economic empowerment approach to HIV treatment adherence. Available at: https://clinicaltrials.gov/ct2/show/NCT01790373; 2013. [Google Scholar]
- 16.Hillis S, Mercy J, Amobi A, Kress H. Global prevalence of past-year violence against children: a systematic review and minimum estimates. Pediatrics 2016; 137:e20154079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shiva Kumar AK, Stern V, Subrahmanian R, Sherr L, Burton P, Guerra N, et al. Ending violence in childhood: a global imperative. Psychol Health Med 2017; 22 suppl 1:1–16. [DOI] [PubMed] [Google Scholar]
- 18.Ward C, Dawes A, Matzopoulos R. Youth violence: sources and solutions in South Africa. Cape Town: UCT Press; 2012. [Google Scholar]
- 19.Burton P, Ward CL, Artz L. The optimus study on child abuse, violence and neglect in South Africa. Cape Town: The Centre for Justice and Crime Prevention; 2015. [Google Scholar]
- 20.Wood K, Jewkes R. Blood blockages and scolding nurses: barriers to adolescent contraceptive use in South Africa. Reprod Health Matters 2006; 14:109–118. [DOI] [PubMed] [Google Scholar]
- 21.Kim MH, Mazenga AC, Yu X, Ahmed S, Paul ME, Kazembe PN, et al. High self-reported nonadherence to antiretroviral therapy amongst adolescents living with HIV in Malawi: barriers and associated factors. J Int AIDS Soc 2017; 20:21437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kacanek D, Malee K, Mellins CA, Tassiopoulos K, Smith R, Grant M, et al. Exposure to violence and virologic and immunological outcomes among youth with perinatal HIV in the pediatric HIV/AIDS cohort study. J Adolesc Health 2016; 59:30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maniglio R. The impact of child sexual abuse on health: a systematic review of reviews. Clin Psychol Rev 2009; 29:647–657. [DOI] [PubMed] [Google Scholar]
- 24.Norman RE, Byambaa M, De R, Butchart A, Scott J, Vos T. The long-term health consequences of child physical abuse, emotional abuse, and neglect: a systematic review and meta-analysis. PLoS Med 2012; 9:e1001349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shemesh E, Annunziato RA, Yehuda R, Shneider BL, Newcorn JH, Hutson C, et al. Childhood abuse, nonadherence, and medical outcome in pediatric liver transplant recipients. J Am Acad Child Adolesc Psychiatry 2007; 46:1280–1289. [DOI] [PubMed] [Google Scholar]
- 26.Hillis SD, Anda RF, Felitti VJ, Marchbanks PA. Adverse childhood experiences and sexual risk behaviors in women: a retrospective cohort study. Fam Plann Perspect 2001; 33:206–211. [PubMed] [Google Scholar]
- 27.Dube SR, Anda RF, Felitti VJ, Chapman DP, Williamson DF, Giles WH. Childhood abuse, household dysfunction, and the risk of attempted suicide throughout the life span: findings from the Adverse Childhood Experiences Study. JAMA 2001; 286:3089–3096. [DOI] [PubMed] [Google Scholar]
- 28.Richter L, Komarek A, Desmond C, Celentano D, Morin S, Sweat M, et al. Reported physical and sexual abuse in childhood and adult HIV risk behaviour in three African countries: findings from Project Accept (HPTN-043). AIDS Behav 2014; 18:381–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown MJ, Thacker LR, Cohen SA. Association between adverse childhood experiences and diagnosis of cancer. PLoS One 2013; 8:e65524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Devries KM, Child JC, Allen E, Walakira E, Parkes J, Naker D. School violence, mental health, and educational performance in Uganda. Pediatrics 2014; 133:e129–e137. [DOI] [PubMed] [Google Scholar]
- 31.Jewkes R, Dunkle K, Nduna M, Shai N. Intimate partner violence, relationship power inequity, and incidence of HIV infection in young women in South Africa: a cohort study. Lancet 2010; 376:41–48. [DOI] [PubMed] [Google Scholar]
- 32.Statistics SA. Gross domestic product: annual estimates 2002-2010, regional estimates 2002-2010, third quarter 2011. Pretoria: Statistics South Africa; 2012. [Google Scholar]
- 33.Bezabhe W, Chalmers L, Bereznicki L, Peterson G. Adherence to antiretroviral therapy and virologic failure: a meta-analysis. Medicine (Baltimore) 2016; 95:e3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fenner L, Atkinson A, Boulle A, Fox MP, Prozesky H, Zürcher K, et al. HIV viral load as an independent risk factor for tuberculosis in South Africa: collaborative analysis of cohort studies. J Int AIDS Soc 2017; 20:21327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duong M, Piroth L, Grappin M, Forte F, Peytavin G, Buisson M, et al. Evaluation of the Patient Medication Adherence Questionnaire as a tool for self-reported adherence assessment in HIV-infected patients on antiretroviral regimens. HIV Clin Trials 2001; 2:128–135. [DOI] [PubMed] [Google Scholar]
- 36.Lowenthal E, Haruna B, Mmapula L, Keofentse M, Tshume O, Anabwani G. Disclosure of HIV status to HIV-infected children in a large African treatment center: lessons learned in Botswana. Child Youth Serv Rev 2014; 45:143–149. [Google Scholar]
- 37.Paterson DL, Swindells S, Mohr J, Brester M, Vergis EN, Squier C, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med 2000; 133:21–30. [DOI] [PubMed] [Google Scholar]
- 38.WHO. Technical and operational considerations for implementing HIV viral load testing: Interim technical update [Internet]. Geneva, Switzerland: World Health Organization; Available at: http://apps.who.int/iris/bitstream/10665/128121/1/9789241507578_eng.pdf?ua=1&ua=1 2014. [Google Scholar]
- 39.Corbett EL, Bandason T, Duong T, Dauya E, Makamure B, Churchyard GJ, et al. Comparison of two active case-finding strategies for community-based diagnosis of symptomatic smear-positive tuberculosis and control of infectious tuberculosis in Harare, Zimbabwe (DETECTB): a cluster-randomised trial. Lancet 2010; 376:1244–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.World Health Organisation Guidance for national tuberculosis programmes on the management of tuberculosis in children. Geneva: World Health Organisation; 2006. [Google Scholar]
- 41.Snider L, Dawes A. Psychosocial vulnerability and resilience measures for national-level monitoring of orphans and other vulnerable children: recommendations for revision of the UNICEF psychological indicator. Cape Town: UNICEF; 2006. [Google Scholar]
- 42.Finkelhor D, Hamby S, Turner H, Ormrod R. The Juvenile Victimization Questionnaire: 2nd Revision (JVQ-R2). Durham: NH; 2011. [Google Scholar]
- 43.Ruchkin V, Schwab-Stone M, Vermeiren R. Social and Health Assessment (SAHA) psychometric development summary. New Haven: Yale University; 2004. [Google Scholar]
- 44.Martinez P, Richters J. The NIMH community violence project: II. Children's distress symptoms associated with violence exposure. Psychiatry 1993; 56:22–35. [DOI] [PubMed] [Google Scholar]
- 45.Statistics South Africa. Census 2011: Household questionnaire. Pretoria: Statistics South Africa; 2011. [Google Scholar]
- 46.Cluver L, Orkin M, Boyes ME, Sherr L, Makasi D, Nikelo J. Pathways from parental AIDS to child psychological, educational and sexual risk: developing an empirically-based interactive theoretical model. Soc Sci Med 2013; 87:185–193. [DOI] [PubMed] [Google Scholar]
- 47.Hosmer D, Lemeshow S. Applied logistic regression. New York: John Wiley & Sons; 1989. [Google Scholar]
- 48.Bekker LG, Venter F, Cohen K, Goemare E, Van Cutsem G, Boulle A, et al. Provision of antiretroviral therapy in South Africa: the nuts and bolts. Antivir Ther 2014; 19 suppl 3:105–116. [DOI] [PubMed] [Google Scholar]
- 49.International Advisory Panel on HIVCCO. IAPAC guidelines for optimizing the HIV care continuum for adults and adolescents. J Int Assoc Provid AIDS Care 2015; 14 suppl 1:S3–S34. [DOI] [PubMed] [Google Scholar]
- 50.MacPherson P, Munthali C, Ferguson J, Armstrong A, Kranzer K, Ferrand R, et al. Service delivery interventions to improve adolescents’ linkage, retention and adherence to antiretroviral therapy and HIV care. Trop Med Int Health 2015; 20:1015–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fatti G, Shaikh N, Eley B, Grimwood A. Improved virological suppression in children on antiretroviral treatment receiving community-based adherence support: a multicentre cohort study from South Africa. AIDS Care 2014; 26:448–453. [DOI] [PubMed] [Google Scholar]
- 52.UNICEF. Hidden in plain sight: a statistical analysis of violence against children. New York: UNICEF; 2014. [Google Scholar]
- 53.Wachira J, Naanyu V, Genberg B, Koech B, Akinyi J, Kamene R, et al. Health facility barriers to HIV linkage and retention in Western Kenya. BMC Health Serv Res 2014; 14:646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chimbindi N, Barnighausen T, Newell ML. Patient satisfaction with HIV and TB treatment in a public programme in rural KwaZulu-Natal: evidence from patient-exit interviews. BMC Health Serv Res 2014; 14:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vale B, Hodes R, Cluver L, Thabeng M. Bureaucracies of blood and belonging: documents, HIV-positive youth and the state in South Africa. Dev Change 2016; 48:1287–1309. [Google Scholar]
- 56.Mark D, Armstrong A, Andrade C, Penazzato M, Hatane L, Taing L, et al. HIV treatment and care services for adolescents: a situational analysis of 218 facilities in 23 sub-Saharan African countries. J Int AIDS Soc 2017; 20 suppl 3:21591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hatcher AM, Smout EM, Turan JM, Christofides N, Stockl H. Intimate partner violence and engagement in HIV care and treatment among women: a systematic review and meta-analysis. AIDS 2015; 29:2183–2194. [DOI] [PubMed] [Google Scholar]
- 58.Kosia A, Kakoko D, Semakafu AM, Nyamhanga T, Frumence G. Intimate partner violence and challenges facing women living with HIV/AIDS in accessing antiretroviral treatment at Singida Regional Hospital, central Tanzania. Glob Health Action 2016; 9:32307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hampanda KM. Intimate partner violence and HIV-positive women's nonadherence to antiretroviral medication for the purpose of prevention of mother-to-child transmission in Lusaka, Zambia. Soc Sci Med 2016; 153:123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kidman R, Violari A. Dating violence against HIV-infected youth in South Africa: associations with sexual risk behavior, medication adherence, and mental health. J Acquir Immune Defic Syndr 2018; 77:64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mikton C, Maguire H, Shakespeare T. A systematic review of the effectiveness of interventions to prevent and respond to violence against persons with disabilities. J Interpers Violence 2014; 29:3207–3226. [DOI] [PubMed] [Google Scholar]
- 62.Emerson E, Brigham P. Exposure of children with developmental delays to social determinants of poor health: cross-sectional case record reveiw study. Child Care Health Dev 2015; 41:249–257. [DOI] [PubMed] [Google Scholar]
- 63.Jones L, Bellis M, Wood S, Hughes K, McCoy E, Eckley L, et al. Prevalence and risk of violence against children with disabilities: a systematic review and meta-analysis of observational studies. Lancet 2012; 380:899–907. [DOI] [PubMed] [Google Scholar]
- 64.Orrell C, Cohen K, Leisegang R, Bansberg D, Wood R, Maartens G. Comparison of six methods to estimate adherence in an ART-naïve cohort in a resource-poor setting: which best predicts virological and resistance outcomes?. AIDS Res Ther 2017; 14:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mudhune V, Gvetadze R, Girde S, Ndivo R, Angira F, Zeh C, et al. Correlation of adherence by pill count, self-report, MEMS and plasma drug levels to treatment response among women receiving ARV therapy for PMTCT in Kenya. AIDS Behav 2018; 22:918–928. [DOI] [PubMed] [Google Scholar]
- 66.Usitalo A, Leister E, Tassiopoulos K, Allison S, Malee K, Paul ME, et al. Relationship between viral load and self-report measures of medication adherence among youth with perinatal HIV infection. AIDS Care 2014; 26:107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Buscher A, Hartman C, Kallen MA, Giordano TP. Validity of self-report measures in assessing antiretroviral adherence of newly diagnosed, HAART-naive, HIV patients. HIV Clin Trials 2011; 12:244–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Devries KM, Knight L, Child JC, Mirembe A, Nakuti J, Jones R, et al. The Good School Toolkit for reducing physical violence from school staff to primary school students: a cluster-randomised controlled trial in Uganda. Lancet Glob Health 2015; 3:e378–e386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cluver L, Meinck F, Steinert J, Shenderovich Y, Doubt J, Romero RH, et al. Parenting for lifelong health: a pragmatic cluster randomised controlled trial of a non-commericalised parenting programme for adolescents and their families in South Africa. BMJ Glob Health 2017; 3:e000539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cluver L, Meinck F, Shenderovich Y, Ward CL, Romero RH, Redfern A, et al. A parenting programme to prevent abuse of adolescents in South Africa: study protocol for a randomised controlled trial. Trials 2016; 17:328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cluver L, Meinck F, Yakubovich A, Doubt J, Redfern A, Ward C, et al. Reducing child abuse amongst adolescents in low- and middle- income countries: a prepost trial in South Africa. BMC Public Health 2016; 16:567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mathews C, Sander ME, Townsend L, Aaro L, de Vries P, Mason-Jones A, et al. Effects of PREPARE, a multicomponent, school-based HIV and Intimate Partner Violence (IPV) Prevention Programme on Adolescent Sexual Risk Behaviour and IPV: Cluster Randomised Controlled Trial. AIDS Behav 2016; 20:1821–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Abramsky T, Devries KM, Michau L, Nakuti J, Musuya T, Kyegombe N, et al. The impact of SASA!, a community mobilisation intervention, on women's experiences of intimate partner violence: secondary findings from a cluster randomised trial in Kampala, Uganda. J Epidemiol Community Health 2016; 70:818–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kuo C, Mathews C, Cluver L, Operario D, Atujuna M, Beardslee W, et al. Feasibility and acceptability of the ‘Our Family Our Future’ intervention: a pilot study of a family-based intervention for preventing adolescent HIV risk behavior. International AIDS Conference, 19–22 July 2016; Durban, South Africa; 2016. [Google Scholar]
- 75.Dwamena F, Holmes-Rovner M, Gaulden CM, Jorgenson S, Sadigh G, Sikorskii A, et al. Interventions for providers to promote a patient-centred approach in clinical consultations. Cochrane Database Syst Rev 2012; 12:CD003267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Global Partnership to End Violence against Children. INSPIRE: seven strategies for ending violence against children. Geneva: World Health Organisation; 2016. [Google Scholar]


