Abstract
Purpose of review
To provide an overview of the mechanistic and clinical evidence for the use of nonspecific immunomodulators in paediatric respiratory tract infection (RTI) and wheezing/asthma prophylaxis.
Recent findings
Nonspecific immunomodulators have a long history of empirical use for the prevention of RTIs in vulnerable populations, such as children. The past decade has seen an increase in both the number and quality of studies providing mechanistic and clinical evidence for the prophylactic potential of nonspecific immunomodulators against both respiratory infections and wheezing/asthma in the paediatric population. Orally administered immunomodulators result in the mounting of innate and adaptive immune responses to infection in the respiratory mucosa and anti-inflammatory effects in proinflammatory environments. Clinical data reflect these mechanistic effects in reductions in the recurrence of respiratory infections and wheezing events in high-risk paediatric populations. A new generation of clinical studies is currently underway with the power to position the nonspecific bacterial lysate immunomodulator OM-85 as a potential antiasthma prophylactic.
Summary
An established mechanistic and clinical role for prophylaxis against paediatric respiratory infections by nonspecific immunomodulators exists. Clinical trials underway promise to provide high-quality data to establish whether a similar role exists in wheezing/asthma prevention.
Keywords: asthma, bacterial lysate, nonspecific immunomodulators, recurrent respiratory infection, wheezing
INTRODUCTION
Despite being overwhelmingly viral in nature, respiratory tract infections (RTIs) are a major source of antibiotic misuse and create a significant burden of care [1–4,5▪]. Immunological immaturity and environmental factors (e.g. frequent social contacts, exposure to pollution and lack of breastfeeding) put children at increased risk of recurrent RTI (RRTI) [4,5▪,6,7,8▪▪]. RTIs early in life, and episodes of viral-induced wheezing in particular, are a significant risk factor for asthma in later life [4,6,7]. Patient interventions and parental education have a role in prevention of RRTI and its consequences, as does active immunization in cases where vaccines are available [5▪]. However, the difficulties inherent in effecting behavioural change and the lack of vaccines against most pathogenic organisms responsible for RTI create the need for other prophylactic strategies [5▪,9]. The use of nonspecific immunomodulation to boost the body's natural defences against infection offers a strategy with proven efficacy and tolerability in preventing RTIs in children [8▪▪].
Box 1.
no caption available
AIM AND SEARCH STRATEGY
The aim of this publication was to summarize the mechanistic and clinical evidence for the use of nonspecific oral immunomodulators in the prevention of RTIs, wheezing and asthma exacerbations in childhood. A systematic literature search was performed to identify articles with either mechanistic or clinical evidence on nonspecific immunomodulators between 1997 and 2017. Search terms and article disposition is shown in Supplementary Figure 1A. Only therapies with both mechanistic data and clinical data from paediatric double-blind randomized controlled trials were included in the analysis. A hand search of eligible articles and author expertise were used to supplement the included articles.
RATIONALE FOR THE USE OF IMMUNOMODULATORS IN RESPIRATORY TRACT INFECTION, WHEEZING AND ASTHMA CONTROL
The empiric use of immunomodulators has over a century of clinical history, with publications investigating their efficacy dating back to at least the 1950s. The mechanistic rationale for the use of the orally administered immunomodulators for the prevention of respiratory conditions centres on the gut–lung immune axis (Fig. 1) [10]. Antigen sampling by M cells and dendritic cells resident in the Peyer's patches of the gut-associated lymphoid tissue leads to maturation of dendritic cells into an antigen-presenting cell phenotype [11]. The subsequent dendritic cell-initiated immune cascade involves homing of cells from both innate and adaptive branches of the immune system to the mucosal-associated lymphoid tissue of the lungs and subsequent antibody production.
FIGURE 1.
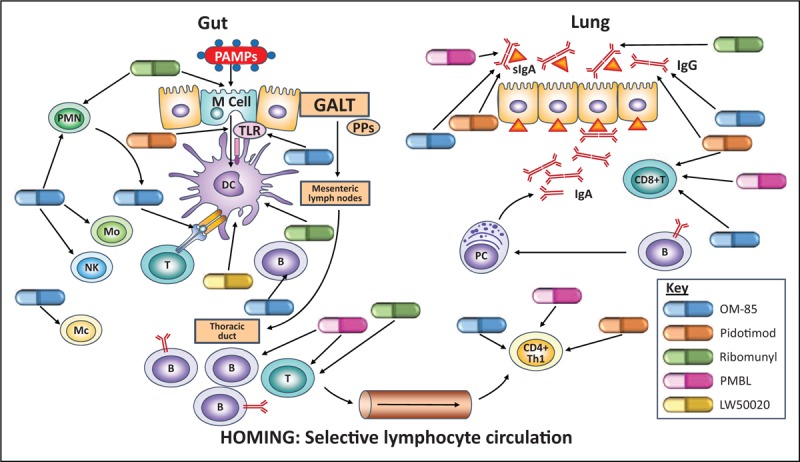
The gut–lung immune axis illustrating points of immunomodulator activity in RTI prophylaxis. B, B cell; GALT, gut-associated immune tissue; Mc, macrophage; Mo, monocyte; NK, natural killer cell; PAMPS, pathogen-associated molecular patterns; PC plasma cell; PMN, polymorphonuclear neutrophil; PPs, Peyer's patches; T, T cell. Based on [10].
Data suggest that immunomodulators may also act to aid maturation of the immune system in children, correcting T helper cell (Th) Th1/Th2 imbalance through activation of T regulatory (Treg) cells (for review, see Kearney et al.[12]). The correction of this Th2-oriented imbalance and other antiinflammatory activity may reduce atopic responses related to wheezing and asthma. These effects combined with the reduced risk of RTIs, which predispose towards asthma and cause exacerbations, form the mechanist framework for a reduced risk of wheezing events and asthma.
MECHANISM OF ACTION FOR IMMUNOMODULATORS IN RESPIRATORY TRACT INFECTION
Five immunomodulators eligible for inclusion in this review had mechanistic data pertaining to the prophylaxis of RTIs: OM-85, pidotimod, ribomunyl, LW50020 and polyvalent mechanical bacterial lysate (PMBL) (Table 1) [13,14,15▪,16▪▪,17–20,21▪,22–28,29▪,30–36,37▪,38–51].
Table 1.
Characteristics of included immunomodulators and proposed mechanisms of action for infection prevention
| Therapy | Constituents | Antigen-presenting cells | Innate immunity | Adaptive |
| OM-85 | Alkaline lysis of 21 strains of eight species of respiratory tract pathogens: Haemophilus influenzae, Streptococcus pneumoniae, Klebsiella pneumoniae, Klebsiella ozaenae, Staphylococcus aureus, Streptococcus pyogenes, Streptococcus viridans and Moraxella catarrhalis | Maturation of mesenteric DCs [13,14,15▪]Modulated activation suggesting prealert anti-infective state [14,16▪▪]Innate and adaptive cytokine release [17]PRR yet to be determined [14,16▪▪,18,19] | Release of antimicrobial peptides [human beta-defensin-1 (hβD-1)] and C1q-R) [20,21▪]ICAM downregulated in lung epithelium [21▪] Rapid neutrophil recruitment in murine model of influenza infection [15▪]Cytokines promoting NK-cells, monocytes, phagocytosis, neutrophils (CCL2, CCL3, CXCL1, CXCL5, CXCL6 and CXCL8) [14,17]Macrophage activation (IL-1b, IL-6 and TNFα mRNA) [16▪▪,18,19] Antiviral cytokine release (INFβ) [16▪▪] | DC-induced T-cell activation [22,23]Airway CD8+ T cells in murine influenza model [15▪]Pro-B-cell cytokines (IL-6, BAFF and IL-10) [14,23]Serum IgA/IgG (murine/human) [15▪,18,20]B-cell maturation from mouse splenocytes [15▪]Airway/salivary murine IgA/IgG [15▪,18,24]Immune maturation (pro-INFy and IgG2/anti-IL-4) [25]Release of antiviral cytokines INFα/INFy [14,21▪,25,26] |
| Pidotimod | Synthetic thymic dipeptide (3-L-pyroglutamyl-L-thiaziolidine-4 carboxylic acid) | Mucosal DC maturation and increased antigen presentation [27,28,29▪,30]Increased TLR2 and TLR4 [29▪,30]Innate and adaptive cytokine release [28,29▪] | Increased TLR2 expression in lung epithelial cells in vitro [31]Release of antimicrobial peptides (CAMP, LCN2, LTF and MPO) [29▪]Improved mucociliary transport [32]Cytokines promoting macrophages, monocytes, NK cells and neutrophils (CCL3, CXCL1, CXCL2, IL-18 and IL-8) [29▪,33] | Activation of cytotoxic and helper T cells (CD3+, CD4+ and CD4+/CD8+) [34]Immune maturation (pro-IL-12, IFNy, IL-10 and IL-18/anti-IL-4) [33,34,35,36,37▪,38]Increased mucosal sIgA [39]Release of antiviral cytokines INFy [39] |
| Ribomunyl | Bacterial proteoglycans and ribosomes of common respiratory tract pathogens: K. pneumoniae (proteoglycans and ribosomes) and K. pneumoniae, S. pneumoniae, S. pyogenes and H. influenza (ribosomes) | DC maturation [13,40,41]Innate and adaptive cytokine release [41] | Increased neutrophil adhesion molecules (+CD11c and +CD103) and phagocytosis [42,43] | DCs-induced T cells activation causing release of antiviral INFy (CD4+) [13,41]Possible release of pro-TH1 cytokines (IL-12, IL-10) [13,40]Increase in CD4+ and CD8+ T cells [44]B cell production (humoural, tonsils, mesenteric/cervical lymph nodes) [44]Salivary sIgA [45,46]Serum IgA and IgG [44,47,48] |
| PMBL | Bacterial lysates of eight bacterial species: S. aureus, S. viridans, S. pyogenes, K. pneumoniae, K. ozaenae, H. influenzae serotype B, M. catarrhalis and S. pneumoniae | -- | Putative macrophage activation (pro-IL-12) [49] | T cell activation (CD4+ and CD8+) [49]B cell activation [49]IgM memory B cell expansion [50]Immune maturation (+IL-2, +IL-10, IL-12 and +IFNy) [49]Release of antiviral cytokines INFy [49]Release of salivary sIgA [51] |
| LW50020 | Bacterial lysates of seven bacterial species: S. aureus, Streptococcus mitis, S. pyogenes, S. pneumoniae, K. pneumoniae, M. catarrhalis and H. influenza | DC maturation [13] | DC-induced T cells activation [13] |
TNFα, tumour necrosis factor-alpha.
OM-85
OM-85 is the product of alkaline lysis of 21 strains of common bacterial respiratory tract pathogens (Table 1) [14,52]. The active ingredients of OM-85 are resistant to gastric transit and cause maturation of mucosal dendritic cells in gastrointestinal Peyer's patches, a key step in orally induced respiratory immunity (Table 1; Fig. 1) [14,15▪,17,22]. OM-85-induced dendritic cell activation occurs in a modulated manner, resulting in a putative prealert antiinfective state in the mucosal immune system (Table 1) [11,14,16▪▪,22]. There are conflicting data on the identity of the dendritic cell-expressed pattern-recognition receptors (PRRs) activated by pathogen-associated molecular patterns comprising OM-85, possibly because of species differences (Table 1) [14,16▪▪,18,19,22]. We identified a single eligible study reporting no dendritic cell maturation with OM-85 at concentrations below those affecting cell viability (<100 μg); however, these results conflict with other cell viability data [13,17].
Dendritic cells are the nexus of the innate and adaptive mucosal immune response, and OM-85-activated dendritic cells have been shown to directly activate cellular constituents of both immune-system branches (Table 1; Fig. 1) [14,22]. OM-85-induced dendritic cells release chemokines that act on monocytes and natural killer (NK) cells, as well as prophagocytic chemokines which induce polymorphonuclear neutrophil migration (Table 1; Fig. 1) [14]. The downstream effects on OM-85 on the innate immune system include the release of antimicrobial peptides and the activation of macrophages resulting in expression of proinflammatory and antiviral cytokines (Table 1; Fig. 1) [16▪▪,18–20,21▪]. In line with these antiviral actions, OM-85 reduced rhinovirus infection of lung epithelial cells and cell death in vitro[21▪]. Data also suggest that OM-85 causes more rapid neutrophil recruitment in response to viral infection, reducing viral load (Table 1) [15▪].
OM-85-induced dendritic cells activate T cells in vitro and oral OM-85 increases antiviral CD8+ T-cell response in the airways of mice following influenza infection (Table 1; Fig. 1) [15▪,22,23]. In neonatal rats, oral OM-85 promotes immune system maturation by acting to correct the Th1/Th2 imbalance, and the release of antiviral Th1-related cytokines has been demonstrated both in vivo and in vitro (Table 1; Fig. 1) [14,25,26]. OM-85-induced dendritic cells also produce B-cell-related cytokines and OM-85 causes B-cell maturation in vitro[14,15▪,23], leading to increases in serum and airway immunoglobulins (Ig) in both children and mice [16▪▪,18,20,25,53] (Table 1; Fig. 1). In murine models of bacterial, viral and viral/bacterial respiratory infections, OM-85 reduced clinical symptoms and improved survival [15▪,53]. However, one similar mouse study failed to show an effect on bacterial clearance, neutrophil recruitment or survival. The authors postulated that the virulence of the Klebsiella pneumoniae infection may have led to masking of the effect of OM-85 in this study [54].
Pidotimod
Unlike the other immunomodulators included in this review, pidotimod is a synthetic thymic dipeptide rather than a bacterial derivative [52,55] (Table 1). However, orally administered pidotimod appears to share a number of mechanistic similarities with bacterial immunomodulators. Pidotimod causes maturation of mucosal dendritic cells and increases antigen presentation [27,28,29▪,30], likely via PRRs toll-like receptor 2 (TLR2) and TLR4 [29▪,30,56] (Table 1; Fig. 1). Activated dendritic cells release cytokines and chemokines related to the innate immune response (Table 1) [28,29▪]. Pidotimod-induced innate immune responses include increased expression of TLR2 in lung epithelial cells in vitro, increases in the release of antimicrobial peptides and improved mucociliary transport (Table 1; Fig. 1) [29▪,31,32]. In a model of Mycoplasma pneumoniae infection, NK cell markers were down regulated; however, data suggest that this may improve resistance to further infection [34,57]. Furthermore, in a small group of adults with community acquired pneumonia, pidotimod reduced the number of cells producing tumour necrosis factor-alpha, a proinflammatory cytokine associated with a negative prognosis in this condition [30].
Pidotimod-induced dendritic cells promote proliferation of T cells and, along with induced monocytes, release cytokines promoting adaptive Th1-mediated immunity (Fig. 1; Table 1) [28,29▪]. Pidotimod also directly increases levels of Th1-related cytokines and suppresses Th2 cytokines in various settings including in children with frequent infections (Fig. 1; Table 1) [29▪,35,36,37▪,38]. In addition, markers of both cytotoxic and helper T cells were elevated following the treatment of M. pneumoniae pneumonia though not in a study of combined treatment with loratadine for RRTI (Table 1; Fig. 1) [33,34]. However, we identified a single in-vitro study where pidotimod failed to promote differentiation of Th0 to Th1 [35]. Direct data showing pidotimod acting on B cells are lacking; however, increased production of nasopharyngeal and salivary secretory IgA (sIgA) in children with RTI has been demonstrated (Table 1; Fig. 1) [39]. Although, pidotimod therapy did not increase antibody titres in two studies on children with bacterial pneumonia or RRTI [33,34]. Finally, novel data suggest that pidotimod may have positive effects on the metabolic profile of children suffering RRTI [58].
Ribomunyl
Ribomunyl is a mixture of bacterial proteoglycans and ribosomes which are delivered to lymphoid cells resident in Peyer's patches via uptake by mucosal M cells, resulting in dendritic cell maturation (Table 1; Fig. 1) [13,40,41,52,59,60]. Data on innate immune system effects are sparse, with studies showing increased expression of adhesion molecules and phagocytic activity in peripheral-blood neutrophils in response to ribomunyl [42,43] (Table 1; Fig. 1).
Ribomunyl-induced dendritic cells stimulate T cells causing antiviral interferon gamma (IFN-γ) release; however, there are conflicting data on ribomunyl-induced release of pro-Th1 cytokines (Table 1) [13,40,41]. Proliferation of T cells is evident in patients with otitis media treated with ribomunyl (Table 1; Fig. 1) [44]. Furthermore, oral administration causes expansion of constituent-specific B cells and sIgA production [45,46,61], and increased serum IgA and IgG in children with RRTI [44,47,48]. Increased sIgA concentrations in healthy volunteers were associated with reduced adhesion of a constituent strain, Streptococcus pneumoniae (Table 1; Fig. 1) [46].
Others
PMBL (Ismigen) is a sublingually delivered lysate made using a mechanical process that preserves the structure of the bacterial antigens [52]. It activates both T and B cells and causes release of pro-Th1 and macrophage activating cytokines in vitro[49] (Fig. 1; Table 1). PMBL induces memory B-cell expansion which correlates with RTI prophylaxis in patients suffering recurrence and sIgA response in healthy children and adults [50,51].
The immunomodulator LW50020 is and orally delivered bacterial lysate which induces dendritic cell maturation, and activated dendritic cells are capable of stimulating T lymphocytes (Table 1; Fig. 1) [13].
Summary
The available mechanistic data on oral immunomodulators fit the proposed model of gut-mediated respiratory mucosal immunity. A relatively full mechanistic picture is available of OM-85 from activation of gut immunity, downstream activation of both innate and adaptive immune responses, trafficking of immune cells to the airway and release of airway sIg. Pidotimod appears to work via a similar mechanism derived from activation of mesenteric dendritic cell, leading to the activation of innate and adaptive immune branches and subsequent mounting of a response in the airway. The data on ribomunyl are more sparse particularly regarding innate immune action, though what data are available fit the current understanding of gut–lung immune axis. Little data on PMBL and LW50020 were available, though, importantly, effector activity in the lung has been demonstrated following oral immunization with PMBL.
EFFICACY OF IMMUNOMODULATORS IN CHILDREN WITH RESPIRATORY TRACT INFECTION
All the above immunomodulators have evidence of efficacy in paediatric RTI-prophylaxis, to varying degrees.
OM-85
OM-85 has demonstrated efficacy in a number of forms of paediatric RTI (Supplementary Table 1) [8▪▪,62,63▪]. Oral therapy reduced the incidence, prevalence and/or duration of infections in children with a history of RRTI compared with placebo [64–67] and versus probiotic therapy [24]. In a study of children with recurrent tonsillitis, OM-85 prophylaxis improved outcomes in the majority of patients and, importantly, removed the need for surgery in a significant proportion of those treated [66]. In children with subacute sinusitis, OM-85 prophylaxis sped recovery and reduced infections [68], whereas children with chronic rhinosinusitis had a reduced symptom burden and a lower incidence of attacks [69]. OM-85 has also shown efficacy in high-risk environs, reducing the incidence and prevalence of infections in a Mexican orphanage [70].
Reductions in antibiotic and drug treatment following prophylactic therapy with OM-85 have also been demonstrated in children with a history of RRTI, subacute sinusitis and in high-risk environments (orphanages) [64,68,69]. OM-85 therapy reduced school absenteeism both in children with a history or RRTI and in those living within an orphanage [67,69]. Efficacy was unaffected by coadministration with antibiotic therapy or the influenza vaccine, with OM-85 conferring additional benefit in terms of absenteeism and prevalence of infections in both cases [67,68].
OM-85 is well tolerated with a frequency of adverse events comparable to that seen with placebo in clinical trials. Undesirable events are mainly mild and transient, with manageable risks. This safety profile appears to be stable in nature and frequency over long-standing use [24,64–67,69,70].
Pidotimod
Research into the efficacy of pidotimod in the paediatric population has increased in recent years, particularly in the Russian Federation. The incidence and/or prevalence of infections was reduced in children with a history of RTI in a number of studies without an active control [32,71–74] versus another immunomodulator [75] and versus antibiotic therapy [37▪]. In children with RRTI, pidotimod reduced fever, cough and pulmonary rales [76], and duration of symptoms was also reduced compared with spleen amino peptide [36]. In children about to enter the high-risk day care environment, pidotimod showed nonsignificant reductions in RTI and antibiotic use. The lack of power in this study may have contributed to the failure to demonstrate significant efficacy [77].
Pidotimod prophylaxis also results in less antibiotic use [37▪,71,74], hospitalization and paediatric visits [71,72] and school absenteeism [72]. Where reported, adverse events were infrequent, mild and transient [71,72,75–77].
Ribomunyl
In a number of trials on children with RRTI, including those with adenoiditis, pharyngotonsillitis and otitis media, ribomunyl reduced the incidence, prevalence and/or duration of infections [44,47,48,78,79]. The use of antibiotics/ancillary therapy [44,47,48,79], school absenteeism [44,47,79] and medical visits [48] was also reduced. In a comparison of ribomunyl prophylaxis for patients with more or less than five RTIs in the past year, only those with five or less showed a decrease in incidence of infection, and physicians did not rate a significant improvement in either group. Neither was there a difference in school absenteeism in this study [78]. Ribomunyl is generally well tolerated and treatment-related adverse events are uncommon.
Other
LW50020 reduced clinical severity score, infection rates and duration of infections in children with RRTI compared with placebo [80]. Furthermore, the rate, duration and severity of RTIs, and the use of antibiotics reduced compared to pretreatment values in a dose comparison study in children with RRTI [81]. PMBL reduced the incidence of RTI and the use of both antibiotic and antiviral drugs, in children with a history of RRTI and was well tolerated [82]. Adverse drug reactions were infrequent, transient and nonserious with both therapies [81,82].
Summary
The eligible immunomodulators demonstrated efficacy in preventing RTI in children with a history of RRTI, and in a number of specific upper RTI subtypes. Numerous definitions of RRTI were used and standardization of the definition of this condition is desirable. Although a quantitative assessment of trial quality was not undertaken, observations of reporting quality were in line with data from the 2012 Cochrane review suggesting articles assessing OM-85 were of higher quality [8▪▪]. Studies on pidotimod in particular lacked reporting of safety, control groups or information on the definition of RRTI, particularly in foreign language abstract-only publications.
MECHANISM OF ACTION OF IMMUNOMODULATORS IN WHEEZING AND ASTHMA
Mechanistic data relating to OM-85, pidotimod, ribomunyl, PMBL in wheezing, asthma or related conditions were available.
OM-85
The Th2 immune response is key to the airway hyper-reactivity that occurs during asthma exacerbations. Data from mouse models show that orally administered OM-85 activates gut dendritic cells which induce trafficking of pro-Th1/anti-Th2 Treg cells to the lung (Table 2) [16▪▪,18,37▪,83▪▪,84▪,85–87,88▪,90▪,91–101]. OM-85 also downregulates Th2-associated markers on gut dendritic cells (Table 2) [16▪▪]. In the lung, OM-85-induced Tregs inhibit the Th2-associated response, likely via modulation of the response of lung dendritic cells (Table 2) [83▪▪,84▪]. This immunoregulatory activity results in reduced allergen-induced airway inflammation and hyper-reactivity in sensitized mice over a time course which reflects OM-85's cellular effects (Table 2) [83▪▪,88▪].
Table 2.
Proposed mechanisms of action for the prevention of wheezing and asthma exacerbation
| Therapy | Dendritic cells/monocytes | Th1-Th2 Balance | Airway inflammation | Immunoglobulins |
| OM-85 | Increased T-reg-related CD103+ DCs in mesenteric lymph nodes [83▪▪]Reduced Th2-associated markers on induced DCs (ICOSL) [15▪]Accelerated resolution of airway DCs reaction to allergen in a mouse model of asthma [84▪] | Trafficking of IL-10 producing Tregs from gut to airway [84▪]Reduced CD4+ Th2-type cells and inflammatory cytokines (IL-4, IL-5, IL-6, IL-10 and IL-13) in lungs of sensitized mice [84▪]Allograft of induced Tregs blocks Th2 and inflammatory cytokine production in sensitized mice (IL-5 and IL-13) [84▪]Induces pro-Th1/anti-Th2 cytokine induction in mouse models of allergy/asthma (Pro-IFN-γ and IL-10/anti IL-1b, IL-4, IL-5, IL-13 and TGF-b1) [18,85,86,87,88▪]Induced IL-10 release from human PBMCs increased under inflammatory conditions [15▪]Pro-Th1 and anti-Th2 cytokine release in children with asthma (Pro-IL-10 IFN-γ/anti-IL-4, IL-17 and IL-1b) [16▪▪,89,90▪,91] | Blocks infiltration of eosinophils, neutrophils, macrophages and lymphocytes in mouse models of asthma/allergic rhinitis [83▪▪,84▪,88▪]Allograft of induced Tregs blocks eosinophilia in sensitized mice [84▪]Reduced mucus metaplasia, hypersectretion and tissue remodelling [83▪▪,84▪,88▪] | Reduced specific-serum and nonspecific serum IgE and IgG1 in a mouse model of asthma and allergic rhinitis [16▪▪,18,84▪,85,86,88▪] |
| Pidotimod | Upregulates anti-inflammatory NOD-like receptor NLRP12 in monocytes [92]Inhibits proinflammatory MCP-1 [92] | Downregulates Th2-associated CD-30 in cells from normal and atopic individuals [93] | – | Reduced IgE in a mixed group of patients with RRTI some of whom were atopic [37▪] |
| Ribomunyl | – | Pro-Th1/anti-Th2 cytokine changes (pro-INFy/anti-IL-4, IL-5) [94,95] | – | – |
| PMBL | – | Anti-Th2 cytokine change (IL4) [96]Increased Treg cells [97] | – | – |
DC, dendritic cell.
Shifting of the cytokine balance in a pro-Th1/anti-Th2 direction has been demonstrated in murine models of both asthma and allergy [17,19,85–87,88▪], in human peripheral blood mononuclear cells (PBMCs) [15▪] and in children with asthma and related conditions [89,90▪,91,102]. Notably, this anti-inflammatory effect is increased in the presence of proinflammatory mediators [15▪]. In line with these changes in cytokine balance, OM-85 reduces the infiltration of proinflammatory cells in murine inflammatory models of asthma and allergic rhinitis [17,83▪▪,84▪,85,86,88▪]; suppresses mucus metaplasia and hypersecretion [84▪,85,88▪]; and attenuates airway remodelling [88▪].
The Th2 response is characterized by increased serum IgG1 and IgE, an effect inhibited by OM-85 in mouse models of allergic rhinitis [86] and asthma [18,84▪,88▪] (Table 2). This effect was not seen in all the studies we identified, however [83▪▪,89]. Furthermore, in a low-dose study using murine asthma model, OM-85 did not reduce inflammatory cell infiltration, serum IgE or lung histopathologic findings, though proinflammatory cytokines were reduced and there was no increase in airway resistance in OM-85-treated mice [87]. In a response to these results, it was suggested that reduced levels of the key eosinophil activator interleukin-5 may result in these cells being in a quiescent state and account for the absence of increased airway resistance [103].
Other
Data on the other eligible immunomodulators related to wheezing, asthma or allergy were sparse. Pidotimod reduced inflammatory response to TLR ligands via the upregulation of a member of the nod-like receptor family of PRRs in human monocytes [92]. In PBMCs from children with or without atopic asthma, in-vitro pidotimod causes downregulation of a Th2-related marker, but does not appear to affect Th1/Th2 cytokine balance [37▪,93]. In addition, pidotimod reduced serum IgE in 50% of a group of RTI patients, some of whom had atopy [37▪]. However, in a recent study on the ovalbumin mouse model of asthma, pidotimod treatment leads to a significant increase in both IgE and eosinophil infiltration compared to mice treated with ovalbumin alone. This proinflammatory effect was reflected in increases in proinflammatory cytokine release and a failure to downregulate markers of asthma severity and airway remodelling [104].
Ribomunyl shifted the Th1/Th2 cytokine balance in favour of Th1 in two studies, although this was less marked and slower in patients with atopy than in those without [94,95]. In patients with allergic rhinitis who received PMBL, there was a decrease in IL-4, although IFN-γ and IgE were not affected [96]. Finally, in children with partially controlled or uncontrolled asthma, PMBL treatment increased the numbers of CD8+ cytotoxic cells, Treg cells and NK cells [97].
Summary
There was a substantial gap in the depth of mechanistic data on wheezing/asthma between OM-85 and the other eligible immunomodulators. OM-85 appears to inhibit Th2-related inflammation via activation of gut dendritic cells and subsequent trafficking of Treg cells to the lung. A number of studies demonstrated anti-inflammatory effects in inflammatory or atopic physiological environments and reductions in cell infiltration and atopy-related immunoglobulins. The other immunomodulators showed some anti-inflammatory and proTh1 effects but lacked the data to obtain a clear mechanistic understanding of their potential influence on wheezing/asthma.
EFFICACY OF IMMUNOMODULATORS IN WHEEZING AND ASTHMA
Studies into the efficacy of immunomodulators in wheezing and asthma were relatively sparse in comparison to those studying RTIs in general; however, an increasing number of studies have been published over the last 10 years (Supplementary Table 2).
OM-85
OM-85 prophylaxis reduced the duration and incidence of wheezing/asthma exacerbations in children with a history or recurrent wheezing or asthma [90▪,91,105▪▪,106], as well as hospitalizations related to asthma [89]. The reductions in exacerbations appear to be related to reduced incidence of RTIs in these studies [89,105▪▪]. In line with this observation, OM-85 also reduced the incidence of RTI [21▪,90▪,91,105▪▪,106] and antibiotic use [90▪]. As expected, OM-85 was well tolerated in all the studies which reported safety data and its addition to corticosteroid therapy caused no apparent issues [21▪,90▪,105▪▪].
Pidotimod
The two studies investigating pidotimod in asthma and the related condition obstructive syndrome did not report data on asthma exacerbations; however, there were reductions in the incidence of RTI in both studies [39,107]. RTI duration was also reduced in children with allergic rhinitis and asthma [107].
Polyvalent mechanical bacterial lysate
In an unpublished trial, PMBL reduced the incidence and prevalence of asthma exacerbations in children with atopic asthma [97]. In addition, PMBL improved symptoms in a mixed group of children and adults with allergic rhinitis [96].
Summary
With some notable exceptions (e.g. Razi et al.[105▪▪]), the quality of the identified studies on wheezing/asthma identified was low with important design and safety criteria frequently not reported. Trials currently underway are likely to provide higher quality data in the near future. Data available suggest that the reductions in exacerbations achieved were related to reductions in RTI. Again, future studies may provide insights on the contribution of the anti-inflammatory effects detailed herein towards prophylaxis.
FUTURE RESEARCH
A search of the major clinical trial registries (EU Clinical Trials Register; Australian New Zealand Clinical Trial Registry; ClinicalTrials.gov) revealed ongoing trials for OM-85 only.
OM-85 in respiratory tract infection
A phase 4 trial (NCT03243565) investigating the efficacy of OM-85 in children with a history of RRTIs and symptoms of adenoid hypertrophy began recruiting in 2017. The primary outcome will be the number of RTIs, and secondary outcomes will include symptoms of adenoiditis [108].
OM-85 in wheezing/asthma
Four trials investigating OM-85 in asthma and wheezing are currently ongoing. The OM-85 in Prevention of Asthma in Children (OMPAC) randomised controlled trial (RCT) (ACTRN12612000518864) will assess outcomes in infants who have a sibling with asthma/atopy, treated with OM-85 in two cycles during their first two winter seasons. Outcomes will include prevention of symptomatic lower RTIs, persistent asthma development and allergen sensitization [109]. Results are expected in June 2019. The Italian OMPeR RCT (EudraCT: 2016-002705-19) will investigate OM-85 for the prevention of upper respiratory tract infections (URTIs) in children with mild immunodeficiency (IgA and IgG), atopy or recurrent wheezing [110]. Standard and longer term dosing will be explored. The trial outcomes include URTIs prophylaxis, school days lost, use of antibiotics and wheezing, with results expected in 2018. In the BREATHE RCT (EudraCT: 2016-001213-24), adolescents and adults with uncontrolled asthma will receive OM-85 for two consecutive October–March winter seasons [111]. The primary outcome will be the incidence of asthma exacerbations. Trial results are expected in 2020.
The ORal Bacterial EXtracts for the prevention of wheezing lower respiratory tract illness (ORBEX) trial represents a step change in immunomodulator research [112▪▪]. This large, multicentre, National Institute of Health-funded RCT (NCT02148796) will enrol upwards of 1000 infants at high asthma risk due to having atopic eczema and/or parents or siblings with asthma. Participants will receive long-term OM-85 prophylaxis (3.5 mg/day for 10 days/month for 2 years). The primary outcome will be time to first wheezing episode in the third observational year when children are not receiving prophylaxis. Preliminary results of the ORBEX trial are expected by December 2022.
Alongside the above clinical outcomes, the effects of OM-85 on microbiota, immunological, inflammatory and genetic markers will be assessed in these trials [109–111,112▪▪].
CONCLUSION
Mechanistic data, in particular for OM-85, support the rational for use of immunomodulators in both prophylaxis against RTIs and wheezing/asthma exacerbations in children. The included immunomodulatory compounds appear to act on both adaptive and innate portions of the immune system conferring both immunoglobulin-related and cell-mediated immunity to the respiratory system. Maturation of the immune system via the redressing of the Th1/Th2 imbalance appears to be a route by which immunomodulators can both reduce RTIs and potentially reduce atopy. In addition, under inflammatory conditions, immunomodulators, particularly OM-85, appear to reduce inflammation via immunoregulatory mechanisms and reduce hyper-reactivity. Efficacy data in patients at risk of both RTI and asthma support the above mechanist rational, with reductions in both RTIs and asthma exacerbations. The large upcoming ORBEX study has the potential to verify the role of OM-85 as antiwheezing prophylactic.
Acknowledgements
We would like to thank Jacques Bauer of OM Pharma, part of the Vifor Pharma Group, for his support in designing and carrying out the literature search. Prof. Alberto Ciceran of the University of Buenos Aires, Argentina, for providing the inspiration for our mechanistic diagram; Stefania Ballarini of Vifor Pharma for her time and insightful comments; and Ewen Legg of Halcyon Medical Writing for editorial and writing support.
Financial support and sponsorship
Editorial and writing support was funded by OM Pharma, part of the Vifor Pharma Group.
M.S. received speaker honoraria from Vifor Pharma, Sandoz, Genzyme, GSK and Novartis.
W.F. received speaker honoraria from Vifor Pharma, GSK, Pierre-Fabre Medicament and Sandoz.
M.J. received speakers honoraria from Abbvie, Astrazeneca, Boehringer and OM Vifor Pharma.
U.S. received research support and honoraria for conference attendance from OM Vifor Pharma.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
Supplementary Material
REFERENCES
- 1.Schroeck JL, Ruh CA, Sellick JA, Jr, et al. Factors associated with antibiotic misuse in outpatient treatment for upper respiratory tract infections. Antimicrob Agents Chemother 2015; 59:3848–3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gonzales R, Steiner JF, Sande MA. Antibiotic prescribing for adults with colds, upper respiratory tract infections, and bronchitis by ambulatory care physicians. JAMA 1997; 278:901–904. [PubMed] [Google Scholar]
- 3.Jain S, Williams DJ, Arnold SR, et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med 2015; 372:835–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Toivonen L, Karppinen S, Schuez-Havupalo L, et al. Burden of recurrent respiratory tract infections in children: a prospective cohort study. Pediatr Infect Dis J 2016; 35:e362–e369. [DOI] [PubMed] [Google Scholar]
- 5▪.Schaad UB, Esposito S, Razi CH. Diagnosis and management of recurrent respiratory tract infections in children: a practical guide. Arch Pediatr Infect Dis 2016; 4:1–10. [Google Scholar]; Recent review discussing the place of immunomodulators in the management of recurrent respiratory tract infections.
- 6.Simon AK, Hollander GA, McMichael A. Evolution of the immune system in humans from infancy to old age. Proc Biol Sci 2015; 282:20143085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Busse WW, Lemanske RF, Gern JE. The role of viral respiratory infections in asthma and asthma exacerbations. Lancet 2010; 376:826–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8▪▪.Del-Rio-Navarro BE, Espinosa-Rosales FJ, Flenady V, Sienra-Monge JJL. Immunostimulants for preventing respiratory tract infection in children. Evid Based Child Health 2012; 7:629–717. [DOI] [PubMed] [Google Scholar]; Meta-analysis of the efficacy of available immunomodulators in children with recurrent respiratory tract infections.
- 9.Greenberg HB, Piedra PA. Immunization against viral respiratory disease: a review. Pediatr Infect Dis J 2004; 23:S254–S261. [DOI] [PubMed] [Google Scholar]
- 10.Kiyono H, Fukuyama S. NALT- versus Peyer's-patch-mediated mucosal immunity. Nat Rev Immunol 2004; 4:699–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marsland BJ, Trompette A, Gollwitzer ES. The gut-lung axis in respiratory disease. Ann Am Thorac Soc 2015; 12 (Suppl 2):S150–S156. [DOI] [PubMed] [Google Scholar]
- 12.Kearney SC, Dziekiewicz M, Feleszko W. Immunoregulatory and immunostimulatory responses of bacterial lysates in respiratory infections and asthma. Ann Allergy Asthma Immunol 2015; 114:364–369. [DOI] [PubMed] [Google Scholar]
- 13.Spisek R, Brazova J, Rozkova D, et al. Maturation of dendritic cells by bacterial immunomodulators. Vaccine 2004; 22:2761–2768. [DOI] [PubMed] [Google Scholar]
- 14.Parola C, Salogni L, Vaira X, et al. Selective activation of human dendritic cells by OM-85 through a NF-kB and MAPK dependent pathway. PLoS One 2013; 8:e82867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15▪.Pasquali C, Salami O, Taneja M, et al. Enhanced mucosal antibody production and protection against respiratory infections following an orally administered bacterial extract. Front Med 2014; 1:41. [DOI] [PMC free article] [PubMed] [Google Scholar]; Preclinical study covering a number of aspects of the immune response, in particular the effects of immunomodulation on adaptive immune response to bacterial/viral super-infection.
- 16▪▪.Dang AT, Pasquali C, Ludigs K, Guarda G. OM-85 is an immunomodulator of interferon-β production and inflammasome activity. Sci Rep 2017; 7:43844. [DOI] [PMC free article] [PubMed] [Google Scholar]; Key mechanistic study showing evidence of both the anti-infective and anti-inflammatory actions of OM-85.
- 17.Manolova V, Flace A, Jeandet P, et al. Biomarkers induced by the immunomodulatory bacterial extract OM-85: unique roles for Peyer's patches and intestinal epithelial cells. J Clin Cell Immunol 2017; 8:494. [Google Scholar]
- 18.Huber M, Mossmann H, Bessler WG. Th1-orientated immunological properties of the bacterial extract OM-85-BV. Eur J Med Res 2005; 10:209–217. [PubMed] [Google Scholar]
- 19.Luan H, Zhang Q, Wang L, et al. OM-85-BV induced the productions of IL-1 beta, IL-6, and TNF-alpha via TLR4-and TLR2-mediated ERK1/2/NF-kappa B pathway in RAW264.7 cells. J Interfer Cytok Res 2014; 34:526–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liao JY, Zhang T. Influence of OM-85 BV on hBD-1 and immunoglobulin in children with asthma and recurrent respiratory tract infection. Zhongguo Dang Dai Er Ke Za Zhi 2014; 16:508–512. [PubMed] [Google Scholar]
- 21▪.Roth M, Pasquali C, Stolz D, Tamm M. Broncho Vaxom (OM-85) modulates rhinovirus docking proteins on human airway epithelial cells via Erk1/2 mitogen activated protein kinase and cAMP. PLoS One 2017; 12:e0188010. [DOI] [PMC free article] [PubMed] [Google Scholar]; Mechanistic study showing the stimulation of innate antiviral activity by OM-85 at the level of the lung epithelium.
- 22.Zelle-Rieser C, Ramoner R, Bartsch G, Thurnher M. A clinically approved oral vaccine against pneumotropic bacteria induces the terminal maturation of CD83(+) immunostimulatory dendritic cells. Immunol Lett 2001; 76:63–67. [DOI] [PubMed] [Google Scholar]
- 23.Roth M, Block LH. Distinct effects of Broncho-Vaxom (OM-85 BV) on gp130 binding cytokines. Thorax 2000; 55:678–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gu S, Wu C, Xie G. Efficacy of bacterial lysates in the adjuvant therapy for children with recurrent respiratory tract infections. Chin J Pract Pediatr 2015; 30:207–210. [Google Scholar]
- 25.Bowman LM, Holt PG. Selective enhancement of systemic Th1 immunity in immunologically immature rats with an orally administered bacterial extract. Infect Immun 2001; 69:3719–3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Byl B, Libin M, Gérard M, et al. Bacterial extract OM85-BV induces interleukin-12-dependent IFN-gamma production by human CD4(+) T cells. J Interfer Cytok Res 1998; 18:817–821. [DOI] [PubMed] [Google Scholar]
- 27.Hu X, Zhang W, Wang L, et al. The detailed analysis of the changes of murine dendritic cells (DCs) induced by thymic peptide: pidotimod(PTD). Hum Vaccine Immunother 2012; 8: 1250e1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giagulli C, Noerder M, Avolio M, et al. Pidotimod promotes functional maturation of dendritic cells and displays adjuvant properties at the nasal mucosa level. Int Immunopharmacol 2009; 9:1366–1373. [DOI] [PubMed] [Google Scholar]
- 29▪.Esposito S, Garziano M, Rainone V, et al. Immunomodulatory activity of pidotimod administered with standard antibiotic therapy in children hospitalized for community-acquired pneumonia. J Translat Med 2015; 13:288.https://www.ncbi.nlm.nih.gov/pubmed/26335787 [DOI] [PMC free article] [PubMed] [Google Scholar]; Key mechanistic study showing immodulatory activity of pidotimod in childhood respiratory tract infections.
- 30.Trabattoni D, Clerici M, Centanni S, et al. Immunomodulatory effects of pidotimod in adults with community-acquired pneumonia undergoing standard antibiotic therapy. Pulm Pharmacol Ther 2017; 44:24–29. [DOI] [PubMed] [Google Scholar]
- 31.Carta S, Silvestri M, Rossi GA. Modulation of airway epithelial cell functions by Pidotimod: NF-kB cytoplasmatic expression and its nuclear translocation are associated with an increased TLR-2 expression. Ital J Pediatr 2013; 39:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aivazis V, Hatzimichail A, Papachristou A, et al. Clinical evaluation and changes of the respiratory epithelium function after administration of Pidotimod in Greek children with recurrent respiratory tract infections. Minerva Pediatr 2002; 54:315–319. [PubMed] [Google Scholar]
- 33.He S, Hong Y. Clinical efficacy of loratadine combined with pidotimod in the treatment of children with recurrent respiratory tract infection and effect on immune function. Zhongguo Yaoshi 2015; 18:1904–1906. [Google Scholar]
- 34.Zhang SL, Man D, Lei YG. Changes of immune function in children with Mycoplasma pneumoniae pneumonia and effects of pidotimod on the disease. J Hainan Med Univ; 2013-12. Available at: http://en.cnki.com.cn/Article_en/CJFDTOTAL-HNYY201312039.htm. [Google Scholar]
- 35.Meng M, Li CX, Hong Y, et al. Effects of C-pseudonucleosides bearing thiazolidin-4-one as immunostimulants on differentiations of human lymphocytes. Chin J Microbiol Immunol 2012; 32:486–490. [Google Scholar]
- 36.Zhou Y, Dai Y. Comparison of effects of pidotimod and spleen aminopeptide on clinical symptoms and Th1/Th2 cytokine in children with RRI. Chin J Biochem Pharm 2012; 33:64–66. [Google Scholar]
- 37▪.Namazova-Baranova LS, Alekseeva AA, Kharit SM, et al. Efficacy and safety of pidotimod in the prevention of recurrent respiratory infections in children: a multicentre study. Int J Immunopathol Pharmacol 2014; 27:413–419. [DOI] [PubMed] [Google Scholar]; Key study showing the efficacy of pidotimod in childhood respiratory tract infections.
- 38.Grigoryan SS, Ivanova AM. Effect of pidotimod on production of pro- and anti-inflammatory cytokines ex vivo. Curr Pediatr 2001; 10:129–132. [Google Scholar]
- 39.Lokshina EE, Kravchenko OV, Zaytseva OV. Pidotimod in treatment of children with acute respiratory infection with concomitant recurrent obstructive syndrome. Curr Pediatr 2011; 10:34–41. [Google Scholar]
- 40.Boccaccio C, Jacod S, Kaiser A, et al. Identification of a clinical-grade maturation factor for dendritic cells. J Immunother 2002; 25:88–96. [DOI] [PubMed] [Google Scholar]
- 41.Peng JC, Thomas R, Nielsen LK. Generation and maturation of dendritic cells for clinical application under serum-free conditions. J Immunother 2005; 28:599–609. [DOI] [PubMed] [Google Scholar]
- 42.Villa-Ambriz J, Rodríguez-Orozco AR, Béjar-Lozano C, Cortés-Rojo C. The Increased Expression of CD11c and CD103 Molecules in the Neutrophils of the Peripheral Blood Treated With a Formula of Bacterial Ribosomes and Proteoglycans of Klebsiella pneumoniae. Archivos De Bronconeumologia 2012; 48:316–319. [DOI] [PubMed] [Google Scholar]
- 43.Litovskaia AV, Penknovich AA, Lavreniuk NA, et al. Experience of ribomunyl application in patients with chronic bronchitis. Klin Med 2005; 83:53–57. [PubMed] [Google Scholar]
- 44.Mora R, Ralli G, Passali FM, et al. Short ribosomal prophylaxis in the prevention of clinical recurrences of chronic otitis media in children. Int J Pediatr Otorhinolaryngol 2004; 68:83–89. [DOI] [PubMed] [Google Scholar]
- 45.Béné MC, Faure GC. From Peyer's patches to tonsils. Specific stimulation with ribosomal immunotherapy. Drugs 1997; 54:24–28. [DOI] [PubMed] [Google Scholar]
- 46.Hbabi-Haddioui L, Roques C. Inhibition of Streptococcus pneumoniae adhesion by specific salivary IgA after oral immunisation with a ribosomal immunostimulant. Drugs 1997; 54:29–32. [DOI] [PubMed] [Google Scholar]
- 47.Mora R, Dellepiane M, Crippa B, Salami A. Ribosomal therapy in the prophylaxis of recurrent pharyngotonsillitis in children. Int J Pediatr Otorhinolaryngol 2007; 71:257–261. [DOI] [PubMed] [Google Scholar]
- 48.Mora R, Dellepiane M, Crippa B, et al. Ribosomal therapy in the treatment of recurrent acute adenoiditis. Eur Arch Otorhinolaryngol 2010; 267:1313–1318. [DOI] [PubMed] [Google Scholar]
- 49.Lanzilli G, Falchetti R, Tricarico M, et al. In vitro effects of an immunostimulating bacterial lysate on human lymphocyte function. Int J Immunopathol Pharmacol 2005; 18:245–254. [DOI] [PubMed] [Google Scholar]
- 50.Lanzilli G, Falchetti R, Cottarelli A, et al. In vivo effect of an immunostimulating bacterial lysate on human B lymphocytes. Int J Immunopathol Pharmacol 2006; 19:551–559. [DOI] [PubMed] [Google Scholar]
- 51.Rossi GA, Peri C, Raynal ME, et al. Naturally occurring immune response against bacteria commonly involved in upper respiratory tract infections: analysis of the antigen-specific salivary IgA levels. Immunol Lett 2003; 86:85–91. [DOI] [PubMed] [Google Scholar]
- 52.Cazzola M, Capuano A, Rogliani P, Matera MG. Bacterial lysates as a potentially effective approach in preventing acute exacerbation of COPD. Curr Opin Pharmacol 2012; 12:300–308. [DOI] [PubMed] [Google Scholar]
- 53.Bessler WG, Vor dem Esche U, Masihi N. The bacterial extract OM-85 BV protects mice against influenza and salmonella infection. Int Immunopharmacol 2010; 10:1086–1090. [DOI] [PubMed] [Google Scholar]
- 54.Broug-Holub E, Kraal G. In vivo study on the immunomodulating effects of OM-85 BV on survival, inflammatory cell recruitment and bacterial clearance in Klebsiella pneumonia. Int J Immunopharmacol 1997; 19:559–564. [DOI] [PubMed] [Google Scholar]
- 55.Ferrario BE, Garuti S, Braido F, Canonica GW. Pidotimod: the state of art. Clin Mol Allergy 2015; 13:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim CH, Kim DJ, Lee SJ, et al. Toll-like receptor 2 promotes bacterial clearance during the initial stage of pulmonary infection with Acinetobacter baumannii. Mol Med Rep 2014; 9:1410–1414. [DOI] [PubMed] [Google Scholar]
- 57.Bodhankar S, Woolard MD, Sun X, Simecka JW. NK cells interfere with the generation of resistance against mycoplasma respiratory infection following nasal-pulmonary immunization. J Immunol 2009; 183:2622–2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bozzetto S, Pirillo P, Carraro S, et al. Metabolomic profile of children with recurrent respiratory infections. Pharmacol Res 2017; 115:162–167. [DOI] [PubMed] [Google Scholar]
- 59.Portalès P, Clot J. Immunostimulants revisited: focus on the pharmacology of Ribomunyl. BioDrugs 2006; 20:81–84. [DOI] [PubMed] [Google Scholar]
- 60.Caliot E, Libon C, Kernéis S, Pringault E. Translocation of ribosomal immunostimulant through an in vitro-reconstituted digestive barrier containing M-like cells. Scand J Immunol 2000; 52:588–594. [DOI] [PubMed] [Google Scholar]
- 61.Kolopp-Sarda MN, Béné ME, Allaire JM, et al. Kinetics of specific salivary IgA responses in man after oral challenge by ribosomal immunostimulant. Int J Immunopharmacol 1997; 19:181–186. [DOI] [PubMed] [Google Scholar]
- 62.Schaad UB. Prevention of paediatric respiratory tract infections: emphasis on the role of OM-85. Eur Respir Rev 2005; 14:74–77. [Google Scholar]
- 63▪.Yin J, Xu B, Zeng X, Shen K. Broncho-Vaxom in pediatric recurrent respiratory tract infections: a systematic review and meta-analysis. Int Immunopharmacol 2018; 54:198–209. [DOI] [PubMed] [Google Scholar]; Most recent meta-analysis of 54 RCTs of OM-85.
- 64.Gutierrez-Tarango MD, Berber A. Safety and efficacy of two courses of OM-85 BV in the prevention of respiratory tract infections in children during 12 months. Chest 2001; 119:1742–1748. [DOI] [PubMed] [Google Scholar]
- 65.Schaad UB, Mütterlein R, Goffin H. BV-Child Study Group. Immunostimulation with OM-85 in children with recurrent infections of the upper respiratory tract: a double-blind, placebo-controlled multicenter study. Chest 2002; 122:2042–2049. [DOI] [PubMed] [Google Scholar]
- 66.Bitar MA, Saade R. The role of OM-85 BV (Broncho-Vaxom) in preventing recurrent acute tonsillitis in children. Int J Pediatr Otorhinolaryngol 2013; 77:670–673. [DOI] [PubMed] [Google Scholar]
- 67.Esposito S, Marchisio P, Prada E, et al. Impact of a mixed bacterial lysate (OM-85 BV) on the immunogenicity, safety and tolerability of inactivated influenza vaccine in children with recurrent respiratory tract infection. Vaccine 2014; 2:2546–2552. [DOI] [PubMed] [Google Scholar]
- 68.Gomez Barreto D, De la Torre C, Alvarez A, et al. Safety and efficacy of OM-85-BV plus amoxicillin/clavulanate in the treatment of subacute sinusitis and the prevention of recurrent infections in children. Allergol Immunopathol 1998; 26:17–22. [PubMed] [Google Scholar]
- 69.Chen J, Zhou Y, Nie J, et al. Bacterial lysate for the prevention of chronic rhinosinusitis recurrence in children. J Laryngol Otol 2017; 131:523–528. [DOI] [PubMed] [Google Scholar]
- 70.Jara-Perez JV, Berber A. Primary prevention of acute respiratory tract infections in children using a bacterial immunostimulant: a double-masked, placebo-controlled clinical trial. Clin Ther 2000; 22:748–759. [DOI] [PubMed] [Google Scholar]
- 71.Caramia G, Clemente E, Solli R, et al. Efficacy and safety of pidotimod in the treatment of recurrent respiratory infections in children. Curr Pediatr 2008; 7:72–76. [PubMed] [Google Scholar]
- 72.Licari A, De Amici M, Nigrisoli S, et al. Pidotimod may prevent recurrent respiratory infections in children. Minerva Pediatr 2014; 66:363–367. [PubMed] [Google Scholar]
- 73.Kharit SM, Nacharova EP, Namazova-Baranova LS, Fridman IV. Prophylactic efficacy of pidotimod for prophylaxis of respiratory diseases in children. Russian J Child Infect 2010; 9:46–50. [Google Scholar]
- 74.Migatcheva NB, Kaganova TI. Relapsing respiratory infections in children: differential approach to management. Curr Pediatr 2012; 11:99–105. [Google Scholar]
- 75.Di Filippo S, Varacalli S, Zardo F. Pidotimod in treatment of recurrent pharyngotonsillitis. Curr Pediatr 2008; 7:20–22. [Google Scholar]
- 76.Yu X, Wang L, Xu C. Clinical efficacy of pidotimod in adjuvant treatment of recurrent respiratory tract infections in children. Chin J Nosocomiol 2014; 24:4361–4362. [Google Scholar]
- 77.Mameli C, Pasinato A, Picca M, et al. Pidotimod for the prevention of acute respiratory infections in healthy children entering into daycare: a double blind randomized placebo-controlled study. Pharmacol Res 2015; 97:79–83. [DOI] [PubMed] [Google Scholar]
- 78.Fiocchi A, Omboni S, Mora R, et al. Efficacy and safety of ribosome-component immune modulator for preventing recurrent respiratory infections in socialized children. Allergy Asthma Proc 2012; 33:197–204. [DOI] [PubMed] [Google Scholar]
- 79.Mora R, Barbieri M, Passali GC, et al. A preventive measure for otitis media in children with upper respiratory tract infections. Int J Pediatr Otor-hinolaryngol 2002; 63:111–118. [DOI] [PubMed] [Google Scholar]
- 80.Rutishauser M, Pitzke P, Grevers G, et al. Use of a polyvalent bacterial lysate in patients with recurrent respiratory tract infections: results of a prospective, placebo-controlled, randomized, double-blind study. Adv Ther 1998; 15:330–341. [PubMed] [Google Scholar]
- 81.Ruah SB, Ruah C, van Aubel A, et al. Efficacy of a polyvalent bacterial lysate in children with recurrent respiratory tract infections. Adv Ther 2001; 18:151–162. [DOI] [PubMed] [Google Scholar]
- 82.Zaplatnikov AL, Leonidovich A, Girina AA, et al. Polyvalent mechanical bacterial lysate in children with recurrent infections of the respiratory system: using experience, efficacy and safety. Pediatriya Zhurnal im GN Speranskogo 2016; 95:96–101. [Google Scholar]
- 83▪▪.Strickland DH, Judd S, Thomas JA, et al. Boosting airway T-regulatory cells by gastrointestinal stimulation as a strategy for asthma control. Mucosal Immunol 2011; 4:43–52. [DOI] [PubMed] [Google Scholar]; A study demonstrating the gut-lung axis and the role of Treg cells in prevention of aeroallergen sensitization.
- 84▪.Navarro S, Cossalter G, Chiavaroli C, et al. The oral administration of bacterial extracts prevents asthma via the recruitment of regulatory T cells to the airways. Mucosal Immunol 2011; 4:53–65. [DOI] [PubMed] [Google Scholar]; Key study covering mechanistic aspects of OM-85's immunoregulatory activity under inflammatory conditions and the action of the gut lung axis.
- 85.Fu R, Li J, Zhong H, et al. Broncho-Vaxom attenuates allergic airway inflammation by restoring GSK3β-related T regulatory cell insufficiency. PLoS One 2014; 9:e92912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Han L, Zheng CP, Sun YQ, et al. A bacterial extract of OM-85 Broncho-Vaxom prevents allergic rhinitis in mice. Am J Rhinol Allergy 2014; 28:110–116. [DOI] [PubMed] [Google Scholar]
- 87.Rodrigues A, Gualdi LP, de Souza RG, et al. Bacterial extract (OM-85) with human-equivalent doses does not inhibit the development of asthma in a murine model. Allergol Immunopathol 2016; 44:504–511. [DOI] [PubMed] [Google Scholar]
- 88▪.Zhong H, Wei J, Yao Y, et al. A bacterial extract of OM-85 Broncho-Vaxom suppresses ovalbumin-induced airway inflammation and remodeling in a mouse chronic allergic asthma model. Int J Clin Exp Pathol 2017; 10:1149–1157. [Google Scholar]; Study illustrating a number of anti-inflammatory mechanisms with potential to affect wheezing/asthma.
- 89.Chen ZG, Ji JZ, Li M, et al. Effect and analysis of clinical efficacy of immunomodulator on serum levels of IL-4 and IFN-gamma in asthmatic children. J Sun Yat-sen Univ Med Sci 2009; 30:100–103. [Google Scholar]
- 90▪.Lu Y, Li Y, Xu L, et al. Bacterial lysate increases the percentage of natural killer T cells in peripheral blood and alleviates asthma in children. Pharmacology 2015; 95:139–144. [DOI] [PubMed] [Google Scholar]; Recent study showing both mechanistic and clinical efficacy of OM-85 in children with respiratory tract infections and asthma.
- 91.Han RF, Li HY, Wang JW, Cong XJ. Study on clinical effect and immunologic mechanism of infants capillary bronchitis secondary bronchial asthma treated with bacterial lysates Broncho-Vaxom. Eur Rev Med Pharmacol Sci 2016; 20:2151–2155. [PubMed] [Google Scholar]
- 92.Fogli M, Caccuri F, Iaria ML, et al. The immunomodulatory molecule pidotimod induces the expression of the NOD-like receptor NLRP12 and attenuates TLR-induced inflammation. J Biol Regul Homeost Agents 2014; 28:753–766. [PubMed] [Google Scholar]
- 93.Gourgiotis D, Papadopoulos NG, Bossios A, et al. Immune modulator pidotimod decreases the in vitro expression of CD30 in peripheral blood mononuclear cells of atopic asthmatic and normal children. J Asthma 2004; 41:285–287. [DOI] [PubMed] [Google Scholar]
- 94.Bystroň J, a Heřmanová Z, Szotkovská J, et al. Comparison of the effect of ribosomal immunotherapy on plasma levels of total IgE and cytokines IL-4, IL-5, IL-12 and IFNγ in adult atopic and non-atopic patients during the Pollen season. Clin Drug Invest 2004; 24:755–760. [DOI] [PubMed] [Google Scholar]
- 95.Bystroň J, b Heřmanová Z, Szotkovská J, et al. Effect of ribosomal immunotherapy on the clinical condition and plasma levels of cytokines IL-4, IL-5, IL-12 and IFNγ and total IgE in patients with seasonal allergy during the Pollen season. Clin Drug Invest 2004; 24:761–764. [DOI] [PubMed] [Google Scholar]
- 96.Banche G, Allizond V, Mandras N, et al. Improvement of clinical response in allergic rhinitis patients treated with an oral immunostimulating bacterial lysate: in vivo immunological effects. Int J Immunopathol Pharmacol 2007; 20:129–138. [DOI] [PubMed] [Google Scholar]
- 97.Bartkowiak-Emeryk M. The influence of polyvalent mechanical bacterial lysate on immunological parameters in asthmatic children. European Academy of Allergy and Clinical Immunology Congress 17–21 June 2017 Helsinki (Abstract 0078). [Google Scholar]
- 98.Evans-Marin HL, Cao AT, Yao S, et al. Unexpected regulatory role of CCR9 in regulatory T cell development. PLoS One 2015; 10:e0134100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Matloubian M, Lo CG, Cinamon G, et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 2004; 427:355–360. [DOI] [PubMed] [Google Scholar]
- 100.Graler MH, Goetzl EJ. The immunosuppressant FTY720 down-regulates sphingosine 1-phosphate G-protein-coupled receptors. FASEB J 2005; 18:551–553. [DOI] [PubMed] [Google Scholar]
- 101.Wei SH, Rosen H, Matheu MP, et al. Sphingosine 1-phosphate type 1 receptor agonism inhibits transendothelial migration of medullary T cells to lymphatic sinuses. Nat Immunol 2005; 6:1228–1235. [DOI] [PubMed] [Google Scholar]
- 102.McGeachie MJ, Yates KP, Zhou X, et al. Patterns of growth and decline in lung function in persistent childhood asthma. N Engl J Med 2016; 374:1842–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Holt PG, Strickland DH. Low dose treatment of mice with bacterial extract (OM-85) for attenuation of experimental atopic asthma in mice. Allergol Immunopathol 2017; 45:310–311. [DOI] [PubMed] [Google Scholar]
- 104.Fu LQ, Li YL, Fu AK, et al. Pidotimod exacerbates allergic pulmonary infection in an OVA mouse model of asthma. Mol Med Rep 2017; 16:4151–4158. [DOI] [PubMed] [Google Scholar]
- 105▪▪.Razi CH, Harmancı K, Abaci A, et al. The immunostimulant OM-85 BV prevents wheezing attacks in preschool children. J Allergy Clin Immunol 2010; 126:763–769. [DOI] [PubMed] [Google Scholar]; Key study showing efficacy of OM-85 in preventing viral wheezing during childhood.
- 106.Chen ZG, Ji JZ, Li M, et al. Immunoregulants improves the prognosis of infants with wheezing. Nan Fang Yi Ke Da Xue Xue Bao 2007; 27:1612–1613. [PubMed] [Google Scholar]
- 107.Vargas Correa JB, Espinosa Morales S, Bolanos Ancona JC, et al. Pidotimod in recurring respiratory infection in children with allergic rhinitis, asthma, or both conditions. Rev Alerg Mexico 2002; 49:27–32. [PubMed] [Google Scholar]
- 108. Clinicaltrials.gov: NCT03243565. Available from: https://clinicaltrials.gov/show/NCT03243565. Accessed 31 July 2017. [Google Scholar]
- 109. Australia New Zealand Clinical Trial Registry (ANZCTR):ACTRN12612000518864. Available from: https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=362459&isReview=true. Accessed 31 June 2007. [Google Scholar]
- 110. EU Clinical Trials Registry. EudraCT: 2016-002705-19. Available from: https://www.clinicaltrialsregister.eu/ctr-search/trial/2016-002705-19/IT. Accessed 30 January 2018. [Google Scholar]
- 111. EU Clinical Trials Registry. EudraCT: 2016-001213-24. Available from: https://www.clinicaltrialsregister.eu/ctr-search/search?query=espid. Accessed 30 June 2017. [Google Scholar]
- 112▪▪. Clinicaltrials.gov: NCT02148796. Available from: https://clinicaltrials.gov/ct2/show/NCT02148796. Accessed 31 July 2017. [Google Scholar]; Large ongoing National Institutes for Health-sponsored trial investigating the prophylactic potential of OM-85 against wheezing in at risk children.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


