Abstract
Purpose of review
Advances in diagnostic methods mean that co-infections are increasingly being detected in clinical practice, yet their significance is not always obvious. In parallel, basic science studies are increasingly investigating interactions between pathogens to try to explain real-life observations and elucidate biological mechanisms.
Recent findings
Co-infections may be insignificant, detrimental, or even beneficial, and these outcomes can occur through multiple levels of interactions which include modulation of the host response, altering the performance of diagnostic tests, and drug–drug interactions during treatment. The harmful effects of chronic co-infections such as tuberculosis or Hepatitis B and C in association with HIV are well established, and recent studies have focussed on strategies to mitigate these effects. However, consequences of many acute co-infections are much less certain, and recent conflicting findings simply highlight many of the challenges of studying naturally acquired infections in humans.
Summary
Tackling these challenges, using animal models, or careful prospective studies in humans may prove to be worthwhile. There are already tantalizing examples where identification and treatment of relevant co-infections seems to hold promise for improved health outcomes.
Keywords: co-infection, diagnosis, interactions, pathogenesis, susceptibility, treatment
INTRODUCTION
Globally, co-infections are almost certainly the norm rather than a rare curiosity. We are continuously exposed to multiple potential pathogens, most people are chronically or latently infected (be it with herpes viruses, helminths, or tuberculosis), and we all carry potential pathogens in our colonizing microbial flora. This means that nearly every new incident infection is likely to constitute some sort of co-infection. Nevertheless we know relatively little about which combinations of co-infections matter the most for our health. Here, using examples from the recent literature, we illustrate situations in which co-infections have important implications, both harmful and beneficial, and explain why it is sometimes difficult to be sure.
Box 1.
no caption available
DOUBLE TROUBLE?
One might expect that infection with two or more pathogens would always be worse than infection with one. Even if co-infection is just bad luck, the adverse effect on health might be expected to be additive. But interactions do occur, on many levels, and these are not always detrimental (Fig. 1). Three examples involving malaria illustrate this well. Deliberate malaria co-infection (so-called, malaria therapy) was used as a treatment for neurosyphilis in the preantibiotic era, and is thought to have been moderately effective because of antitreponemal effects of the fever and cytokine response to malaria [1]. There is evidence that some helminth infections reduce malaria severity, possibly through immunomodulation [2]. Somewhat unexpectedly, antiretroviral therapy (ART) protected children with HIV from malaria, by prolonging the half-life of the antimalarial lumefantrine and effectively turning short courses of treatment into medium-term prophylaxis [3].
FIGURE 1.
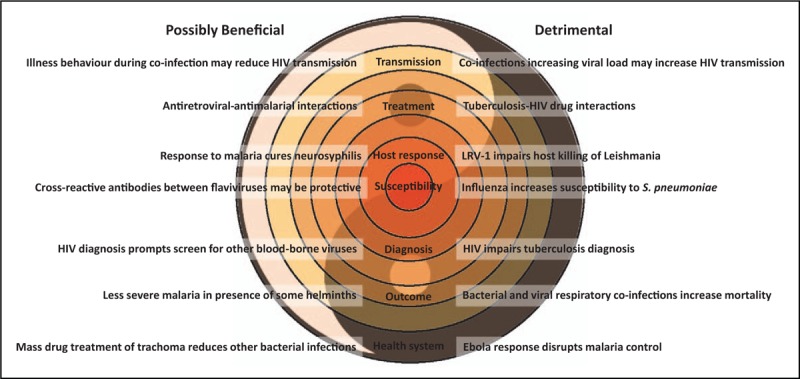
The good and the bad of co-infections. Co-infections can effect health through interactions at multiple levels. Examples are given where these interactions may be detrimental or sometimes beneficial. LRV-1, Leishmania RNA virus-1.
Unfortunately, determining the consequences of co-infection through observation of natural infections in humans is rife with problems because there are so many possible confounders. These include the presence of additional infections other than those being studied; the order, timing, and natural history of each infection; shared risk factors for acquisition of infections; and shared risk factors for their adverse outcomes. Interactions between infections which influence their likelihood of detection at earlier or later disease stages may also introduce bias. These challenges in human studies mean that co-infections are increasingly being investigated in animal models where conditions can be tightly controlled, and with careful consideration these can be useful to help to explain observations in humans.
CO-INFECTIONS IN HIV
In people living with HIV (PLHIV) co-infections usually do matter, and most have adverse consequences. Despite providing some of the most obvious examples for every level of interaction illustrated in Fig. 1, HIV co-infection is really a special case because of lifelong infection and acquired immunodeficiency. The burden of co-infections in PLHIV is hard to quantify. Some insights come from intervention studies such as the recent randomised controlled trial of combined antifungal, antituberculous, antihelminthic, and antibacterial prophylaxis started at the time of ART initiation in African children and adults with profound immunosuppression. This regime prevented one death for every 30 patients treated in comparison to standard prophylaxis with cotrimoxazole alone over a 24-week period [4▪▪].
HIV and tuberculosis
Tuberculosis is the leading cause of opportunistic infection and death among PLHIV. HIV is a potent risk factor for tuberculosis and complicates every aspect of tuberculosis care from prevention to diagnosis and treatment, whereas tuberculosis increases progression of HIV and contributes to slower CD4 recovery and faster virological failure on ART [5]. Recent work has shown tuberculosis incidence after ART initiation is significantly lower in PLHIV with CD4 more than 500 cells/μl compared to their counterparts with lower CD4 counts [6▪]. Ongoing HIV replication is an important risk factor for tuberculosis, regardless of CD4 cell counts [7▪], but tuberculosis risk does not differ before and after ART initiation when appropriately controlled for laboratory values and ART exposure [8▪].
Timely tuberculosis diagnosis is challenging in PLHIV because of high rates of smear negative and extrapulmonary tuberculosis. Clinical screening performs poorly in PLHIV and may miss up to 25% of all laboratory-confirmed tuberculosis cases and up to 70% among HIV-infected pregnant women [9▪▪]. Molecular and lateral flow diagnostics with greater sensitivity are showing promise for improving the situation [10,11▪▪,12,13▪▪,14].
Treatment of tuberculosis in PLHIV is challenging because of drug–drug interactions and overlapping toxicities with ART. Despite this, early ART initiation within the first 8 weeks of antituberculous therapy was associated with favourable outcomes in a large multinational cohort study in children [15▪▪]. Preventing tuberculosis in PLHIV is also complicated – although isoniazid preventive therapy (IPT) has been shown to be effective there are concerns that widespread use will drive the spread of isoniazid resistance. New estimates suggest that in the context of a declining/controlled tuberculosis epidemic, tuberculosis incidence and mortality benefits of continuous IPT for PLHIV outweigh the potential resistance risks [16▪]. A systematic review of universal IPT in children with no known tuberculosis exposure showed reduction of tuberculosis among children not receiving ART but, perhaps surprisingly, no clear benefit for children on ART [17▪].
HIV and hepatitis B and C viruses
Viral hepatitis is associated with increased morbidity in PLHIV. End-stage liver disease is most common in patients with hepatitis B virus hepatitis C virus (HCV) HIV co-infection, then in dual infections, and much less common in HIV monoinfection [18▪▪]. Viral hepatitis is also associated with extrahepatic complications in PLHIV such as increased risk of non-Hodgkin lymphoma [19▪], kidney disease [20,21▪], osteoporosis and fractures [21▪], and more severe cognitive impairment [22]. Hepatitis virus co-infection also slows immunological recovery in pregnant women [23▪] and children [24▪] with HIV, and HCV contributes to an ongoing immune activation and immune dysfunction even in controlled HIV infection [25▪,26,27].
Directly acting antivirals now allow more than 95% HCV cure rates, regardless of HIV co-infection [28,29,30,31▪,32▪,33], and HCV eradication reduces mortality, HIV progression, liver-related events, and diabetes mellitus [21▪,34▪▪]. Well tolerated and effective regimes for PLHIV on ART are now achievable [35,36,37▪▪,38].
BEYOND HIV
Chronic co-infections with HIV clearly demonstrate many potentially harmful impacts, but the evidence can be much less clear when acute infections are considered.
Ebola–malaria co-infection
The 2014–2015 West African Ebola virus disease (EVD) epidemic ravaged countries which already suffered a high burden of malaria and bacterial infections. Differentiating EVD from other causes of febrile illness and identifying co-infections was problematic so pragmatic guidelines advised empirical antimalarial and antibiotic treatment [39]. Subsequent studies have tried to characterize the burden and consequences of co-infection. Of four large studies (albeit employing quite different methodologies), three concluded that malaria co-infection resulted in increased mortality in individuals with EVD [40▪,41▪▪,42], whereas one study concluded the opposite [43▪]. These discordant findings highlight some key challenges for studying acute co-infections.
Malaria-associated co-infections are particularly difficult to study because Plasmodium can cause repeated acute, chronic, and asymptomatic infections, and individuals in endemic countries develop a degree of naturally acquired immunity which accumulates over many years. Asymptomatic infection with Plasmodium falciparum is common in highly endemic settings, but in a febrile individual coinfected with an additional potential pathogen it is almost impossible to know whether P. falciparum detected in blood is the sole cause of illness, contributing to illness, or just a bystander. Higher parasite load and younger age generally associate with greater likelihood of symptomatic disease, allowing the attributable fraction of febrile illness because of malaria to be calculated at a population level by comparison with parasite loads detected in appropriately matched healthy community controls [44]. In contrast to Plasmodium infection, it is assumed that almost all individuals with Ebola virus infection will manifest EVD, and it remains controversial whether Ebola virus infection may produce minimal or no symptoms [39]. It is conceivable that presymptomatic EVD may be detected in an individual with malaria, particularly when there is active surveillance for febrile illness in EVD contacts. None of the four studies of EVD and P. falciparum co-infection had appropriate control groups to determine malaria attributable fractions of febrile illness, so they are all likely to be confounded by relationships between parasite load, age, and coincidence of exposure and comorbidities. However, the apparent protective effect of P. falciparum in one study led to the suggestion that malaria therapy might be used to treat EVD [45]. Although the other studies would caution against this, the urgent need for effective treatments against EVD makes it important to resolve the controversy and explore possible underlying biological mechanisms.
Helminths and tuberculosis
Helminths are among the most prevalent pathogens globally. As they stimulate a type 2 helper T cell (Th2)-biased immune response, whereas protection from tuberculosis requires a type 1 helper T cell (Th1) response, the question has arisen whether co-infection may compromise defence against tuberculosis. In latent tuberculosis, co-infection with Strongyloides stercoralis reduced systemic and tuberculosis antigen-stimulated type 1 and type 17 cytokines, and increased systemic type 2 and regulatory cytokines [46▪]. Following treatment for Strongyloides stercoralis, type 1 and type 17 cytokine responses increased, along with increases in Mycobacterium tuberculosis-specific immunolgobulin M and immunoglobulin G [46▪,47▪]. However, real-world evidence that helminth-tuberculosis interactions are clinically important is less convincing. A large cross-sectional study of tuberculosis patients and uninfected household contacts in Tanzania, showed that tuberculosis infection was associated with Schistosoma mansoni infection, though this was just one of many helminths studied and the significance was borderline [48▪]. Interesting, and of greater statistical significance, was the finding that tuberculosis patients who did have S. mansoni infection had lower sputum bacterial loads, hinting at more complex interactions than those predicted from the Th1/Th2 paradigm. Consistent with this, Mycobacterium bovis bacterial loads were also decreased in cattle by co-infection with the fluke Fasciola hepatica, and co-infection was associated with reduced phagocytosis of mycobacteria [49▪].
Another practical concern is whether the presence of helminths may influence immune-based diagnostic tests for tuberculosis infection. Although there is some evidence that helminth infection reduces reactivity to purified protein derivative and increases the proportion of indeterminate interferon-γ release assay results in human tuberculosis, findings are far from conclusive [50]. However, in experimental bovine tuberculosis, co-infection with Fasciola hepatica reduced interferon-γ responses [49▪], consistent with earlier discovery of reduced intradermal purified protein derivative positivity, and estimates of a one-third reduction in ascertainment [51].
Helminths and other co-infections
A recent public health success story in dealing with the challenge of co-infections comes from two neglected tropical infections: Loa loa and Onchocercha volvulus. Onchocerciasis is a common cause of blindness (so-called, river blindness), and the burden of disease can be reduced by mass administration of ivermectin. Yet mass treatment can cause severe encephalopathy in communities where there is also a high-burden of Loa loa filarial infections [52]. As a result, some of the worst affected communities have been excluded from mass-treatment programmes because of excessive risks. Automated video microscopy screening of blood samples to detect and quantitatively measure Loa loa burden allowed just over 2% of individuals to be excluded, and ivermectin treatment to be reintroduced without serious adverse events [53▪▪].
Helminth infection has also revealed an interesting perspective on the complexity of interactions occurring during co-infections. Rather than mediating its effects directly through modulation of the host immune response, Heligmosomoides polygyrus was found to modify colonization and virulence of Salmonella Typhimurium by modulating the mouse gut metabolome [54▪]. This suggests an intermediary role for the microbiota in the interaction between pathogens, and implies that current approaches to studying co-infections may be far too simplistic.
Gastrointestinal and respiratory co-infections
Molecular diagnostics have increased pathogen detection, particularly in gastrointestinal and respiratory samples, and inevitably this has increased the detection of co-infections. In many cases the clinical implications of these co-infections have been hard to establish. Reanalysis of the Global Enteric Multicentre study, using molecular detection and quantification by qRT PCR, found that half of cases had more than one pathogen detected at a diarrhoea-associated load [55▪▪]. Shigella spp. and rotavirus, were most frequently detected as sole pathogens in diarrhoea-associated quantities, meaning that they were often true pathogens. Many other pathogens were not detected in diarrhoea-associated quantities or were associated with diarrhoea only in combination with other pathogens with stronger causal relationships. Therefore, simple detection of co-infection is not enough to understand its consequences, and even with quantification attribution is difficult.
Similar results come from studies of respiratory viruses in children: multiple viruses are often detected but few are consistently associated with disease. In a recent study of children with acute respiratory infection, 82% had a respiratory virus detected, 59% had a single virus, and 23% had co-infections [56▪]. Detection of multiple respiratory viruses was not associated with any difference in severity or outcomes compared to monoinfections in this population. However, in longer follow-up studies asthma features were more common in 6–8-year-old children with a previous admission with multiple respiratory viruses when compared with those with single respiratory viruses, even when accounting for age at previous admission [57]. We do not yet know whether co-infection causes later asthma symptoms or acts as a marker of those who are susceptible.
Despite limited evidence that co-infections between different respiratory viruses are important, there is a well established association between respiratory viral and bacterial co-infection which corresponds with more severe illness [58▪▪]. Beyond simple additive effects, mechanistic evidence comes from studying influenza and pneumococcal co-infections. Influenza often precedes pneumococcal pneumonia, at least partly because it causes a depletion alveolar macrophages which allows a smaller inoculum of bacteria to establish productive infection [59▪▪]. Interestingly, the extent of depletion of alveolar macrophages and consequent severity of the bacterial co-infection may be exacerbated by preexisting host factors such as obesity [60▪].
Pathogen as host for co-infections
Co-infection usually implies two or more pathogens infecting the human (or animal) host, but nature is full of surprises and one clinically important type of co-infection turns out to involve viral infection of the principal pathogen. Leishmania parasites infected with endosymbiont Leishmania RNA virus 1 are more virulent in rodent models [61] and human patients [62▪,63▪] because the virus induces type 1 interferon production in host macrophages, impairing intracellular killing of Leishmania [64▪▪]. Targeting viral clearance improves cure in mice suggesting a potential therapeutic avenue for humans with this disease [65▪▪,66▪].
CONCLUSION
Accumulating evidence indicates that co-infections frequently do matter, but it is often difficult to predict how and when they matter. The examples highlighted in this review illustrate the potential complexity of interactions between infections and their effects on the host. It is also clear that studying co-infections is challenging, particularly in the context of natural infection. Conflicting results and conclusions from the study of the same infections serve to illustrate that there must be many as yet unknown factors involved. The interactions between the blurred boundaries of infection and colonization will undoubtedly need to be considered in the future. Perhaps a complete understanding of the relevance of co-infections will only come when large-scale unbiased approaches like metagenomic sequencing are applied longitudinally and in conjunction with other omics approaches which characterize the host and microbiome, and are interpreted with machine-learning strategies rather than standard clinician classifications. For now, animal models and human challenge studies may offer an intermediate step for identifying specific pathogen–pathogen interactions.
Acknowledgements
None.
Financial support and sponsorship
A.J.C. receives funding from the UK Medical Research Council (MRC) and the UK Department for International Development (DFID) under the MRC/DFID Concordat agreement and is also part of the EDCTP2 programme supported by the European Union (MR/L006529/1).
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Gartlehner G, Stepper K. Julius Wagner-Jauregg: pyrotherapy, simultanmethode, and ’racial hygiene’. J R Soc Med 2012; 105:357–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nacher M. Interactions between worms and malaria: good worms or bad worms? Malar J 2011; 10:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Achan J, Kakuru A, Ikilezi G, et al. Antiretroviral agents and prevention of malaria in HIV-infected Ugandan children. N Engl J Med 2012; 367:2110–2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4▪▪.Hakim J, Musiime V, Szubert AJ, et al. Enhanced prophylaxis plus antiretroviral therapy for advanced HIV infection in Africa. N Engl J Med 2017; 377:233–245. [DOI] [PMC free article] [PubMed] [Google Scholar]; Prophylaxis with isoniazid pyridoxine, fluconazole, azithromycin, albendazole, and trimethoprim sulfamethoxazole, improved survival in advanced HIV.
- 5.Tornheim JA, Dooley KE. Tuberculosis associated with HIV infection. Microbiol Spectr 2017; 5: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6▪.Bock P, Jennings K, Vermaak R, et al. Incidence of tuberculosis among HIV-positive individuals initiating antiretroviral treatment at higher CD4 counts in the HPTN 071 (PopART) trial in South Africa. J Acquir Immune Defic Syndr 2018; 77:93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]; Incidence of tuberculosis was lower amongst individuals starting ART at CD4 count about 500 cells/μl.
- 7▪.Fenner L, Atkinson A, Boulle A, et al. HIV viral load as an independent risk factor for tuberculosis in South Africa: collaborative analysis of cohort studies. J Int AIDS Soc 2017; 20:21327. [DOI] [PMC free article] [PubMed] [Google Scholar]; Higher HIV viral load is a risk factor for tuberculosis, independent of CD count.
- 8▪.Pettit AC, Mendes A, Jenkins C, et al. Timing of antiretroviral treatment, immunovirologic status, and TB risk: implications for testing and treatment. J Acquir Immune Defic Syndr 2016; 72:572–578. [DOI] [PMC free article] [PubMed] [Google Scholar]; Tuberculosis incidence is not increased by starting ART.
- 9▪▪.Modi S, Cavanaugh JS, Shiraishi RW, et al. Performance of clinical screening algorithms for tuberculosis intensified case finding among people living with HIV in western Kenya. PLoS One 2016; 11:e0167685. [DOI] [PMC free article] [PubMed] [Google Scholar]; Clinical algorithms for tuberculosis diagnosis perform poorly in PLHIV.
- 10.Li S, Liu B, Peng M, et al. Diagnostic accuracy of Xpert MTB/RIF for tuberculosis detection in different regions with different endemic burden: a systematic review and meta-analysis. PLoS One 2017; 12:e0180725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11▪▪.Kendall EA, Schumacher SG, Denkinger CM, et al. Estimated clinical impact of the Xpert MTB/RIF Ultra cartridge for diagnosis of pulmonary tuberculosis: a modeling study. PLoS Med 2017; 14:e1002472. [DOI] [PMC free article] [PubMed] [Google Scholar]; More sensitive molecular diagnostics are predicted to improve case detection in settings with high rates of HIV-tuberculosis co-infection.
- 12.Suwanpimolkul G, Kawkitinarong K, Manosuthi W, et al. Utility of urine lipoarabinomannan (LAM) in diagnosing tuberculosis and predicting mortality with and without HIV: prospective TB cohort from the Thailand Big City TB Research Network. Int J Infect Dis 2017; 59:96–102. [DOI] [PubMed] [Google Scholar]
- 13▪▪.Lawn SD, Kerkhoff AD, Burton R, et al. Diagnostic accuracy, incremental yield and prognostic value of Determine TB-LAM for routine diagnostic testing for tuberculosis in HIV-infected patients requiring acute hospital admission in South Africa: a prospective cohort. BMC Med 2017; 15:67. [DOI] [PMC free article] [PubMed] [Google Scholar]; Urine-lipoarabinomannan testing produces incremental benefits in tuberculosis detection in admitted patients with HIV.
- 14.Huerga H, Ferlazzo G, Bevilacqua P, et al. Incremental yield of including determine-TB LAM assay in diagnostic algorithms for hospitalized and ambulatory HIV-positive patients in Kenya. PLoS One 2017; 12:e0170976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15▪▪.Carlucci JG, Blevins Peratikos M, Kipp AM, et al. Tuberculosis treatment outcomes among HIV/TB-coinfected children in the international epidemiology databases to evaluate AIDS (IeDEA) network. J Acquir Immune Defic Syndr 2017; 75:156–163. [DOI] [PMC free article] [PubMed] [Google Scholar]; Early initiation of ART was associated with favourable outcomes in children with HIV-tuberculosis co-infection.
- 16▪.Kunkel A, Crawford FW, Shepherd J, et al. Benefits of continuous isoniazid preventive therapy may outweigh resistance risks in a declining tuberculosis/HIV coepidemic. AIDS 2016; 30:2715–2723. [DOI] [PMC free article] [PubMed] [Google Scholar]; Overall benefits are predicted for IPT if tuberculosis controls remain strong.
- 17▪.Zunza M, Gray DM, Young T, et al. Isoniazid for preventing tuberculosis in HIV-infected children. Cochrane Database Syst Rev 2017; 8:CD006418. [DOI] [PMC free article] [PubMed] [Google Scholar]; HIV-infected children not on ART stand to gain most from IPT.
- 18▪▪.Klein MB, Althoff KN, Jing Y, et al. Risk of end-stage liver disease in HIV-viral hepatitis coinfected persons in north America from the early to modern antiretroviral therapy eras. Clin Infect Dis 2016; 63:1160–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]; Rates of end-stage liver diseases have not declined in coinfected individuals despite ART.
- 19▪.Wang Q, De Luca A, Smith C, et al. Chronic hepatitis B and C virus infection and risk for non-Hodgkin lymphoma in HIV-infected patients: a cohort study. Ann Intern Med 2017; 166:9–17. [DOI] [PubMed] [Google Scholar]; Co-infection with hepatitis B or HCV increases risk of non-Hodgkin lymphoma in association with HIV.
- 20.Rossi C, Raboud J, Walmsley S, et al. Hepatitis C coinfection is associated with an increased risk of incident chronic kidney disease in HIV-infected patients initiating combination antiretroviral therapy. BMC Infect Dis 2017; 17:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21▪.Kovari H, Rauch A, Kouyos R, et al. Hepatitis C infection and the risk of non-liver-related morbidity and mortality in HIV-infected persons in the Swiss HIV cohort study. Clin Infect Dis 2017; 64:490–497. [DOI] [PubMed] [Google Scholar]; Treatment of hepatitis C co-infection reduced liver disease, liver-associated death, and diabetes.
- 22.Fialho R, Pereira M, Bucur M, et al. Cognitive impairment in HIV and HCV co-infected patients: a systematic review and meta-analysis. AIDS Care 2016; 28:1481–1494. [DOI] [PubMed] [Google Scholar]
- 23▪.Floridia M, Masuelli G, Tamburrini E, et al. HBV coinfection is associated with reduced CD4 response to antiretroviral treatment in pregnancy. HIV Clin Trials 2017; 18:54–59. [DOI] [PubMed] [Google Scholar]; Hepatitis B co-infection impaired CD4 recovery following ART in pregnancy.
- 24▪.Majekodunmi AO, Thorne C, Malyuta R, et al. Modelling CD4 T cell recovery in hepatitis C and HIV co-infected children receiving antiretroviral therapy. Pediatr Infect Dis J 2017; 36:e123–e129. [DOI] [PMC free article] [PubMed] [Google Scholar]; Hepatitis C co-infection impaired CD4 T cell recovery.
- 25▪.Keating SM, Dodge JL, Norris PJ, et al. The effect of HIV infection and HCV viremia on inflammatory mediators and hepatic injury-the women's interagency HIV study. PLoS One 2017; 12:e0181004. [DOI] [PMC free article] [PubMed] [Google Scholar]; Hepatitis C co-infection was associated with greater immune activation.
- 26.Stafford KA, Rikhtegaran Tehrani Z, Saadat S, et al. Long-term follow-up of elite controllers: higher risk of complications with HCV coinfection, no association with HIV disease progression. Medicine (Baltimore) 2017; 96:e7348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaczmarek DJ, Kokordelis P, Kramer B, et al. Alterations of the NK cell pool in HIV/HCV co-infection. PLoS One 2017; 12:e0174465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meissner EG. Update in HIV-hepatitis C virus coinfection in the direct acting antiviral era. Curr Opin Gastroenterol 2017; 33:120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martinello M, Hajarizadeh B, Grebely J, et al. HCV cure and reinfection among people with HIV/HCV coinfection and people who inject drugs. Curr HIV/AIDS Rep 2017; 14:110–121. [DOI] [PubMed] [Google Scholar]
- 30.Soriano V, Benitez-Gutierrez L, Arias A, et al. Evaluation of sofosbuvir, velpatasvir plus voxilaprevir as fixed-dose co-formulation for treating hepatitis C. Expert Opin Drug Metab Toxicol 2017; 13:1015–1022. [DOI] [PubMed] [Google Scholar]
- 31▪.Wyles D, Saag M, Viani RM, et al. TURQUOISE-I Part 1b: ombitasvir/paritaprevir/ritonavir and dasabuvir with ribavirin for hepatitis C virus infection in HIV-1 coinfected patients on darunavir. J Infect Dis 2017; 215:599–605. [DOI] [PubMed] [Google Scholar]; Effective treatment of both HIV and Hepatitis C in coinfected individuals.
- 32▪.Ingiliz P, Christensen S, Kimhofer T, et al. Sofosbuvir and ledipasvir for 8 weeks for the treatment of chronic hepatitis C virus (HCV) infection in HCV-monoinfected and HIV-HCV-coinfected individuals: results from the german hepatitis C cohort (GECCO-01). Clin Infect Dis 2016; 63:1320–1324. [DOI] [PubMed] [Google Scholar]; High rates of treatment success in coinfected individuals outside of clinical trials.
- 33.Milazzo L, Lai A, Calvi E, et al. Direct-acting antivirals in hepatitis C virus (HCV)-infected and HCV/HIV-coinfected patients: real-life safety and efficacy. HIV Med 2017; 18:284–291. [DOI] [PubMed] [Google Scholar]
- 34▪▪.Berenguer J, Rodriguez-Castellano E, Carrero A, et al. Eradication of hepatitis C virus and nonliver-related nonacquired immune deficiency syndrome-related events in human immunodeficiency virus/hepatitis C virus coinfection. Hepatology 2017; 66:344–356. [DOI] [PMC free article] [PubMed] [Google Scholar]; Multiple benefits of hepatitis C eradication in HIV coinfected individuals.
- 35.Smolders EJ, Smit C, TMM de Kanter C, et al. Brief report: high need to switch cART or comedication with the initiation of DAAS in elderly HIV/HCV-coinfected patients. J Acquir Immune Defic Syndr 2017; 76:193–199. [DOI] [PubMed] [Google Scholar]
- 36.Bonora S, Puoti M. Use of daclatasvir in HCV/HIV-coinfected patients in a real-life setting. AIDS Rev 2017; 19:24–34. [PubMed] [Google Scholar]
- 37▪▪.Lacombe K, Fontaine H, Dhiver C, et al. Real-world efficacy of daclatasvir and sofosbuvir, with and without ribavirin, in HIV/HCV coinfected patients with advanced liver disease in a French early access cohort. J Acquir Immune Defic Syndr 2017; 75:97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]; Highly effective and well tolerated regime in HIV hepatitis C coinfected individuals.
- 38.Castells L, Llaneras J, Campos-Varela I, et al. Sofosbuvir and daclatasvir in mono- and HIV-coinfected patients with recurrent hepatitis C after liver transplant. Ann Hepatol 2017; 16:86–93. [DOI] [PubMed] [Google Scholar]
- 39.Rojek A, Horby P, Dunning J. Insights from clinical research completed during the west Africa Ebola virus disease epidemic. Lancet Infect Dis 2017; 17:e280–e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40▪.Waxman M, Aluisio AR, Rege S, Levine AC. Characteristics and survival of patients with Ebola virus infection, malaria, or both in Sierra Leone: a retrospective cohort study. Lancet Infect Dis 2017; 17:654–660. [DOI] [PubMed] [Google Scholar]; Malaria co-infection was common and increased mortality in an Ebola Treatment Unit.
- 41▪▪.2017; Carroll MW, Haldenby S, Rickett NY, et al. Deep sequencing of RNA from blood and oral swab samples reveals the presence of nucleic acid from a number of pathogens in patients with acute Ebola virus disease and is consistent with bacterial translocation across the gut. mSphere. 2: [DOI] [PMC free article] [PubMed] [Google Scholar]; Patients with EVD have multiple pathogens detected in their blood, and presence of Plasmodium was associated with adverse outcome.
- 42.Kerber R, Krumkamp R, Diallo B, et al. Analysis of Diagnostic findings from the European mobile laboratory in Gueckedou, Guinea, March 2014 through March 2015. J Infect Dis 2016; 214:S250–S257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43▪.Rosenke K, Adjemian J, Munster VJ, et al. Plasmodium parasitemia associated with increased survival in Ebola virus-infected patients. Clin Infect Dis 2016; 63:1026–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]; Molecular detection of Plasmodium was associated with greater chance of surviving EVD.
- 44.Hendriksen IC, White LJ, Veenemans J, et al. Defining falciparum-malaria-attributable severe febrile illness in moderate-to-high transmission settings on the basis of plasma PfHRP2 concentration. J Infect Dis 2013; 207:351–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Drancourt M, Raoult D. Malaria therapy for Ebola virus infection. Clin Infect Dis 2017; 64:696–697. [DOI] [PubMed] [Google Scholar]
- 46▪.Anuradha R, Munisankar S, Bhootra Y, et al. Anthelmintic therapy modifies the systemic and mycobacterial antigen-stimulated cytokine profile in Helminth-latent mycobacterium tuberculosis coinfection. Infect Immun 2017; 85: [DOI] [PMC free article] [PubMed] [Google Scholar]; Mycobacterial cellular immune responses are reversibly altered in individuals with strongyloides co-infection.
- 47▪.Anuradha R, Munisankar S, Bhootra Y, et al. Modulation of mycobacterium tuberculosis-specific humoral immune responses is associated with strongyloides stercoralis co-infection. PLoS Negl Trop Dis 2017; 11:e0005569. [DOI] [PMC free article] [PubMed] [Google Scholar]; Mycobacterial humoral immune responses are reversibly altered in individuals with strongyloides co-infection.
- 48▪.Mhimbira F, Hella J, Said K, et al. Prevalence and clinical relevance of helminth co-infections among tuberculosis patients in urban Tanzania. PLoS Negl Trop Dis 2017; 11:e0005342. [DOI] [PMC free article] [PubMed] [Google Scholar]; S. mansoni was associated with increased risk of tuberculosis.
- 49▪.Garza-Cuartero L, O'Sullivan J, Blanco A, et al. Fasciola hepatica infection reduces Mycobacterium bovis burden and mycobacterial uptake and suppresses the pro-inflammatory response. Parasite Immunol 2016; 38:387–402. [DOI] [PMC free article] [PubMed] [Google Scholar]; Mycobacterial loads are lower in liver fluke coinfected cows, associated with a lower inflammatory response.
- 50.Babu S, Nutman TB. Helminth-tuberculosis co-infection: an immunologic perspective. Trends Immunol 2016; 37:597–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Claridge J, Diggle P, McCann CM, et al. Fasciola hepatica is associated with the failure to detect bovine tuberculosis in dairy cattle. Nat Commun 2012; 3:853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gardon J, Gardon-Wendel N, Demanga N, et al. Serious reactions after mass treatment of onchocerciasis with ivermectin in an area endemic for Loa loa infection. Lancet 1997; 350:18–22. [DOI] [PubMed] [Google Scholar]
- 53▪▪.Kamgno J, Pion SD, Chesnais CB, et al. A test-and-not-treat strategy for onchocerciasis in Loa loa-endemic areas. N Engl J Med 2017; 377:2044–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]; Mass drug treatment for river blindness can be reintroduced when Loa loa coinfected individuals can be detected avoided.
- 54▪.Reynolds LA, Redpath SA, Yurist-Doutsch S, et al. Enteric helminths promote salmonella coinfection by altering the intestinal metabolome. J Infect Dis 2017; 215:1245–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]; The gut metabolic environment plays and intermediary role in allowing one pathogen to enhance infection by another
- 55▪▪.Liu J, Platts-Mills JA, Juma J, et al. Use of quantitative molecular diagnostic methods to identify causes of diarrhoea in children: a reanalysis of the GEMS case-control study. Lancet 2016; 388:1291–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]; Quantitative molecular diagnostics reveal the frequency of co-infections and attributable burden of diarrhoea.
- 56▪.Wishaupt JO, van der Ploeg T, de Groot R, et al. Single- and multiple viral respiratory infections in children: disease and management cannot be related to a specific pathogen. BMC Infect Dis 2017; 17:62. [DOI] [PMC free article] [PubMed] [Google Scholar]; Detecting multiple respiratory viruses in children does not correlate with clinical course.
- 57.Garcia-Garcia ML, Calvo C, Ruiz S, et al. Role of viral coinfections in asthma development. PLoS One 2017; 12:e0189083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58▪▪.Voiriot G, Visseaux B, Cohen J, et al. Viral-bacterial coinfection affects the presentation and alters the prognosis of severe community-acquired pneumonia. Crit Care 2016; 20:375. [DOI] [PMC free article] [PubMed] [Google Scholar]; Outcome is worse if viral and bacterial respiratory pathogens are both detected.
- 59▪▪.Smith AM, Smith AP. A Critical, nonlinear threshold dictates bacterial invasion and initial kinetics during influenza. Sci Rep 2016; 6:38703. [DOI] [PMC free article] [PubMed] [Google Scholar]; Depletion of alveolar macrophages allows secondary bacterial infection by reducing the inoculum required to establish productive infection.
- 60▪.Karlsson EA, Meliopoulos VA, van de Velde NC, et al. A Perfect storm: increased colonization and failure of vaccination leads to severe secondary bacterial infection in influenza virus-infected obese mice. MBio 2017; 8: [DOI] [PMC free article] [PubMed] [Google Scholar]; Influenza effects on bacterial susceptibility are even worse in obese mice.
- 61.Ives A, Ronet C, Prevel F, et al. Leishmania RNA virus controls the severity of mucocutaneous leishmaniasis. Science 2011; 331:775–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62▪.Adaui V, Lye LF, Akopyants NS, et al. Association of the endobiont double-stranded RNA virus lrv1 with treatment failure for human leishmaniasis caused by leishmania Braziliensis in Peru and Bolivia. J Infect Dis 2016; 213:112–121. [DOI] [PMC free article] [PubMed] [Google Scholar]; An RNA virus infecting Leishmania parasites is associated with worse clinical outcomes.
- 63▪.Bourreau E, Ginouves M, Prevot G, et al. Presence of leishmania RNA virus 1 in leishmania guyanensis increases the risk of first-line treatment failure and symptomatic relapse. J Infect Dis 2016; 213:105–111. [DOI] [PubMed] [Google Scholar]; An RNA virus infecting Leishmania parasites is associated with worse clinical outcomes.
- 64▪▪.Rossi M, Castiglioni P, Hartley MA, et al. Type I interferons induced by endogenous or exogenous viral infections promote metastasis and relapse of leishmaniasis. Proc Natl Acad Sci U S A 2017; 114:4987–4992. [DOI] [PMC free article] [PubMed] [Google Scholar]; An RNA virus infecting Leishmania parasites subverts the protective host response.
- 65▪▪.Brettmann EA, Shaik JS, Zangger H, et al. Tilting the balance between RNA interference and replication eradicates Leishmania RNA virus 1 and mitigates the inflammatory response. Proc Natl Acad Sci U S A 2016; 113:11998–12005. [DOI] [PMC free article] [PubMed] [Google Scholar]; Targeting an RNA virus infecting Leishmania parasites reduces virulence, identifying a potential therapeutic strategy.
- 66▪.Kuhlmann FM, Robinson JI, Bluemling GR, et al. Antiviral screening identifies adenosine analogs targeting the endogenous dsRNA Leishmania RNA virus 1 (LRV1) pathogenicity factor. Proc Natl Acad Sci U S A 2017; 114:E811–E819. [DOI] [PMC free article] [PubMed] [Google Scholar]; Potential adjunctive therapies for mucocutaneous Leishmaniasis.


