Abstract
The explosive spread of Zika virus is the most recent example of the threat imposed to human health by flaviviruses. High-resolution structures are available for several of these arthropod-borne viruses, revealing alternative icosahedral organizations of immature and mature virions. Incomplete proteolytic maturation, however, results in a cloud of highly heterogeneous mosaic particles. This heterogeneity is further expanded by a dynamic behavior of the viral envelope glycoproteins. The ensemble of heterogeneous and dynamic infectious particles circulating in infected hosts offers a range of alternative possible receptor interaction sites at their surfaces, potentially contributing to the broad flavivirus host-range and variation in tissue tropism. The potential synergy between heterogeneous particles in the circulating cloud thus provides an additional dimension to understand the unanticipated properties of Zika virus in its recent outbreaks.
Introduction
Flaviviruses are perpetuated in nature by cycling between vertebrate and invertebrate hosts. They comprise a number of important human pathogens transmitted by mosquitos or ticks [1]. Some of these viruses have the potential to spread dramatically across the planet, as exemplified by the global expansion of dengue viruses (DENV) and the recent explosive outbreak of Zika virus (ZIKV) [2,3]. Quasi-atomic resolution structures of DENV and ZIKV particles have been determined recently, taking advantage of new instrumentation and methodological developments in electron cryo-microscopy [4–9]. These structures show regular icosahedral virions in which the viral membrane is covered by a highly symmetric shell formed by the flavivirus envelope glycoproteins. The resulting picture is, however, an average structure obtained through a strict selection of the particles present in a virus preparation. Indeed, a number of studies have demonstrated that flaviviruses released from infected cells are highly heterogeneous - in particular those of DENV, which have been most studied [10–12]. This heterogeneity results in large part from an incomplete proteolytic maturation process during virus exocytosis, which is subject to virus-specific and host cell-specific modulation [13]. In addition, increasing evidence shows that even completely mature particles display a highly dynamic behavior, with the envelope proteins exhibiting considerable “breathing” movements that can expose the lipid membrane and otherwise cryptic protein surfaces [14,15]. The resulting overall heterogeneity – especially when considered at the particle population level – has the potential to have a strong impact on virus-host interactions, which cannot be explained by considering static, regular particles like those described in the available structures.
Since its discovery in Uganda in 1947, rare and mild ZIKV infections of humans were reported in Africa and Asia [16]. The virus first showed its epidemic potential in an outbreak on Yap Island in 2007 [17] and later in other Pacific Ocean islands further east (2013/14), to end with its emergence in the Americas since 2015 [3,16]. This ZIKV epidemic was not only surprising because of its explosive geographical spread but also because it provided evidence for features of pathogenicity that had not been observed previously in infections by flaviviruses. Most notably, ZIKV was shown to be able to cross the placental barrier to cause congenital infections and to be transmitted sexually among humans [18,19]. These newly observed pathogenic properties have previously neither been linked to infections with ZIKV nor with other human-pathogenic flaviviruses such as yellow fever (YF), DEN, West Nile (WN), Japanese encephalitis, or tick-borne encephalitis (TBE) viruses. An important question therefore is whether these traits are due to specific mutations that could explain the increased pathogenicity and dissemination of ZIKV or whether they already existed before and became apparent through the unprecedented high number of infections [20].
In this review, we discuss particle heterogeneity in the context of virus entry, with a focus on comparative aspects of DEN and ZIK viruses. Incorporating these considerations into experimental analyses will be essential for a more complete understanding of the mechanisms of virus uptake into cells. The implications for the induction of antibodies and their interaction with flaviviruses are beyond the scope and limits of this review.
Flavivirus assembly and maturation
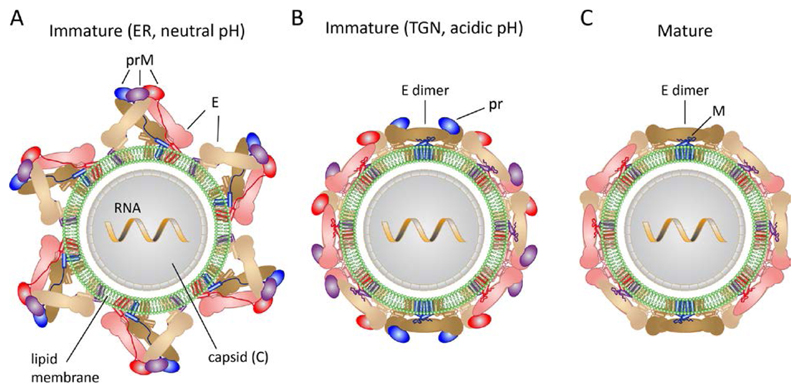
Flaviviruses are small enveloped viruses that have only three structural proteins, C, prM and E in immature and C, M and E in mature virions (Fig. 1). The complex flavivirus morphogenetic pathway leads first to formation of immature virus particles by budding into the neutral-pH environment of the ER lumen [21]. These particles are non-infectious and have icosahedral symmetry, with180 prM/E heterodimers associated into 60 (prM/E)3 trimeric projections (Fig. 1A and 2A) [5,9,22,23]. In the particles, each prM/E protomer displays extensive intra- and inter-trimer interactions to make a highly intertwined glycoprotein shell, resulting in a spiky particle exposing the viral membrane at the icosahedral symmetry axes – and most prominently at the 5-fold icosahedral axes (Fig. 2A).
Figure 1. Schematics of flavivirus particles, with protein E in pastel colors (sand, brown and pink) and prM in bright colors (purple, blue and red).
A. Immature virion after budding in the ER (neutral pH)
B. Immature virion after exposure to low pH in the TGN and rearrangement of envelope proteins
C. Mature virion after secretion from infected cells
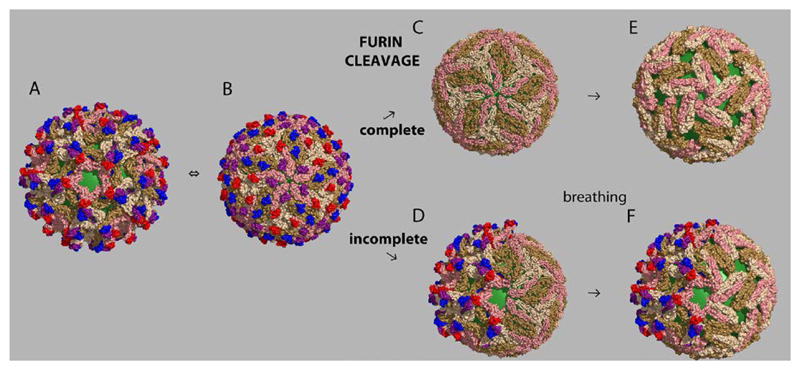
Figure 2. Flavivirus particle maturation and dynamics.
View down a 5-fold icosahedral axis of the particle, with the underlying viral membrane in green, and the prM/E protomers colored as in Fig. 1. There are three independent prM/E heterodimers in the icosahedral asymmetric unit, colored red/pink (by the 5-fold axes), blue/brown (2-fold axes) and purple/sand (3-fold axes). prM has a globular N-terminal half, which binds to the tip of domain II of E, and an extended C-terminal half. A) Immature flavivirus particle at neutral pH, displaying 60 (prM/E)3 “tipi” shaped trimeric projections. This panel used the immature structure of DENV serotype 1 [5] (PDB code 4B03). B) Rearrangement at acidic pH into a lattice of 90 (prM/E)2 dimers, completely hiding the viral membrane. The double arrow between panels A and B indicates that this change is reversible with pH. This panel was made with the immature structure of DENV serotype 2 at low pH [22] (PDB code 3C6R). C) The mature virion. Upon furin clavage, exposure to neutral pH leads to shedding of pr, leaving the virus particle in the same “herringbone” arrangement of E dimers as in the low pH immature particles. The arrow indicates that the change from B to C is irreversible. This panel used the structure of mature DENV serotype 2 [4] (PDB code 3j27).
D. Partially mature mosaic particle resulting from incomplete prM cleavage in the TGN
E. Illustration of breathing of a mature virion, using a “bumpy” particle as example
F. Potential aspect of a partially mature virion after breathing in the mature side, further exposing the viral membrane.
Exposure to the acidic milieu of the Trans-Golgi-Network (TGN) during exocytosis results in trimer dissociation and disassembly of the immature lattice, followed by a reorganization of the protomers into 90 (prM/E)2 dimers interacting laterally to form a very different glycoprotein shell (Fig. 1B and Fig. 2B), which completely coats the viral membrane [22]. At the same time, prM exposes a sequence motif specific for cleavage by the TGN-resident protease furin in the linker between the N-terminal globular head of prM (termed “pr”) and the membrane-anchored C-terminal half (termed protein M) (Fig 1B and 1C). At acidic pH, pr remains associated with the particles, but exposure to neutral pH upon secretion into the extracellular fluid results in its shedding from the virion, leaving an “activated” mature particle coated by 90 dimers of protein E in a metastable conformation (Fig. 1C) organized in the same “herringbone” pattern made at acidic pH by the immature particle [22]. When up-taken by a cell by receptor-mediated endocytosis, the acidic pH in the endosome triggers an irreversible structural change, which converts E into homotrimers - the final, lowest-energy conformation of E – in order to drive fusion between viral and endosomal membranes. The fusogenic conformational change, essential for entry, explains the requirement to maintain E in a metastable state until it reaches the endosome of the cell to be infected.
Particle heterogeneity and structural dynamics
Flavivirus particles released from infected cells display important deviations from the highly symmetric organization illustrated in Fig. 2A-C. Two main features are responsible:
1. Incomplete furin cleavage: depending on the extent of furin expression in the infected cell or on variations in the prM amino acid sequence at the furin cleavage site, only partial proteolytic maturation may take place [13]. The resulting particles exhibit a heterogeneous and “mosaic” architecture, exposing a mature lattice on one side (capable of mediating endosomal membrane fusion), and an immature lattice on the other (Fig. 2D) [11], the extent of which varies from particle to particle. The immature side exposes not only prM and E but also the viral membrane, all of which have the potential to interact with cell-entry factors (see below). Such mosaic particles are likely to be infectious because of the presence of sufficient E molecules in their mature conformation which can mediate membrane fusion. Consistent with this notion, experiments with West Nile virus have shown that the infectivity of virions containing considerable amounts of uncleaved prM is insensitive to treatment of cells with a potent furin inhibitor and therefore does not seem to depend on further prM cleavage by a furin-like protease during virus entry [24]. Dengue viruses are particularly rich in partially mature particles, as they have evolved a sub-optimal furin cleavage motif in prM, presenting a conserved acidic residue at cleavage position P3 (Table 1), which was shown to have a negative effect on the efficiency of furin cleavage [25]. Poor maturation thus appears important to maintain dengue viruses in their natural ecological cycle.
Table 1.
Amino acid alignment of furin cleavage sites of different flaviviruses
| Virus | Strain | Sequence pr | Sequence M | GenBank Accession no. | |
|---|---|---|---|---|---|
| P14a P1 | P1‘b P6‘ | ||||
| DENV-1 | SG/07K3640DK1/2008 | GTC-SQTGEHRRDKR | ↓C | SVALAPH | GQ398255 |
| DENV-2 | 16681 | GTC-TTMGEHRREKR | ↓ | SVALVPH | U87411 |
| DENV-3 | SG/05K863DK1/2005 | GTC-NQAGEHRRDKR | ↓ | SVALAPH | EU081190 |
| DENV-4 | SG/06K2270DK1/2005 | GTC-TQNGERRREKR | ↓ | SVALTPH | GQ398256 |
| ZIKV | H/PF/2013 | GTCHHKKGEARRSRR | ↓ | AVTLPSH | KJ776791 |
| WNV | NY_99 | GRC-TKTRHSRRSRR | ↓ | SLTVQTH | KC407666 |
| YFV | Asibi | GKC-DSAGRSRRSRR | ↓ | AIDLPTH | AY640589 |
| TBEV | Neudoerfl | GRCGKQEGS--RTRR | ↓ | SVLIPSH | U27495 |
Amino acid sequence positions 1 to 14 (dengue virus numbers) upstream of cleavage site (pr part), position 3 (P3) in bold letters
Amino acid sequence positions 1 to 6 downstream of cleavage site (protein M)
The arrow indicates the proteolytic cleavage site.
2. The required metastability of the E dimers gives rise to a highly dynamic “breathing” behavior, in which E transiently exposes otherwise buried surfaces - either buried within the dimer or at the inter-dimer contact region (Fig. 2E). Particle dynamics of fully and partially mature particles may thus facilitate interactions with cellular attachment factors and entry receptors. DENV serotype 2 (strains 16681 and NGC) were shown to undergo an irreversible change to adopt a “bumpy” appearance upon incubation at temperatures above 34°C [14,15]. In this case, the “breathing” E dimers appear to become trapped through an intermediate set of contacts in an expanded particle, leaving space in between them and exposing the underlying membrane. It is likely that breathing of both smooth and bumpy particles takes place continuously – albeit with a smaller amplitude than that observed between the two forms. In mammalian cells, the bumpy dengue virus particles appeared to be even more infectious than the original smooth particles, and it was therefore speculated that they might represent the dominant infectious forms of the virus during human infections (14). However, an irreversible temperature-dependent conversion into “bumpy” particles was not observed for strain PVP94/07 of DENV serotype 2 [8]. Likewise, bumpy particles were neither observed for strain PVP159 of DENV serotype 1, strain SG/05K863DK1/2005 of DENV serotype 3, nor strain SG/K2270DK1/2005 of DENV serotype 4 [6,8,26]. The possible role of particles trapped irreversibly in an infectious bumpy structure in the course of natural dengue infections of humans therefore requires further study. Nevertheless, the fact that in some cases an expanded state of the particle can be trapped after a certain temperature threshold suggests that similar movements - albeit with a smaller amplitude - may take place continuously.
The dynamic behavior of the virions also appears to be responsible for the kinetics of their inactivation, as the amplitude of the dynamic motions may lead to irreversible change not only into bumpy infectious virions but also into non-functional forms [27]. This so-called intrinsic decay is highly accelerated with temperature [27]. Single amino acid changes in E can modulate breathing dynamics and concomitantly affect virus stability [28]. Comparative thermal inactivation analyses of DEN, ZIK and WN viruses have shown that ZIKV occupies a position of intermediate stability among these viruses, with WNV being the most stable [29, 58].
The observed deviations from symmetry of the flavivirus particles - modulated by the efficiency of prM cleavage and/or breathing behavior of E - may thus be important determinants of flavivirus biology. So far, most of the data on incomplete cleavage of prM is derived from in vitro studies in cultured cells. Almost no information is available about the actual heterogeneity of flavivirus populations synthesized in their natural hosts and tissues.
Virus entry
The role of protein E
Flaviviruses have been shown to enter cells by receptor-mediated endocytosis [30]. Protein E, exposed prominently at the surface of all virus particles (Fig.2), has always been considered as being responsible for receptor binding. It is important to distinguish here between entry receptor - the interactions with which will result in virion uptake by the cell - and attachment factors, which merely retain virus particles at the cell surface until there is interaction with an entry receptor. Important efforts led to the identification of multiple flavivirus attachment and entry factors, including cell surface proteins, lectins (such as DC-SIGN), carbohydrates, and lipids, although not all of them have been validated to a similar extent (for extensive reviews, see [31–33]). DC-SIGN, which is present at the surface of dermal dendritic cells and certain macrophages was also shown recently to mediate ZIKV infection of cells [34]. The virus used in these studies was grown in mosquito cells, which is likely to result in the presence of high mannose N-linked glycans at the single carbohydrate attachment site of E. Elegant studies conducted with West Nile virus have shown previously that the capacity to use DC-SIGN for virus entry was restricted to virus grown in C6/36 cells and dependent on the presence of a mannose-rich glycan that was added in these cells [59]. Virus grown in mammalian cells, however, have complex carbohydrates at the same site, and are unable to use DC-SIGN for cell entry. Both types of glycans, however, were able to promote infection of cells expressing DC SIGNR a homologue of DC-SIGN that is expressed on sinusoidal endothelial cells in the liver, endothelial cells in lymph node sinuses, and capillary endothelial cells in placenta [60]. It was therefore proposed that DC-SIGN could promote cellular attachment of the initial, mosquito-derived virus inoculum only, whereas DC-SIGNR might be involved in subsequent rounds of replication in infected hosts [59].
None of the identified entry factors directly interacting with protein E has so far been established as bona fide entry receptor, and most could be only attachment factors helping to concentrate virus particles at the cell surface until interaction with poorly accessible or low abundance receptors that allow particle internalization [31–33]. They also could be involved in the entry process indirectly, by triggering an allosteric change of the particle that would only then expose a relevant surface for interaction with an entry receptor. It is also possible that various combinations of attachment and entry factors are used to infect different hosts and tissues.
Different structural elements of E have been implicated in the interaction with cellular attachment factors, including positively charged patches for binding to glycosaminoglycans [35,36] and the immunoglobulin-like domain III of the E protein [1] although the identity of the receptor to which DIII would bind has remained elusive. In this context, it is interesting to note that a single mutation in E of ZIKV (D393E) differentiates the Pacific islands strains since the outbreak in Yap Island in 2007 and contemporary American strains from earlier Asian and all African strains [37]. Although this is a conservative mutation, it is located in the FG loop of DIII, which has been shown to be critical for infection of Aedes aegypti mosquito midguts and mammalian cells [38]. It is important to note that not only surface-exposed residues but also seemingly cryptic internal sites of the E dimer can become exposed during virus breathing – or through a potential allosteric change triggered by a first ligand – which may then interact with an entry receptor [39]. This is an additional feature related to particle dynamics that has not yet been fully addressed in studies of flavivirus-cell interactions.
Entry mediated by viral lipid interactions: Apoptotic mimicry
Recent studies with several different flaviviruses convincingly show that virus entry can be mediated by interactions that do not involve E but occur between negatively charged lipids such as phosphatidylserine (PS) in the viral membrane. The anionic lipid receptors identified in flavivirus entry are proteins of the TIM (T cell immunoglobulin mucin domain) and TAM (Tyro3, Axl and Mer) receptor families [34]. The physiological function of these receptors is to recognize negatively charged lipids in apoptotic cells and to trigger their endocytic engulfment by phagocytic cells (they are accordingly termed “eat me receptors’). The hijacking of this process - identified also for a number of unrelated enveloped viruses – has therefore been termed ‘apoptotic mimicry’ [34,40]. As flaviviruses bud into the ER lumen during morphogenesis, the viral membrane reflects the composition of the ER membrane, which has PS in its luminal leaflet [41,42]. The plasma membrane of living cells does not normally have such negatively charged lipids in the outer leaflet [43], due to the presence of specific enzymes termed “lipid flippases” that ensure plasma membrane asymmetry by maintaining these lipids only in the inner leaflet (reviewed in reference [44]).
The role of “eat-me” receptors for entry of flaviviruses into certain cell types was originally identified in studies with DENV [45], but several lines of direct and indirect evidence have suggested that ZIKV may also use apoptotic mimicry for gaining access to different tissues that are involved in its spread throughout the human body, including skin cells, endothelial cells, neural cells and cells of the placenta [34,46–51]. Recent studies in wild type and TAM receptor knock-out mice, however, have shown that these molecules are not required for Zika virus infectivity in this animal model through several different routes of infection, and virus replication was unaffected in spleen, placenta, vagina and brain [52]. The data corroborate previous observations [53] and suggest that there may be substantial functional redundancy of ligands (e.g. TIM receptors or other molecules) that can be used for the entry of Zika virus (and possibly other flaviviruses) into host cells.
Particle heterogeneity and/or breathing appear to be absolute requirements for using apoptotic mimicry in cell entry, because the viral membrane would not be accessible in static mature virions (Fig. 2C). So far, there are no studies specifically monitoring the maturation status and breathing behavior of viral particles in relation to their capacity for entry mediated by TIM and TAM (Fig. 2E, F). It is likely that different viruses or even strains of the same virus show different degrees of membrane exposure (see section above) and therefore may differ in their use of lipid receptors for entry.
prM as a putative receptor-binding protein
The release of partially mature but infectious flavivirus particles from infected cells (Fig. 2D) suggests that prM or the prM-E complex could mediate receptor binding in certain cases. It was indeed shown for WNV that a single N-linked glycan on prM alone (in the absence of E glycosylation) could mediate infection through its interaction with DC-SIGNR, albeit at somewhat lower efficiency than in the presence of another glycan on E [54]. In this context it may be relevant that there are potential N-linked glycosylation sites in prM at different sites in flaviviruses: N69 and N70 in DEN and ZIK viruses, respectively, N13, N29 and N51 in YFV, N15 in WNV, and N28 in TBEV.
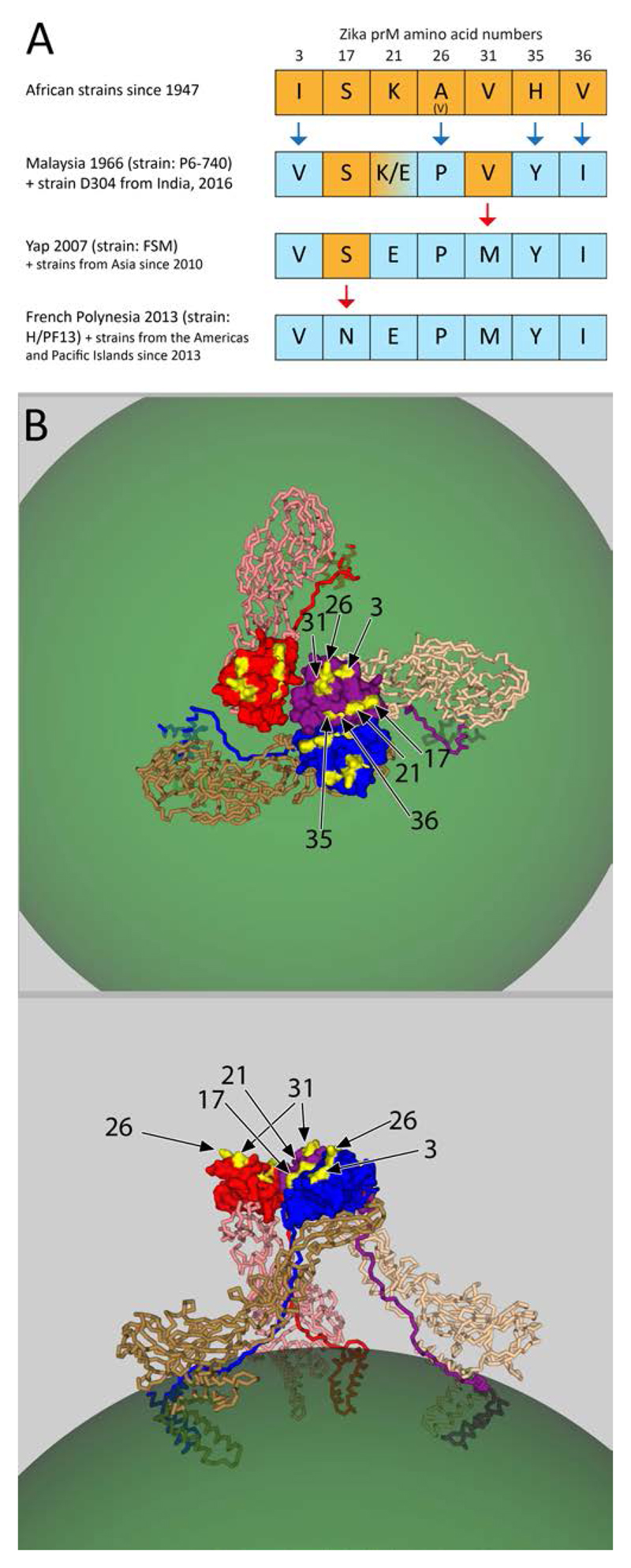
The comparison of ZIKV sequences from Africa, Asia, Micronesia and the Americas revealed a noticeable concentration of mutations that occurred in prM since its African origins, whereas proportionately fewer changes were observed in E [37]. An analysis of the appearance of these mutations in the history of ZIKV is displayed in Figure 3A. African strains (mostly isolated between 1947 and 1984) differ from Malaysia 1966 at 4 positions, including two drastic alterations (A26P and H35Y). The K/E polymorphism observed at position 21 in different sequence deposits of the same Malaysian strain may be due to adaptive mutation during passaging, as described for other flaviviruses [35,36]. The Yap 2007 strain, as well as all Asian strains since 2010, display an additional mutation (V31M), which is maintained in all Pacific island and American strains since 2013. The latter, however, differ from Yap 2007 by an S to N mutation at position 17 (Fig. 3A).
Fig. 3. Changes in ZIKV prM in the strains circulating in the Americas compared to African strains.
A) Graphical representation of mutations in prM related to time and location of isolation. Changes in the ZIKV prM amino acid sequence over time was observed at seven positions, as indicated at the top of the figure. The results displayed are deduced from a comparison based on a ClustalO alignment (http://www.ebi.ac.uk/Tools/msa/clustalo/) of 210 complete prM sequences downloaded from the Virus Pathogen Resource (ViPR) database (www.viprbrc.org) on 4 January, 2017.
Row 1: With a single exception, all 29 African strain sequences in the ViPR data base have identical amino acids at these positions. The exception is strain IbH-30656 with a valine at position 26.
Row 2: The first Asian isolate from Malaysia 1966 (strain P6-740) differs from the African strains at 4 positions, indicated in blue. Position 21 was K in two deposits and E in two further deposits of the same strain, which may reflect different passaging histories. The same sequence with E at position 21 was recently submitted for an Indian strain.
Row 3: Strain FSM from the outbreak in Yap Island 2007 is identical to 7 strains isolated from SE Asia since 2010, all of which differ from the Malaysia 1966 strain at position 31 (V31M).
Row 4: All strains from the Pacific Islands since 2013 and the Americas (a total of 145 sequences) had the same sequence and differed from the Yap and Asian strains in Row 3 at position 17 (S17N).
B) Structure of a trimer of prM-E heterodimers (PDB code 4B03). Color code is the same as in Fig. 1 and 2. The mutations observed in the ZIKV prM protein over time since its discovery in Uganda 1947 (see panel A) are displayed in yellow and labelled with arrows by their amino acid numbers.
It is striking that the seven residues that are different between the virulent strains circulating now in South America and the older strains from Africa cluster at a prM surface exposed prominently at the top of the spikes in immature patches of the virion (Fig. 3B). Hypothetically, this prM surface could contribute to interaction with a receptor and influence cell tropism and pathogenesis of the virus. It is therefore important to expand the scope of the search for receptors by using prM or the prM-E complex as well as E as potential receptor-interacting proteins.
Conclusions
In summary, the current studies suggest that flaviviruses produce an ensemble of structurally different virions circulating in an organism, collectively contributing to tissue tropism and virus dissemination. Some of the particles in the ensemble may be more suitable for infection of certain cells – in analogy to the genome quasi-species notion that posits that RNA virus particles circulate as a cloud of different genomic species, which together act synergistically in order to allow the virus to replicate in different cells and reach certain tissues [55]. The flavivirus quasi-species cloud appears to be centered around a consensus sequence that gives rise to a constellation of mosaic and dynamic particles able to infect a range of tissues in vertebrate and invertebrate hosts, as required to maintain these viruses in their natural ecological cycle. This heterogeneity provides the potential to further expand cell, tissue and host tropisms and to influence the interactions with the immune system [56,57]. Mutations affecting prM (including the efficiency of furin cleavage) and/or particle dynamics could thus be important determinants of changes in biological properties such as host range, efficiency of replication in vectors and vertebrates, transmission dynamics, tissue tropism, and pathogenesis. Some of the most pressing unresolved questions in this context therefore relate to the degree of maturation in the range of hosts and cells in which the virus replicates during natural infections, and the required interactions with relevant receptors. The important progress made recently in interpreting flavivirus structure now suggests a virus population-based framework to understand certain characteristics of flavivirus dissemination and tissue tropism. This new framework will undoubtedly stimulate new research towards a more complete understanding of how particular features in the observed particle heterogeneity distribution relate to variations in the entry mechanism and modulate the outcome of flavivirus infections.
Highlights.
Virion heterogeneity arising from incomplete maturation and particle dynamics
Heterogeneous particle populations may synergistically expand flavivirus tropism
Partially mature viruses appear as important players in natural transmission cycles
Exposure of viral membrane patches is crucial for entry via apoptotic mimicry
Concentration of putative adaptive changes in the immature Zika virus protein prM
Acknowledgements
FAR acknowledges funding from the ANR and from the LABEX IBEID; KS and FXH from the Austrian Science Fund (FWF).
References
- 1.Pierson TC, Diamond MS. Flaviviruses. In: Knipe DM, Howley PM, Cohen JI, Griffin DE, Lamb RA, Martin MA, Racaniello VR, Roizman B, editors. Fields virology. Lippincott. Williams & Wilkins; Philadelphia: 2013. pp. 747–794. [Google Scholar]
- 2.Messina JP, Brady OJ, Scott TW, Zou C, Pigott DM, Duda KA, Bhatt S, Katzelnick L, Howes RE, Battle KE, Simmons CP, et al. Global spread of dengue virus types: Mapping the 70 year history. Trends in microbiology. 2014;22(3):138–146. doi: 10.1016/j.tim.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weaver SC, Costa F, Garcia-Blanco MA, Ko AI, Ribeiro GS, Saade G, Shi P-Y, Vasilakis N. Zika virus: History, emergence, biology, and prospects for control. Antiviral Research. 2016;130:69–80. doi: 10.1016/j.antiviral.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang X, Ge P, Yu X, Brannan JM, Bi G, Zhang Q, Schein S, Zhou ZH. Cryo-em structure of the mature dengue virus at 3.5-a resolution. Nat Struct Mol Biol. 2013;20(1):105–110. doi: 10.1038/nsmb.2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kostyuchenko VA, Zhang Q, Tan JL, Ng TS, Lok SM. Immature and mature dengue serotype 1 virus structures provide insight into the maturation process. J Virol. 2013;87(13):7700–7707. doi: 10.1128/JVI.00197-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kostyuchenko VA, Chew PL, Ng TS, Lok SM. Near-atomic resolution cryo-electron microscopic structure of dengue serotype 4 virus. Journal of virology. 2014;88(1):477–482. doi: 10.1128/JVI.02641-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sirohi D, Chen Z, Sun L, Klose T, Pierson TC, Rossmann MG, Kuhn RJ. The 3.8 å resolution cryo-em structure of zika virus. Science. 2016;352(6284):467–470. doi: 10.1126/science.aaf5316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kostyuchenko VA, Lim EXY, Zhang S, Fibriansah G, Ng T-S, Ooi JSG, Shi J, Lok S-M. Structure of the thermally stable zika virus. Nature. 2016;533(7603):425–428. doi: 10.1038/nature17994. [DOI] [PubMed] [Google Scholar]
- 9.Prasad VM, Miller AS, Klose T, Sirohi D, Buda G, Jiang W, Kuhn RJ, Rossmann MG. Structure of the immature zika virus at 9 a resolution. Nat Struct Mol Biol. 2017 doi: 10.1038/nsmb.3352. advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cherrier MV, Kaufmann B, Nybakken GE, Lok SM, Warren JT, Chen BR, Nelson CA, Kostyuchenko VA, Holdaway HA, Chipman PR, Kuhn RJ, et al. Structural basis for the preferential recognition of immature flaviviruses by a fusion-loop antibody. Embo J. 2009 doi: 10.1038/emboj.2009.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Plevka P, Battisti AJ, Junjhon J, Winkler DC, Holdaway HA, Keelapang P, Sittisombut N, Kuhn RJ, Steven AC, Rossmann MG. Maturation of flaviviruses starts from one or more icosahedrally independent nucleation centres. EMBO Rep. 2011;12(6):602–606. doi: 10.1038/embor.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Junjhon J, Edwards TJ, Utaipat U, Bowman VD, Holdaway HA, Zhang W, Keelapang P, Puttikhunt C, Perera R, Chipman PR, Kasinrerk W, et al. Influence of pr-m cleavage on the heterogeneity of extracellular dengue virus particles. Journal of virology. 2010;84(16):8353–8358. doi: 10.1128/JVI.00696-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pierson TC, Diamond MS. Degrees of maturity: The complex structure and biology of flaviviruses. Curr Opin Virol. 2012;2(2):168–175. doi: 10.1016/j.coviro.2012.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang X, Sheng J, Plevka P, Kuhn RJ, Diamond MS, Rossmann MG. Dengue structure differs at the temperatures of its human and mosquito hosts. Proc Natl Acad Sci U S A. 2013;110(17):6795–6799. doi: 10.1073/pnas.1304300110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fibriansah G, Ng TS, Kostyuchenko VA, Lee J, Lee S, Wang J, Lok SM. Structural changes in dengue virus when exposed to a temperature of 37°c. J Virol. 2013;87(13):7585–7592. doi: 10.1128/JVI.00757-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kindhauser MK, Allen T, Frank V, Santhana R, Dye C. Zika: The origin and spread of a mosquito-borne virus. Bulletin of the World Health Organization. 2016 doi: 10.2471/BLT.16.171082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duffy MR, Chen T-H, Hancock WT, Powers AM, Kool JL, Lanciotti RS, Pretrick M, Marfel M, Holzbauer S, Dubray C, Guillaumot L, et al. Zika virus outbreak on yap island, federated states of micronesia. New England Journal of Medicine. 2009;360(24):2536–2543. doi: 10.1056/NEJMoa0805715. [DOI] [PubMed] [Google Scholar]
- 18.Coyne CB, Lazear HM. Zika virus - reigniting the torch. Nat Rev Micro. 2016;14(11):707–715. doi: 10.1038/nrmicro.2016.125. [DOI] [PubMed] [Google Scholar]
- 19.Lessler J, Chaisson LH, Kucirka LM, Bi Q, Grantz K, Salje H, Carcelen AC, Ott CT, Sheffield JS, Ferguson NM, Cummings DAT, et al. Assessing the global threat from zika virus. Science. 2016;353(6300) doi: 10.1126/science.aaf8160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weaver SC. Emergence of epidemic zika virus transmission and congenital zika syndrome: Are recently evolved traits to blame? mBio. 2017;8(1) doi: 10.1128/mBio.02063-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindenbach BD, Murray CL, Thiel HJ, Rice CM. Flaviviridae. In: Knipe DM, Howley PM, Cohen JI, Griffin DE, Lamb RA, Martin MA, Racaniello VR, Roizman B, editors. Fields virology. Lippincott. Williams & Wilkins; Philadelphia: 2013. pp. 712–746. [Google Scholar]
- 22.Yu IM, Zhang W, Holdaway HA, Li L, Kostyuchenko VA, Chipman PR, Kuhn RJ, Rossmann MG, Chen J. Structure of the immature dengue virus at low ph primes proteolytic maturation. Science. 2008;319(5871):1834–1837. doi: 10.1126/science.1153264. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y, Corver J, Chipman PR, Zhang W, Pletnev SV, Sedlak D, Baker TS, Strauss JH, Kuhn RJ, Rossmann MG. Structures of immature flavivirus particles. Embo J. 2003;22(11):2604–2613. doi: 10.1093/emboj/cdg270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mukherjee S, Lin TY, Dowd KA, Manhart CJ, Pierson TC. The infectivity of prm-containing partially mature west nile virus does not require the activity of cellular furin-like proteases. J Virol. 2011;85(22):12067–12072. doi: 10.1128/JVI.05559-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Junjhon J, Lausumpao M, Supasa S, Noisakran S, Songjaeng A, Saraithong P, Chaichoun K, Utaipat U, Keelapang P, Kanjanahaluethai A, Puttikhunt C, et al. Differential modulation of prm cleavage, extracellular particle distribution, and virus infectivity by conserved residues at nonfurin consensus positions of the dengue virus pr-m junction. J Virol. 2008;82(21):10776–10791. doi: 10.1128/JVI.01180-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fibriansah G, Tan JL, Smith SA, de Alwis R, Ng TS, Kostyuchenko VA, Jadi RS, Kukkaro P, de Silva AM, Crowe JE, Lok SM. A highly potent human antibody neutralizes dengue virus serotype 3 by binding across three surface proteins. Nature communications. 2015;6(6341) doi: 10.1038/ncomms7341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuhn RJ, Dowd KA, Beth Post C, Pierson TC. Shake, rattle, and roll: Impact of the dynamics of flavivirus particles on their interactions with the host. Virology. 2015;479–480C:508–517. doi: 10.1016/j.virol.2015.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dowd KA, DeMaso CR, Pierson TC. Genotypic differences in dengue virus neutralization are explained by a single amino acid mutation that modulates virus breathing. MBio. 2015;6(6) doi: 10.1128/mBio.01559-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goo L, Dowd KA, Smith ARY, Pelc RS, DeMaso CR, Pierson TC. Zika virus is not uniquely stable at physiological temperatures compared to other flaviviruses. mBio. 2016;7(5) doi: 10.1128/mBio.01396-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smit JM, Moesker B, Rodenhuis-Zybert I, Wilschut J. Flavivirus cell entry and membrane fusion. Viruses. 2011;3(2):160–171. doi: 10.3390/v3020160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Acosta EG, Kumar A, Bartenschlager R. Revisiting dengue virus–host cell interaction: New insights into molecular and cellular virology. In: Karl M, Frederick AM, editors. Advances in virus research. Vol. 88. Academic Press; 2014. pp. 1–109. [DOI] [PubMed] [Google Scholar]
- 32.Perera-Lecoin M, Meertens L, Carnec X, Amara A. Flavivirus entry receptors: An update. Viruses. 2014;6(1):69–88. doi: 10.3390/v6010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cruz-Oliveira C, Freire JM, Conceicao TM, Higa LM, Castanho MA, Da Poian AT. Receptors and routes of dengue virus entry into the host cells. FEMS Microbiol Rev. 2015;39(2):155–170. doi: 10.1093/femsre/fuu004. [DOI] [PubMed] [Google Scholar]
- 34.Hamel R, Dejarnac O, Wichit S, Ekchariyawat P, Neyret A, Luplertlop N, Perera-Lecoin M, Surasombatpattana P, Talignani L, Thomas F, Cao-Lormeau VM, et al. Biology of zika virus infection in human skin cells. Journal of virology. 2015;89(17):8880–8896. doi: 10.1128/JVI.00354-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Allison SL, Schalich J, Stiasny K, Mandl CW, Heinz FX. Mutational evidence for an internal fusion peptide in flavivirus envelope protein e. J Virol. 2001;75(9):4268–4275. doi: 10.1128/JVI.75.9.4268-4275.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee E, Hall RA, Lobigs M. Common e protein determinants for attenuation of glycosaminoglycan-binding variants of japanese encephalitis and west nile viruses. Journal of Virology. 2004;78(15):8271–8280. doi: 10.1128/JVI.78.15.8271-8280.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang L, Valderramos SG, Wu A, Ouyang S, Li C, Brasil P, Bonaldo M, Coates T, Nielsen-Saines K, Jiang T, Aliyari R, et al. From mosquitos to humans: Genetic evolution of zika virus. Cell Host & Microbe. 2016;19(5):561–565. doi: 10.1016/j.chom.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Erb SM, Butrapet S, Moss KJ, Luy BE, Childers T, Calvert AE, Silengo SJ, Roehrig JT, Huang CYH, Blair CD. Domain-iii fg loop of the dengue virus type 2 envelope protein is important for infection of mammalian cells and aedes aegypti mosquitoes. Virology. 2010;406(2):328–335. doi: 10.1016/j.virol.2010.07.024. [DOI] [PubMed] [Google Scholar]
- 39.Cockburn JJ, Navarro Sanchez ME, Fretes N, Urvoas A, Staropoli I, Kikuti CM, Coffey LL, Arenzana Seisdedos F, Bedouelle H, Rey FA. Mechanism of dengue virus broad cross-neutralization by a monoclonal antibody. Structure. 2012;20(2):303–314. doi: 10.1016/j.str.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 40.Mercer J, Helenius A. Apoptotic mimicry: Phosphatidylserine-mediated macropinocytosis of vaccinia virus. Annals of the New York Academy of Sciences. 2010;1209(1):49–55. doi: 10.1111/j.1749-6632.2010.05772.x. [DOI] [PubMed] [Google Scholar]
- 41.Fairn GD, Schieber NL, Ariotti N, Murphy S, Kuerschner L, Webb RI, Grinstein S, Parton RG. High-resolution mapping reveals topologically distinct cellular pools of phosphatidylserine. The Journal of Cell Biology. 2011;194(2):257. doi: 10.1083/jcb.201012028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kay JG, Koivusalo M, Ma X, Wohland T, Grinstein S. Phosphatidylserine dynamics in cellular membranes. Molecular Biology of the Cell. 2012;23(11):2198–2212. doi: 10.1091/mbc.E11-11-0936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Meer G, Voelker DR, Feigenson GW. Membrane lipids: Where they are and how they behave. Nat Rev Mol Cell Biol. 2008;9(2):112–124. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Andersen JP, Vestergaard AL, Mikkelsen SA, Mogensen LS, Chalat M, Molday RS. P4-atpases as phospholipid flippases—structure, function, and enigmas. Frontiers in Physiology. 2016;7(275) doi: 10.3389/fphys.2016.00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meertens L, Carnec X, Lecoin MP, Ramdasi R, Guivel-Benhassine F, Lew E, Lemke G, Schwartz O, Amara A. The tim and tam families of phosphatidylserine receptors mediate dengue virus entry. Cell host & microbe. 2012;12(4):544–557. doi: 10.1016/j.chom.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu S, DeLalio LJ, Isakson BE, Wang TT. Axl-mediated productive infection of human endothelial cells by zika virus. Circulation Research. 2016;119(11):1183. doi: 10.1161/CIRCRESAHA.116.309866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Retallack H, Di Lullo E, Arias C, Knopp KA, Laurie MT, Sandoval-Espinosa C, Mancia Leon WR, Krencik R, Ullian EM, Spatazza J, Pollen AA, et al. Zika virus cell tropism in the developing human brain and inhibition by azithromycin. Proceedings of the National Academy of Sciences. 2016 doi: 10.1073/pnas.1618029113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tabata T, Petitt M, Puerta-Guardo H, Michlmayr D, Wang C, Fang-Hoover J, Harris E, Pereira L. Zika virus targets different primary human placental cells, suggesting two routes for vertical transmission. Cell Host & Microbe. 2016;20(2):155–166. doi: 10.1016/j.chom.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tang H, Hammack C, Ogden Sarah C, Wen Z, Qian X, Li Y, Yao B, Shin J, Zhang F, Lee Emily M, Christian Kimberly M, et al. Zika virus infects human cortical neural progenitors and attenuates their growth. Cell Stem Cell. 2016;18(5):587–590. doi: 10.1016/j.stem.2016.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wells Michael F, Salick Max R, Wiskow O, Ho Daniel J, Worringer Kathleen A, Ihry Robert J, Kommineni S, Bilican B, Klim Joseph R, Hill Ellen J, Kane Liam T, et al. Genetic ablation of axl does not protect human neural progenitor cells and cerebral organoids from zika virus infection. Cell Stem Cell. 2016;19(6):703–708. doi: 10.1016/j.stem.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 51.Meertens L, Labeau A, Dejarnac O, Cipriani S, Sinigaglia L, Bonnet-Madin L, Le Charpentier T, Hafirassou ML, Zamborlini A, Cao-Lormeau V-M, Coulpier M, et al. Axl mediates zika virus entry in human glial cells and modulates innate immune responses. Cell Reports. 2017;18(2):324–333. doi: 10.1016/j.celrep.2016.12.045. [DOI] [PubMed] [Google Scholar]
- 52.Hastings AK, Yockey LJ, Jagger BW, Hwang J, Uraki R, Gaitsch HF, Parnell LA, Cao B, Mysorekar IU, Rothlin CV, Fikrig E, et al. Tam receptors are not required for zika virus infection in mice. Cell Rep. 2017;19(3):558–568. doi: 10.1016/j.celrep.2017.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Govero J, Esakky P, Scheaffer SM, Fernandez E, Drury A, Platt DJ, Gorman MJ, Richner JM, Caine EA, Salazar V, Moley KH, et al. Zika virus infection damages the testes in mice. Nature. 2016;540(7633):438–442. doi: 10.1038/nature20556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Davis CW, Mattei LM, Nguyen H-Y, Ansarah-Sobrinho C, Doms RW, Pierson TC. The location of asparagine-linked glycans on west nile virions controls their interactions with cd209 (dendritic cell-specific icam-3 grabbing nonintegrin) Journal of Biological Chemistry. 2006;281(48):37183–37194. doi: 10.1074/jbc.M605429200. [DOI] [PubMed] [Google Scholar]
- 55.Lauring AS, Andino R. Quasispecies theory and the behavior of rna viruses. PLoS Pathogens. 2010;6(7):e1001005. doi: 10.1371/journal.ppat.1001005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dowd KA, Jost CA, Durbin AP, Whitehead SS, Pierson TC. A dynamic landscape for antibody binding modulates antibody-mediated neutralization of west nile virus. PLoS Pathog. 2011;7(6):e1002111. doi: 10.1371/journal.ppat.1002111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pierson TC, Diamond MS. A game of numbers: The stoichiometry of antibody-mediated neutralization of flavivirus infection. Progress in molecular biology and translational science. 2015;129:141–166. doi: 10.1016/bs.pmbts.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goo L, VanBlargan LA, Dowd KA, Diamond MS, Pierson TC. A single mutation in the envelope protein modulates flavivirus antigenicity, stability, and pathogenesis. PLOS Pathogens. 2017;13:e1006178. doi: 10.1371/journal.ppat.1006178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Davis CW, Nguyen HY, Hanna SL, Sanchez MD, Doms RW, Pierson TC. West Nile virus discriminates between DC-SIGN and DC-SIGNR for cellular attachment and infection. J Virol. 2006;80:1290–1301. doi: 10.1128/JVI.80.3.1290-1301.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pohlmann S, Soilleux EJ, Baribaud F, Leslie GJ, Morris LS, Trowsdale J, Lee B, Coleman N, Doms RW. DC-SIGNR, a DC-SIGN homologue expressed in endothelial cells, binds to human and simian immunodeficiency viruses and activates infection in trans. Proc Natl Acad Sci USA. 2001;98:2670–2675. doi: 10.1073/pnas.051631398. [DOI] [PMC free article] [PubMed] [Google Scholar]