Abstract
Importance
Delirium is associated with accelerated cognitive decline. The pathologic substrates of this association are not yet known, that is, whether they are the same as those associated with dementia, are independent, or are interrelated.
Objective
To examine whether the accelerated cognitive decline observed after delirium is independent of the pathologic processes of classic dementia.
Design, Setting, and Participants
Harmonized data from 987 individual brain donors from 3 observational cohort studies with population-based sampling (Vantaa 85+, Cambridge City Over-75s Cohort, Cognitive Function and Ageing Study) performed from January 1, 1985, through December 31, 2011, with a median follow-up of 5.2 years until death, were used in this study. Neuropathologic assessments were performed with investigators masked to clinical data. Data analysis was performed from January 1, 2012, through December 31, 2013. Clinical characteristics of brain donors were not different from the rest of the cohort. Outcome ascertainment was complete given that the participants were brain donors.
Exposures
Delirium (never vs ever) and pathologic burden of neurofibrillary tangles, amyloid plaques, vascular lesions, and Lewy bodies. Effects modeled using random-effects linear regression and interactions between delirium and pathologic burden were assessed.
Outcomes
Change in Mini-Mental State Examination (MMSE) scores during the 6 years before death.
Results
There were 987 participants (290 from Vantaa 85+, 241 from the Cambridge City Over-75s Cohort, and 456 from the Cognitive Function and Ageing Study) with neuropathologic data; mean (SD) age at death was 90 (6.4) years, including 682 women (69%). The mean MMSE score 6 years before death was 24.7 points. The 279 individuals with delirium (75% women) had worse initial scores (−2.8 points; 95% CI, −4.5 to −1.0; P < .001). Cognitive decline attributable to delirium was −0.37 MMSE points per year (95% CI, −0.60 to −0.13; P < .001). Decline attributable to the pathologic processes of dementia was −0.39 MMSE points per year (95% CI, −0.57 to −0.22; P < .001). However, the combination of delirium and the pathologic processes of dementia resulted in the greatest decline, in which the interaction contributed an additional −0.16 MMSE points per year (95% CI, −0.29 to −0.03; P = .01). The multiplicative nature of these variables resulted in individuals with delirium and the pathologic processes of dementia declining 0.72 MMSE points per year faster than age-, sex-, and educational level–matched controls.
Conclusions and Relevance
Delirium in the presence of the pathologic processes of dementia is associated with accelerated cognitive decline beyond that expected for delirium or the pathologic process itself. These findings suggest that additional unmeasured pathologic processes specifically relate to delirium. Age-related cognitive decline has many contributors, and these findings at the population level support a role for delirium acting independently and multiplicatively to the pathologic processes of classic dementia.
Understanding the pathologic basis of cognitive impairment in whole populations is a prerequisite to mitigating the increasing public health burden of dementia.1 Many strands of investigation presuppose that Alzheimer, vascular, and Lewy body pathologic features are the predominant causes of dementia. This paradigm has directed the search for biomarkers, treatments, and potential prevention strategies. However, evidence indicates that these classic pathologic processes do not fully account for the clinical syndrome,2 especially in unselected populations of the oldest-old.3,4 For example, older people may have a large burden of the pathologic processes of classic dementia but no associated clinical dementia and vice versa.
Delirium is a syndrome of acute brain dysfunction characterized by inattention and other mental status impairments. It is a major public health problem that affects at least 20% of older inpatients and has well-documented adverse associations.5 An emerging literature reveals that delirium is a strong predictor of new-onset dementia and acceleration of existing cognitive decline.6–10 These results are consistent across several different settings: after hospitalization,11 in those with dementia,6,12 in postoperative patients,13 and in a community population.8 In multiple animal models of neurodegeneration, triggers of acute cognitive dysfunction, such as systemic inflammation, also exacerbate the pathologic processes14,15 and accelerate functional decline during longer periods.16,17 This finding implies that delirium and/or its causes can contribute to the overall burden of dementia. Moreover, research indicates that 3 of 10 cases are preventable,18 which in turn suggests that delirium interventions might reduce at least some cognitive decline and dementia.
Although delirium is now established as a strong predictor of cognitive decline in older adults,6,8,12 whether it accounts for additional, interrelated, or unexplained pathologic injury that contributes to dementia has not previously been examined. It is possible that when dementia follows delirium it has a different pathologic profile compared with dementia that develops without delirium. Therefore, understanding how delirium affects the evolution of dementia in the context of a particular burden of pathologic findings may offer new insights into independent mechanisms that explain cognitive decline after delirium.
In this study, the challenge was to examine a key hypothesis: that faster cognitive decline associated with delirium would act independently of the cognitive decline associated with the pathologic processes of classic dementia. Accordingly, we investigated the extent to which delirium and the pathologic processes of classic dementia contributed to associated cognitive decline in 3 unselected, population-based cohort studies with neuropathologic autopsy data: the Medical Research Council Cognitive Function and Ageing Study (CFAS), the Cambridge City Over-75s Cohort (CC75C), and the Vantaa 85+ study. These studies represent the entirety of such studies conducted in Europe and provide a unique opportunity to increase the understanding of the clinical significance of delirium and its interrelation with the pathologic processes of dementia in the general population.
Methods
The individual studies have previously been described in detail,19–21 and participant-level data have been harmonized as the Epidemiological Clinicopathological Studies in Europe (EClipSE) collaboration.22 Briefly, participants were sampled from general practitioners’ registers (CFAS [1991-2011] and CC75C [1985-2011] in the United Kingdom) and the Population Register Centre (Vantaa 85+ in Finland [1991-2001]) from January 1, 1985, through December 31, 2011. Data analysis was performed from January 1, 2012, through December 31, 2013. The CFAS recruited persons 65 years or older, the CC75C recruited persons 75 years or older, and Vantaa 85+ recruited persons 85 years or older. Individuals were assessed mostly at 2- to 4-year intervals, with some subsamples having annual evaluation. The Mini-Mental State Examination (MMSE)23 was performed in all 3 studies. Additional neuropsychological batteries were also performed, with some differences among the studies (eAppendix in the Supplement). Table 1 summarizes the characteristics of each cohort. Previous work found that participants in the brain donor programs had no systematic differences in clinical characteristics compared with other participants in the cohorts,24 although donors in the CFAS were selected by stratified random sampling, weighted to those who were older and cognitively impaired. Each study had local ethical approval (CFAS centers: Cambridge: North West Anglia Health Authority Local Research Ethics Committee [Peterborough]; Huntingdon Local Research Ethics Committee; Cambridge Local Research Ethics Committee; Gwynedd: Gwynedd Hospitals National Health Service Trust–North West Health Authority Research Ethics Committee (West); Liverpool: Liverpool Local Research Ethics Committee; Newcastle: Newcastle & North Tyneside Health Authority–Joint Ethics Committee; Northumberland and Tyne & Wear Health Authority–Local Research Ethics Committee; Nottingham: Queen’s Medical Centre National Health Service Trust Ethics Committee; Nottingham University Medical School Ethical Committee; City Hospital Ethics Committee; Oxford: Oxfordshire Health Authority: Central Oxford Research Ethics Committee; CC75C: Cambridge Research Ethics Committee; and Vantaa 85+: Ethics Committee of the City of Vantaa). Written informed consent was provided for each study, and all analyses were conducted with deidentified data.
Table1. Characteristics of Studies Comprising the EClipSE Database.
| Source | Total No. of Patients | Site | Age, y | Baseline Survey Year | Follow-up, y | No. of Surveysa | Donors, No. (%) |
|---|---|---|---|---|---|---|---|
| Vantaa 85+ | 553 | Vantaa, Finland | ≥85 | 1991 | 10 | 5 | 290 (52.4) |
| CC75C | 2166 | Cambridge, England | ≥75 | 1985 | 25 | 9 | 241 (11.1) |
| CFAS | 18 226 | UK multicenterb | ≥65 | 1993 | 10 | 7 | 456 (2.5) |
Abbreviations: CC75C, Cambridge City Over-75s Cohort; CFAS, Cognitive Function and Ageing Study; EClipSE, Epidemiological Clinicopathological Studies in Europe.
Number of surveys refers to the maximum number of times a participant could have been seen up to the most recent follow-up point.
The CFAS sampled from 6 geographic areas: 4 urban (Newcastle, Nottingham, Liverpool, and Oxford) and 2 rural (Cambridgeshire, Gwynedd).
Delirium Assessments
In the CFAS and CC75C, delirium symptoms were a feature of the standardized interview schedules administered by trained interviewers to participants and informants. These schedules assigned diagnostic groups based on validated, structured algorithms for psychiatric disorders, themselves based on DSM-III-R or related classifications.25 Questions included the following: “Were there brief episodes during the 24 hours when s/he seemed much worse and then times when quite clear?” “Were there marked fluctuations in his/her level of attention or alertness?” “Could a physical illness…be sufficient explanation for the subject’s mental or psychiatric symptoms (eg, delirious due to acute infection)?” A full list of relevant questions is given in the eAppendix in the Supplement.
At each interview in the Vantaa 85+ study, the examining neurologists assessed participants and informant(s) for a history of any episodes of delirium, with reference to a checklist of DSM-III-R criteria for delirium diagnosis.26 The reported history was corroborated with medical case records that were available at the time of assessment such that the study ascertainment of delirium was retrospectively derived from multiple sources and the overall diagnosis accepted if the examining neurologists judged there was sufficient evidence from participant and informant recall and/or indication in the medical records.
Neuropathologic Analyses
Paraffin-embedded brain tissue samples were used to assess neuropathologic markers with investigators masked to clinical data. Each study reported Braak stage as a semiquantitative measure of τ neurofibrillary tangles and neocortical amyloid plaque burden from the Consortium to Establish a Registry for Alzheimer’s Disease protocol.27 The presence of infarcts (>10 mm), lacunes, and hemorrhage was histologically assessed using hematoxylin-eosin. Lewy bodies in the substantia nigra were assessed with hematoxylin-eosin but also included immunohistochemical staining against α-synuclein (or ubiquitin in some of the earlier CC75C specimens) (eAppendix in the Supplement).
Statistical Analysis
Consistent with previous approaches, delirium exposure was operationalized as never or ever.8 Change in MMSE score before death was modeled using a time-to-death random-effects (random slopes) model.28 We were interested in estimating the final trajectory toward death because this approach makes associations with pathologic data easier to define. The mean time from the start of the trajectory identified by the model to death was 5.2 years; therefore, the start point (intercept) for this trajectory was set (centered) at 6 years. This start point is not so near the point of death that rates of change (slopes) cannot be estimated yet not so far from death that the pathologic findings at autopsy might not plausibly be related to the estimated parameters. Six years before death is also comparable to start points from change-point models of the final trajectory of cognitive decline29–31 and in the range observed in other analyses (3-8 years).32 Models were adjusted for baseline MMSE score, age at death (centered at a mean age of 90 years), sex (0 for men, 1 for women), years of education (0-3, 4-7, 8-11, or ≥12), and study. Missing data were assumed to be missing at random given that outcome ascertainment was essentially complete in this brain donor cohort.
The 4 neuropathologic variables of classic dementia that contribute the greatest population-attributable risk for dementia4 were examined: Braak stage (neurofibrillary tangles), neocortical amyloid plaques, vascular pathologic findings (large artery infarcts, lacunes, or hemorrhage), and Lewy bodies in the substantia nigra. In keeping with previous methods, neuropathologic variables were dichotomized (0, none to mild; 1, moderate to severe).3,8,24 This approach allows for simpler interpretation and is more likely to be robust. Individuals were assigned a pathologic burden score based on the number of times they scored in the higher category for each of the 4 markers. Therefore, the overall pathologic burden score ranged from 0 to 4 (ie, being in the lower category for all markers [pathologic burden score of 0], in the upper category of all 4 markers [pathologic burden score of 4], or some combination). Finally, interactions between delirium and pathologic burden ([delirium history] × [pathologic score]) in terms of their effect on both the start point (−6 years before death) and rate of change of MMSE scores were calculated. Full details are given in the eAppendix in the Supplement. All analyses were conducted with STATA statistical software, version 12.1 (StataCorp). P values were calculated though tests of maximum likelihood, where P < .05 was considered significant. All tests were 2-tailed.
Results
There were 987 participants (290 from Vantaa 85+, 241 from the CC75C, and 456 from the CFAS) with neuropathologic data (mean [SD] age at death, 90 [6.4] years; 472 females [67%] without delirium and 210 [75%] with delirium). Table 2 describes the characteristics of the sample. Persons with delirium were slightly older, more likely to be women, and more likely to have more years of education. Neocortical amyloid plaques, vascular pathologic findings, or Lewy bodies were not significantly different in individuals with and without a history of delirium.
Table 2. Characteristics of Study Participants According to History of Deliriuma.
| Characteristic | No Delirium (n = 708)b |
Delirium (n = 279)b |
P Valuec |
|---|---|---|---|
| Follow-up, median (IQR), y | 4.3 (2.0-7.1) | 4.7 (2.5-7.8) | NA |
| No. of assessments in last 6 y, median (IQR)d | 2 (1-3) | 3 (2-4) | NA |
| Study | |||
| Vantaa 85+ (n = 290) | 232 (80.0) | 58 (20.0) | NA |
| CC75C (n = 241) | 142 (58.9) | 99 (41.1) | |
| CFAS (n = 456) | 334 (73.2) | 122 (26.8) | |
| Age at death, mean (SD), y | 89 (6.7) | 90 (5.8) | .03 |
| Female | 472 (66.7) | 210 (75.3) | <.001 |
| Years of education, median (IQR) | 9 (6-13) | 9 (8-14) | <.001 |
| Pathologic findinge | |||
| Braak stage (n = 978) | 346 (50.6) | 166 (56.5) | .09 |
| Neocortical amyloid plaques (n = 960) | 344 (49.7) | 138 (51.5) | .62 |
| Vascular (infarcts, lacunes, or hemorrhages) (n = 884) | 358 (55.6) | 139 (57.9) | .54 |
| Lewy bodies in substantia nigra (n = 967) | 67 (9.7) | 27 (9.7) | .99 |
| Pathologic burden scoref | |||
| 0 | 132 (18.6) | 40 (14.3) | .10 |
| 1 | 207 (29.2) | 72 (25.8) | |
| 2 | 220 (31.1) | 107 (38.4) | |
| 3 or 4 | 149 (21.0) | 60 (21.5) | |
| Any moderate to severe pathologic burdeng | 576 (81.4) | 239 (85.7) | .20 |
Abbreviations: CC75C, Cambridge City Over-75s Cohort; CFAS Cognitive Function and Ageing Study; IQR, interquartile range; NA, not applicable.
Data are presented as number (percentage) of patients unless otherwise indicated.
Delirium means evidence of delirium at any time compared with those with no history of delirium.
P values for differences in means and medians (continuous measures) were obtained by 2-sample t test or Wilcoxon test, and proportions were tested using χ2 tests.
Six years is the chosen intercept for this model describing the final trajectory of cognitive decline.
Pathologic measures are dichotomized. Numbers given here are for the higher category (Braak stage ranges from 0 to 6); figures are those scoring 4, 5, or 6. Neocortical amyloid plaques scored as none, mild, moderate, or severe; figures are those scoring moderate to severe. Vascular indicates the presence (yes/no) of infarcts in arteries larger than 10 mm, lacunar lesions, or hemorrhage. Lewy bodies scored as none, mild, moderate, or severe; figures are those scoring moderate to severe. Full details are given in the eAppendix in the Supplement.
Pathologic burden score refers to the number of pathologic measures in a higher category for an individual.
Any moderate to severe pathologic findings were scored as a pathologic burden score of 1 or higher.
Results from the random-effects models that described delirium and cognitive decline are presented in Table 3. The median number of longitudinal observations for participants in the model was 2 (interquartile range, 1-4). In the fully adjusted model (including delirium and pathologic burden), the start point was estimated at 24.7 MMSE points. The start point should be interpreted as the estimated MMSE score 6 years before death in persons in whom all covariates are in the reference category (eg, youngest age, no delirium). For the typical 90-year-old, the mean base rate of decline was 0.35 points per year (base rate indicates all covariates in the reference category, eg, no delirium, lowest pathologic score). There was no significant influence of study source (Vantaa 85+, CC75C, or CFAS) on the model estimates (eAppendix in the Supplement).
Table 3. Quantifying Trajectories of Mini-Mental State Examination Change in Relation to Delirium and Dementia Pathologic Burdena.
| Variable | Clinical (n = 877 Cases and 2570 Observations) |
Clinical and Delirium (n = 877 Cases and 2570 Observations) |
Clinical and Pathologic Burden (n = 872 Cases and 2558 Observations) |
Clinical, Delirium, and Pathologic Burden (n = 872 Cases and 2558 Observations) |
||||
|---|---|---|---|---|---|---|---|---|
| β (95% CI) | P Value | β (95% CI) | P Value | β (95% CI) | P Value | β (95% CI) | P Value | |
| Intercept | 21.73 (19.98 to 23.48) | <.001 | 22.18 (20.51 to 23.85) | <.001 | 24.76 (22.84 to 26.67) | <.001 | 24.65 (22.77 to 26.53) | <.001 |
| Slope | −0.86 (−0.93 to −0.78) | <.001 | −0.66 (−0.74 to −0.58) | <.001 | −0.45 (−0.60 to −0.31) | <.001 | −0.35 (−0.51 to −0.20) | <.001 |
| Age | −0.27 (−0.33 to −0.20) | <.001 | −0.25 (−0.31 to −0.19) | <.001 | −0.23 (−0.29 to −0.16) | <.001 | −0.21 (−0.27 to −0.15) | <.001 |
| Age × slope | −0.02 (−0.03 to −0.01) | <.001 | −0.02 (−0.03 to −0.00) | <.001 | −0.01 (−0.02 to −0.00) | <.001 | −0.01 (−0.02 to −0.00) | .05 |
| Sex | −2.08 (−2.81 to −1.34) | <.001 | −1.96 (−2.69 to −1.24) | <.001 | −2.08 (−2.80 to −1.35) | <.001 | −1.98 (−2.70 to −1.27) | <.001 |
| Educational level, y | ||||||||
| 0-3 | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | ||||
| 4-7 | 0.99 (−0.94 to 2.78) | .33 | 1.17 (−0.66 to 2.88) | .22 | 0.88 (−0.91 to 2.66) | .34 | 1.06 (−0.66 to 2.77) | .23 |
| 8-11 | 1.93 (−0.91 to 4.77) | .12 | 1.22 (−1.51 to 3.95) | .24 | 1.49 (−1.24 to 4.22) | .20 | 0.73 (−1.90 to 3.37) | .57 |
| ≥12 | 5.55 (2.68 to 8.43) | <.001 | 4.56 (1.79 to 7.33) | <.001 | 5.18 (2.41 to 7.94) | <.001 | 4.16 (1.49 to 6.83) | <.001 |
| Delirium | −3.84 (−4.62 to −3.06) | <.001 | −2.75 (−4.49 to −1.01) | <.001 | ||||
| Delirium × slope | −0.62 (−0.77 to −0.48) | <.001 | −0.37 (−0.60 to −0.13) | <.001 | ||||
| Pathologic burden score | ||||||||
| 0 | 1 [Reference] | .04 | 1 [Reference] | .24 | ||||
| 1 | −1.30 (−2.33 to −0.26) | <.01 | −0.67 (−1.79 to 0.45) | <.001 | ||||
| 2 | −2.83 (−3.86 to −1.79) | <.01 | −2.22 (−3.34 to −1.09) | <.001 | ||||
| 3 or 4 | −4.81 (−6.04 to −3.58) | <.01 | −4.40 (−5.71 to −3.10) | <.001 | ||||
| Pathologic burden × slope | −0.51 (−0.68 to −0.35) | −0.39 (−0.57 to −0.22) | <.001 | |||||
| Delirium × pathologic burden interaction (intercept) | −0.86 (−2.75 to 1.03) | .37 | ||||||
| Delirium × pathologic burden interaction (slope) | −0.16 (−0.29 to −0.03) | .01 | ||||||
The term dementia pathologic burden refers to classic dementia pathologic variables known to contribute to cognitive impairment (ie, Braak stage, amyloid plaques, infarcts, and Lewy bodies). Observations refers to the total number of longitudinal outcomes in the model. Each of the 4 columns represents a model of cognitive trajectories adjusted by study source. The intercept and slope are given for each model. These variables indicate the estimated Mini-Mental State Examination scores 6 years before death (intercept) and the rate of decline per year (slope). The intercept from 6 years before death was chosen because the mean time before death was 5.2 years, and the model is centered just before the mean. The figures given in this row are for the baseline group, that is, where all other variables in the model are in the lowest category.
Effect of Delirium on Start Point and Rate of Change
Delirium was associated with a mean 2.8-point lower MMSE score (P < .001) 6 years before death. For these persons, the rate of change was an additional 0.37 points per year (P < .001). These coefficients are additive. Therefore, for the typical individual aged 90 years at death with delirium, the estimated MMSE score is 24.7 points (baseline) with −2.8 points equaling 21.9 MMSE points, declining at 0.35 points (base rate) with −0.37 (attributable to delirium) equaling 0.72 points per year.
Effect of Pathologic Burden on Start Point and Rate of Change
An increasing pathologic burden score was associated with a lower MMSE score (−0.7 for 1 instance of high dementia pathologic marker, −2.2 point for 2 markers, and −4.4 for 3 or more markers; P < .001). Pathologic burden conferred an additional 0.39-point decline in MMSE score over and above the effects of age and delirium (P < .001).
Interaction Between Delirium and Pathologic Burden
A significant interaction between delirium and pathologic burden estimated an additional decline of 0.16 MMSE points per year (P = .01). Therefore, individuals with delirium and high dementia pathologic burden had estimated rates of decline of −0.35 points (base rate), −0.37 points (attributable to delirium), −0.39 points (attributable to pathologic burden), and −0.16 points (attributable to interaction), which equals 1.27 points per year. In comparison, the independent effect of age alone on the rate of MMSE score change was 0.01 points per year (ie, MMSE score difference of 0.05 between the ages of 85 and 90 years).
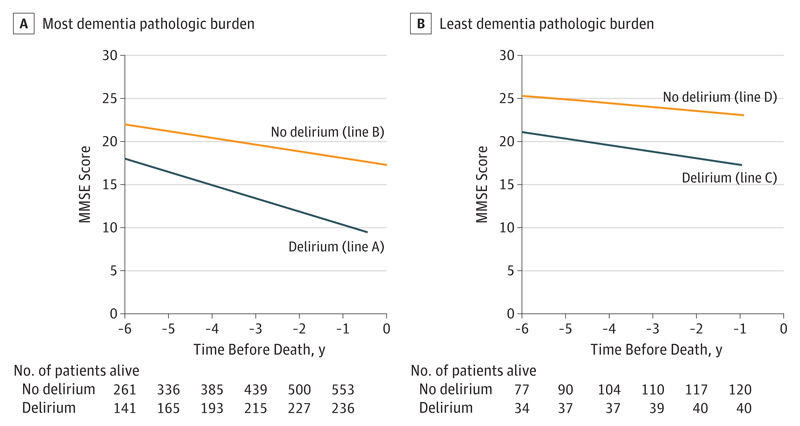
The Figure shows how the rate of cognitive decline varies by delirium and pathologic status. The slowest decline was seen in persons with no history of delirium and least dementia pathologic burden. The fastest decline was seen in persons with a history of delirium and most dementia pathologic burden. Intermediate rates of decline were observed in individuals with delirium but least dementia pathologic burden and in those with no delirium history but most dementia pathologic burden.
Figure. Trajectory of Cognitive Decline in Relation to Delirium and Dementia Pathologic Burden at Autopsy.
Trajectories of cognitive decline in individuals with the most (A) and least (B) dementia pathologic burden (based on Braak stage, cortical amyloid plaques, infarcts, and Lewy bodies) according to delirium status. Individuals with delirium and more dementia pathologic burden have the fastest decline (line A), whereas individuals with no delirium and little dementia pathologic burden have slowest decline (line D). For some individuals, cognitive decline is driven by dementia pathologic burden (no delirium, high pathologic burden) (line B). For other individuals, cognitive decline is associated with delirium (delirium, little pathologic burden) (line C), and this condition is distinct from, but contributory to, classic dementia pathologic burden. P < .001 for line A vs B, P < .001 for line C vs D, and P = .01 for line A vs C. MMSE indicates Mini-Mental State Examination.
Discussion
This is the first report, to our knowledge, that people with delirium and higher levels of pathologic processes of classic dementia have the greatest cognitive decline. Delirium in the presence of dementia-related neuropathologic processes was associated with cognitive decline beyond that expected for delirium or the neuropathologic process itself. This finding means that delirium may be independently associated with pathologic processes that drive cognitive decline, which are different from the pathologic processes of classic dementia. These findings suggest new possibilities regarding the pathologic correlates of cognitive impairment, positioning delirium, and/or its precipitants as a critically interrelated mechanism.
These results are in keeping with other studies identified in a systematic review33 reporting that delirium is associated with faster trajectories of cognitive decline.6,8,13 A previous report8 from the Vantaa 85+ study raised the possibility that the pathologic processes of classic dementia might not mediate the observed association between delirium and dementia, although the analysis was underpowered. In this study, the larger sample size and the more precise determination of cognitive change in the 6 years before death allow us to be more conclusive about the interrelated effect of delirium on clinicopathologic correlations in dementia. Experimental data from mouse models suggest that delirium may arise through the interaction between systemic or central nervous system inflammation and existing neurodegenerative pathologic processes,34 and acute exacerbation of inflammation clearly leads to neuronal death,14 synaptic changes,35 and accelerated decline.36 These changes occur independently of increased extracellular amyloid. However, we now need to know whether individuals with delirium superimposed on dementia have different patterns of inflammation, synaptic loss, axonal pathologic findings, and/or differential loss of key neuronal populations of the hippocampus and cortex and of cholinergic and noradrenergic projection areas.37
Strengths and Limitations
This analysis has a number of strengths. It focuses on a major question arising from the prevalence of cognitive impairment and aging. In terms of study design, the 3 cohorts have high generalizability for the oldest-old populations, who are underrepresented in dementia research despite having the highest prevalence of dementia.38 This is also the first article, to our knowledge, to examine delirium and the pathologic correlates of cognitive decline at the end of life in the general population; the other analysis comes from a leading study in this area, the Religious Orders cohort study,30 which is, however, focused on specific populations. Modeling change in cognitive outcomes as continua rather than simply the presence or absence of dementia allows for an exploration of the effect of delirium across the whole spectrum of cognitive function (ie, from no baseline impairment through mild cognitive impairment to more severe dementia severity). The power to assess such effects as interactions between delirium and neuropathologic processes is unique.
A number of limitations should be taken into account. Delirium was retrospectively ascertained and by slightly different methods. In the Vantaa 85+ study, assessments for history of delirium occurred at each visit, using information from participants, informants, and medical records. Ascertainment of data in the CFAS and CC75C relied on diagnostic interviews at each study visit, but these data are likely to underestimate delirium in the intervening period. The diagnostic classification criteria also varied, although the different diagnostic schedules for delirium have good agreement with DSM-III-R.39 Despite these differences, the results appear to be consistent across the cohorts. The implication, either way, is that core symptoms in delirium—acute fluctuating change in attention in association with acute illness—represent an adverse state for subsequent cognitive trajectories regardless of the exact methods for operationalizing the syndrome. As with other prospective cohort data, the possibility remains that residual confounding contributes to these observed associations. Another consideration is that only a limited range of pathologic markers and comorbidities could be examined in this harmonized data set. Finally, although recent research based on neuroimaging and neuropathologic examination suggests that insults in earlier life can also be malignant,40–42 this hypothesis could not be examined within this study.
Conclusions
Our results indicate that delirium interacts with underlying pathologic processes of classic dementia and so represents a potential independent but interrelated pathologic pathway to chronic cognitive impairment and dementia. If delirium prevention could lead to consequent prevention of dementia,43,44 it will be essential to understand whether certain dimensions of the delirium syndrome might have a greater effect on cognitive trajectories than others. For example, duration, severity, and/or cause (eg, medications vs acute illness, surgery vs sepsis) may be differently important. The degree of preexisting multimorbidity or frailty may have a significant bearing. Animal studies modeling different causes and severities have some scope to elucidate some of these questions, but greater clarity on these issues must also come from careful prospective studies in representative populations. Nonetheless, our findings indicate that clinicians need to be alert to older people’s cognitive changes during acute episodes and in follow-up across all settings and therefore support wider implementation of best practice in delirium prevention.45
Supplementary Material
Key Points.
Question What is the association among delirium, the pathologic processes of dementia, and cognitive decline in older persons?
Findings In this cohort of 987 autopsied brains from 3 population-based cohort studies, delirium and the pathologic processes of dementia were associated with cognitive decline; however, the combination of delirium and the pathologic processes of dementia interacted to give the fastest trajectory of cognitive decline.
Meaning During cognitive decline in the oldest-old, delirium appears to act independently and multiplicatively to the neuropathologic processes of classic dementia.
Funding/Support
The Cognitive Function and Ageing Study (CFAS) and Cambridge City Over-75s Cohort (CC75C) are member studies of the Cambridgeshire and Peterborough Collaboration for Leadership in Applied Health Research and Care funded by the United Kingdom’s National Institute for Health Research. The Cambridge Brain Bank Laboratory (which processed all the CC75C and some of the CFAS cases) is supported by the National Institute for Health Research Cambridge BioMedical Research Centre. The original Epidemiological Clinicopathological Studies in Europe (EClipSE) harmonization project was established through grant RHAG/094 from the Bupa UK Foundation and a European Union Marie Curie International Incoming Fellowship (Dr Keage). Dr Fleming is supported by grant RHAG/058 from the Bupa UK Foundation, which included the latest CC75C fieldwork funding. Dr Davis is supported by grants 090661/Z/09/Z and 107467/Z/15/Z from the Wellcome Trust. Drs Davis and MacLullich are members of The University of Edinburgh Centre for Cognitive Ageing and Cognitive Epidemiology, part of the cross-council Lifelong Health and Wellbeing Initiative funded by grant G0700704/84698. Funding from the Biotechnology and Biological Sciences Research Council, Engineering and Physical Sciences Research Council, Economic and Social Research Council, and the Medical Research Council is gratefully acknowledged. Dr Keage is supported by Australian National Health and Medical Research Council Fellowship grant 568890. Dr Cunningham is supported by grant 09/09/07 from the Wellcome Trust Senior Research Fellowship.
Role of the Funder/Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions: Dr Davis had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Davis, Ince, Matthews, Ely, Brayne.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Davis, Keage, Ely, MacLullich.
Critical revision of the manuscript for important intellectual content: Davis, Muniz-Terrera, Stephan, Fleming, Ince, Matthews, Cunningham, Ely, MacLullich, Brayne.
Statistical analysis: Davis, Muniz-Terrera, Matthews.
Obtained funding: Davis, Fleming, Ince, Matthews, Brayne.
Administrative, technical, or material support: Keage, Fleming, Ince, Ely.
Study supervision: MacLullich.
Conflict of Interest Disclosures: None reported.
Additional Contributions: We thank the CFAS, CC75C, and Vantaa 85+ teams; their study participants; and relatives.
Contributor Information
Daniel H. J. Davis, Department of Public Health and Primary Care, University of Cambridge, Cambridge, England; Medical Research Council Unit for Lifelong Health and Ageing at University College London, London, England.
Graciela Muniz-Terrera, Centre for Dementia Prevention, University of Edinburgh, Edinburgh, Scotland.
Hannah A. D. Keage, School of Psychology, SocialWork and Social Policy, University of South Australia, Adelaide, Australia.
Blossom C. M. Stephan, Institute of Health and Society, Newcastle University, Newcastle, England.
Jane Fleming, Department of Public Health and Primary Care, University of Cambridge, Cambridge, England.
Paul G. Ince, Sheffield Institute for Translational Neuroscience, University of Sheffield, Sheffield, England.
Fiona E. Matthews, Institute of Health and Society, Newcastle University, Newcastle, England.
Colm Cunningham, School of Biochemistry and Immunology, Trinity College, Dublin, Ireland.
E. Wesley Ely, Department of Medicine, Vanderbilt University Medical Center, Nashville, Tennessee; Tennessee Valley Veterans Affairs Geriatric Research Education Clinical Center, Nashville.
Alasdair M. J. MacLullich, Edinburgh Delirium Research Group, University of Edinburgh, Edinburgh, Scotland.
Carol Brayne, Department of Public Health and Primary Care, University of Cambridge, Cambridge, England.
References
- 1.Brayne C, Davis D. Making Alzheimer’s and dementia research fit for populations. Lancet. 2012;380(9851):1441–1443. doi: 10.1016/S0140-6736(12)61803-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyle PA, Wilson RS, Yu L, et al. Much of late life cognitive decline is not due to common neurodegenerative pathologies. Ann Neurol. 2013;74(3):478–489. doi: 10.1002/ana.23964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Savva GM, Wharton SB, Ince PG, Forster G, Matthews FE, Brayne C, Medical Research Council Cognitive Function and Ageing Study Age, neuropathology, and dementia. N Engl J Med. 2009;360(22):2302–2309. doi: 10.1056/NEJMoa0806142. [DOI] [PubMed] [Google Scholar]
- 4.Matthews FE, Brayne C, Lowe J, McKeith I, Wharton SB, Ince P. Epidemiological pathology of dementia: attributable-risks at death in the Medical Research Council Cognitive Function and Ageing Study. PloS Med. 2009;6(11):e1000180. doi: 10.1371/journal.pmed.1000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siddiqi N, House AO, Holmes JD. Occurrence and outcome of delirium in medical in-patients: a systematic literature review. Age Ageing. 2006;35(4):350–364. doi: 10.1093/ageing/afl005. [DOI] [PubMed] [Google Scholar]
- 6.Fong TG, Jones RN, Shi P, et al. Delirium accelerates cognitive decline in Alzheimer disease. Neurology. 2009;72(18):1570–1575. doi: 10.1212/WNL.0b013e3181a4129a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.MacLullich AMJ, Beaglehole A, Hall RJ, Meagher DJ. Delirium and long-term cognitive impairment. Int Rev Psychiatry. 2009;21(1):30–42. doi: 10.1080/09540260802675031. [DOI] [PubMed] [Google Scholar]
- 8.Davis DH, Muniz Terrera G, Keage H, et al. Delirium is a strong risk factor for dementia in the oldest-old: a population-based cohort study. Brain. 2012;135(pt 9):2809–2816. doi: 10.1093/brain/aws190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pandharipande PP, Girard TD, Ely EW. Long-term cognitive impairment after critical illness. N Engl J Med. 2014;370(2):185–186. doi: 10.1056/NEJMc1313886. [DOI] [PubMed] [Google Scholar]
- 10.Fong TG, Davis D, Growdon ME, Albuquerque A, Inouye SK. The interface between delirium and dementia in elderly adults. Lancet Neurol. 2015;14(8):823–832. doi: 10.1016/S1474-4422(15)00101-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Witlox J, Eurelings LSM, de Jonghe JFM, Kalisvaart KJ, Eikelenboom P, van Gool WA. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA. 2010;304(4):443–451. doi: 10.1001/jama.2010.1013. [DOI] [PubMed] [Google Scholar]
- 12.Gross AL, Jones RN, Habtemariam DA, et al. Delirium and long-term cognitive trajectory among persons with dementia. Arch Intern Med. 2012;172(17):1324–1331. doi: 10.1001/archinternmed.2012.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saczynski JS, Marcantonio ER, Quach L, et al. Cognitive trajectories after postoperative delirium. N Engl J Med. 2012;367(1):30–39. doi: 10.1056/NEJMoa1112923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cunningham C, Wilcockson DC, Campion S, Lunnon K, Perry VH. Central and systemic endotoxin challenges exacerbate the local inflammatory response and increase neuronal death during chronic neurodegeneration. J Neurosci. 2005;25(40):9275–9284. doi: 10.1523/JNEUROSCI.2614-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kitazawa M, Oddo S, Yamasaki TR, Green KN, LaFerla FM. Lipopolysaccharide-induced inflammation exacerbates tau pathology by a cyclin-dependent kinase 5-mediated pathway in a transgenic model of Alzheimer’s disease. J Neurosci. 2005;25(39):8843–8853. doi: 10.1523/JNEUROSCI.2868-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cunningham C, Campion S, Lunnon K, et al. Systemic inflammation induces acute behavioral and cognitive changes and accelerates neurodegenerative disease. Biol Psychiatry. 2009;65(4):304–312. doi: 10.1016/j.biopsych.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cerejeira J, Firmino H, Vaz-Serra A, Mukaetova-Ladinska EB. The neuroinflammatory hypothesis of delirium. Acta Neuropathol. 2010;119(6):737–754. doi: 10.1007/s00401-010-0674-1. [DOI] [PubMed] [Google Scholar]
- 18.Inouye SK, Bogardus ST, Jr, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340(9):669–676. doi: 10.1056/NEJM199903043400901. [DOI] [PubMed] [Google Scholar]
- 19.Brayne C, McCracken C, Matthews FE, Medical Research Council Cognitive Function and Ageing Study (CFAS) Cohort profile: the Medical Research Council Cognitive Function and Ageing Study (CFAS) Int J Epidemiol. 2006;35(5):1140–1145. doi: 10.1093/ije/dyl199. [DOI] [PubMed] [Google Scholar]
- 20.Fleming J, Zhao E, O'Connor DW, Pollitt PA, Brayne C. Cohort profile: the Cambridge City Over-75s Cohort (CC75C) Int J Epidemiol. 2007;36(1):40–46. doi: 10.1093/ije/dyl293. [DOI] [PubMed] [Google Scholar]
- 21.Polvikoski T, Sulkava R, Rastas S, et al. Incidence of dementia in very elderly individuals: a clinical, neuropathological and molecular genetic study. Neuroepidemiology. 2006;26(2):76–82. doi: 10.1159/000090252. [DOI] [PubMed] [Google Scholar]
- 22.EClipSE Collaborative Members. Cohort profile: Epidemiological Clinicopathological Studies in Europe (EClipSE) J Alzheimers Dis. 2009;18(3):659–663. doi: 10.3233/JAD-2009-1181. [DOI] [PubMed] [Google Scholar]
- 23.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 24.Brayne C, Ince PG, Keage HA, et al. EClipSE Collaborative Members. Education, the brain and dementia: neuroprotection or compensation? Brain. 2010;133(pt 8):2210–2216. doi: 10.1093/brain/awq185. [DOI] [PubMed] [Google Scholar]
- 25.Davis DH, Barnes LE, Stephan BC, et al. MRC Cognitive Function and Ageing Study. The descriptive epidemiology of delirium symptoms in a large population-based cohort study: results from the Medical Research Council Cognitive Function and Ageing Study (MRC CFAS) BMC Geriatr. 2014;14:87. doi: 10.1186/1471-2318-14-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rahkonen T, Eloniemi-Sulkava U, Halonen P, et al. Delirium in the non-demented oldest old in the general population: risk factors and prognosis. Int J Geriatr Psychiatry. 2001;16(4):415–421. doi: 10.1002/gps.356. [DOI] [PubMed] [Google Scholar]
- 27.Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD), part II: standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41(4):479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 28.Piccinin AM, Muniz G, Matthews FE, Johansson B. Terminal decline from within- and between-person perspectives, accounting for incident dementia. J Gerontol B Psychol Sci Soc Sci. 2011;66(4):391–401. doi: 10.1093/geronb/gbr010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilson RS, Beck TL, Bienias JL, Bennett DA. Terminal cognitive decline: accelerated loss of cognition in the last years of life. Psychosom Med. 2007;69(2):131–137. doi: 10.1097/PSY.0b013e31803130ae. [DOI] [PubMed] [Google Scholar]
- 30.Wilson RS, Segawa E, Hizel LP, Boyle PA, Bennett DA. Terminal dedifferentiation of cognitive abilities. Neurology. 2012;78(15):1116–1122. doi: 10.1212/WNL.0b013e31824f7ff2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MacDonald SW, Hultsch DF, Dixon RA. Aging and the shape of cognitive change before death: terminal decline or terminal drop? J Gerontol B Psychol Sci Soc Sci. 2011;66(3):292–301. doi: 10.1093/geronb/gbr001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muniz-Terrera G, Matthews FE, Stephan B, Brayne C, CC75C Collaboration Group Are terminal decline and its potential indicators detectable in population studies of the oldest old? Int J Geriatr Psychiatry. 2011;26(6):584–592. doi: 10.1002/gps.2566. [DOI] [PubMed] [Google Scholar]
- 33.Davis DH, Kreisel SH, Muniz Terrera G, et al. The epidemiology of delirium: challenges and opportunities for population studies. Am J Geriatr Psychiatry. 2013;21(12):1173–1189. doi: 10.1016/j.jagp.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cunningham C, MacLullich AM. At the extreme end of the psychoneuroimmunological spectrum: delirium as a maladaptive sickness behaviour response. Brain Behav Immun. 2013;28:1–13. doi: 10.1016/j.bbi.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Richwine AF, Parkin AO, Buchanan JB, et al. Architectural changes to CA1 pyramidal neurons in adult and aged mice after peripheral immune stimulation. Psychoneuroendocrinology. 2008;33(10):1369–1377. doi: 10.1016/j.psyneuen.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 36.Field R, Campion S, Warren C, Murray C, Cunningham C. Systemic challenge with the TLR3 agonist poly I:C induces amplified IFNα/β and IL-1β responses in the diseased brain and exacerbates chronic neurodegeneration. Brain Behav Immun. 2010;24(6):996–1007. doi: 10.1016/j.bbi.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davis DH, Skelly DT, Murray C, et al. Worsening cognitive impairment and neurodegenerative pathology progressively increase risk for delirium. Am J Geriatr Psychiatry. 2015;23(4):403–415. doi: 10.1016/j.jagp.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schoenmaker N, Van Gool WA. The age gap between patients in clinical studies and in the general population: a pitfall for dementia research. Lancet Neurol. 2004;3(10):627–630. doi: 10.1016/S1474-4422(04)00884-1. [DOI] [PubMed] [Google Scholar]
- 39.Treloar AJ, Macdonald AJ. Outcome of delirium, part 1: outcome of delirium diagnosed by DSM-III-R, ICD-10 and CAMDEX and derivation of the Reversible Cognitive Dysfunction Scale among acute geriatric inpatients. Int J Geriatr Psychiatry. 1997;12(6):609–613. doi: 10.1002/(sici)1099-1166(199706)12:6<609::aid-gps553>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 40.Gunther ML, Morandi A, Krauskopf E, et al. VISIONS Investigation, VISualizing Icu SurvivOrs Neuroradiological Sequelae. The association between brain volumes, delirium duration, and cognitive outcomes in intensive care unit survivors: the VISIONS cohort magnetic resonance imaging study. Crit Care Med. 2012;40(7):2022–2032. doi: 10.1097/CCM.0b013e318250acc0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morandi A, Rogers BP, Gunther ML, et al. VISIONS Investigation, VISualizing Icu SurvivOrs Neuroradiological Sequelae. The relationship between delirium duration, white matter integrity, and cognitive impairment in intensive care unit survivors as determined by diffusion tensor imaging: the VISIONS prospective cohort magnetic resonance imaging study*. Crit Care Med. 2012;40(7):2182–2189. doi: 10.1097/CCM.0b013e318250acdc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Janz DR, Abel TW, Jackson JC, Gunther ML, Heckers S, Ely EW. Brain autopsy findings in intensive care unit patients previously suffering from delirium: a pilot study. J Crit Care. 2010;25(3):538.e7–538.e12. doi: 10.1016/j.jcrc.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Inouye SK. Delirium in older persons. N Engl J Med. 2006;354(11):1157–1165. doi: 10.1056/NEJMra052321. [DOI] [PubMed] [Google Scholar]
- 44.MacLullich AMJ, Hall RJ. Who understands delirium? Age Ageing. 2011;40(4):412–414. doi: 10.1093/ageing/afr062. [DOI] [PubMed] [Google Scholar]
- 45.National Institute for Health and Care Excellence. Delirium: diagnosis, prevention and management. [Accessed December 13, 2016]; https://www.nice.org.uk/guidance/cg103. Published July 2010.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.