Abstract
Fibroblasts play a central role in tumor invasion, recurrence, and metastasis in head and neck squamous cell carcinoma. The aim of this study was to investigate the influence of tumor cell self-produced factors and paracrine fibroblast–secreted factors in comparison to indirect co-culture on cancer cell survival, growth, migration, and epithelial–mesenchymal transition using the cell lines SCC-25 and human gingival fibroblasts. Thereby, we particularly focused on the participation of the fibroblast-secreted transforming growth factor beta-1.Tumor cell self-produced factors were sufficient to ensure tumor cell survival and basic cell growth, but fibroblast-secreted paracrine factors significantly increased cell proliferation, migration, and epithelial–mesenchymal transition–related phenotype changes in tumor cells. Transforming growth factor beta-1 generated individually migrating disseminating tumor cell groups or single cells separated from the tumor cell nest, which were characterized by reduced E-cadherin expression. At the same time, transforming growth factor beta-1 inhibited tumor cell proliferation under serum-starved conditions. Neutralizing transforming growth factor beta antibody reduced the cell migration support of fibroblast-conditioned medium. Transforming growth factor beta-1 as a single factor was sufficient for generation of disseminating tumor cells from epithelial tumor cell nests, while other fibroblast paracrine factors supported tumor nest outgrowth. Different fibroblast-released factors might support tumor cell proliferation and invasion, as two separate effects.
Keywords: Desmoplakin, N-cadherin, scratch assay, survival, serum-starved
Introduction
Recent findings suggest that the tumor microenvironment (TME) surrounding the tumor cells plays a remarkable role in tumor initiation, growth, and metastasis.1,2 Several lines of evidence demonstrated that the stroma surrounding the tumors plays an important role in the growth and progression of head and neck squamous cell carcinoma (HNSCC).3 Moreover, the strongest independent risk factor of early cancer death is a feature of stroma rather than tumor cells.4
There is a continuous interplay between internal oncogenic stimuli of tumor cells and numerous signals from the microenvironment.5 Moreover, different levels of dependence of tumor cells on the microenvironment would be expected. Mounting evidence suggests that in solid cancers, activated fibroblasts acquire the capacity to provide fertile soil for tumor progression.6 In this soil, fibroblasts play a significant role in the metastatic development of HNSCC.7,8 This specific role of fibroblasts is highlighted by experimental evidence showing that metastatic HNSCC cells started to form tumor nodules when fibroblasts were co-injected in vivo.9 Moreover, in the presence of fibroblasts, additional growth factors are not needed to induce tumor cell invasion.10,11 Fibroblastic stroma might support both the release of mesenchymal transdifferentiated circulating tumor cells from the primary tumor site as well as the attachment, re-epithelialization, and outgrowth of tumor cells at the secondary site.12 These even contradicting effects can be imagined as fibroblasts might be re-programmed by tumor cells and other cellular components through cytokine or chemokine signals or as fibroblasts might release more different signals in distinct conditions. A subset of re-programming signals create cancer-associated fibroblasts (CAFs) from normal fibroblast.13 Nevertheless, due to these distinct signals, CAFs demonstrate a remarkable heterogeneity with activation and senescence being their common responses.14 Previous works from our group15–18 and from others revealed that tumor cells induce transdifferentiation of primary normal fibroblasts to myofibroblasts, whereas, in turn, myofibroblast-secreted factors stimulate tumor cell proliferation.3
Transforming growth factor beta-1 (TGF-β1) was reported as major factor responsible for the transition of normal fibroblasts into CAFs.3,19 Several authors argue that CAFs differ from normal fibroblasts (NFs) in their phenotype, faster proliferation,20 increased collagen production,19 and secretion of a distinct set of molecules.20 Furthermore, CAFs share characteristics with myofibroblasts,11 which differentiate from fibroblasts on response to TGF-β1.21 It is commonly accepted that CAFs contribute to tumor cell motility, invasion, angiogenesis, extracellular matrix remodeling, and the initiation of epithelial–mesenchymal transition (EMT) by the secretion of diverse factors and cytokines critical to tumorigenesis.11,15,22 While many studies observed that fibroblasts could promote HNSCC progression via paracrine and/or autocrine signaling,23–25 another co-culture experiment showed that for the secretion of matrix remodeling metalloproteinase enzymes, a direct contact between tumor cells and fibroblasts was required.26
It is not only experimentally, but also clinically evidenced that CAFs contribute to poor outcome of squamous cell carcinoma.27–29 As previously mentioned, and repeatedly revealed in several studies, CAFs are recognized by the myofibroblast marker alpha smooth muscle actin (SMA).30 Abundant presence of myofibroblasts significantly correlated with N stage, disease stage, regional recurrence, and proliferative potential of the tumor cells.3 In addition, myofibroblasts are functionally recognized by the production of collagen fibers. Interestingly, myofibroblast appearance increases with increasing tumor invasiveness, more importantly, invasive tumors contain fibrous stroma.31 In conclusion, SMA-positive, myofibroblastic stroma is the strongest predictor of tumor mortality.4
In contrast, not only supportive, but also tumor-suppressive effects of normal fibroblasts and CAFs have been published,32 which grounds the urgent need to elucidate whether the tumor-promoting or suppressive effects of fibroblasts arise from communication with tumor cells by paracrine signaling or by direct cell–cell contact, and in particular, which signaling molecules and pathways are involved in this interplay. Several (myo)fibroblast populations develop in interaction to tumor cells, and not all of them are supporting the tumor growth.32
Previously, we applied an indirect co-culture system using semipermeable inserts between fibroblasts and HNSCC tumor cells. Using this culture system, we demonstrated induction of EMT-like gene expression changes,15 increase of cell growth,17 and induction of matrix metalloproteinases (MMPs) as MMP-918 and cell invasion in HNSCC tumor cells. This experimental system contains too many unknown parameters because of the continuous interaction between these two cell types, and even contradictive effects can be imagined. To simplify this experimental approach, we treated HNSCC cells with conditioned medium (CM) collected from normal fibroblasts or cancer cells or from cancer cells directly co-cultured with fibroblasts together called mix culture. The culture and treatment systems were adapted to serum-free conditions, replacing all serum protein components with bovine serum albumin (BSA). In this system for growth and survival factors, the cells relied on their own production or on the supplementation by co-culture or CM treatments. We used three important readout parameters, namely, tumor cell growth, migration, and EMT-like phenotype changes in cancer cells and compared the outcomes between the different experimental approaches. As mentioned before, TGF-β1 is a key factor both in induction of EMT in tumor cells33–36 and in induction of CAFs from normal fibroblasts,3,21 which qualified this cytokine for detailed investigation in the above-mentioned readout conditions. We asked the question what happens if we remove TGF-β1 from the CAF–tumor cell interaction?
Materials and methods
Cell lines
Human gingival fibroblasts (FIBs) were purchased from the German Cell Line Service (CLS, Eppelheim, Germany) and routinely cultured in Dulbecco’s modified Eagle’s medium (DMEM; with 1 g/L glucose; Biowhittaker®, Verviers, Belgium) supplemented with 10% fetal bovine serum (FBS; Gibco®, Auckland, New Zealand), 2 mM l-glutamine (Gibco®, Paisley, UK), 100 units/mL penicillin, and 100 μg/mL streptomycin (PAA Laboratories, Pasching, Austria). SCC-25 cells were acquired from the German Collection of Microorganisms and Cell Cultures (DSMZ, Braunschweig, Germany, DSMZ no.: ACC 617). As described above, FIB cells were routinely cultured in serum-containing DMEM medium with low glucose and SCC-25 cells in serum-containing DMEM/F12 medium with high glucose, whereas for experimental purposes both the FIB cells and SCC-25 cells were cultured in an albumin-containing medium (1:1 ratio of DMEM-low glucose and DMEM-high glucose/Ham’s F12 supplemented with 4.4 g/L BSA from PAA Laboratories, Pasching, Austria, 2 mM l-glutamine, 100 units/mL penicillin, and 100 μg/mL streptomycin) to replace the whole protein content of the full culture medium.
Research using human material including the used cell lines was approved by the Ethic Commission of the Medical University of Innsbruck (ref. number: UN3678).
Preparation of CM
For the production of FIB CM, 6 × 104 FIB cells/mL were plated in 75 cm2 tissue culture flasks (Orange Scientific, Braine-l’Alleud, Belgium) and cultured for 72 h in 15 mL serum-containing DMEM. Then, the cells were washed twice with Dulbecco’s phosphate-buffered saline (DPBS; Biowhittaker®) and the serum-containing medium was replaced by 15 mL albumin-supplemented medium.37 The albumin-containing medium was left 48 h on the FIB cells leading to FIB CM. Afterwards, the FIB CM was collected, portioned, and diluted: 1 mL CM represented 0.75 × 105 cells.37,38 In case of production of SCC-25-CM: 5 × 104 cells/mL; mixed-culture CM: 4 × 104 SCC-25 cells/mL and 1 × 104 FIB cells/mL were plated in 75 cm2 tissue culture flasks and cultured for 72 h in 15 mL serum-containing DMEM/F12 or in 1:1 ratio of DMEM and DMEM/F12. Further steps were performed as described above.37
Cell treatment with CM
For the treatment with conditioned media: 1 × 105 SCC-25 cells/mL were plated in six-well plates (Falcon™, Durham, NC, USA) and cultured for 24 h in 2 mL/well serum-containing DMEM/F12. Then, the cells were washed twice with DPBS and treated for 72 h with 2 mL/well albumin-supplied or CM by exchanges every 24 h. After completion of treatments, the supernatants were taken from the SCC-25 cells and frozen at −20°C to be used for the human TGF-β1 PicoKine™ enzyme-linked immunosorbent assay (ELISA Kit; Boster Biological Technology, Pleasanton, CA, USA) later on. The SCC-25 cells themselves were used for cell counting in a Neubauer chamber (Paul Marienfeld GmbH & Co. KG, Lauda-Königshofen, Germany) for RNA or protein isolation.
Co-culture of SCC-25 and FIB cells
In the case of co-culture, 1 × 105 SCC-25 cells/mL were plated in six-well plates and cultured for 24 h in 2 mL serum-containing DMEM/F12/well. 2.5 × 104 FIB cells were plated in inserts (0.4 μm pore size; Greiner Bio-One®, Corning, NY, USA) and cultured for 24 h in 2 mL serum-containing DMEM medium. Then, both SCC-25 cells and FIB cells were washed twice with DPBS and put together leading to a co-culture.15 Subsequently, the co-culture was kept for 72 h in 4 mL albumin-containing medium by pipetting 2 mL on SCC-25 and 2 mL on FIB cells.15 The albumin-containing medium was exchanged every 24 h. In the end, the supernatants were stored at −20°C for the human TGF-β1 ELISA and SCC-25 cells were used for cell counting, RNA, and protein isolation.
Anti-TGF-β treatment of SCC-25 cells
A volume of 1 × 105 SCC-25 cells/mL were plated in six-well plates and cultured in 2 mL/well serum-containing DMEM medium. After 24 h, the cells were washed twice with DPBS and treated with 2 mL albumin-containing or CM supplemented with neutralizing 1.5 μg/mL TGF-β 1, -2, and -3 antibody (R&D Systems™, Minneapolis, MN, USA; Catalog no. MAB1835). After treatment, the cells were used as described above.
Cell migration
For the scratch assay, SCC-25 cells were plated in six-well plates at 1.5 × 105 cells/mL with 2 mL serum-containing DMEM medium over a period of 4 days and allowed to grow to confluence. In case of co-culture, 3.75 × 104 FIB cells/mL were plated in inserts and cultured in 2 mL serum-containing DMEM medium for 4 days as well. The cell monolayer was scraped using a sterile 200 μL pipet tip. After removing the debris, the SCC-25 cells were incubated with 10 μg/mL Mitomycin C (Sigma-Aldrich®, St. Louis, MO, USA) in albumin-containing medium for 30 min at 37°C ensuring cell-cycle arrest.39 Then, the SCC-25 cells were washed twice with albumin-containing medium and subsequently treated in the same way as described before. The treatment lasted 96 h and the media were replaced every 24 h. Eight different parallels per well were observed and photographed at 0, 24, 48, 72, and 96 h with a phase-contrast microscope (Primo Vert Microscope; Zeiss, Jena, Germany) connected to a camera (AxioCam ERc 5s, Zeiss). The area covered by migrating cells was calculated using the software Fiji-win32 (NIH, Bethesda, MA, USA).
Alternatively, cells were plated and cultured as described above, treated with Mitomycin C, scraped, and treated with albumin-supplemented medium containing TGF-β1 or FIB CM for 48 h. The migratory behavior of the cells was filmed by a Juli BR live cell imaging system (Peqlab, Erlangen, Germany). Movements of individual cells were measured in micrometer in Axiovision program of Zeiss (Jena, Germany).
RNA isolation
For RNA isolation, cells were collected, lysed in TRIzol® Reagent (Ambion®, Life technologies™, Carlsbad, CA, USA), and RNA was isolated as instructed by the manufacturer of TRIzol. RNA concentrations were determined by photometric measurements (BioPhotometer plus 6132, Eppendorf, Germany).
Reverse transcription and real-time quantitative polymerase chain reaction
Total RNA was reverse transcribed by M-MuLV Reverse Transcriptase (GeneON, Ludwigshafen am Rhein, Germany) in a MyiQ™ cycler (Bio-Rad Laboratories, Inc., Hercules, CA, USA) following the manufacturer’s instructions. Real-time quantitative polymerase chain reaction (qPCR) of complementary DNA (cDNA) transcripts was performed in a MyiQ™ cycler (Bio-Rad Laboratories, Inc.) using iTaq™ Universal SYBR® Green Supermix (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The primer sequences are summarized in Supplementary Table 1. β-actin, N-cadherin, and Desmoplakin primers were synthesized by Invitrogen™ (Darmstadt, Germany), whereas E-cadherin and Vimentin primers were purchased from Eurofins MWG Operon, Inc. (Ebersberg, Germany). β-actin was applied as a housekeeping gene.40 The data analysis was performed according to the instructions of Haller et al.40
Protein extraction and western blot analysis
After completion of treatments, cells of one plate were scraped into 400 μL radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris-HCl pH 8.0, 1 mM ethylenediaminetetraacetic acid (EDTA), 0.5 mM ethylene glycol tetraacetic acid (EGTA), 1% Triton X-100, 0.25% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS), 150 mM NaCl, 10 mM NaF, and 1 mM phenylmethylsulfonyl fluoride (PMSF)). Then, they were incubated on ice for 3 × 15 min and were vortexed after each incubation period. Subsequently, extracts were homogenized and cleared by centrifugation: 15.000g, 15 min, and 4°C. The protein concentration was determined by Pierce 660 Protein Assay (Rockford, IL, USA). Western blot was done as published before,15–18 following the suggestions of antibody providers. Antibody sources and dilutions are listed in Supplementary Table 2. Detection was done by peroxidase-conjugated secondary antibodies and ECL select (Amersham, Fisher Scientific, Vienna, Austria) in an Azure C500 (Biomedica, Vienna, Austria). The band intensities were measured in ImageJ (NIH, Bethesda, MA, USA) software using mean intensities/bands for β-actin as loading control for vimentin or E-cadherin as proteins of interest. The images were background subtracted and the mean intensity values of vimentin were normalized to loading control and normalized to intensities in reference samples (cultured in albumin medium, which were set as “1”). A logarithmic scale (2-log) graph was used for showing upregulation or downregulation; 10 repeats were used for western blot quantification purposes. The limitation of the quantification of chemiluminescent detection with reduced linearity between the signal and the detected protein quantity has been taken into consideration.41 The quantification was done for comparison of treatment conditions and in order to present data of 10 repeats.
Statistical analysis
All experiments were repeated at least three times. For statistical analysis, the means and the standard errors were calculated using GraphPad Prism 4.03 (GraphPad Software Inc., San Diego, CA, USA). Normal distributions were tested by the D’Agostino and Pearson omnibus normality test. If the distribution of the samples was normal, Student’s t-test was performed. In the case of a non-normal distribution, we applied the Mann–Whitney U test for comparison of treatments to controls. If the samples were normally distributed but the variances were significantly different, the Student’s t-test with Welch’s correction was used. All analysis has been done at a confidence interval of 95%. Significant differences have been claimed if the p values were under 0.05 (labeled with *). If the p value was lower than 0.01, it was labeled with **, and a p value lower than 0.001 was marked by ***.
Results
Fibroblast CM increases proliferation and lateral migration of HNSCC cells
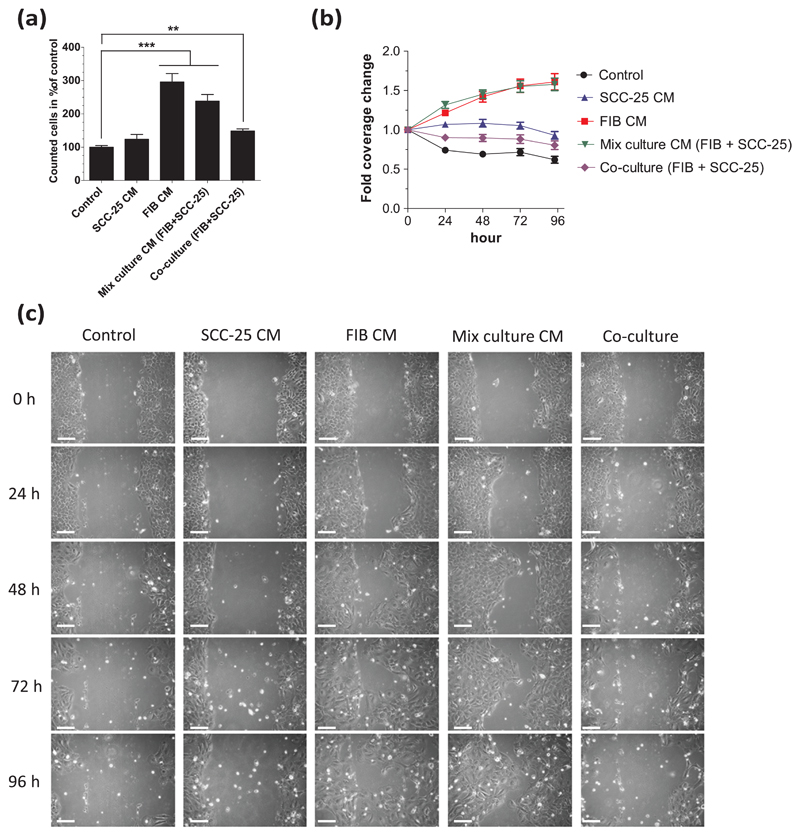
The influence of fibroblasts on the proliferation was investigated by cell counting after 72 h treatment period with conditioned media or after 72-h indirect co-culture. The cells were kept in serum-free conditions for this time period, by supplementing all serum proteins by albumin. The cell numbers of cells kept in albumin-containing medium were set at 100%. The application of CM from SCC-25 cells did not induce significant changes of cell numbers compared with the cells treated with albumin-containing medium, whereas indirect co-culture and mixed-culture CM treatment induced a significant increase, as well as FIB CM leads to a three times increase in SCC-25 cell numbers (p < 0.001, Figure 1(a)).
Figure 1.
Proliferation, viability, and migration of conditioned-medium-treated or co-cultured SCC-25 cells. After 3 days of treatment, SCC-25 cells were counted and cell migration was investigated using a scratch assay. (a) The treatment with FIB and mixed-culture CM (p < 0.001) and co-culture (p < 0.01) leads to significantly higher cell numbers compared to control (albumin-medium-treated cells, which was set to 100%). (b) FIB CM and mixed-culture CM significantly (p < 0.001) increased the lateral migration of SCC-25 cells even in the first 24 h. SCC-25 cells treated with SCC-25 CM or co-cultured with FIBs exhibited a significant higher cell coverage than albumin-medium-treated (control) cells (SCC-25 CM: p < 0.001, co-culture: p < 0.01). (c) These effects could also be observed on the images taken every 24 h, where SCC-25 cells migrated toward empty space when treated with FIB CM or mixed-culture CM (CM: conditioned medium; FIB: human gingival fibroblasts; **p < 0.01; ***p < 0.001). Cells treated with FIB CM or mixed-culture CM or co-cultured SCC-25 cells showed elongated, mesenchymal-like morphology, especially in the scratched area (bars: 100 μm).
For investigation of cell migration, at first, the cell cultures reached confluence and then were scraped, treated with Mitomycin C to ensure cell migration without cell division, and treated with albumin-containing or conditioned media or were indirectly co-cultured for up to 4 days. This method investigates two effects. First, the cells must survive after Mitomycin C induced cell-cycle arrest just by production of their own factors (albumin-medium treatments) or they rely on the co-culture or CM treatment. The second is the cell migration, by which the cells repopulate the scratched area, while cell division is impaired by Mitomycin C. These two effects were recorded by live video imaging and by quantification of the covered scratched area. The use of albumin-containing medium (“control”) after Mitomycin C treatment did not offer sufficient survival of the SCC-25 cells and induced withdrawal of cells from the scratch line and shrinkage of the cell culture (Supplementary Figure 1). These changes are also clearly evidenced in Figure 1(b)–(c). In contrast, the cell culture shrinkage was compensated by the addition of conditioned media or co-culture with fibroblasts (Figure 1(b)–(c)).
To quantify the migration of SCC-25 cells via scratch assay, the covered scratched area by cells in each image was calculated in percent. To guarantee the same start point in every single treatment, the calculated covered area at 0 h was set at 1 and the covered areas at other time points were related to this start point, stated as “fold coverage change” (Figure 1(b)). Closing the gap of SCC-25 cells got most enhanced (p < 0.001) when treated with FIB CM (Figure 1(b)) or mixed-culture CM in comparison to the albumin-containing medium treatment (control) in which the covered area of tumor cells drastically decreased over time. Both the FIB CM and mixed-culture CM lead to an up to 1.6-fold gap coverage increase within 96 h (Figure 1(b)) by extensive lateral migration of SCC-25 cells toward the free-scraped area (Figure 1(c)). The area covered by SCC-25 cells slightly decreased when co-cultured with FIB cells but was significantly higher (p < 0.01) than in the albumin-containing medium treatment (control) at 24 and 48 h. When treating SCC-25 cells with SCC-25 CM, the cell coverage did not change strikingly; it was decreasing slightly within 96 h, but exhibited significant differences from the only albumin-containing medium treatment (control; p < 0.001) at each sampled time point.
TGF-β1 supports migration of HNSCC cells
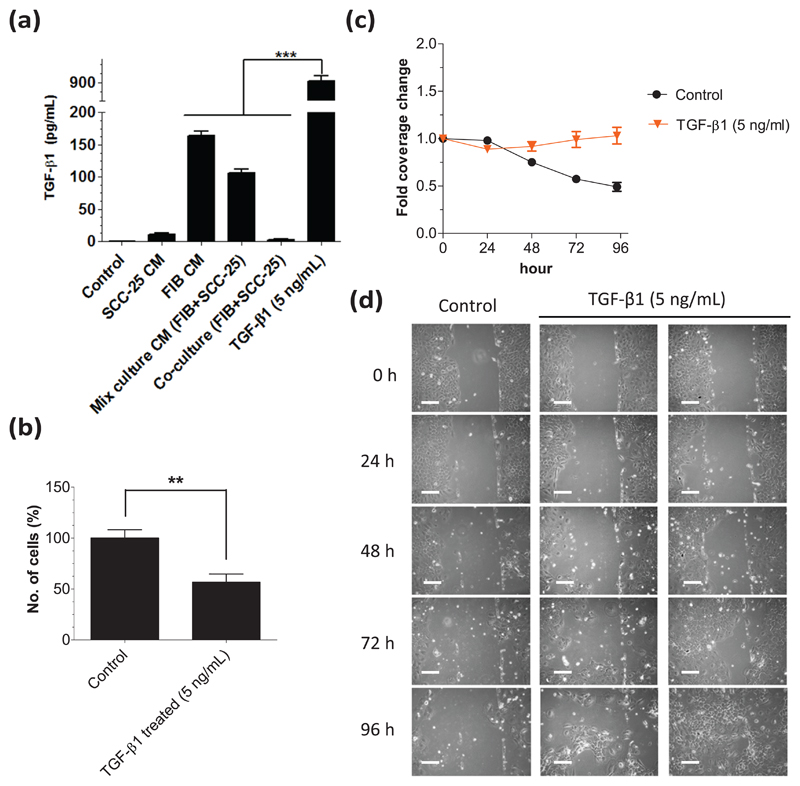
Since the growth factor TGF-β1 is considered as major determinant in HNSCC progression, we were interested in the levels of TGF-β1 in the cell culture supernatants. After 3 days of treatment with CM or after 3 days of indirect coculture, the supernatants were taken from the SCC-25 cells and used to measure the amount of TGF-β1 with ELISA (Figure 2(a)). Albumin-containing medium-treated (control) SCC-25 cells contained almost no TGF-β1 in the supernatant (0.3 ± 0.12 pg/mL). As expected, the highest amount of TGF-β1 (917.1 ± 38.12 pg/mL, p < 0.001) was measured in the supernatant of the SCC-25 cells that were treated with 5 ng/mL TGF-β1 serving as a positive control. The second highest concentration of TGF-β1 was detected in the supernatant of FIB CM-treated SCC-25 cells (164.1 ± 6.85 pg/mL). When treating the tumor cells with mixedculture CM, the TGF-β1 concentration was 106.0 ± 5.82 pg/mL, which was significantly less than in FIB CM (p < 0.001). Interestingly, the TGF-β1 concentrations in supernatants of FIB CM and mixed-culture CM treated SCC-25 cells were significantly higher than in the co-culture system (p < 0.001) whose supernatant only contained 2.3 ± 0.94 pg/mL TGF-β1. SCC-25 cells treated with SCC-25 CM exhibited 10.4 ± 2.53 pg/mL TGF-β1 in the supernatant, indicating that SCC-25 themselves produced low levels of TGF-β1, but it was 16 times less than the production in fibroblasts, which was delivered to the SCC-25 cells by treatments with FIB CM.
Figure 2.
FIB and SCC-25 cells produce TGF-β1 and the effects of TGF-β1 on SCC-25 cell proliferation and migration. (a) The TGF-β1 concentrations in the supernatants were measured using ELISA after 3 days of treatment. SCC-25 cells treated with 5 ng/mL TGF-β1 contained 917.1 ± 38.12 pg/mL in the supernatant. In the supernatant of the albumin-medium-treated (control) SCC-25 cells, there was almost no detectable TGF-β1 (0.3 ± 0.12 pg/mL). Significant levels of TGF-β1 were measured in the supernatants of FIB CM (164.1 ± 6.85 pg/mL) and mixed-culture CM (106.0 ± 5.82 pg/mL)-treated SCC-25 cells, whereas the TGF-β1 levels in co-culture (2.3 ± 0.94 pg/mL) were low. In the supernatant of SCC-25-CM-treated cells 10.4 ± 2.53 pg/mL TGF-β1 was measured. (b) After treatment with 5 ng/mL TGF-β1, there were significantly (p < 0.01) less SCC-25 cells growing than in the albumin-medium treated (control). (c) 0–96 h treatment with 5 ng/mL TGF-β1 leads to no change in coverage of the scraped area, while in the albumin-medium treated (control; p < 0.001), it was decreased. (d) 48–96 h treatment with 5 ng/mL TGF-β1 cells were also distributed over the scraped area (CM: conditioned medium; FIB: human gingival fibroblasts; **p < 0.01; ***p < 0.001). Cells treated with 5 ng/mL TGF-β1 showed elongated, mesenchymal-like morphology at 72–96 h (bars: 100 μm).
On the basis of the ELISA results, we were wondering whether TGF-β1 is a key effector in the enhanced proliferation and migration elicited by FIB CM and mixed-culture CM and whether the sole addition of TGF-β1 was sufficient to induce these effects. To verify this assumption, we treated SCC-25 cells with 5 ng/mL recombinant human TGF-β1 (suggested IC50 of the commercial TGF-β1) for 3 days. In contrast to the CM, SCC-25 cells treated with 5 ng/mL TGF-β1 exhibited significantly less (p < 0.01) cell numbers in comparison to the albumin-containing medium–treated control (Figure 2(b)). TGF-β1 in the concentration range of 50–250 pg/mL had no effect on SCC-25 cell proliferation, and 500-1000 pg/mL showed a significant 30% growth reduction (p = 0.034) compared to albumin medium (not shown). Based on the ELISA concentration determination, the 500–1000 pg/mL range of TGF-β1 is comparable with the production level of fibroblasts and mixed culture.
In the scratch assay, the addition of 5 ng/mL TGF-β1 maintained the coverage of the scraped area, and it slightly increased over time (Figure 2(c)). The images taken from TGF-β1-treated SCC-25 cells and monitoring the cells during the scratch assay (Supplementary Figures 1 and 2) revealed that the migratory behavior of SCC-25 differed between 5 ng/mL TGF-β1-treated cells and cells treated with FIB or mixed-culture CM. While FIB and mixed-culture CM provoked the cell layer to laterally move as a whole front (Figure 1), the addition of TGF-β1 triggered the movement of individual cell groups in an unpredictable way leading to extremely variable distribution patterns of SCC-25 cells at 96 h (Figure 2(d)) ranging from no change in gap coverage to an almost gap closure. This also means that TGF-β1 treatment induced not only the migration of SCC-25 cells but also the separation of individual cells from the cell layer front (Supplementary Figure 1). Those separated individual cells or cell groups were detected even within 24 h after treatment with as low as 250 pg/mL TGF-β1 (not shown).
Fibroblasts and TGF-β1 have different effects on EMT-like gene expression in HNSCC cells
Since epithelial-to-mesenchymal transition (EMT) has been linked to HNSCC invasion and metastasis, we investigated the messenger RNA (mRNA) expression of two mesenchymal (vimentin and N-cadherin) and two epithelial (E-cadherin and desmoplakin) marker genes. SCC-25 cells were treated with albumin-containing medium (control), SCC-25 CM, FIB CM, mix culture CM, and 5 ng/mL TGF-β1 or co-cultured with FIB cells for 3 days after which the RNA was isolated from the cells, reverse transcribed, and used for real-time PCR analysis. The treatment with 5 ng/mL TGF-β1 lead to the strongest upregulation of vimentin and N-cadherin (p < 0.001) and significant downregulation of E-cadherin (p < 0.05) and desmoplakin (p < 0.001) mRNA in SCC-25 (Table 1). The FIB CM (human gingival fibroblast (HGF) fibroblast CM) and mixed-culture CM caused significantly higher vimentin expression (p < 0.001) and lower E-cadherin (p < 0.05) and desmoplakin mRNA (p < 0.001) expression than the albumin-containing medium (control). However, the mesenchymal marker N-cadherin was significantly downregulated (p < 0.001). In the co-culture, the expression of both the mesenchymal markers vimentin (p < 0.05) and N-cadherin (p < 0.001) and the epithelial markers E-cadherin and desmoplakin (p < 0.001) were significantly decreased. When treating SCC-25 with SCC-25 CM, vimentin (p < 0.01) and N-cadherin (p < 0.001) were significantly downregulated and E-cadherin and desmoplakin remained stable.
Table 1.
Fold change EMT-related gene expressions in SCC-25 cells related to the albumin-medium-treated control.
| SCC-25 CM |
HGF CM |
Mixed-culture CM |
Co-culture |
TGF-β1 (5 ng/mL) |
|
|---|---|---|---|---|---|
| N = 18 | N = 22 | N = 20 | N = 22 | N = 20 | |
| Vimentin | |||||
| Mean ± SD | 0.65 ± 0.12 | 2.63 ± 0.84 | 2.08 ± 0.82 | 0.85 ± 0.66 | 8.79 ± 3.19 |
| p | 0.0012** | <0.0001*** | <0.0001*** | 0.0487* | <0.0001*** |
| N-cadherin | |||||
| Mean ± SD | 0.62 ± 0.18 | 0.54 ± 0.24 | 0.37 ± 0.17 | 0.51 ± 0.36 | 1.80 ± 0.85 |
| p | <0.0001*** | <0.0001*** | <0.0001*** | <0.0001*** | <0.0004*** |
| E-cadherin | |||||
| Mean ± SD | 1.19 ± 0.63 | 0.68 ± 0.47 | 0.71 ± 0.39 | 0.36 ± 0.26 | 0.69 ± 0.41 |
| p | 0.5107 | 0.0174* | 0.0161* | <0.0001*** | 0.0119* |
| Desmoplakin | |||||
| Mean ± SD | 1.05 ± 0.42 | 0.19 ± 0.07 | 0.27 ± 0.11 | 0.43 ± 0.30 | 0.38 ± 0.17 |
| p | 0.6296 | <0.0001*** | <0.0001*** | <0.0001*** | <0.0001*** |
CM: conditioned medium; HGF: human gingival fibroblast; SD: standard deviation; N: number of values; PCR: polymerase chain reaction.
SCC-25 cells were treated for 3 days with albumin-containing medium (control), SCC-25 CM, FIB CM (= HGF CM), mixed-culture CM, and 5 ng/mL TGF-β1 or co-cultured with FIB cells. The gene expressions of the mesenchymal markers vimentin and N-cadherin and the epithelial markers E-cadherin and desmoplakin were determined using real-time PCR. The expression of the genes of interest in the control normalized to the house-keeping gene β-actin was set to 1 and the expressions of the genes of interest were related to this reference (downregulation <1, upregulation >1).
The mean ± SD, p value, and N are depicted.
*p < 0.05; **p < 0.01; ***p < 0.001.
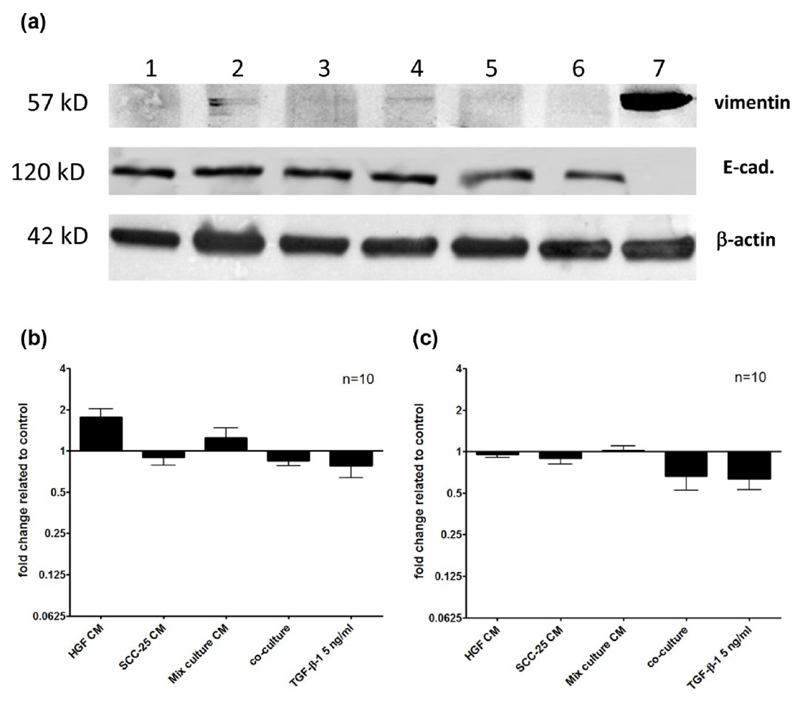
We further tried to confirm the mRNA expression changes of vimentin and E-cadherin on the protein level via western blot analysis (Figure 3(a)). The expression of the housekeeping protein β-actin stayed constant across the different treatments and cells. Fibroblasts served as control cells and showed a strong vimentin band. Vimentin expression could be slightly detected in SCC-25 cells that were treated with FIB CM and mixed-culture CM. E-cadherin showed an intensive detectable band in all SCC-25 cell–related samples, while it was absent in cultured fibroblasts. TGF-β1 treatment and co-culture showed slight reduction of the E-cadherin band in SCC-25 cells, which was revealed by densitometry. The intensity of the bands was determined via densitometric analysis (Figure 3(b)–(c)) using ImageJ as described in “Materials and methods” section. The protein synthesis levels were normalized to loading control and the different treatments were related to control cells (treated with albumin-containing medium only) whose protein expression was set to “1.” Densitometry revealed two times upregulation of vimentin by treatment with FIB CM (Figure 3(b)) and 40%–50% decrease of E-cadherin in co-culture and after TGF-β1 treatment (Figure 3(c)).
Figure 3.
Fold change of vimentin and E-cadherin protein related to the control. The protein synthesis of vimentin, E-cadherin and β-actin in SCC-25 cells was determined using western blot analysis. β-actin was applied as a loading control. Fibroblasts (a, lane 7) served as positive control cells for vimentin, since this protein was contained in undetectable levels in parental SCC-25 cells. (a) Typical western blot of 57 kDa vimentin, 120 kDa E-cadherin, and 42 kDa β-actin in samples: (1) control SCC-25 treated with albumin medium; (2) SCC-25 treated with FIB CM; (3) SCC-25 treated with SCC-25 CM; (4) SCC-25 treated with mixed-culture CM; (5) SCC-25 co-cultured with fibroblasts; (6) SCC-25 treated with TGF-β1 (5 ng/mL); and (7) cultured fibroblasts, positive control for vimentin and negative control for E-cadherin (CM: conditioned medium). In FIB and mixed-culture CM-treated SCC-25 cells, a faint vimentin band appeared. (b–c) The band intensities were analyzed with densitometry. The different treatments were related to control cells treated with albumin-containing medium only. Densitometry graphs covered 10 comparable western blot experiments. (b) Two times upregulation of vimentin was shown by treatment with FIB CM. (c) E-cadherin showed decrease in co-culture and after TGF-β1 treatment.
TGF-β1 neutralization reduces migration support and influences EMT-inducing effects in fibroblast secretome
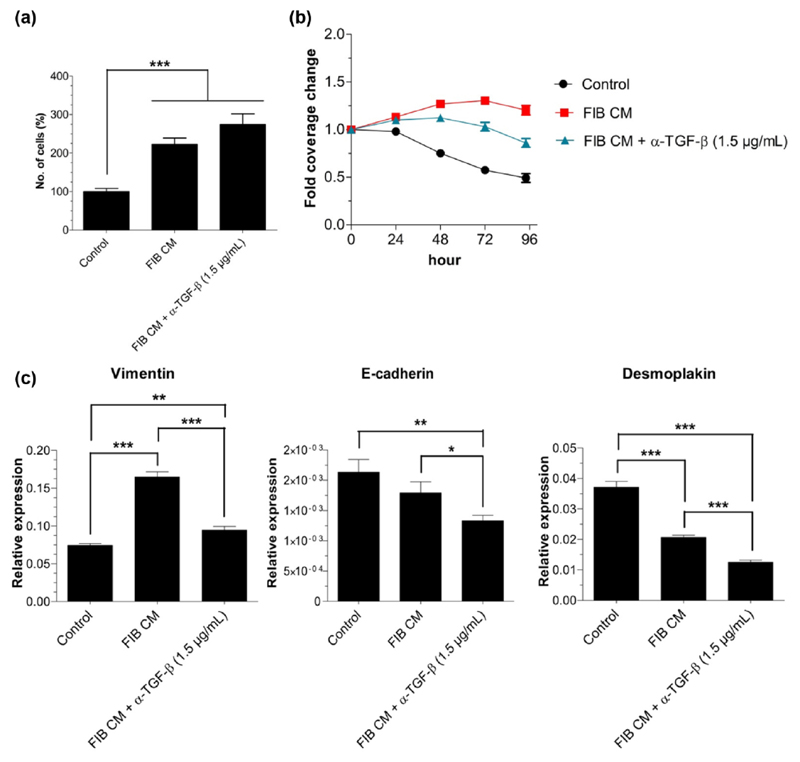
To further elucidate the importance of TGF-β1 in proliferation, migration, and EMT-related gene expression changes in SCC-25 cells, we added a neutralizing TGF-β antibody (α-TGF-β) to the FIB CM. Regarding cell numbers, α-TGF-β did not significantly change SCC-25 proliferation, that is, the FIB CM also leads to a drastic increase (p < 0.001) in cell numbers in the presence of the TGF-β antibody (Figure 4(a)) compared with albumin-medium-treated cells. When adding α-TGF-β to the albumin-containing medium treatment of the cells, the proliferation slightly decreased at 72 and 96 h compared with albumin-containing medium treatment (data not shown). This effect was not significant and was considered as not significant self-effect of α-TGF-β. Concerning migration, the addition of α-TGF-β to the FIB CM significantly antagonized (p < 0.01) the stimulatory effect of the FIB CM, but the gap area covered by the SCC-25 cells was still significantly higher (p < 0.001) than in the albumin-medium-treated cells (control; Figure 4(b)). To test whether α-TGF-β has an intrinsic effect on the SCC-25 cells, the antibody was added to the albumin-containing medium of the control cells and significantly decreased (p < 0.05) the coverage of SCC-25 at 72 h and 96 h (data not shown). The gene expression of vimentin was not as much upregulated in the presence of α-TGF-β as when treated with FIB CM (p < 0.001), but in comparison to the albumin-medium-treated cells (control), the vimentin expression was still significantly higher (p < 0.05, Figure 4(c)). Both the epithelial marker E-cadherin (p < 0.05) and desmoplakin (p < 001) were dramatically further downregulated when TGF-β was neutralized in the FIB CM leading to a significant lower E-cadherin (p < 0.01) and desmoplakin (p < 0.001) expression compared to SCC-25 control cells. The N-cadherin expression was not influenced by the TGF-β antibody (data not shown).
Figure 4.
The effects of anti-TGF-β neutralizing antibody on SCC-25 proliferation, migration, and EMT-related gene expression. Cell numbers were counted in a Neubauer chamber, migration was investigated via scratch assay, and EMT was examined using real-time PCR. (a) The neutralization of TGF-β in the FIB CM did not cause a significant change in proliferation of SCC-25 (number of counted cells represented as % of albumin-medium-treated (control) cell number). (b) The migratory behavior stimulated by FIB CM significantly decreased (p < 0.01) in the presence of 1.5 μg/mL α-TGF-β. (c) When adding the TGF-β antibody to the FIB CM, the gene expression of both vimentin and the epithelial markers E-cadherin and desmoplakin significantly decreased (p < 0.05–0.001) compared to FIB CM (CM: conditioned medium; FIB: human gingival fibroblasts; *p < 0.05, **p < 0.01, and ***p < 0.001).
Discussion
In this work, we used secretomes of tumor cells and fibroblasts, utilized factors derived from direct co-culture, performed indirect co-cultures, and evaluated the following parameters: cancer cell proliferation, migration, and EMT in HNSCC tumor cells. In comparison, we examined the effects of the sole addition of a recombinant human transforming growth factor-β1 (TGF-β1) on these parameters and further analyzed the functions of TGF-β1 by adding a neutralizing antibody in the fibroblast CM. Since the whole protein supplementation of the cell culture media was replaced by BSA we additionally got insight into the survival of the tumor cells in growth factor-free condition. In particular, also other serum protein components were replaced with albumin.
Consistent with the most recent results of several authors7,11,3,42 that paracrine factors secreted by tumor-associated fibroblasts increased the proliferation and migration of HNSCC cells, the conditioned media derived from fibroblasts or fibroblast tumor cells direct co-culture (mixed-culture containing CAFs) also lead to a significantly higher proliferation and migration in our HNSCC cell line. These data are in consistence with the observation that cancer invasion can be promoted by CAF or myofibroblasts factors,42 which are concentrated at the invasive margin.11 Interestingly, the factors secreted by normal fibroblasts had the same strong stimulating effect on proliferation and migration as the direct co-culture-CM (with no significant differences between FIB and Mix CM treatments). In the indirect co-culture system, the proliferation of HNSCC cells was also increased as already reported before,17 but to a significantly lower extent than in the secretome-treated cells.23 The migration of our HNSCC cells was not enhanced when they were indirectly co-cultured with fibroblasts. Intriguingly, the tumor cell–covered gap area of the indirect co-culture in the migration assay was significantly higher than in the albumin-medium-treated control. The same effect was observed in the case of HNSCC cells treated with tumor epithelial–derived CM that did not cause any changes in HNSCC proliferation and migration. This means that the indirect co-culture system and tumor-cell-CM did not enhance the migratory behavior of tumor cells but might secreted factors that supported tumor cell survival.
The action of TGF-β1 is considered to be a double-edged sword,36 inhibiting HNSCC growth in the early stages, but fostering invasion and metastasis at later stages.43,44 In this study, TGF-β1 had a negative effect on tumor cell proliferation. This was further confirmed by adding a neutralizing TGF-β antibody in the fibroblast-CM, which seemed to increase cell numbers, but not significantly. These data are consistent with the anti-proliferative effects of TGF-β1 observed in tamoxifen-treated HNSCC cell lines.45 In contrast, Freudlsperger et al.46 found a proliferation supportive role of TGF-β. In another study, TGF-β1 caused keratinocyte hyperproliferation in vivo which is most likely a secondary effect of inflammation and angiogenesis.47 In contrast to proliferation, the migratory behavior of HNSCC cells seemed to be enhanced by TGF-β1. Filming tumor cells during the scratch assay and further investigating covered distances of TGF-β1-treated tumor cells (see supplementary material) revealed that TGF-β1 strongly enhanced the motility of some individual tumor cells and supported the separation of individual cell groups from the tumor cell layer. The separation of tumor cells from the epithelial cell nest is also confirmed by the TGF-β1-induced reduction of E-cadherin levels, indicating a loss of protein that is required for epithelial cell–cell adhesion.48 Interestingly, this migration supportive role of TGF-β1 differed from the effect obtained with fibroblast or mixedculture CM treatment. In the two latter cases, it appeared to induce tumor cells to migrate laterally toward the empty space as a whole cell front. Neutralizing TGF-β in the fibroblast-CM significantly decreased the migration of HNSCC cells approving the assumption that TGF-β1 is a key effector in tumor cell motility. These findings are consistent with published evidence on cancer cell invasion in which TGF-β1 was found to increase MMP-9 expression in HNSCC cell lines,49 and metastatic cells have been shown to invade through a basement membrane layer in response to TGF-β.50
In addition to cell migration, TGF-β1 is known to be capable of inducing EMT in epithelial cells in vitro.51,52 Similarly, we observed that the addition of TGF-β1 lead to upregulation of mesenchymal marker gene (mRNA) expressions (vimentin and N-cadherin) and to significant downregulation of the epithelial markers (E-cadherin and desmoplakin) in HNSCC cells. Vimentin was detected in SCC-25 cells treated with fibroblast or mixed CM; nevertheless, TGF-β1 treatment did not allow a reproducible detection of a vimentin band at western blot level or required overloading of protein in the gels. Adding a neutralizing TGF-β antibody to the fibroblast-CM significantly decreased vimentin gene expression, emphasizing the importance of TGF-β1 in the upregulation of vimentin. Intriguingly, the gene expression of the epithelial markers was further downregulated when neutralizing TGF-β in the fibroblast-CM; TGF-β1 seems to function in concert with other factors by partially repressing negative regulators of epithelial genes. The isoforms TGF-β2 and TGF-β3 are also blocked through the antibody, which might be considerably involved in this regulatory network. FIB and mixed-culture-derived secretome seemed to be sufficient to increase the vimentin expression on the protein level, nevertheless the treatment time and the used experimental conditions did not allow the appearance of strong convincing western blot bands. In indirect co-culture, the vimentin upregulation was not observed. In contrast, E-cadherin was downregulated. The different conditions, FIB CM, mixed-culture, or indirect co-culture might contain different levels of activated fibroblast and myofibroblasts, which ensures distinct effects in stimulation of cell growth, cell migration, and EMT induction in tumor cells. This work also suggests that there is an epithelial–mesenchymal cross-talk (EMC), which defines cell proliferation, cell migration, and invasion, and most importantly, cell survival conditions of cellular components, which are also subject of dynamic changes including mesenchymal transdifferentiation of epithelial tumor cells as well differentiation of fibroblasts to a myofibroblast-like CAF phenotype. Deciphering of EMC is a key requirement for significant improvement of treatment of HNSCC.
Conclusion
Taken together, tumor cell–produced autocrine factors seem to be sufficient to ensure tumor cell survival, maintain tumor epithelial phenotype, and sustain basic tumor cell proliferation. Nevertheless, fibroblast-secreted factors further enhanced tumor cell growth and were capable to induce tumor cell migration (Table 2). A particular form of tumor cell migration, the clinically most important dissemination of small-cell groups or individual cells, was induced by TGF-β1 and was connected to reduction of E-cadherin expression, EMT-phenomena. In accordance, previous published data also revealed that the (myo)fibroblasts-conditioned media might support tumor cell proliferation and invasion as two separate effects.42 In addition to SCC-25 cells we also performed our investigations in HNSCC cells derived from malignant pleural effusion of a pharyngeal carcinoma37 (Detroit 562). In Detroit 562 cells fibroblast CM did not change cancer cell proliferation or EMT-related gene expressions. The investigation of the HNSCC cell responsiveness to secreted factors is complex and depends also on the cell type,53 which is an objective of our future investigation. Nevertheless, the establishment of the different experimental systems in this study serves to examine the EMC in HNSCC models and to investigate the factors and signaling molecules being involved in HNSCC progression.
Table 2.
Comparison of the main changes observed in the secretome or co-culture treatments of tumor cells.
| SCC-25 tumor cells’ autocrine effects | Normal fibroblast effects | CAFs’ effects from direct co-culture | TGF-β1 | Normal fibroblasts’ effects when TGF-β1 was neutralized | |
|---|---|---|---|---|---|
| Proliferation | Maintains basic survival | Three times induction of proliferation | Induces proliferation | Up to 50% reduction of cell growth | Even more proliferation than with Fibs CM |
| Migration | Only maintenance | Induction of cell migration in all cells | Induction of cell migration in all cells | Individual cell groups migrate | Cell migration reduced |
| EMT-like gene expression | Epithelial factors maintained | Mesenchymal vimentin increased | Mesenchymal vimentin increased | E-cadherin decreased | Increase in vimentin is lower |
| Main effects | Maintenance of epithelial phenotype | Induction of mesenchymal genes, proliferation, and cell migration | Induction of mesenchymal genes, proliferation, and cell migration | Cell loss, reduction of E-cadherin gene expression, and dissemination of tumor cells | More proliferation, less migration, and less vimentin |
TGF-β1: transforming growth factor beta 1; CAF: cancer-associated fibroblast; EMT: epithelial–mesenchymal transition; CM: conditioned medium.
Supplementary Material
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Austrian Science Fund (FWF P 25869-B13). Role of the Funding Source: The funding source has no influence on the direction and the outcome of the study, and it was an independent granting.
Footnotes
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Markwell SM, Weed SA. Tumor and stromal-based contributions to head and neck squamous cell carcinoma invasion. Cancers. 2015;7(1):382–406. doi: 10.3390/cancers7010382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koontongkaew S. The tumor microenvironment contribution to development, growth, invasion and metastasis of head and neck squamous cell carcinomas. J Cancer. 2013;4(1):66–83. doi: 10.7150/jca.5112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kellermann MG, Sobral LM, da Silva SD, et al. Mutual paracrine effects of oral squamous cell carcinoma cells and normal oral fibroblasts: induction of fibroblast to myofibroblast transdifferentiation and modulation of tumor cell proliferation. Oral Oncol. 2008;44(5):509–517. doi: 10.1016/j.oraloncology.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Marsh D, Suchak K, Moutasim KA, et al. Stromal features are predictive of disease mortality in oral cancer patients. J Pathol. 2011;223(4):470–481. doi: 10.1002/path.2830. [DOI] [PubMed] [Google Scholar]
- 5.Sutton LA, Rosenquist R. The complex interplay between cell-intrinsic and cell-extrinsic factors driving the evolution of chronic lymphocytic leukemia. Semin Cancer Biol. 2015;34:22–35. doi: 10.1016/j.semcancer.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 6.Kuzet SE, Gaggioli C. Fibroblast activation in cancer: when seed fertilizes soil. Cell Tissue Res. 2016;365:607–619. doi: 10.1007/s00441-016-2467-x. [DOI] [PubMed] [Google Scholar]
- 7.Wheeler SE, Shi H, Lin F, et al. Enhancement of head and neck squamous cell carcinoma proliferation, invasion, and metastasis by tumor-associated fibroblasts in preclinical models. Head Neck. 2014;36(3):385–392. doi: 10.1002/hed.23312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leef G, Thomas SM. Molecular communication between tumor-associated fibroblasts and head and neck squamous cell carcinoma. Oral Oncol. 2013;49(5):381–386. doi: 10.1016/j.oraloncology.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoo GH, Piechocki MP, Lonardo F, et al. In vivo characteristics of HPV-immortalized and carcinogen transformed oral keratinocytes. Laryngoscope. 2002;112(9):1672–1679. doi: 10.1097/00005537-200209000-00027. [DOI] [PubMed] [Google Scholar]
- 10.Zhang W, Matrisian LM, Holmbeck K, et al. Fibroblast-derived MT1-MMP promotes tumor progression in vitro and in vivo. BMC Cancer. 2006;6:52. doi: 10.1186/1471-2407-6-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lewis MP, Lygoe KA, Nystrom ML, et al. Tumour-derived TGF-beta1 modulates myofibroblast differentiation and promotes HGF/SF-dependent invasion of squamous carcinoma cells. Br J Cancer. 2004;90(4):822–832. doi: 10.1038/sj.bjc.6601611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramakrishna R, Rostomily R. Seed, soil, and beyond: The basic biology of brain metastasis. Surg Neurol Int. 2013;4(Suppl 4):S256–S264. doi: 10.4103/2152-7806.111303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Öhlund D, Elyada E, Tuveson D. Fibroblast heterogeneity in the cancer wound. J Exp Med. 2014;211(8):1503–1523. doi: 10.1084/jem.20140692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prime SS, Cirillo N, Hassona Y, et al. Fibroblast activation and senescence in oral cancer. J Oral Pathol Med. 2016;46:82–88. doi: 10.1111/jop.12456. [DOI] [PubMed] [Google Scholar]
- 15.Dudás J, Bitsche M, Schartinger V, et al. Fibroblasts produce brain-derived neurotrophic factor and induce mesenchymal transition of oral tumor cells. Oral Oncol. 2011;47(2):98–103. doi: 10.1016/j.oraloncology.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dudás J, Fullár A, Bitsche M, et al. Tumor-produced, active interleukin-1β regulates gene expression in carcinoma-associated fibroblasts. Exp Cell Res. 2011;317(15):2222–2229. doi: 10.1016/j.yexcr.2011.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dudás J, Fullár A, Romani A, et al. Curcumin targets fibroblast–tumor cell interactions in oral squamous cell carcinoma. Exp Cell Res. 2013;319(6):800–809. doi: 10.1016/j.yexcr.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fullár A, Kovalszky I, Bitsche M, et al. Tumor cell and carcinoma-associated fibroblast interaction regulates matrix metalloproteinases and their inhibitors in oral squamous cell carcinoma. Exp Cell Res. 2012;318(13):1517–1527. doi: 10.1016/j.yexcr.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bagordakis E, Sawazaki-Calone I, Macedo CC, et al. Secretome profiling of oral squamous cell carcinoma-associated fibroblasts reveals organization and disassembly of extracellular matrix and collagen metabolic process signatures. Tumour Biol. 2016;37(7):9045–9057. doi: 10.1007/s13277-015-4629-y. [DOI] [PubMed] [Google Scholar]
- 20.Madar S, Goldstein I, Rotter V. “Cancer associated fibroblasts”—more than meets the eye. Trends Mol Med. 2013;19(8):447–453. doi: 10.1016/j.molmed.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 21.Li J, Zhang W, Jiao R, et al. DIM attenuates TGF-β1-induced myofibroblast differentiation in neonatal rat cardiac fibroblasts. Int J Clin Exp Pathol. 2015;8(5):5121–5128. [PMC free article] [PubMed] [Google Scholar]
- 22.Curry JM, Sprandio J, Cognetti D, et al. Tumor microenvironment in head and neck squamous cell carcinoma. Semin Oncol. 2014;41(2):217–234. doi: 10.1053/j.seminoncol.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 23.Liu Z, Hartman YE, Warram JM, et al. Fibroblast growth factor receptor mediates fibroblast-dependent growth in EMMPRIN-depleted head and neck cancer tumor cells. Mol Cancer Res. 2011;9(8):1008–1017. doi: 10.1158/1541-7786.MCR-11-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sweeny L, Zimmermann TM, Liu Z, et al. Evaluation of tyrosine receptor kinases in the interactions of head and neck squamous cell carcinoma cells and fibroblasts. Oral Oncol. 2012;48(12):1242–1249. doi: 10.1016/j.oraloncology.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen S-F, Nieh S, Jao S-W, et al. The paracrine effect of cancer-associated fibroblast-induced interleukin-33 regulates the invasiveness of head and neck squamous cell carcinoma. J Pathol. 2013;231(2):180–189. doi: 10.1002/path.4226. [DOI] [PubMed] [Google Scholar]
- 26.Koontongkaew S, Amornphimoltham P, Monthanpisut P, et al. Fibroblasts and extracellular matrix differently modulate MMP activation by primary and metastatic head and neck cancer cells. Med Oncol. 2012;29(2):690–703. doi: 10.1007/s12032-011-9871-6. [DOI] [PubMed] [Google Scholar]
- 27.Fujii N, Shomori K, Shiomi T, et al. Cancer-associated fibroblasts and CD163-positive macrophages in oral squamous cell carcinoma: their clinicopathological and prognostic significance. J Oral Pathol Med. 2012;41(6):444–451. doi: 10.1111/j.1600-0714.2012.01127.x. [DOI] [PubMed] [Google Scholar]
- 28.Bello IO, Vered M, Dayan D, et al. Cancer-associated fibroblasts, a parameter of the tumor microenvironment, overcomes carcinoma-associated parameters in the prognosis of patients with mobile tongue cancer. Oral Oncol. 2011;47(1):33–38. doi: 10.1016/j.oraloncology.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 29.Kellermann MG, Sobral LM, da Silva SD, et al. Myofibroblasts in the stroma of oral squamous cell carcinoma are associated with poor prognosis. Histopathology. 2007;51(6):849–853. doi: 10.1111/j.1365-2559.2007.02873.x. [DOI] [PubMed] [Google Scholar]
- 30.Ding L, Zhang Z, Shang D, et al. α-Smooth muscle actin-positive myofibroblasts, in association with epithelial-mesenchymal transition and lymphogenesis, is a critical prognostic parameter in patients with oral tongue squamous cell carcinoma. J Oral Pathol Med. 2014;43(5):335–343. doi: 10.1111/jop.12143. [DOI] [PubMed] [Google Scholar]
- 31.Kawashiri S, Tanaka A, Noguchi N, et al. Significance of stromal desmoplasia and myofibroblast appearance at the invasive front in squamous cell carcinoma of the oral cavity. Head Neck. 2009;31(10):1346–1353. doi: 10.1002/hed.21097. [DOI] [PubMed] [Google Scholar]
- 32.Augsten M. Cancer-associated fibroblasts as another polarized cell type of the tumor microenvironment. Front Oncol. 2014;4:62. doi: 10.3389/fonc.2014.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bergmann C, Strauss L, Wang Y, et al. T regulatory type 1 cells in squamous cell carcinoma of the head and neck: mechanisms of suppression and expansion in advanced disease. Clin Cancer Res. 2008;14(12):3706–3715. doi: 10.1158/1078-0432.CCR-07-5126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosenthal E, McCrory A, Talbert M, et al. Elevated expression of TGF-beta1 in head and neck cancer-associated fibroblasts. Mol Carcinog. 2004;40(2):116–121. doi: 10.1002/mc.20024. [DOI] [PubMed] [Google Scholar]
- 35.Davies M, Robinson M, Smith E, et al. Induction of an epithelial to mesenchymal transition in human immortal and malignant keratinocytes by TGF-beta1 involves MAPK, Smad and AP-1 signalling pathways. J Cell Biochem. 2005;95(5):918–931. doi: 10.1002/jcb.20458. [DOI] [PubMed] [Google Scholar]
- 36.Prime SS, Davies M, Pring M, et al. The role of TGF-beta in epithelial malignancy and its relevance to the pathogenesis of oral cancer (part II) Crit Rev Oral Bio Med. 2004;15(6):337–347. doi: 10.1177/154411130401500603. [DOI] [PubMed] [Google Scholar]
- 37.Steinbichler TB, Metzler V, Pritz C, et al. Tumor-associated fibroblast-conditioned medium induces CDDP resistance in HNSCC cells. Oncotarget. 2015;7:2508–2518. doi: 10.18632/oncotarget.6210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hassona Y, Cirillo N, Heesom K, et al. Senescent cancer-associated fibroblasts secrete active MMP-2 that promotes keratinocyte dis-cohesion and invasion. Br J Cancer. 2014;111(6):1230–1237. doi: 10.1038/bjc.2014.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Draper BK, Komurasaki T, Davidson MK, et al. Epiregulin is more potent than EGF or TGFalpha in promoting in vitro wound closure due to enhanced ERK/MAPK activation. J Cell Biochem. 2003;89(6):1126–1137. doi: 10.1002/jcb.10584. [DOI] [PubMed] [Google Scholar]
- 40.Haller F, Kulle B, Schwager S, et al. Equivalence test in quantitative reverse transcription polymerase chain reaction: confirmation of reference genes suitable for normalization. Anal Biochem. 2004;335(1):1–9. doi: 10.1016/j.ab.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 41.Mathews ST, Graff E, Judd RL, et al. Comparison of chemiuminescence vs. infrared techniques for detection of fetuin-A in saliva. Methods Mol Biol. 2015;1314:333–348. doi: 10.1007/978-1-4939-2718-0_34. [DOI] [PubMed] [Google Scholar]
- 42.Sobral LM, Bufalino A, Lopes MA, et al. Myofibroblasts in the stroma of oral cancer promote tumorigenesis via secretion of activin A. Oral Oncol. 2011;47(9):840–846. doi: 10.1016/j.oraloncology.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 43.Akhurst RJ, Derynck R. TGF-beta signaling in cancer—a double-edged sword. Trends Cell Biol. 2001;11(11):S44–51. doi: 10.1016/s0962-8924(01)02130-4. [DOI] [PubMed] [Google Scholar]
- 44.White RA, Malkoski SP, Wang X-J. TGFβ signaling in head and neck squamous cell carcinoma. Oncogene. 2010;29(40):5437–5446. doi: 10.1038/onc.2010.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tavassoli M, Soltaninia J, Rudnicka J, et al. Tamoxifen inhibits the growth of head and neck cancer cells and sensitizes these cells to cisplatin induced-apoptosis: role of TGF-beta1. Carcinogenesis. 2002;23(10):1569–1575. doi: 10.1093/carcin/23.10.1569. [DOI] [PubMed] [Google Scholar]
- 46.Freudlsperger C, Bian Y, Contag Wise S, et al. TGF-β and NF-κB signal pathway cross-talk is mediated through TAK1 and SMAD7 in a subset of head and neck cancers. Oncogene. 2013;32(12):1549–1559. doi: 10.1038/onc.2012.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Silberstein RM. The child psychiatrist’s view of learning problems. J Med Soc New Jersey. 1967;64(8):467–471. [PubMed] [Google Scholar]
- 48.Nagar B, Overduin M, Ikura M, et al. Structural basis of calcium-induced E-cadherin rigidification and dimerization. Nature. 1996;380(6572):360–364. doi: 10.1038/380360a0. [DOI] [PubMed] [Google Scholar]
- 49.Sinpitaksakul SN, Pimkhaokham A, Sanchavanakit N, et al. TGF-beta1 induced MMP-9 expression in HNSCC cell lines via Smad/MLCK pathway. Biochem Biophys Res Commun. 2008;371(4):713–718. doi: 10.1016/j.bbrc.2008.04.128. [DOI] [PubMed] [Google Scholar]
- 50.Miyazaki H, Patel V, Wang H, et al. Growth factor-sensitive molecular targets identified in primary and metastatic head and neck squamous cell carcinoma using microarray analysis. Oral Oncol. 2006;42(3):240–256. doi: 10.1016/j.oraloncology.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 51.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119(6):1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yu J, Xie F, Bao X, et al. miR-300 inhibits epithelial to mesenchymal transition and metastasis by targeting Twist in human epithelial cancer. Mol Cancer. 2014;13:121. doi: 10.1186/1476-4598-13-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sweeny L, Liu Z, Lancaster W, et al. Inhibition of fibroblasts reduced head and neck cancer growth by targeting fibroblast growth factor receptor. Laryngoscope. 2012;122(7):1539–1544. doi: 10.1002/lary.23266. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.