Abstract
Systemic mastocytosis (SM) is a mast cell (MC) neoplasm with complex pathology and a variable clinical course. In aggressive SM (ASM) and MC leukemia (MCL) responses to conventional drugs are poor and the prognosis is dismal. R763 is a multi-kinase inhibitor that blocks the activity of Aurora-kinase-A/B, ABL1, AKT and FLT3. We examined the effects of R763 on proliferation and survival of neoplastic MC. R763 produced dose-dependent inhibition of proliferation in the human MC lines HMC-1.1 (IC50 5-50 nM), HMC-1.2 (IC50 1-10 nM), ROSAKIT WT (IC50 1-10 nM), ROSAKIT D816V (IC50 50-500 nM) and MCPV-1.1 (IC50 100-1000 nM). Moreover, R763 induced growth inhibition in primary neoplastic MC in patients with ASM and MCL. Growth-inhibitory effects of R763 were accompanied by signs of apoptosis and a G2/M cell cycle arrest. R763 also inhibited phosphorylation of KIT, BTK, AKT and STAT5 in neoplastic MC. The most sensitive target appeared to be STAT5. In fact, tyrosine phosphorylation of STAT5 was inhibited by R763 at 10 nM. At this low concentration, R763 produced synergistic growth-inhibitory effects on neoplastic MC when combined with midostaurin or dasatinib. Together, R763 is a novel promising multi-kinase inhibitor that blocks STAT5 activation and thereby overrides drug-resistance in neoplastic MC.
Introduction
Systemic mastocytosis (SM) is a stem cell-derived, myeloid neoplasm defined by abnormal expansion and accumulation of tissue mast cells (MC) in the bone marrow (BM) and other internal organs (1–6). Non-aggressive and advanced variants of the disease have been defined (1–6). Depending on MC burden, organ involvement, disease-subtype and comorbidities, the clinical course and prognosis vary greatly among patients (1–7). In contrast to indolent SM (ISM), patients with aggressive SM (ASM) and MC leukemia (MCL) have a poor prognosis. The response to conventional drugs is particularly poor in these patients (4–7). Therefore, current research is seeking novel therapeutic targets in neoplastic MC in advanced SM (8,9). One major regulator of growth and survival of MC is the tyrosine kinase receptor KIT (4–9). In a majority of all SM patients, including those with ASM and MCL, neoplastic MC exhibit the D816V-mutated variant of KIT (10–14). This mutation leads to stem cell factor-independent activation of KIT and triggers autonomous, uncontrolled growth of neoplastic MC (15). Therefore, the effects of several KIT-targeting tyrosine kinase inhibitors (TKI) have been tested in vitro and in vivo, with the aim to control neoplastic expansion of MC in advanced SM (16–19). However, although many patients benefit from treatment and show long-lasting clinical responses to midostaurin, no long-term hematologic remissions are induced by this drug (20,21). Moreover, recent data have shown that apart from KIT, other oncogenic kinases and pathways may play an important role in growth and survival of neoplastic MC (22–26).
More recent data suggest that neoplastic cells in advanced SM display one or more molecular lesion(s) (mutations) that may be responsible for oncogenic signaling and drug resistance (23–26). Other studies have shown that activation of STAT5 is a crucial event contributing to KIT D816V-dependent proliferation of neoplastic MC (27–29). All these signaling molecules and pathways may act together to promote abnormal growth of neoplastic MC in ASM and MCL. However, most drugs applied so far are directed against a limited number of kinase targets.
Aurora-kinases (AURK) are serine/threonine kinases that serve as important regulators of mitosis and are overexpressed in neoplastic cells in various solid tumors and hematologic malignancies (30–31). More recently, AURK inhibitors have been developed and were applied in preclinical studies as well as in clinical trials in cancer patients (30,32–34). Most of these inhibitors are directed against a number of additional targets, such as BCR-ABL1, FLT3, or PDGFR which may explain their broad and quite impressive anti-neoplastic effects in diverse cancer types (33,34).
In the present study, we investigated the effects of a broadly acting AURK inhibitor, R763, on proliferation, cell cycle progression, and survival in neoplastic MC. We found that R763 induces major growth-inhibitory effects by blocking the activity of several different molecular targets in neoplastic MC. The most sensitive target appeared to be STAT5.
Materials and Methods
Reagents
Reagents used in this study are described in the Supplement.
Isolation of primary neoplastic MC and culture of MC lines
Primary neoplastic cells were obtained from 14 patients with SM. Patients were classified as indolent SM (ISM; n=6), (ISM-AHN, n=1), ASM (n=5), and MCL (n=2) according to published criteria and the WHO classification 2016 (35,36). The patients´ characteristics are shown in Supplemental Table S1. Heparinized bone marrow (BM) or peripheral blood (PB) cells were layered over Ficoll to isolate mononuclear cells (MNC). The study was approved by the ethic committee of the Medical University of Vienna and conducted in accordance with the declaration of Helsinki. All patients gave written informed consent before BM puncture. Cell lines used in this study were the human MC lines HMC-1.1 (17,19,37), HMC-1.2 (17,19,37), ROSAKIT WT (38), ROSAKIT D816V (38) and MCPV-1.1 (39), and the canine MC lines C2 (40) and NI-1 (41). A detailed description of cell lines is provided in the supplement.
Detection of AURKA and AURKB mRNA and protein
Quantitative real time (RT) PCR was performed using cDNA from MC lines and primary neoplastic MC, and primers specific for AURKA and AURKB (Supplemental Table S2). Immunocytochemistry (ICC) was performed on cytospin-slides prepared with human MC lines and antibodies against AURKA and AURKB (Supplemental Table S3) essentially as described (42). Immunohistochemistry (IHC) was performed on sections prepared from paraffin-embedded (formalin-fixed) BM biopsy specimens (ISM, n=5; ASM, n=3, MCL n=2) according to standard methodology. A detailed description is provided in the supplement.
Western blotting and Immunoprecipitation (IP)
Western blotting and IP were performed following standard techniques (41–44). Technical details are provided in the Supplement.
Determination of proliferation, cell cycle progression and apoptosis
Proliferation and cell cycle progression of drug-exposed MC were determined following standard techniques. Apoptosis of drug-exposed MC lines was determined by light microscopy, TUNEL assay and Annexin V/PI staining as reported (45). Technical details are described in the Supplement.
Silencing of AURKA and AURKB by shRNA
Knockdown experiments were performed with shRNA against AURKA or AURKB (Supplemental Table S4) and HMC-1.2 cells following published methods (46,47). Technical details are described in the Supplement.
Statistical analysis
To determine the level of significance in the results obtained the paired Student´s t-test was applied. Results were considered to be significantly different when p was <0.05.
Results
Detection of AURKA in neoplastic MC
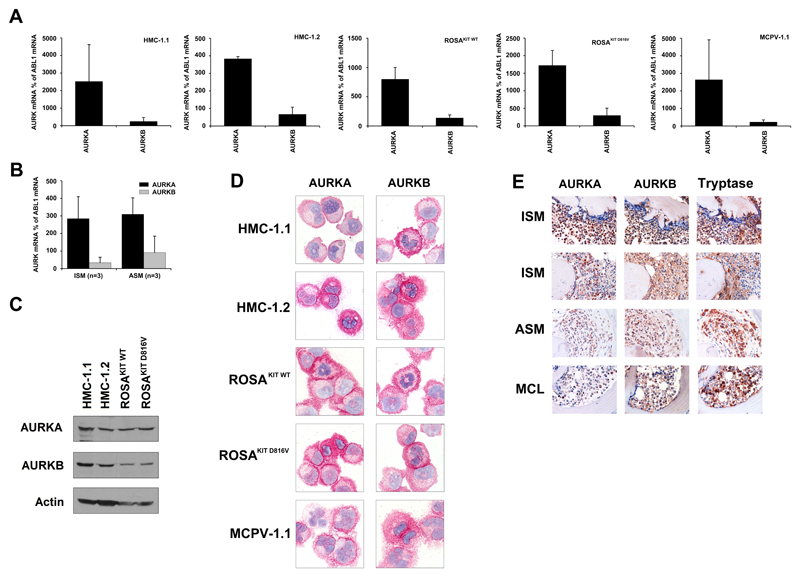
As assessed by qPCR, HMC-1, ROSA, and MCPV-1.1 cells expressed substantial amounts of AURKA mRNA, whereas AURKB mRNA was expressed at lower levels (Figure 1A). Similar results were obtained with primary neoplastic MC in indolent or advanced SM (Figure 1B). We were also able to detect AURKA and AURKB mRNA in the canine MC lines C2 and NI-1 (Supplemental Figure S1). As assessed by Western blotting, HMC-1 and ROSA cells expressed the AURKA and AURKB protein (Figure 1C). ICC staining experiments confirmed that all MC lines tested display AURKA and AURKB (Figure 1D). Finally, we found that primary neoplastic MC in patients with ISM, ASM, and MCL exhibit AURKA and AURKB (Figure 1E). In patients with ISM, neoplastic MC expressed substantial amounts of AURKA and AURKB, whereas in advanced SM (ASM, MCL), MC expressed lower amounts of AURKA, but retained substantial amounts of AURKB. Other BM cells tested, including immature myeloid progenitors also displayed AURKA and AURKB. A summary of antibody-staining results is shown in Supplemental Table S5.
Figure 1. Expression of AURKA and AURKB in neoplastic mast cells (MC).
A-C: Expression of AURKA mRNA and AURKB mRNA in HMC-1 cells, ROSA cells, MCPV-1.1 cells (A) and primary neoplastic MC (B) was analyzed by qPCR using specific primers as described in the text. AURK mRNA levels are expressed as percent of ABL1 mRNA levels. Results represent the mean±S.D. of 3 independent experiments. C: Expression of AURKA and AURKB in HMC-1 cells and ROSA cells determined by Western blotting. Actin served as loading control. D: Expression of AURKA and AURKB in neoplastic MC assessed by immunocytochemistry using antibodies against AURKA (left panels) and AURKB (right panels). Original magnification, x100. E: Immunhistochemical detection of AURKA (left panels), AURKB (middle panels) and tryptase (right panels) in neoplastic MC in bone marrow biopsy sections in 2 patients with indolent systemic mastocytosis (ISM), one with aggressive SM (ASM), and one with MC leukemia (MCL). Original magnification, x60.
shRNA-induced knockdown of AURKA and AURKB results in reduced proliferation of neoplastic MC
To study the functional role of AURKA/B in neoplastic MC, experiments with shRNA-transduced HMC-1.2 cells were performed. In these experiments, knock-down of AURKA resulted in a decreased proliferation compared to a control shRNA (Supplemental Figure S2A). Knock-down of AURKB was also followed by reduced proliferation, but the effect on HMC-1.2 was less pronounced compared to the effect of shRNA against AURKA (Supplemental Figure S2A). shRNA-induced knock-down of AURKA and AURKB was confirmed by qPCR (Supplemental Figure S2B).
R763 inhibits growth of neoplastic MC
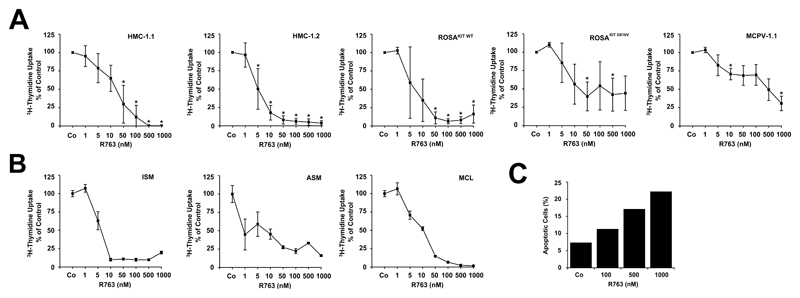
As determined by 3H-thymidine uptake, R763 was found to inhibit the proliferation in HMC-1, ROSA, and MCPV-1.1 cells. Interestingly, IC50 values were higher in HMC-1.1 cells (5-50 nM) compared to HMC-1.2 cells (1-10 nM), and higher in ROSAKIT D816V cells (50-500 nM) and MCPV-1.1 cells (100-1,000 nM) compared to ROSAKIT WT cells (1-10 nM) (Figure 2A). Confirming our previous data (48) R763 was also found to suppress growth of C2 and NI-1 cells (Supplemental Figure S3A). In addition, R763 induced dose-dependent inhibition of proliferation in primary neoplastic MC in most patients with ISM, ASM and MCL (Figure 2B, Supplemental Table S1). In normal BM cells, R763 also produced growth-inhibitory effects, but was less effective than in neoplastic MC in ASM/MCL (Supplemental Figure S3B).
Figure 2. R763 inhibits the proliferation and survival of neoplastic mast cells (MC).
A, B: HMC-1.1, HMC-1.2, ROSAKIT WT, ROSAKIT D816V, and MCPV-1.1 cells (A), and primary neoplastic MC obtained from patients with various subtypes of SM (B) were incubated with control medium or various concentrations of R763 as indicated at 37°C for 48 hours. After incubation, 0.5 µCi 3H-thymidine was added. After 16 hours, cells were harvested and bound radioactivity was measured in a β-counter. Results in ´A´ are expressed as percent of control and represent the mean±S.D. from at least 3 independent experiments Asterisk (*): p<0.05. Results in ´B´ are expressed as percent of control and represent the mean±S.D. of triplicates. C: Primary neoplastic MC were incubated in control medium or various concentrations of R763 for 48 hours. Then, the percentage of apoptotic MC (CD45+/CD34-/CD117+ cells) was analyzed by flow cytometry. Apoptotic cells were defined as DAPI-/Annexin V+ cells.
R763 induces a G2/M cell cycle arrest in neoplastic MC
Since AURKA/B play an essential role in cell cycle progression and mitosis, we assessed the effects of R763 on cell cycle progression. R763 induced a substantial G2/M cell cycle arrest at low nanomolar concentrations in all MC lines tested (Supplemental Figure S4). R763 also induced endoreduplication in C2 and NI-1 cells after 24 hours, whereas no substantial endoreduplication was observed in drug-exposed HMC-1 cells (Supplemental Figure S5).
R763 induces apoptosis in neoplastic MC
As assessed by light microscopy, R763 induced apoptosis in HMC-1.1, HMC-1.2, C2, and NI-1 cells in a dose-dependent manner (Supplemental Figure S6A). The effects of R763 on viability of MC lines were confirmed by TUNEL assay (Supplemental Figure S6B). In addition, R763 induced cleavage of caspase 3 in both HMC-1 sub-clones as well as in C2 and NI-1 cells as evidenced by Western blotting (Supplemental Figure S6C). Moreover, R763 was found to induce time-dependent apoptosis in HMC-1 and ROSA cells in our flow cytometry experiments (Supplemental Figure S6D). Finally, we were able to demonstrate that R763 induces apoptosis in primary neoplastic MC obtained from a patient with MCL (Figure 2C).
R763 inhibits phosphorylation of various signaling molecules, including STAT5
Recent data suggest that R763 is not specific for AURK but also blocks other major regulators of proliferation and survival in neoplastic cells (33). Therefore, we examined the effects of R763 on other key signaling molecules expressed in neoplastic MC, including KIT, BTK, and STAT5. In these experiments, we were able to show that R763 inhibits phosphorylation of KIT, AKT, BTK, and STAT5 in HMC-1 cells (Supplemental Figure S7A). Phosphorylation of KIT, AKT and BTK decreased at 100-1000 nM of R763. By contrast, STAT5 phosphorylation was completely inhibited at 10 nM R763 (Supplemental Figure S7B). In IP experiments, R763 was found to inhibit phosphorylation of STAT5A and STAT5B in HMC-1.2 cells (Supplemental Figure S7C). A decrease in phosphorylation of STAT5A and STAT5B was already detected after 30 to 60 minutes of incubation with 10 nM R763 (Supplemental Figure S7C). Moreover, R763 decreased STAT5 phosphorylation in primary neoplastic MC (Supplemental Figure S7D). These data suggest that STAT5 is an important and sensitive target of R763.
R763 cooperates with PKC412 (midostaurin) and with dasatinib in producing growth-inhibition and apoptosis in neoplastic MC
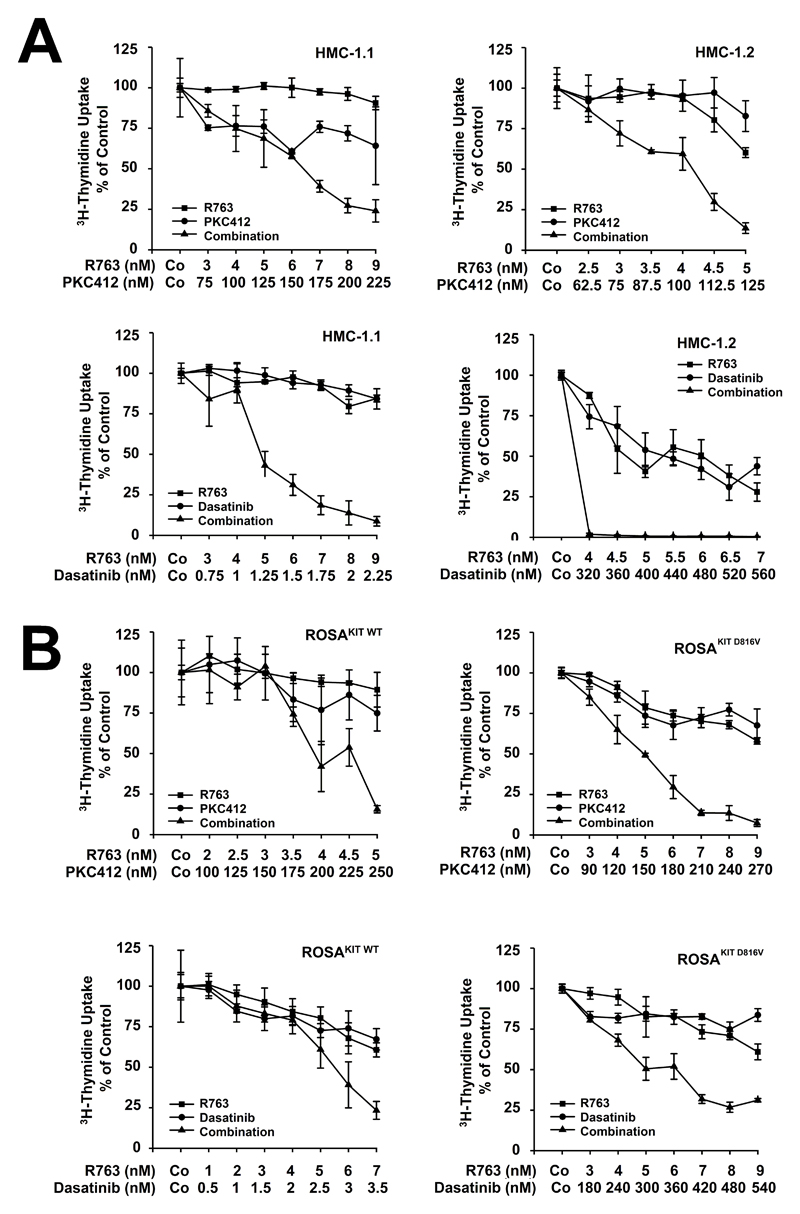
Since R763 did not inhibit proliferation of neoplastic MC in all patients we were interested to know whether the effect of R763 can be augmented by addition of other anti-neoplastic drugs. In these experiments, we combined R763 with either PKC412 or dasatinib, two KIT D816V-targeting drugs exhibiting a broad target interaction profile (22,49). We found that PKC412 and dasatinib cooperate with R763 in producing growth inhibition in HMC-1.1, HMC-1.2, ROSAKIT WT, and ROSAKIT D816V cells (Figure 3). Moreover, R763 was found to cooperate with PKC412 in producing growth-inhibition in primary neoplastic MC (Supplemental Figure S8A). Finally, we were able to show that R763 cooperates with PKC412 and with dasatinib in producing apoptosis in HMC-1 cells (Supplemental Figure S8B).
Figure 3. R763 cooperates with PKC412 and with dasatinib in producing growth inhibition in neoplastic mast cells (MC).
A: HMC-1.1 and HMC-1.2 cells (upper panel) were incubated with various concentrations of R763, PKC412, or a combination of both drugs a fixed ratio of drug concentrations for 48 hours. Lower panel: HMC-1.1 and HMC-1.2 cells were incubated in various concentrations of R763, dasatinib, or a combination of both drugs at a fixed ratio of drug concentrations for 48 hours. Results are expressed as percent of control and represent the mean±S.D. of triplicates. B: ROSA KIT WT and ROSAKIT D816V cells (upper panel) were incubated with various concentrations of R763, PKC412, or a combination of both drugs at a fixed ratio of drug concentrations for 48 hours. Lower panel: ROSA KIT WT and ROSAKIT D816V cells were incubated with various concentrations of R763, dasatinib, or a combination of both drugs at a fixed ratio of drug concetrations for 48 hours. Results are expressed as percent of control and represent the mean±S.D. of triplicates.
Discussion
AURKA and AURKB are serine/threonine kinases that serve as major regulators of cell cycle progression in neoplastic cells (30–33). However, little is known about expression and function of AURK in neoplastic MC. We found that neoplastic MC in SM express AURKA and AURKB, and that the AURK-targeting drug R763 acts as a potent inhibitor of growth and survival of neoplastic MC. In addition, R763 suppressed the activity of additional targets in neoplastic MC, including KIT, BTK and AKT. Most significantly, R763 was identified as an extremely potent inhibitor of STAT5-activation in neoplastic MC.
So far, little is known about expression and function of AURK in human MC and about the effects of AURK blockers (50). In an initial phase of our study, we examined the expression of AURKA and AURKB in primary neoplastic MC in SM and in various human MC lines. Interestingly, although neoplastic MC expressed AURKA mRNA in excess over AURKB mRNA, the AURKA and AURKB proteins were both expressed at detectable levels in MC. This observation may be explained by higher production- and turn-over rates of AURKA in MC.
In a next step we were able to show that AURKA and AURKB are functionally relevant molecules in neoplastic MC. In particular, our data show that shRNA-mediated knock-down of AURKA or AURKB leads to decreased proliferation in HMC-1.2 cells. Interestingly, the knock-down of AURKA resulted in stronger growth-inhibitory effects compared to AURKB knock-down. These data may suggest that AURKA is more important for the viability or growth of MC. Differences in the transfection- or knock-down efficacy of the shRNA applied could be excluded. All in all, our data show that AURKA and AURKB contribute to growth of neoplastic MC, which confirms the role of these kinase targets observed in other neoplasms (30–34).
Over the past 10 years, several different AURK inhibitors have been developed and applied in preclinical and clinical studies (30,32–34). R763 is a multi-kinase blocker that exerts major inhibitory effects on AURKA and AURKB. We found that R763 is a potent inhibitor of growth and survival of neoplastic MC. The effects of this drug on MC proliferation were seen in primary neoplastic MC and in all human and canine MC lines tested. An interesting observation was that the effects of R763 on proliferation were stronger in HMC-1.2 than in HMC-1.1 cells, and stronger in ROSAKIT WT cells compared to ROSAKIT D816V cells. The reason for the differential response of various MC lines to R763 remains unknown. Concerning ROSA cells, the differential response may be explained by a higher sensitivity of wild type (WT) KIT against R763 compared to KIT D816V. In this regard the higher sensitivity of HMC-1.2 cells (expressing KIT D816V) is difficult to explain. One possibility could be that additional molecular targets of R763 are selectively expressed by HMC-1.2 cells. For example, HMC-1.2 cells display higher levels of pSTAT5 compared to HMC-1.1 cells (27). An alternative explanation would be that HMC-1.1 cells exhibit additional molecular pathways leading to relative resistance against R763. Major differences in expression of the key targets, AURKA and AURKB, were excluded in our experiments.
STAT5 is an established target in KIT D816V+ neoplastic MC (27–29). In the present study, we were able to confirm that STAT5 is constitutively phosphorylated in neoplastic MC. In addition, we found that R763 suppresses STAT5 phosphorylation in both HMC-1 sub-clones. An interesting observation was that R763 inhibits tyrosine phosphorylation of STAT5A and STAT5B at very low concentrations (10 nM) in HMC-1.2 cells. Interestingly, these low drug concentrations were not sufficient to block activation of other signaling molecules in HMC-1 cells, which may have several explanations. One possibility may be that R763 directly acts on STAT5 or a STAT5-related signaling complex. Another possibility would be that the incomplete but simultaneous inhibition of several different upstream targets leads to a rapid decrease of STAT5 tyrosine phosphorylation. Alternatively, STAT5 inhibition may be a secondary effect mediated by another sensitive (unknown) target of R763 in neoplastic MC.
A number of different mechanisms may underly R763-induced growth inhibition in neoplastic MC. We found that R763 induces a G2/M cell cycle arrest as well as apoptosis in neoplastic MC.
A clinically relevant question to address was whether the effects of R763 can be confirmed using primary human MC. In a first step, we were able to show that primary neoplastic MC express AURKA and AURKB. Moreover, R763 was found to inhibit the proliferation of primary neoplastic MC in most samples tested. In addition, R763 was found to induce apoptosis in primary neoplastic MC. Growth-inhibitory effects of R763 on neoplastic MC were observed in all categories of advanced SM, including ASM and MCL. It is also noteworthy that in 2 patients with ISM, neoplastic cells did not respond to R763. This may be due to the fact that in the BM samples analyzed, a majority of cells were non-clonal (normal) cells that did not respond, whereas in most ASM- and MCL samples examined, most or even all cells were clonal cells expressing KIT D816V. An important observation was that R763 did not exert major growth-inhibitory effects on normal BM cells.
Recent data suggest that antineoplastic drugs, when used as single agents, may not be sufficient to completely (and durably) suppress growth of neoplastic MC in ASM and MCL. Therefore, combinations of various targeted drugs have been tested (19,22,42). In the present study, we applied drug combinations consisting of R763 and the KIT D816V-targeting drugs PKC412 and dasatinib. Both combinations were found to produce synergistic growth-inhibitory effects in HMC-1 and ROSA cells. These data suggest that such combinations may be useful and should be considered for testing in consecutive studies. The primary mechanisms underlying the synergistic drug interactions remain unknown. Based on our data, it is tempting to speculate that R763-induced inhibition of STAT5 activation plays a role in synergistic drug effects. This hypothesis was supported by the observation that very low concentrations of R763 were sufficient to block STAT5 activation (but not other signaling molecules) and produced synergistic anti-neoplastic effects.
In summary, our data show that neoplastic MC in SM express AURKA, AURKB, as well as several other targets of R763. In addition, our data show that exposure to R763 is associated with inhibition of STAT5 activation as well as with major growth-inhibition and apoptosis. R763 may be a novel promising agent to treat advanced SM, a hypothesis that needs to be tested in clinical trials.
Supplementary Material
Acknowledgement
This study was supported by Austrian Science Fund (FWF), SFB grants F4701-B20, F4704-B20, F4707-B20 and F4710-B20.
Footnotes
Conflicts of interest
G.H. received honoraria from Novartis and Ariad. W.R.S. received honoraria from Novartis and Celgene, and a research grant from Lipomed. P.V., H-P.H and A.R. served as a Consultant in a global Novartis trial examining the effects of midostaurin in advanced SM. J.Z. received a institutional support from Boehringer-Ingelheim, and is consultant and stock holder at Mirimus Inc. A.R. received a research grant from Novartis, honoraria from Novartis and BMS, and served in advisory boards organized by Novartis. M.A. received a research grants from Blueprint and Deciphera, and received honoraria from Deciphera. P.V. received research grants from Novartis, Blueprint, and Deciphera, and honoraria from Novartis, Celgene, Pfizer, and Deciphera. The authors have no other conflicts of interest to disclose.
References
- 1.Metcalfe DD. Classification and diagnosis of mastocytosis: current status. J Invest Dermatol. 1991;96:2S–4S. [PubMed] [Google Scholar]
- 2.Valent P. Biology, classification and treatment of human mastocytosis. Wien Klin Wochenschr. 1996;108:385–397. [PubMed] [Google Scholar]
- 3.Horny HP, Valent P. Diagnosis of mastocytosis: general histopathological aspects, morphological criteria, and immunohistochemical findings. Leuk Res. 2001;25:543–551. doi: 10.1016/s0145-2126(01)00021-2. [DOI] [PubMed] [Google Scholar]
- 4.Escribano L, Akin C, Castells M, Orfao A, Metcalfe DD. Mastocytosis: current concepts in diagnosis and treatment. Ann Hematol. 2002;81:677–690. doi: 10.1007/s00277-002-0575-z. [DOI] [PubMed] [Google Scholar]
- 5.Valent P, Akin C, Sperr WR, Horny HP, Arock M, Lechner K, et al. Diagnosis and treatment of systemic mastocytosis: state of the art. Br J Haematol. 2003;122:695–717. doi: 10.1046/j.1365-2141.2003.04575.x. [DOI] [PubMed] [Google Scholar]
- 6.Akin C, Metcalfe DD. Systemic mastocytosis. Annu Rev Med. 2004;55:419–432. doi: 10.1146/annurev.med.55.091902.103822. [DOI] [PubMed] [Google Scholar]
- 7.Pardanani A, Tefferi A. Systemic mastocytosis in adults: a review on prognosis and treatment based on 342 Mayo Clinic patients and current literature. Curr Opin Hematol. 2010;17:125–132. doi: 10.1097/MOH.0b013e3283366c59. [DOI] [PubMed] [Google Scholar]
- 8.Valent P, Ghannadan M, Akin C, Krauth MT, Selzer E, Mayerhofer M, et al. On the way to targeted therapy of mast cell neoplasms: identification of molecular targets in neoplastic mast cells and evaluation of arising treatment concepts. Eur J Clin Invest. 2004;34(Suppl 2):41–52. doi: 10.1111/j.0960-135X.2004.01369.x. [DOI] [PubMed] [Google Scholar]
- 9.Tefferi A, Pardanani A. Clinical, genetic, and therapeutic insights into systemic mast cell disease. Curr Opin Hematol. 2004;11:58–64. doi: 10.1097/00062752-200401000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Nagata H, Worobec AS, Oh CK, Chowdhury BA, Tannenbaum S, Suzuki Y, et al. Identification of a point mutation in the catalytic domain of the protooncogene c-kit in peripheral blood mononuclear cells of patients who have mastocytosis with an associated hematologic disorder. Proc Natl Acad Sci (USA) 1995;92:10560–10564. doi: 10.1073/pnas.92.23.10560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feger F, Ribadeau Dumas A, Leriche L, Valent P, Arock M. Kit and c-kit mutations in mastocytosis: a short overview with special reference to novel molecular and diagnostic concepts. Int Arch Allergy Immunol. 2002;127:110–114. doi: 10.1159/000048179. [DOI] [PubMed] [Google Scholar]
- 12.Longley BJ, Tyrrell L, Lu SZ, Ma YS, Langley K, Ding TG, et al. Somatic c-kit activating mutation in urticaria pigmentosa and aggressive mastocytosis: establishment of clonality in a human mast cell neoplasm. Nat Genet. 1996;12:312–314. doi: 10.1038/ng0396-312. [DOI] [PubMed] [Google Scholar]
- 13.Fritsche-Polanz R, Jordan JH, Feix A, Sperr WR, Sunder-Plassmann G, Valent P, et al. Mutation analysis of C-KIT in patients with myelodysplastic syndromes without mastocytosis and cases of systemic mastocytosis. Br J Haematol. 2001;113:357–364. doi: 10.1046/j.1365-2141.2001.02783.x. [DOI] [PubMed] [Google Scholar]
- 14.Arock M, Sotlar K, Akin C, Broesby-Olsen S, Hoermann G, Escribano L, et al. KIT mutation analysis in mast cell neoplasms: recommendations of the European Competence Network on Mastocytosis. Leukemia. 2015;29:1223–1232. doi: 10.1038/leu.2015.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Furitsu T, Tsujimura T, Tono T, Ikeda H, Kitayama H, Koshimizu U, et al. Identification of mutations in the coding sequence of the proto-oncogene c-kit in a human mast cell leukemia cell line causing ligand-independent activation of the c-kit product. J Clin Invest. 1993;92:1736–1744. doi: 10.1172/JCI116761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shah NP, Lee FY, Luo R, Jiang Y, Donker M, Akin C. Dasatinib (BMS-354825) inhibits KITD816V, an imatinib-resistant activating mutation that triggers neoplastic growth in most patients with systemic mastocytosis. Blood. 2006;108:286–291. doi: 10.1182/blood-2005-10-3969. [DOI] [PubMed] [Google Scholar]
- 17.Akin C, Brockow K, D'Ambrosio C, Kirshenbaum AS, Ma Y, Longley BJ, et al. Effects of tyrosine kinase inhibitor STI571 on human mast cells bearing wild-type or mutated c-kit. Exp Hematol. 2003;31:686–692. doi: 10.1016/s0301-472x(03)00112-7. [DOI] [PubMed] [Google Scholar]
- 18.Gleixner KV, Mayerhofer M, Sonneck K, Gruze A, Samorapoompichit P, Baumgartner C, et al. Synergistic growth-inhibitory effects of two tyrosine kinase inhibitors, dasatinib and PKC412, on neoplastic mast cells expressing the D816V-mutated oncogenic variant of KIT. Haematologica. 2007;92:1451–1459. doi: 10.3324/haematol.11339. [DOI] [PubMed] [Google Scholar]
- 19.Gleixner KV, Mayerhofer M, Aichberger KJ, Derdak S, Sonneck K, Böhm A, Gruze, et al. PKC412 inhibits in vitro growth of neoplastic human mast cells expressing the D816V-mutated variant of KIT: comparison with AMN107, imatinib, and cladribine (2CdA) and evaluation of cooperative drug effects. Blood. 2006;107:752–759. doi: 10.1182/blood-2005-07-3022. [DOI] [PubMed] [Google Scholar]
- 20.Gotlib J, Berubé C, Growney JD, Chen CC, George TI, Williams C, et al. Activity of the tyrosine kinase inhibitor PKC412 in a patient with mast cell leukemia with the D816V KIT mutation. Blood. 2005;106:2865–2870. doi: 10.1182/blood-2005-04-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gotlib J, Kluin-Nelemans HC, George TI, Akin C, Sotlar K, Hermine O, et al. Efficacy and Safety of Midostaurin in Advanced Systemic Mastocytosis. N Engl J Med. 2016;374:2530–2541. doi: 10.1056/NEJMoa1513098. [DOI] [PubMed] [Google Scholar]
- 22.Gleixner KV, Mayerhofer M, Cerny-Reiterer S, Hörmann G, Rix U, Bennett KL, et al. KIT-D816V-independent oncogenic signaling in neoplastic cells in systemic mastocytosis: role of Lyn and BTK activation and disruption by dasatinib and bosutinib. Blood. 2011;118:1885–1898. doi: 10.1182/blood-2010-06-289959. [DOI] [PubMed] [Google Scholar]
- 23.Wilson TM, Maric I, Simakova O, Bai Y, Chan EC, Olivares N, et al. Clonal analysis of NRAS activating mutations in KIT-D816V systemic mastocytosis. Haematologica. 2011;96:459–463. doi: 10.3324/haematol.2010.031690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwaab J, Schnittger S, Sotlar K, Walz C, Fabarius A, Pfirrmann M, et al. Comprehensive mutational profiling in advanced systemic mastocytosis. Blood. 2013;122:2460–2466. doi: 10.1182/blood-2013-04-496448. [DOI] [PubMed] [Google Scholar]
- 25.Bibi S, Langenfeld F, Jeanningros S, Brenet F, Soucie E, Hermine O, et al. Molecular defects in mastocytosis: KIT and beyond KIT. Immunol Allergy Clin North Am. 2014;34:239–262. doi: 10.1016/j.iac.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 26.Damaj G, Joris M, Chandesris O, Hanssens K, Soucie E, Canioni D, et al. ASXL1 but not TET2 mutations adversely impact overall survival of patients suffering systemic mastocytosis with associated clonal hematologic non-mast-cell diseases. PLoS One. 2014;9:e85362. doi: 10.1371/journal.pone.0085362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baumgartner C, Cerny-Reiterer S, Sonneck K, Mayerhofer M, Gleixner KV, Fritz R, et al. Expression of activated STAT5 in neoplastic mast cells in systemic mastocytosis: subcellular distribution and role of the transforming oncoprotein KIT D816V. Am J Pathol. 2009;175:2416–2429. doi: 10.2353/ajpath.2009.080953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chaix A, Lopez S, Voisset E, Gros L, Dubreuil P, De Sepulveda P. Mechanisms of STAT protein activation by oncogenic KIT mutants in neoplastic mast cells. J Biol Chem. 2011;286:5956–5966. doi: 10.1074/jbc.M110.182642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harir N, Boudot C, Friedbichler K, Sonneck K, Kondo R, Martin-Lannerée S, et al. Oncogenic Kit controls neoplastic mast cell growth through a Stat5/PI3-kinase signalling cascade. Blood. 2008;112:2463–2473. doi: 10.1182/blood-2007-09-115477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farag SS. The potential role of Aurora kinase inhibitors in haematological malignancies. Br J Haematol. 2011;155:561–579. doi: 10.1111/j.1365-2141.2011.08898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang XX, Liu R, Jin SQ, Fan FY, Zhan QM. Overexpression of Aurora-A kinase promotes tumor cell proliferation and inhibits apoptosis in esophageal squamous cell carcinoma cell line. Cell Res. 2006;16:356–366. doi: 10.1038/sj.cr.7310046. [DOI] [PubMed] [Google Scholar]
- 32.Lok W, Klein RQ, Saif MW. Aurora kinase inhibitors as anti-cancer therapy. Anticancer Drugs. 2010;21:339–350. doi: 10.1097/CAD.0b013e3283350dd1. [DOI] [PubMed] [Google Scholar]
- 33.McLaughlin J, Markovtsov V, Li H, Wong S, Gelman M, Zhu Y, et al. Preclinical characterization of Aurora kinase inhibitor R763 identified through an image-based phenotypic screen. J Cancer Res Clin Oncol. 2010;136:99–113. doi: 10.1007/s00432-009-0641-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cicenas J. The Aurora kinase inhibitors in cancer research and therapy. J Cancer Res Clin Oncol. 2016;142:1995–2012. doi: 10.1007/s00432-016-2136-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Valent P, Horny H-P, Escribano L, Longley BJ, Li CY, Schwartz LB, Marone G, et al. Diagnostic criteria and classification of mastocytosis: a consensus proposal. Leuk Res; Conference Report of “Year 2000 Working Conference on Mastocytosis”; 2001. pp. 603–625. [DOI] [PubMed] [Google Scholar]
- 36.Valent P, Akin C, Metcalfe DD. Mastocytosis: 2016-Updated WHO Classification and Novel Emerging Treatment Concepts. Blood. 2017;129:1420–1427. doi: 10.1182/blood-2016-09-731893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Butterfield JH, Weiler D, Dewald G, Gleich GJ. Establishment of an immature mast cell line from a patient with mast cell leukemia. Leuk Res. 1988;12:345–355. doi: 10.1016/0145-2126(88)90050-1. [DOI] [PubMed] [Google Scholar]
- 38.Saleh R, Wedeh G, Herrmann H, Bibi S, Cerny-Reiterer S, Sadovnik I, et al. A new human mast cell line expressing a functional IgE receptor converts to factor-independence and tumorigenicity by KIT D816V-transfection. Blood. 2014;124:111–120. doi: 10.1182/blood-2013-10-534685. [DOI] [PubMed] [Google Scholar]
- 39.Hoermann G, Blatt K, Greiner G, Putz EM, Berger A, Herrmann H, et al. CD52 is a molecular target in advanced systemic mastocytosis. FASEB J. 2014;28:3540–3551. doi: 10.1096/fj.14-250894. [DOI] [PubMed] [Google Scholar]
- 40.DeVinney R, Gold WM. Establishment of two dog mastocytoma cell lines in continuous culture. Am J Respir Cell Mol Biol. 1990;3:413–420. doi: 10.1165/ajrcmb/3.5.413. [DOI] [PubMed] [Google Scholar]
- 41.Hadzijusufovic E, Peter B, Herrmann H, Rülicke T, Cerny-Reiterer S, Schuch K, Kenner L, Thaiwong T, Yuzbasiyan-Gurkan V, Pickl WF, Willmann M, et al. NI-1: a novel canine mastocytoma model for studying drug resistance and IgER-dependent mast cell activation. Allergy. 2012;67:858–868. doi: 10.1111/j.1398-9995.2012.02833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peter B, Gleixner KV, Cerny-Reiterer S, Herrmann H, Winter V, Hadzijusufovic E, et al. Polo-like kinase-1 as novel target in neoplastic mast cells: demonstration of growth-inhibitory effects of siRNA and the Polo-like kinase-1 targeting drug BI 2536. Haematologica. 2011;96:672–680. doi: 10.3324/haematol.2010.031328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moriggl R, Sexl V, Piekorz R, Topham D, Ihle JN. Stat5 activation is uniquely associated with cytokine signaling in peripheral T cells. Immunity. 1999;11:225–230. doi: 10.1016/s1074-7613(00)80097-7. [DOI] [PubMed] [Google Scholar]
- 44.Friedbichler K, Themanns M, Mueller KM, Schlederer M, Kornfeld JW, Terracciano LM, et al. Growth-hormone-induced signal transducer and activator of transcription 5 signaling causes gigantism, inflammation, and premature death but protects mice from aggressive liver cancer. Hepatology. 2012;55:941–952. doi: 10.1002/hep.24765. [DOI] [PubMed] [Google Scholar]
- 45.Wedeh G, Cerny-Reiterer S, Eisenwort G, Herrmann H, Blatt K, Hadzijusufovic E, et al. Identification of bromodomain-containing protein-4 as a novel marker and epigenetic target in mast cell leukemia. Leukemia. 2015;29:2230–2237. doi: 10.1038/leu.2015.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fellmann C, Hoffmann T, Sridhar V, Hopfgartner B, Muhar M, Roth M, et al. An optimized microRNA backbone for effective single-copy RNAi. Cell Rep. 2013;5:1704–1713. doi: 10.1016/j.celrep.2013.11.020. [DOI] [PubMed] [Google Scholar]
- 47.Hoermann G, Cerny-Reiterer S, Herrmann H, Blatt K, Bilban M, Gisslinger H, et al. Identification of oncostatin M as a JAK2 V617F-dependent amplifier of cytokine production and bone marrow remodeling in myeloproliferative neoplasms. FASEB J. 2012;26:894–906. doi: 10.1096/fj.11-193078. [DOI] [PubMed] [Google Scholar]
- 48.Keller A, Wingelhofer B, Peter B, Bauer K, Berger D, Gamperl S, et al. The JAK2/STAT5 signaling pathway as a potential therapeutic target in canine mastocytoma. Vet Comp Oncol. 2017 doi: 10.1111/vco.12311. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peter B, Winter GE, Blatt K, Bennett KL, Stefanzl G, Rix U, et al. Target interaction profiling of midostaurin and its metabolites in neoplastic mast cells predicts distinct effects on activation and growth. Leukemia. 2016;30:464–472. doi: 10.1038/leu.2015.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tobío A, Alfonso A, Fernández-Araujo A, Alonso E, Botana LM. Protein kinase C modulates Aurora-kinase inhibition induced by CCT129202 in HMC-1560,816 cell line. Antiinflamm Antiallergy Agents Med Chem. 2013;12:265–276. doi: 10.2174/18715230113129990002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.