SUMMARY
SETTING:
Households in Malawi, Mongolia, Myanmar, the Philippines, Rwanda, Tanzania, Viet Nam and Zambia.
OBJECTIVE:
To assess the relationship between household socio-economic level, both relative and absolute, and individual tuberculosis (TB) disease.
DESIGN:
We analysed national TB prevalence surveys from eight countries individually and in pooled multi-country models. Socio-economic level (SEL) was measured in terms of both relative household position and absolute wealth. The outcome of interest was whether or not an individual had TB disease. Logistic regression models were used to control for putative risk factors for TB disease such as age, sex and previous treatment history.
RESULTS:
Overall, a strong and consistent association between household SEL and individual TB disease was not found. Significant results were found in four individual country models, with the lowest socio-economic quintile being associated with higher TB risk in Mongolia, Myanmar, Tanzania and Viet Nam.
CONCLUSIONS:
TB prevalence surveys are designed to assess prevalence of disease and, due to the small numbers of cases usually detected, may not be the most efficient means of investigating TB risk factors. Different designs are needed, including measuring the SEL of individuals in nested case-control studies within TB prevalence surveys or among TB patients seeking treatment in health care facilities.
Keywords: socio-economic level, prevalence survey, asset score, wealth
RESUME
CONTEXTE :
Des foyers au Malawi, en Mongolie, au Myanmar, aux Philippines, au Rwanda, en Tanzanie, au Viet Nam et en Zambie.
OBJECTIF :
Evaluer la relation entre le niveau socioéconomique des ménages, tant absolu que relatif, et la tuberculose (TB) maladie individuelle.
SCHÉMA :
Nous avons analysé les enquêtes nationales de prévalence de la TB de huit pays, individuellement et dans des modèles multi-pays mis en commun. Le niveau socio-économique a été mesuré en termes de position relative à la fois des ménages et de la richesse absolue. Le résultat souhaité était de savoir si une personne était ou non atteinte de TB. Des modèles de régression logistique ont été utilisés pour contrôler les facteurs de risque putatifs de la maladie tuberculeuse comme l’âge, le sexe et les antécédents de traitement.
RÉSULTATS :
Dans l’ensemble, nous n’avons pas constaté d’association solide et cohérente entre le niveau socio-économique des familles et la maladie tuberculeuse. Des résultats significatifs ont été trouvés dans quatre modèles individuels de pays, le plus bas quintile socio-économique étant associé à un risque de TB plus élevé en Mongolie, au Myanmar, en Tanzanie et au Viet Nam.
CONCLUSION :
Les enquêtes de prévalence de la TB sont conçues pour évaluer la prévalence de la maladie et, en raison du petit nombre de cas habituellement détectés, peuvent ne pas être le moyen le plus efficace d’étudier les facteurs de risque de la TB. Différents modèles sont nécessaires, y compris la mesure du niveau socio-économique des individus dans des études castémoins imbriquées dans les enquêtes de prevalence de la TB ou chez les patients atteints de TB qui sollicitent un traitement dans les établissements de soins de santé.
RESUMEN
MARCO DE REFERENCIA:
Los hogares en Malawi, Mongolia, Birmania, Filipinas, Ruanda, Tanzania, Viet Nam y Zambia.
OBJETIVO:
Evaluar la relación entre la situación socioeconómica de los hogares, relativa y absoluta, y los casos individuales de enfermedad tuberculosa.
MÉTODO:
Se analizaron las encuestas nacionales de prevalencia de tuberculosis (TB) de ocho países de manera individual y en modelos combinados multinacionales. Se midió la situación socioeconómica según la posición relativa del hogar y su riqueza absoluta. El criterio de valoración fue la presencia o ausencia de TB en una persona. Se aplicaron modelos de regresión logística, con el fin de ajustar los posibles factores de riesgo enfermedad tuberculosa como la edad, el sexo y los antecedentes de tratamiento.
RESULTADOS:
En general, no se observó una asociación constante y firme entre la situación socioeconómica del hogar y la presencia de TB en una persona. Se encontraron resultados significativos en los modelos de cuatro países, en los cuales se asoció el quintil socioeconómico más bajo con un mayor riesgo de padecer TB en Mongolia, Birmania, Tanzania y Viet Nam.
CONCLUSIÓN:
Las encuestas de prevalencia de TB tienen por finalidad evaluar la prevalencia de la enfermedad y debido al bajo número de casos que se suele detectar, no constituyen el método más eficiente de investigar los factores de riesgo de contraer la TB. Se precisan métodos diferentes que incluyan el nivel socioeconómico de las personas, en estudios de casos y testigos anidados, en el marco de las encuestas de prevalencia o en los pacientes tuberculosos que acuden en busca de tratamiento a los establecimientos de salud.
THE NEWLY ADOPTED END TB STRATEGY has an increased emphasis on the social determinants of tuberculosis (TB).1 It calls for bold policies in the areas of universal health coverage, social protection and poverty alleviation. As attention shifts to interventions outside the medical sphere, it becomes increasingly important to understand how poverty differentially affects TB risk in different countries. A country with great TB disparities between individuals in higher and lower socio-economic groups may want to increase its focus on activities that target the most vulnerable populations, such as through an active case finding programme in slums. Such targeting may yield greater benefit than in a country that has relatively equal TB risk across its socio-economic continuum.
There is considerable literature showing that socio-economic level (SEL) is associated with TB burden. However, much of this work relies on ecological data and does not adjust for well-known individual-level risk factors. Spence et al. analysed 33 districts in the United Kingdom and found a strong correlation between rates of poverty and TB prevalence rates.2 Similar findings have been observed in census tracts in California, USA, and São José do Rio Preto, Brazil, as well as in administrative districts in Cambodia.3–5 In 2008, Janssens and Rieder found a significant inverse relationship between gross domestic product and TB incidence using data from 171 countries, but were limited to 1 year of data and did not control for other confounding factors.6
Many studies that analyse data at the individual level focus only on patients who are already on anti-tuberculosis treatment. Belo et al. examined how TB treatment success rates were associated with SEL among TB patients on treatment in Duque de Caxias, Brazil.7 They found that those from the lowest socio-economic group had over four times the odds of having an unsuccessful treatment outcome compared to those in the highest socio-economic group. Individual level analyses have investigated the relationship between SEL and TB using Demographic and Health Surveys (DHS) from South Africa and India.8,9 These studies found the lowest quintile to have approximately 5 and 12 times the risk of recent tuberculous infection, respectively, relative to the wealthiest quintile after controlling for other risk factors. One major limitation to these studies was that they used self-reported recent tuberculous infection.
To our knowledge, this is the first study to combine individual-level data from numerous countries to examine the relationship between household SEL and bacteriologically confirmed TB disease at the individual level. The conceptual framework for this work has been adapted from a model of determinants of TB developed by Lönnroth et al.10 In this model, poverty does not affect TB directly, but does so indirectly by increasing vulnerability. This analysis represents a reduced-form model, as malnutrition and overcrowding are not directly measured. The rationale is that an individual living in a less impoverished household will have better living conditions, nutrition and other unmeasured risk factors, presumably making them less susceptible to TB exposure and/or progression to disease.
This work improves upon previous research by using a rigorous diagnostic algorithm for TB rather than self-reported disease. Furthermore, not only do we include analyses of SEL within a country and TB risk, we also show how the absolute level of household wealth affects TB risk. Our findings may be useful for anti-tuberculosis treatment programmes and policy makers, as it allows them to allocate resources to best meet a country’s specific TB burden.
STUDY POPULATION AND METHODS
TB prevalence surveys are nationally representative household surveys that aim to estimate the prevalence of bacteriologically confirmed pulmonary TB disease. Methods that incorporate systematic screening of all participants by symptom questionnaire and chest X-ray, followed by case confirmation with at least culture, have been standardised since 2009.11 As TB is a relatively rare disease, prevalence surveys often evaluate up to 90 000 individuals to ensure enough bacteriologically confirmed TB cases are detected to produce reliable burden estimates. In addition, these surveys collect large amounts of individual-level data, including demographics, TB-related symptoms and household assets/characteristics. Between 2007 and 2014, 20 prevalence surveys using standardised methods were completed, eight of which had available individual-level household asset data. These countries include Malawi, Mongolia, Myanmar, the Philippines, Rwanda, Tanzania, Viet Nam and Zambia.
SEL was measured using household asset questions on TB prevalence surveys, which typically ask about the presence of durable goods, the quality and materials used for housing construction, and types of water access and sanitation facilities. Principal component analysis (PCA) was used to create household asset scores from these items. This approach to poverty measurement has recently been gaining popularity, as the DHS and World Bank have favoured this method.12 Furthermore, several studies have shown a correlation between household asset score and directly measured household expenditure.12,13 Once asset scores were derived, households were classified into five wealth quintiles within each survey.* It is worth noting that household asset scores represent each household’s relative SEL within a particular country in a given survey year. Each survey was analysed separately, and these models describe the association between relative SEL and TB within a given country at the time of the prevalence survey. The highest quintile of the asset score (the richest) serves as the reference group. Due to the rarity of TB disease, odds ratios (ORs) approximate relative risk.14
Beyond these models, pooled multicountry analyses that use an absolute wealth estimate (AWE) in constant US dollars for each household were used. This metric utilises the ranking of each household’s asset score within a country as well as information on the country’s gross domestic product per capita and Gini coefficient, a well-known measure of inequality. The method has been validated against World Bank poverty indicators as well as nutritional status indicators known to be linked to poverty.15 The AWE allows us to examine the effect of absolute household wealth, not relative SEL, on the risk of TB disease. As all AWEs are given in 2014-constant US dollars with purchasing power parity, the wealth of households from different countries or time periods are comparable. Mongolia data were excluded from these models because, at the time of this study, the data were not nationally representative—they only included the urban portion of the country. We could not therefore provide accurate input to produce AWEs for these Mongolian households.
The creation of wealth quintiles may be splitting the cases across too many strata. In sensitivity analyses, wealth tertiles were therefore created as well as a comparison between the bottom quintile and the remaining four quintiles. Models that excluded people who had previously been treated for TB were also tested. The logic behind this was that if SEL were to affect TB risk, then including previously treated patients would introduce endogeneity in the models. Stata, version 13 (StataCorp, College Station, TX, USA) was used for all analyses.
Free and informed consent was obtained from all subjects or their legal guardians, and the study was granted exemption from the University of California, Los Angeles (Los Angeles, CA, USA) institutional review board.
RESULTS
Across surveys, the number of participants ranged from 30 667 to 87 413 (Table 1). The proportion of respondents previously treated for TB varied substantially across the surveys, ranging from 0.7% in Zambia to 3.3% in the Philippines. The number of TB cases identified for each survey was quite small relative to the number of those who were not diagnosed with TB (non-cases). Myanmar, with 311 cases, had the highest proportion of TB cases among its participants (0.61%), and Rwanda had the lowest (0.08%).
Table 1.
Characteristics of national TB prevalence surveys analysed
| Country | Malawi n (%) | Mongolia (urban) n (%) | Myanmar n (%) | Philippines n (%) | Rwanda n (%) | Tanzania n (%) | Viet Nam n (%) | Zambia n (%) |
|---|---|---|---|---|---|---|---|---|
| Main year of survey | 2013 | 2014 | 2009 | 2007 | 2012 | 2012 | 2007 | 2014 |
| Participants, n | 31 579 | 46 785 | 51 367 | 30 667 | 43 128 | 50 477 | 87 413 | 46 099 |
| Sex | ||||||||
| Male | 18 480 (58.5) | 21 065 (45.0) | 22 394 (43.6) | 15 549 (50.7) | 18 195 (42.2) | 20 735 (41.1) | 39 654 (45.4) | 19 457 (42.2) |
| Female | 13 099 (41.5) | 25 720 (55.0) | 28 973 (56.4) | 15 118 (49.3) | 24 933 (57.8) | 29 701 (58.9) | 47 759 (54.6) | 26 642 (57.8) |
| Setting | ||||||||
| Urban | 2 889 (9.2) | — | 11 254 (21.91) | 14 745 (48.1) | — | 19 588 (38.8) | 24 400 (27.9) | 30 042 (65.2) |
| Rural | 28 690 (90.9) | — | 40 113 (78.09) | 15 922 (51.9) | — | 30 889 (61.2) | 63 013 (72.1) | 16 057 (34.8) |
| Current/previous anti-tuberculosis treatment | ||||||||
| Yes | 250 (0.8) | 1 268 (2. 7) | 1463 (2.8) | 999 (3.3) | 559 (1.3) | 790 (1.6) | 1558 (1.8) | 644 (1.4) |
| No | 31 329 (99.2) | 45 517 (97.3) | 49 904 (97.2) | 29 668 (96.7) | 42 569 (98.7) | 49 657 (98.4) | 85 292 (98.2) | 45 455 (98.6) |
| Age, years, mean ± SD | 34.65 ± 16.86 | 31.26 ± 19.37 | 38.87 ± 16.46 | 25.73 ± 19.84 | 35.55 ± 16.59 | 38.14 ± 17.83 | 40.11 ± 17.41 | 36.09 ± 16.96 |
| TB status | ||||||||
| Cases | 132 (0.4) | 142 (0.3) | 311 (0.6) | 136 (0.4) | 40 (0.1) | 155 (0.3) | 250 (0.3) | 265 (0.3) |
| Non-cases | 31 447 (99.6) | 46 643 (99.7) | 51 056 (99.4) | 30 531 (99.6) | 43 088 (99.9) | 50 292 (99.7) | 87 163 (99.7) | 45 834 (99.4) |
TB = tuberculosis; SD = standard deviation.
In the single-country analyses, SEL was not predictive of TB disease in the majority of country surveys after adjusting for other risk factors (Table 2). Viet Nam and Myanmar are exceptional in that there is a pattern of higher risk among lower quintile households. In Viet Nam, an individual residing in the lowest quintile household had approximately double the risk (OR 1.94, 95% confidence interval [CI] 1.21–3.11) of TB disease as someone residing in the highest quintile household. This same OR was 1.52 (95%CI 1.03–2.25) in Myanmar. Despite the significant findings in these two settings, the ORs did not align consistently with the socio-economic gradient. For example, the Myanmar model predicts higher associated TB risk with residing in a second quintile home relative to a household in the lowest quintile (OR 1.75 vs. 1.52). In fact, none of the models show consistently decreasing ORs when moving up the socio-economic gradient. Several other quintiles are associated with significantly higher risk relative to the wealthiest quintile: the lowest quintiles in Mongolia and Tanzania, as well as the fourth quintile in Zambia. The significance pattern of these results did not substantially differ in the use of wealth tertiles or when comparing the bottom quintiles to the upper four quintile groups within each country. The results were not significantly different when previously treated patients were excluded from regression models. Other risk factors included in the models were significantly associated with increased risk of TB disease across country settings. Previous anti-tuberculosis treatment was strongly associated with TB risk, with ORs ranging from 2.85 (the Philippines) to 8.07 (Zambia). Males were more likely to have TB in all settings, with OR ranging from 1.31 (Malawi) to 4.89 (Viet Nam). Finally, an additional year of age was significantly associated with increased TB risk, with an additional risk of 2–4% per year.
Table 2.
ORs (95%CIs) of TB cases with separate model for each country
| Country | Malawi OR (95%CI) | Mongolia (urban) OR (95%CI) | Myanmar OR (95%CI) | Philippines OR (95%CI) | Rwanda OR (95%CI) | Tanzania OR (95%CI) | Viet Nam OR (95%CI) | Zambia OR (95%CI) |
|---|---|---|---|---|---|---|---|---|
| Socio-economic level quintile* | ||||||||
| 1 | 1.03 (0.55–1.93) | 1.95 (1.05–3.59)† | 1.52 (1.03–2.25)† | 1.09 (0.61–1.95) | 1.22 (0.54–2.74) | 1.88 (1.05–3.39)† | 1.94 (1.21–3.11)‡ | 1.02 (0.61–1.72) |
| 2 | 1.67 (0.96–2.91) | 0.74 (0.35–1.56) | 1.75 (1.18–2.60)† | 1.48 (0.89–2.49) | 0.52 (0.19–1.41) | 1.37 (0.73–2.57) | 2.15 (1.37–3.37)‡ | 0.85 (0.51–1.40) |
| 3 | 1.61 (0.91–2.84) | 0.87 (0.42–1.78) | 1.33 (0.89–1.98) | 0.93 (0.53–1.65) | 0.43 (0.14–1.34) | 1.26 (0.67–2.36) | 1.75 (1.11–2.76)† | 1.21 (0.79–1.86) |
| 4 | 0.9 (0.49–1.67) | 1.02 (0.52–2.03) | 1.63 (1.11–2.39)† | 0.98 (0.56–1.71) | 0.59 (0.22–1.62) | 0.90 (0.46–1.77) | 1.37 (0.85–2.19) | 1.47 (1.03–2.11)† |
| 5 | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| Sex | ||||||||
| Male | 1.31 (0.93–1.85) | 2.30 (1.48–3.55)§ | 2.60 (2.04–3.32)§ | 2.65 (1.81–3.86)§ | 4.01 (1.95–8.22)§ | 2.04 (1.40–2.97)§ | 4.89 (3.55–6.70)§ | 1.87 (1.44–2.43)§ |
| Female | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| Urban setting | 3.36 (2.12–5.71)§ | — | 1.52 (1.18–1.96)‡ | 1.06 (0.74–1.51) | — | 1.28 (0.87–1.89) | 0.96 (0.70–1.30)§ | 1.83 (1.30–2.58)§ |
| Current/previous anti-tuberculosis treatment | 6.95 (3.62–13.4)§ | 4.77 (2.56–8.90)§ | 5.00 (3.64–6.86)§ | 2.85 (1.78–4.54)§ | 7.11 (2.79–18.7)§ | 5.37 (2.97–9.72)§ | 4.85 (3.29–7.15)§ | 8.07 (5.50–11.8)§ |
| Age, years | 1.04 (1.03–1.05)§ | 1.02 (1.01–1.03)§ | 1.03 (1.03–1.04)§ | 1.04 (1.03–1.05)§ | 1.02 (1.01–1.04)‡ | 1.03 (1.02–1.04)§ | 1.04 (1.03–1.05)§ | 1.02 (1.01–1.02)§ |
Quintiles 1 and 5 are the lowest and highest ranked socio-economic level, respectively.
P < 0.05.
P < 0.01.
P < 0.001.
OR = odds ratio; CI = confidence interval; TB = tuberculosis.
When using a logged scale of AWEs for households within each survey, the distributions appear to be approximately normally distributed, with several being skewed to the right (Figure). The AWEs were used in pooled multicountry analyses of SEL on TB risk (Table 3). The first two models in Table 3 show that when data are pooled together, SEL quintiles are not predictive of TB disease, with or without country-level dummy indicators. Models 3 and 4 show a similar lack of significance between AWE and TB disease, indicating that an additional hundred dollars of household wealth has no association with TB.
Figure.
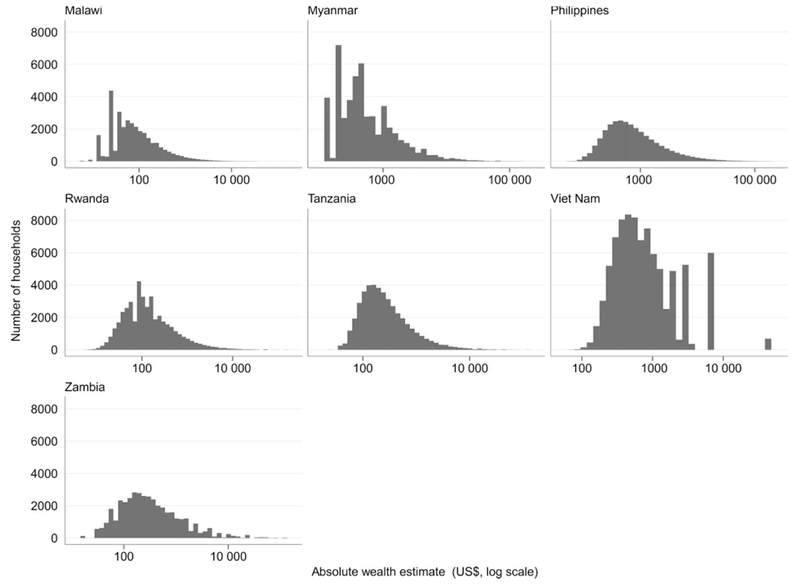
Distribution of household average wealth estimate.
Table 3.
ORs (95%CIs) of TB disease using pooled multicountry models
| Model 1: Wealth quintiles (n = 356 943) | Model 2: Wealth quintiles with country-level dummy variables (n = 356 943) | Model 3: AWE (n = 326 329)* | Model 4: AWE with country-level dummy variables (n = 326 329)* | |
|---|---|---|---|---|
| Socio-economic level quintile† | ||||
| 1 | 1.21 (1.02–1.44)‡ | 1.18 (0.99–1.41) | — | — |
| 2 | 1.17 (0.98–1.40) | 1.17 (0.98–1.40) | — | — |
| 3 | 1.09 (0.91–1.31) | 1.08 (0.90–1.30) | — | — |
| 4 | 1.15 (0.96–1.37) | 1.14 (0.96–1.37) | — | — |
| 5 | Reference | Reference | — | — |
| AWE, 2014 $US hundreds | — | — | 1.00 (1.00–1.00) | 1.00 (1.00–1.00) |
| Sex | ||||
| Male | 2.43 (2.17–2.73)§ | 2.42 (2.16–2.72) § | 2.43 (2.16–2.75)§ | 2.43 (2.15–2.74)§ |
| Female | Reference | Reference | Reference | Reference |
| Current/previous anti-tuberculosis treatment | 5.70 (4.85–6.71)§ | 5.36 (4.55–6.32) § | 5.76 (4.87–6.82)§ | 5.38 (4.54–6.38)§ |
| Age, years | 1.03 (1.03–1.03)§ | 1.03 (1.03–1.03) § | 1.03 (1.03–1.03)§ | 1.03 (1.03–1.03)§ |
Mongolia was excluded from AWE models because this survey only included urban clusters and was therefore not nationally representative.
Quintiles 1 and 5 are respectively the lowest and highest ranked socio-economic level.
P < 0.05.
P < 0.001.
OR = odds ratio; CI = confidence interval; TB = tuberculosis; AWE = absolute wealth estimate.
DISCUSSION
The study set out to document the relationship between TB and SEL. In general, such a relationship was not found, contrary to our hypotheses and previous research. The relationship between a household’s lower SEL, relative to other households, was significant in four of the eight countries studied, but this association did not exhibit a dose-response relationship as expected. Furthermore, when assessing absolute wealth in multi-country pooled analyses, no relationship was found.
Despite these results, we caution that the absence of consistent findings found here should not be taken to mean that household SEL is unrelated to TB risk. There are a number of reasons why such a relationship may not have been detected. First, the relationship between SEL and TB may actually differ among countries due to the presence and level of a range of other confounding factors, such as population density, effectiveness of national TB control programmes, and the ways in which households in different country contexts experience poverty. Potential policy implications of these findings are that the lack of association between relative SEL and TB in some countries may warrant national TB policies that reach households across the entire socio-economic spectrum. For other countries, such as Viet Nam, Myanmar, Tanzania and Mongolia, there does seem to be some justification to allocate policy action to the lower SEL groups. However, the lack of consistently significant findings and several other limitations restrict our ability to make strong recommendations.
Due to the relatively low prevalence of TB in the general population among the 387 515 individuals across the eight prevalence surveys, there were only 1399 cases of bacteriologically confirmed TB. This creates an issue of statistical power due to the small number of cases in certain strata. As a result, it may be true that individuals residing in lower SEL households are at a greater risk of TB, but we are unable to show this relationship without more cases. This is one of the main arguments against using TB prevalence surveys to identify risk factors. Nevertheless, the risk factors age, sex and previous treatment history were very clearly associated with TB disease across the surveys, as well as in the pooled analysis.
This study is also unable to measure SEL prior to an individual’s participation in the survey. Estimating the causal impact of SEL on TB using cross-sectional prevalence surveys is subject to endogeneity bias, as it has been established that TB can lead to poverty.16 TB often strikes in the prime of an individual’s earningy ears, and it has been estimated that up to 60% of the cost of TB can be attributed to lost wages.17 However, the decision to use assets as the main measure of SEL minimises this concern, as assets are considered to be ‘slow moving’.13 It has been shown that even important changes in the household SEL may not affect the ownership of assets in the medium-term.18 Furthermore, this analysis represents the reduced-form model, which does not measure more proximal risk factors associated with both poverty and TB such as undernutrition or crowding. One potential explanation for the lack of significance is that the complex causal pathway from low household SEL to increased vulnerability and eventual TB disease is not fully understood.
CONCLUSIONS
Policy makers across the globe need better population-based evidence to guide allocation of resources beyond the health care sphere to maximise the impact of TB control programmes. This analysis was an attempt to provide that evidence by identifying the TB risk differential for the most impoverished households. Despite a robust measure of TB disease and large, individual-level data sets, this analysis showed limited evidence for an increased risk of TB among individuals residing in households of lower SEL.
If the goal is to assess whether household poverty is a risk factor for TB, collecting household asset data from all participants in TB prevalence surveys may not be an efficient use of resources. These analyses may be underpowered even in the context of these large surveys. The resources freed up by not asking all respondents about household SEL could be used to conduct a nested case-control study within the TB prevalence survey. This smaller analysis would allow for a more in-depth investigation into the social risk factors associated with TB disease. Coker et al. employed this design in the Russian Federation and found a strong link between household asset ownership and TB risk.18 Another alternative would be to routinely collect information on the household SEL of patients in TB treatment centres. This information could be used in conjunction with national household surveys that place more emphasis on SEL (e.g., DHS). The SEL of households from the national survey would serve as a comparator group to TB patients and provide a stronger method of exploring the association between household SEL and TB.
Acknowledgements
The authors would like to thank N Kapata and his contribution to this work, as well as D Hruschka for sharing his code on creating absolute wealth estimates. This research was supported by the National Institutes of Health/National Center for Advancing Translational Science (University of California, Los Angeles, CA, USA), Clinical and Translational Science Institute grant number TL1TR000121.
The authors alone are responsible for the views expressed in this publication and they do not necessarily represent the decisions or policies of the World Health Organization. The authors affirm that they did not enter into an agreement with the funder that may have limited their ability to complete the research as planned, and they have had full control of all primary data.
Footnotes
The lists of items used to create the poverty score in each country can be obtained from the corresponding author on request.
Conflicts of interest: none declared.
References
- 1. Uplekar M Weil D Lönnroth K et al. WHO’s new End TB strategy Lancet 2015. 385 1799–1801 [DOI] [PubMed] [Google Scholar]
- 2. Spence DP Hotchkiss J Williams CS Davies PD Tuberculosis and poverty BMJ 1993. 307 759–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Myers WP Westenhouse JL Flood J Riley LW An ecological study of tuberculosis transmission in California Am J Public Health 2006. 96 685–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vendramini SH Santos ML Gazetta CE Chiaravalloti-Neto F Ruffino-Netto A Villa TC Tuberculosis risks and socio-economic level: a case study of a city in the Brazilian south-east, 1998-2004 Int J Tuberc Lung Dis 2006. 10 1231–1235 [PubMed] [Google Scholar]
- 5. Wong MK Yadav RP Nishikiori N Eang MT The association between household poverty rates and tuberculosis case notification rates in Cambodia, 2010 Western Pac Surveill Response J 2013. 4 25–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Janssens JP Rieder HL An ecological analysis of incidence of tuberculosis and per capita gross domestic product Eur Respir J 2008. 32 1415–1416 [DOI] [PubMed] [Google Scholar]
- 7. Belo MT Luiz RR Teixeira EG Hanson C Trajman A Tuberculosis treatment outcomes and socio-economic status: a prospective study in Duque de Caxias, Brazil Int J Tuberc Lung Dis 2011. 15 978–981 [DOI] [PubMed] [Google Scholar]
- 8. Harling G Ehrlich R Myer L The social epidemiology of tuberculosis in South Africa: a multilevel analysis Soc Sci Med 2008. 66 492–505 [DOI] [PubMed] [Google Scholar]
- 9.Oxlade O, Murray M. Tuberculosis and poverty: why are the poor at greater risk in India? PLOS ONE. 2012;7:e47533. doi: 10.1371/journal.pone.0047533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lönnroth K Jaramillo E Williams BG Dye C Raviglione M Drivers of tuberculosis epidemics: the role of risk factors and social determinants Soc Sci Med 2009. 68 2240–2246 [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization. Tuberculosis prevalence surveys: a handbook. WHO; Geneva, Switzerland: 2011. WHO/HTM/TB/2010.17. [Google Scholar]
- 12. Filmer D Pritchett LH Estimating wealth effects without expenditure data—or tears: an application to educational enrollments in states of India Demography 2001. 38 115–132 [DOI] [PubMed] [Google Scholar]
- 13. Morris SS Carletto C Hoddinott J Christiaensen LJ Validity of rapid estimates of household wealth and income for health surveys in rural Africa J Epidemiol Community Health 2000. 54 381–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang J Yu KF What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes JAMA 1998. 280 1690–1691 [DOI] [PubMed] [Google Scholar]
- 15. Hruschka DJ Gerkey D Hadley C Estimating the absolute wealth of households Bull World Health Organ 2015. 93 483–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tanimura T Jaramillo E Weil D Raviglione M Lönnroth K Financial burden for tuberculosis patients in low- and middle-income countries: a systematic review Eur Respir J 2014. 43 1763–1775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Falkingham J, Namazie C. Measuring health and poverty: a review of approaches to identifying the poor. Department for International Development; London, UK: 2002. [Google Scholar]
- 18. Coker R McKee M Atun R et al. Risk factors for pulmonary tuberculosis in Russia: case-control study BMJ 2006. 332 85–87 [DOI] [PMC free article] [PubMed] [Google Scholar]


