Abstract
Sleep disturbances are prevalent in posttraumatic stress disorder (PTSD) and are associated with a number of adverse health consequences. Few studies have used comprehensive assessment methods to characterize sleep in Operation Iraqi Freedom/Operation Enduring Freedom/Operation New Dawn (OEF/OIF/OND) veterans with PTSD. OEF/OIF/OND veterans with PTSD and sleep disturbance (n = 45) were compared to patients with primary insomnia (n = 25) and healthy control subjects (n = 27). Participants were assessed using questionnaire-based measures as well as daily subjective and objective measures of sleep. The 3 groups were compared with regard to (a) group means, (b) intraindividual (i.e., night-to-night) variability of sleep, and (c) interindividual (i.e., within-group) variability of sleep. In terms of group means, only objective sleep efficiency was significantly worse with PTSD than with primary insomnia (d = 0.54). Those with PTSD differed from those with primary insomnia on measures of intraindividual as well as interindividual variability (d = 0.48–0.73). These results suggested sleep symptoms in OEF/OIF/OND veterans with PTSD are more variable across nights and less consistent across patients relative to sleep symptoms in insomnia patients without PTSD. These findings have implications for research, as well as for personalizing treatment for individuals with PTSD.
Sleep disturbances are highly prevalent in posttraumatic stress disorder (PTSD) and are among the most distressing and chronic symptoms (McLay, Klam, & Volkert, 2010). Specifically, PTSD patients show longer sleep latencies, longer time awake after sleep onset, and shorter total sleep time than healthy controls (Ohayon & Shapiro, 2000). In civilian samples, approximately 70% of PTSD patients experience associated sleep disturbance (Ohayon & Shapiro, 2000). These estimates are even higher for combat veterans (90%; Neylan et al., 1998).
Significant consequences and functional impairment are associated with sleep disturbance. Disrupted sleep is associated with low quality of life (Zammit, Weiner, Damato, Sillup, & McMillan, 1999), poor emotional coping and decreased ability to handle life stressors (Spoormaker & Montgomery, 2008), suicidal ideation (Goodwin & Marusic, 2008), and risky drinking (Swinkels, Ulmer, Beckham, Buse, & Calhoun, 2012). Moreover, insomnia is correlated with functional impairments such as increased risk for work-related accidents (Léger, Guilleminault, Bader, Lévy, & Paillard, 2002).
Given individuals with PTSD also show increased risk for many of the comorbidities listed above (e.g., Mendlowicz & Stein, 2000), sleep disturbances may further complicate the clinical presentation of PTSD. This could be especially true among Operation Iraqi Freedom/Operation Enduring Freedom/Operation New Dawn (OEF/OIF/OND) veterans, because their relatively young age suggests long-term mental and physical health consequences of chronically disturbed sleep could be especially costly, both financially and personally (Hillman, Murphy, Antic, & Pezzullo, 2006).
Although a few studies have investigated the prevalence and consequences of sleep disturbance in OEF/OIF/OND veterans with PTSD (McLay et al., 2010; Swinkels et al., 2012), little is known about whether sleep disturbances in PTSD differ from those in a population with a more “pure” sleep problem. The most commonly reported sleep problems in PTSD (e.g., long sleep latency, large periods of wake in the middle of the night) appear very similar to those of primary insomnia. Nonetheless, issues unique to PTSD suggest sleep disturbances may be worse or different for PTSD patients (Hughes, Jouldjian, Washington, Alessi, & Martin, 2013). For example, individuals with PTSD often report distressing dreams or hyperarousal related to nighttime traumas (Mellman, Kulick-Bell, Ashlock, & Nolan, 1995), both of which may cause different sleep disruptions than primary insomnia and have important treatment implications.
Supporting the hypothesis sleep in PTSD may differ from insomnia alone, one study reported female veterans with insomnia and probable PTSD had worse self-reported sleep quality than veterans with insomnia symptoms alone (Hughes et al., 2013). Notably, this study used only retrospective self-report questionnaires to measure both sleep and PTSD symptoms. Despite these limitations, findings from Hughes et al. (2013) suggested more studies in this area would highlight clinically relevant sleep disruption differences across patient groups.
In addition to comparing across diagnostic groups when examining sleep, it can be informative to examine not only sample means, but also variability within a group and within individuals. Behaviors such as excessive time in bed and irregular sleep/wake timing have been hypothesized to contribute to the development and maintenance of insomnia symptoms. Examination of patients with primary insomnia supports this hypothesis, as these patients show more night-to-night variability than controls (Buysse et al., 2010). Sleep variability may be particularly important in PTSD, as patients with PTSD show additional sleep symptoms not present in primary insomnia (e.g., distressing dreams). These additional factors may make sleep symptoms even more unpredictable from night to night or from individual to individual in patients with PTSD. Recent evidence suggests that sleep/wake inconsistency is more prominent in populations with comorbid insomnia than in patients with insomnia alone (Sánchez-Ortuno, Carney, Edinger, Wyatt, & Harris, 2011), suggesting sleep variability may be particularly salient for PTSD. Additionally, sleep/wake inconsistency may relate to other clinical outcomes such as depression severity (Suh et al., 2012). Because behavioral interventions for insomnia can include a focus on decreasing inconsistencies in sleep/wake schedules (Talbot et al., 2014), examining sleep variability in PTSD, especially relative to that seen in primary insomnia, may have clinical implications.
Our aim here was to provide a detailed characterization of sleep in OEF/OIF/OND veterans with PTSD and insomnia, relative to a group with primary insomnia. We also included a healthy control group, as a point of reference for both patient samples. We hypothesized that (a) patients with PTSD and insomnia would show worse sleep continuity on daily measures, as well as lower retrospective sleep quality than patients with insomnia alone; and (b) patients with PTSD and insomnia would show greater sleep inconsistency (i.e., greater within-group and night-to-night variability), than patients with insomnia alone. The controls were included to provide perspective on how impaired the two patient groups were relative to age-matched healthy controls.
Method
Participants
A pool of 55 U.S. veterans and active duty personnel with PTSD and sleep disturbance were recruited from the VA San Diego Healthcare System (VASDHS) and Naval Medical Center San Diego and gave consent to participate in the current study. They were recruited to participate in a larger clinical trial (Project NITES; NCT01009112), which involved treatments specifically targeting PTSD and sleep symptoms. Thus, the participants were seeking treatment for both PTSD and related sleep problems. Inclusion criteria included (a) being deployed at least once as part of OEF/OIF/OND, (b) having a diagnosis current PTSD from military-related trauma, (c) meeting criteria for comorbid insomnia, and (d) reporting ≥ two distressing dreams/week. Exclusion criteria included (a) unmanaged psychosis or mania, (b) substance abuse or dependence during the prior 6 months, (c) untreated sleep disorder other than insomnia and nightmares, (d) use of hypnotics or prazosin, (e) a history of severe TBI, and (f) concurrent psychotherapy for PTSD. Of the 55 veterans consented, 10 did not meet eligibility criteria and were excluded. The final group comprised veterans (n = 37), active duty service personnel (n = 5), and reserves (n = 3). Participants in this group had a mean age of 35.00 (SD = 9.16). Of the 45 PTSD participants, 57.7% had a history of mild TBI. Comorbid Axis I diagnoses included major depressive disorder (57.7%), past alcohol abuse or dependence (53.3%), past other substance abuse or dependence (15.5%), specific phobia (8.9%), panic disorder (4.4%), social phobia (4.4%), and obsessive-compulsive disorder (2.2%). Of the 45, 14 (31.1%) were taking no medications. Medications for the remainder included antidepressants (40.0%), migraine medications (15.6%), narcotic analgesics (13.3%), beta blockers (6.7%), antipsychotics (4.4%), anxiolytics (2.2%), psychostimulants (2.2%), and other nonpsychotropic medications (31.1%).
Patients with primary insomnia (PI; n = 25) and healthy controls (n = 27) were taken from separate studies in the lab running concurrent to Project NITES and were matched to the PTSD sample according to age. Veteran status was not assessed for these participants. Participants met the following criteria: (a) stable sleep/wake schedule with a preferred sleep phase between 10:00 p.m. and 8:00 a.m., (b) no current Axis I disorder other than insomnia or a specific phobia and no personal history of any Axis I disorder other than a single major depressive episode or a specific phobia, (c) no sleep disorder (other than insomnia, for PI subjects), (d) no use of hypnotics or psychotropic medication, and (e) no history of TBI.
PI patients had to meet the following additional eligibility criteria: (a) sleep difficulties ≥ three nights/week for ≥ 3months as assessed by the Duke Sleep Assessment; (b) ≥45 min total wake time after lights out (i.e., sleep latency + wake after sleep onset), and either <6 h total sleep time or sleep efficiency <80%, as assessed by sleep diaries.
Controls had to additionally report: (a) 7–9 hours total sleep time per night, (b) average sleep efficiency ≥ 90%, (c) taking fewer than one daytime naps per week, and (d) having no daytime performance complaints as assessed by interview and sleep diaries. PI patients had a mean age of 32.28 years (SD = 7.24). Controls had a mean age of 32.15 years (SD = 7.54).
See Table 1 for a summary of participant demographics. Likely because PI patients and controls were taken from other protocols, these groups significantly differed from the PTSD patients on a number of variables including veteran status: gender χ2(2, N = 97) = 11.2, p =.004; race χ2(4, N = 97) = 18.5, p <. 001; and education level χ2(3, N = 97) = 32.5, p < .001. The groups did not significantly differ on age or ethnicity.
Table 1.
Participant Demographics Separately by Group
| PTSD (n = 45) |
PI (n = 25) |
Controls (n = 27) |
|||||
|---|---|---|---|---|---|---|---|
| Variable | M or n | SD or % | M or n | SD or % | M or n | SD or % | Test |
| Age (years) | 35.00 | 9.14 | 32.28 | 7.24 | 32.15 | 7.54 | F = 1.38 |
| Women | 6 | 13.3 | 12 | 48.0 | 5 | 18.5 | χ2 = 11.2* |
| Hispanic/Latino | 13 | 28.9 | 3 | 12.0 | 6 | 22.2 | χ2 = 1.6 |
| Race | χ2 = 18.5* | ||||||
| Caucasian | 28 | 62.2 | 20 | 80.0 | 20 | 74.1 | |
| Asian | 1 | 2.2 | 0 | 0.0 | 5 | 18.5 | |
| Black/African American | 10 | 22.2 | 4 | 16.0 | 1 | 3.7 | |
| Hawaiian or Pacific Islander | 4 | 8.9 | 0 | 0.0 | 0 | 0.0 | |
| Multiple | 2 | 4.4 | 1 | 4.0 | 1 | 3.7 | |
| Education | χ2 = 32.5* | ||||||
| Partial high school | 2 | 4.4 | 0 | 0.0 | 0 | 0.0 | |
| High school graduate | 14 | 31.1 | 1 | 4.0 | 1 | 3.7 | |
| Partial college | 20 | 44.4 | 8 | 32.0 | 4 | 14.8 | |
| ≥College graduate | 9 | 20.0 | 16 | 64.0 | 22 | 81.5 | |
Note. PTSD = posttraumatic stress disorder; PI = primary insomnia.
p < .05.
Measures
The Structured Clinical Interview for DSM-IV (SCID; First, Spitzer, Gibbon, & Williams, 2012) was used with all participants to assess overall clinical symptoms. This standardized interview is designed to obtain diagnostic information according to the Diagnostic and Statistical Manual of Mental Disorders (4th ed., DSM-IV; American Psychiatric Association, 1994) Axis I criteria. For all groups, the SCID was used to identify exclusionary Axis I diagnoses (see above). For the PTSD group, the SCID was also used to assess comorbidity.
All subjects kept sleep diaries and wore actigraphy watches for 1 week.
The sleep diary included typical items, such as total time spent in bed, subjective sleep latency (sSL; i.e., how long it took to fall asleep the first time), and number and duration of awakenings, as well as three calculated variables: subjective total sleep time (sTST), subjective wake after sleep onset (sWASO; i.e., amount of time spent awake during the night after falling asleep initially), and sleep efficiency (sSE; i.e., total sleep time divided by total time in bed). Daily sleep diaries are commonly used in sleep research and in treatment of insomnia (Carney et al., 2012).
Respironics Actiwatch 2 and Actiware software (Respironics) were used to collect and analyze actigraphy data. A single scorer, not blind to participants’ group status, manually set rest intervals corresponding to participants’ time in bed each night. Participants’ diary-reported bed times and wake times were used as guidelines, though rest intervals could be extended up to 60 min on either side to account for obvious sleep outside self-reported time in bed. This procedure was similar to methods used in other sleep studies (e.g., Goldman et al., 2007). Sleep detection settings were set to medium. Automatic algorithms were then used to generate values for objective time in bed (oTIB), objective total sleep time (oTST), objective wake after sleep onset (oWASO), and objective sleep efficiency (oSE).
The Insomnia Severity Index (ISI) was used as a questionnaire-based measure of global insomnia severity (Morin, Belleville, Bélanger, & Ivers, 2011). It provides a total score assessing subjective severity of insomnia symptoms ranging from 0 to 28.
The Pittsburgh Sleep Quality Index (PSQI) is a 19-item questionnaire-based assessment of global sleep quality over the past month (Buysse, Reynolds III, Monk, Berman, & Kupfer, 1989), with scores > 5 indicating clinically significant sleep disturbances.
The following measures were completed by the PTSD patients only; PI and control subjects were recruited specifically to not have psychiatric symptoms. The Clinician Assessed PTSD Scale (CAPS; Blake et al., 1995) was used to diagnose participants with PTSD as well as assess PTSD severity. Participants were assessed for PTSD using the Frequency 1/Intensity 2 scoring method (Weathers, Keane, & Davidson, 2001). Using this method, all participants met full DSM-IV diagnostic criteria, with the exception of three participants who were subthreshold on Criterion C. Exceptions were made for these participants because their CAPS total severity score (> 60) indicated clinically significant PTSD symptoms.
The PTSD Checklist-Specific version (PCL-S) was used as a self-reported measure of PTSD severity (Wilkins, Lang, & Norman, 2011). The PTSD Addendum for the PSQI was also administered (Germain, Hall, Krakow, Shear, & Buysse, 2005). This validated measure assesses sleep disruptive behaviors common in PTSD.
Data Analysis
All daily sleep assessments were compared among the three groups in terms of group means, night-to-night variability, and within-group variability.
Most variables had significantly skewed distributions, so nonparametric tests compared groups on sTST, sSL, sWASO, and sSE. Additionally, groups were compared on oTIB, oTST, oWASO, and oSE. For each variable, omnibus tests were conducted using Kruskal-Wallis one-way analysis of variance, followed by Mann-Whitney U tests for pairwise comparisons. Three pairwise comparisons were conducted to compare PTSD patients to PI patients, PTSD patients to controls, and PI patients to controls. A Bonferroni-adjusted α level of .05/3 = .017 was used for follow-up analyses of each variable. Effect size r was calculated by dividing the Mann-Whitney test statistic Z by √N and using the absolute value of the result. Effect sizes roughly correspond to Cohen’s estimates in terms of small (0.1), medium (0.3), and large (0.5) effects (Field, 2009).
The root mean squared successive difference (RMSSD) served as the index of night-to-night variability due to its ability to detect changes from one night to the next. The RMSSD uses the following formula:
The subscript I refers to the temporal order of each night. RMSSD values were obtained for sTST, sSL, sWASO, and sSE, as well as oTST, oWASO, and oSE. A bootstrapping procedure compared groups statistically. This procedure created a sampling distribution by drawing 250 samples with replacement and calculating the difference in RMSSD between groups. This empirical sampling distribution yielded standard errors and 95% confidence intervals (CIs) for the difference in RMSSD. These procedures compared the PTSD group to both the PI group and to controls.
Box plots showing variable distributions by group were created to visually examine differences among the groups on within-group variability. Then, the difference in variance among groups was statistically compared by using bootstrapping procedures described above.
To examine potential sources of night-to-night and within-group variability in the PTSD group, we conducted several post hoc analyses. For night-to-night variability, frequency, and intensity of distressing dreams as well as mean bed and wake times were correlated with any RMSSD variable showing differences between PTSD patients and PI patients. We also conducted a polynomial regression to examine linear and quadratic effects of sleep schedules on RMSSD measures. Regarding within-group variability, group comparisons between PTSD and PI patients were reexamined including only those PTSD patients who reported relatively few distressing dreams (i.e., on ≤three nights; n = 14), as a means of examining the influence of distressing dreams on within-group variability. To examine within-group variability in sleep schedules, we conducted bootstrapping analyses for bed times and wake times parallel to the others described above. ISI and PSQI scores were compared among groups by using the nonparametric procedures described above. The CAPS, PCL-S, and PSQI Addendum were only collected in the PTSD group. Therefore, only descriptive statistics are reported for these measures.
Results
Kruskal-Wallis tests demonstrated significant differences among groups on all sleep diary (subjective) and actigraphy (objective) variables with the exception of oTIB and oWASO (Table 2). PTSD and PI patients showed more disturbed subjective and objective sleep than controls for all variables except oTIB and oWASO. PTSD patients showed significantly lower oSE than PI patients.
Table 2.
Mean Comparisons by Diagnostic Group Using Kruskal Wallis and Mann-Whitney U Tests
| Variable | PTSD (n = 45) |
PI (n = 25) |
Control (n = 27) |
PTSD vs. PI |
PTSD vs. Control |
PI vs. Control |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | U | r | U | R | U | r | |
| sTSTa | 338.85 | 87.57 | 351.10 | 43.83 | 475.56 | 37.89 | 423.0 | .13 | 101.0* | .69 | 4.0* | .85 |
| sSLa | 50.29 | 47.47 | 44.17 | 26.65 | 12.35 | 6.93 | 462.5 | .06 | 128.5* | .64 | 48.0* | .74 |
| sWASOa | 73.69 | 54.58 | 50.06 | 36.96 | 7.03 | 6.98 | 367.0 | .22 | 34.5* | .79 | 47.0* | .74 |
| sSEa | 73.98 | 14.57 | 79.26 | 9.25 | 95.76 | 2.29 | 400.0 | .17 | 32.0* | .79 | 13.0* | .82 |
| oTIBb | 513.63 | 85.13 | 474.39 | 62.73 | 505.05 | 27.96 | 244.0 | .21 | 338.0 | .03 | 198.0 | .27 |
| oTSTb | 369.85 | 77.33 | 390.29 | 50.93 | 435.44 | 36.73 | 247.5 | .20 | 167.0* | .45 | 139.5* | .45 |
| oWASOb | 50.43 | 22.14 | 54.10 | 18.32 | 41.48 | 13.48 | 253.5 | .16 | 250.5 | .22 | 175.0 | .34 |
| oSEb | 72.49 | 10.29 | 82.48 | 5.22 | 86.21 | 4.60 | 120.0* | .54 | 73.0* | .68 | 166.0* | .36 |
| ISIc | 18.73 | 4.36 | 16.76 | 3.03 | 0.57 | 0.76 | 389.5 | .25 | 0.0* | .73 | 0.0* | .83 |
| PSQI | 14.11 | 3.21 | 11.36 | 2.20 | 2.19 | 1.36 | 272.0* | .43 | 0.0* | .84 | 0.0* | .86 |
Note. PTSD = posttraumatic stress disorder; PI = primary insomnia; sTST = subjective total sleep time; sSL = subjective sleep latency; sWASO = subjective wake after sleep onset; sSE = subjective sleep efficiency; oTIB = objective time in bed, oTST = objective total sleep time, oWASO = objective wake after sleep onset; oSE = objective sleep efficiency; ISI = Insomnia Severity Index; PSQI = Pittsburgh Sleep Quality Index.
For the subjective variables, n = 40 for PTSD, n = 27 for control and n = 25 for PI.
For the objective variables, n = 25 for PTSD and control and n = 21 for PI.
For the ISI, n = 45 for PTSD, n = 25 for PI, and n = 14 for controls.
p < Bonferroni-corrected alpha .05/3 = .017.
Table 3 shows bootstrapped CIs for the differences in night-to-night variability between each pair of groups. On all variables, PTSD patients showed greater night-to-night variability than controls. In addition, PTSD patients showed more night-to-night variability than PI patients on sTST and oSE (see Figure 1). In post hoc analyses, bivariate correlations showed no significant associations between distressing dream frequency/severity and either RMSSD variable, whereas self-reported bedtime was correlated with RMSSD of sTST for the PTSD patients (r = .56, p < .001). Polynomial regression revealed a significant quadratic relationship between PTSD patients’ bedtimes and RMSSD for sTST (p = .030).
Table 3.
Pairwise Variability Comparisons Between Diagnostic Groups Using Bootstrapped 95% Confidence Intervals
| Variable | PTSD (n = 40) - PI (n = 25) | PTSD (n = 40) - Control (n = 27) | PI (n = 25) - Control (n = 27) |
|---|---|---|---|
| Night-to-night variability | |||
| sTST (m) | [1.08, 56.08]* | [58.69, 112.64]** | [25.10, 78.30]** |
| sSL (m) | [−11.00, 18.15] | [17.05, 37.60]** | [15.24, 36.36]** |
| sWASO (m) | [−0.10, 48.66] | [40.02, 78.58]** | [20.27, 44.88]** |
| sSE (%) | [−0.66, 8.02] | [9.80, 15.52]** | [6.86, 12.31]** |
| oTSTa (m) | [−15.41, 37.98] | [36.75, 83.40]** | [23.29, 69.10]** |
| oWASOa (m) | [−13.36, 9.31] | [6.83, 22.44]* | [7.25, 26.33]* |
| oSEa (%) | [1.86, 11.02]* | [8.32, 16.16]** | [0.97, 9.00]* |
| Within-group variability | |||
| sTST (m) | [2372.35, 9121.30]** | [3040.49, 9424.41]*** | [−903.42, 1874.68] |
| sSL (m) | [−96.55,3182.70] | [623.59, 3786.76]** | [90.09, 1234.10]* |
| sWASO (m) | [55.02,3171.75]* | [1662.96, 4197.87]*** | [361.32, 2272.73]** |
| sSE (%) | [12.29, 241.15]* | [104.39, 309.54]*** | [42.14, 118.36]*** |
| oTSTa (m) | [770.35, 6001.86]* | [2304.29, 6957.69]*** | [−25.18, 2514.95] |
| oWASOa (m) | [−327.67, 636.86] | [−113.74, 730.26] | [−23.33, 330.66] |
| oSEa (%) | [35.70, 121.34]*** | [45.14, 124.28]*** | [−14.46, 26.83] |
Note. Root mean squared successive difference was used as the index of night-to-night variability. PTSD = posttraumatic stress disorder; PI = primary insomnia; sTST = subjective total sleep time; sSL = subjective sleep latency; sWASO = subjective wake after sleep onset; sSE = subjective sleep efficiency; oTST = objective total sleep time, oWASO = objective wake after sleep onset; oSE = objective sleep efficiency.
For the objective variables, n = 25 for PTSD and control and n = 21 for PI.
p < .05
p < .01
p < .001
Figure 1.

Night-to-night variability of several sleep parameters by diagnostic group. For all charts, the x-axis represents night of data collection and each line represents one subject.
PTSD patients generally showed greater within-group variability than both other groups (Figure 2 and Table 3). PTSD patients showed more within-group variability than controls on all variables, except oWASO. PTSD patients showed more variability than PI patients on sTST, sWASO, sSE, oTST, and oSE. In post hoc analyses limited to those PTSD patients with distressing nightmares on ≤3 nights, the difference between the PTSD group and the PI group was no longer significant for oTST, 95% CI [−536.00, 7542.05]. Within-group variability for the PTSD group was still greater than the PI group for all other variables (sTST, sSE, oSE). Bootstrap analyses on bed/wake times revealed more within-group variability in PTSD patients than PI patients on self-reported bed times, 95% CI [0.31, 4.64], and wake times, 95% CI [0.88, 5.02], whereas no differences were found between groups on objective bed times, 95% CI [−0.51, 2.98], or wake times, 95% CI [−6.70, 37.15].
Figure 2.
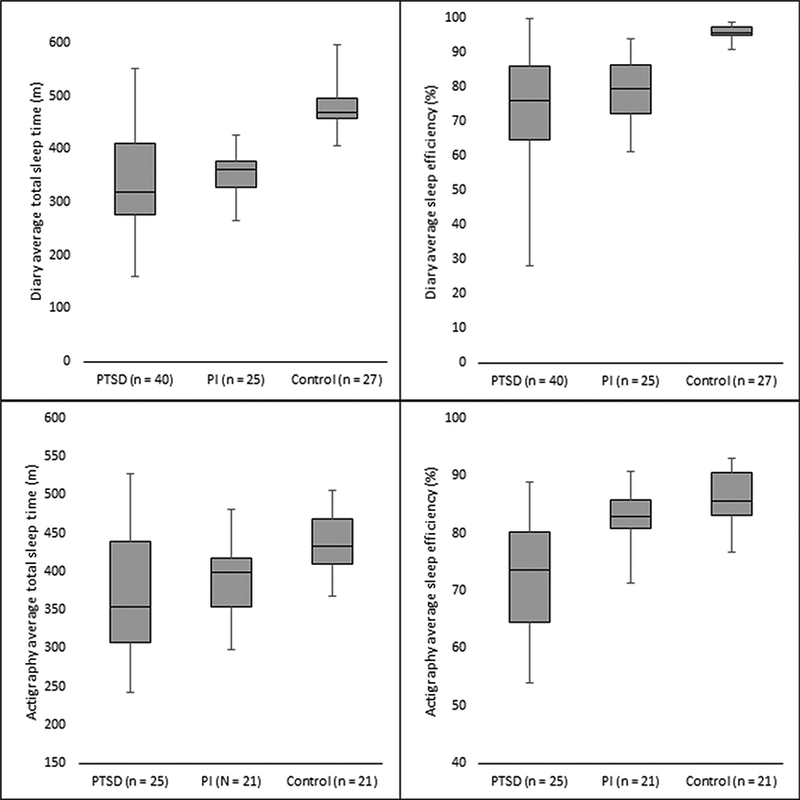
Within-group variability of several sleep parameters by diagnostic group. PTSD = posttraumatic stress disorder, PI = primary insomnia.
On questionnaire-based sleep measures, the patient groups reported more disturbed sleep than controls. Comparing the two clinical groups, PTSD patients reported worse global sleep quality than PI patients on the PSQI. The difference between PTSD patients and PI patients on the ISI was not significant.
On the PTSD assessments, the PTSD patients had an average score of 75.89 (SD = 14.89) on the CAPS, in the severe range of PTSD symptomatology (Blake et al., 2000). Perhaps not surprisingly given the inclusion criteria, PTSD patients rated the Distressing Dreams item with greatest frequency and intensity of all reexperiencing symptoms (Frequency: M = 3.09, SD = 0.70; Intensity: M = 2.76, SD = 0.57) and the Difficulty Sleeping item as the greatest average frequency and intensity of all CAPS items (Frequency: M = 3.93, SD = 0.25; Intensity: M = 2.96, SD = 0.73). Additionally, the PTSD patients had a mean of 59.49 (SD = 10.42) on the PCL-S and a mean of 10.04 (SD = 3.98) on the PSQI Addendum.
Discussion
This study reported several measures of subjective and objective sleep in treatment-seeking OEF/OIF/OND veterans with PTSD and compared them to patients with primary insomnia (sleep disturbance in the absence of other comorbidities) and healthy controls. As expected, PTSD patients had consistently greater sleep complaints, worse sleep quality, greater night-to-night inconsistency, and more within-group variability than controls. PTSD patients also showed greater sleep disturbances than primary insomnia in a few key areas. Specifically, PTSD patients reported greater insomnia symptoms and had lower objectively measured sleep efficiency, as well as greater intra- and interindividual variability than patients with primary insomnia. The PTSD group showed greater night-to-night variability in subjective total sleep time and objective sleep efficiency, as well as greater within-group variability in both objective and subjective total sleep time and sleep efficiency, compared to primary insomnia. Thus, relative to insomnia only, those with PTSD appear to experience worse fragmentation of sleep as well as less predictable sleep. Additionally, sleep disturbance in PTSD appears to be even less uniform than sleep in primary insomnia.
One interesting aspect of our findings was that the PTSD group generally did not differ in mean sleep scores from the primary insomnia group, but showed significantly greater variability in a number of subjective and objective sleep measures. This finding of greater variability in sleep has both research and clinical implications. From a research perspective, it suggests simply reporting mean values from validated measures likely does not accurately reflect sleep in an OEF/OIF/OND PTSD sample. This is particularly notable given we recruited our sample based in part upon sleep parameters, and thus would expect reduced variability. These data suggest future studies should include measures of intra- and interindividual variability. The intraindividual (i.e., night-to-night) variability specifically argues for the use of multi-time-point measurements of sleep, as a single measurement may not fully characterize sleep in a given individual with PTSD. In addition, greater interindividual (i.e., within group) variability suggests when examining sleep, differences between individuals may be more prominent in PTSD than they are in other clinical groups.
The treatment-seeking PTSD patients here showed more night-to-night variability than primary insomnia patients in total sleep time and sleep efficiency. This replicates previous studies showing patients with comorbid insomnia have more night-to-night variability than patients with insomnia alone (Sánchez-Ortuno et al., 2011). Additionally, PTSD patients showed more within-group variability than primary insomnia patients on total sleep time and sleep efficiency. Though it is difficult to make definitive conclusions regarding mechanisms in cross-sectional studies such as this one, several post hoc analyses were conducted to investigate two potential sources of this increased variability in PTSD: distressing dreams and sleep scheduling. Distressing dreams were considered because this is a symptom seen in PTSD, but not present in primary insomnia and thus may have contributed to the differences in variability seen between these two groups. Sleep scheduling (i.e., bed times and wake times) were considered as a potential source of sleep variability in PTSD because this study’s sleep inclusion criteria were slightly more strict for the primary insomnia group (e.g., preferred sleep phase between 10:00 p.m. and 8:00 a.m. than they were for the PTSD group, which may have contributed to variability differences between groups. These post hoc analyses suggest distressing dreams may explain a small portion of the increased within-group sleep variability in PTSD, though limited to oTST. Interestingly, distressing dreams did not appear to contribute to greater night-to-night variability in PTSD. PTSD patients showed more within-group variability for subjective bed times and wake times. This appears to have influenced night-to-night variability in sTST, suggesting patients who sleep during less optimal circadian times (i.e., very early or very late) show increased night-to-night variability in subjective sleep duration. Overall, distressing dreams and sleep schedules would appear to contribute to some of the increased intra- and interindividual variability in PTSD, relative to primary insomnia, though not in a consistent manner. Future studies should examine other potential source(s) of this variability.
Clinically, the similarities in mean sleep parameters between PTSD and primary insomnia patients suggests the goldstandard intervention for insomnia, cognitive behavior therapy for insomnia (CBT-I), should be successful when employed to reduce sleep-related symptoms in PTSD. A recent study (Talbot et al., 2014) tested the full CBT-I protocol in a mostly civilian PTSD sample and found CBT-I improved objective and subjective sleep quality as well as reduced PTSD symptoms and improved overall work and social functioning. Although the Talbot et al. (2014) findings are promising, the significantly greater variability in our sample suggests a straightforward administration of CBT-I may not be fully successful in OEF/OIF/OND veterans with PTSD. The greater interindividual variability in total sleep time and sleep efficiency suggests some individuals will experience particularly fragmented sleep, but an overall sufficient quantity, others may have fairly consolidated sleep but an insufficient quantity, and others may experience problems in both areas. This has implications for personalized medicine. CBT-I, via the 4-factor model of insomnia, provides an opportunity to assess the factors underlying these individual differences (e.g., fear of sleep/nightmares reducing sleep opportunity length, nocturnal hyperarousal interfering with going back to sleep in the middle of the night, cognitive or conditioning effects of nocturnal trauma exposure). Some of these factors may require adjunctive strategies not typically part of CBT-I to provide maximal benefit for the patient.
Inconsistency from night to night is addressed in CBT-I, as patients are encouraged to adhere to a consistent sleep-wake schedule every day. Though not addressing distressing dreams directly, this regularization of sleep timing, along with increased sleep consolidation, would be expected to reduce variability in total sleep time, and thus may be particularly important for reducing sleep disturbances in PTSD. Moreover, intraindividual variability in sleep may be important for PTSD treatment beyond nocturnal symptoms. Future studies may wish to examine the impact of sleep variability on treatment attendance, adherence, and/or outcomes in PTSD.
The fact data from the present study demonstrate sleep in treatment-seeking patients with PTSD and comorbid insomnia was as bad, and in several keys ways worse, than sleep in primary insomnia suggests medical and psychiatric consequences of disturbed sleep most likely generalize to PTSD patients, including negative psychiatric outcomes (Swinkels et al., 2012), impaired emotional coping abilities (Spoormaker & Montgomery, 2008), and functional impairments such as increased risk for accidents (Leger et al., 2002). These consequences are especially concerning given the overall young age of OEF/OIF/OND veterans, as chronic sleep disturbance in this population could result in costly mental and physical health consequences over the long term. It would be valuable for future studies to determine how much sleep contributes, if any, to these comorbidities in PTSD and whether treating sleep (alone or in combination with PTSD-specific interventions) can help reduce the risk of developing these costly problems.
There were a few limitations of this study to highlight. This study recruited OEF/OIF/OND veterans with PTSD who also met criteria for both chronic insomnia and chronic nightmares. Although this represents a large portion of patients with PTSD (as many as 90%; Neylan et al., 1998), it is possible findings may not generalize to veterans of other eras, to civilians, or to those PTSD patients not experiencing sleep difficulties. Also, the PTSD patients were veterans/active duty and mostly men, whereas the other two groups were civilians, and the primary insomnia group had more women. Additionally, the primary insomnia and control groups were more highly educated than the PTSD patients, and had a different racial breakdown (e.g., fewer African Americans and more Asian Americans). These factors may have contributed to the differences observed between the groups. Notably, OEF/OIF/OND active duty soldiers often have irregular sleep schedules and poor sleep quality while deployed (Peterson, Goodie, Satterfield, & Brim, 2008), so veteran status may have influenced the within-group and night-to-night variability seen in this group relative to the two civilian groups. Although that does not diminish the clinical implications of such variability, the group differences may be smaller if compared to an OEF/OIF/OND veteran sample with primary insomnia. Finally, the PTSD and PI samples were not of equal size, and this may have affected our ability to detect subtle differences between the two patient groups. Additionally, there were considerable limitations regarding use of actigraphy in PTSD, as there is no validation study of actigraphy in a PTSD sample. Thus, actigraphy findings in PTSD patients should be interpreted with caution. For example, the lack of differences observed among any of the groups on oWASO may reflect actigraphy’s tendency to overscore wake (and thus WASO) in controls (Short, Gradisar, Lack, Wright, & Carskadon, 2012).
Overall, our characterization of sleep symptoms in a treatment-seeking OEF/OIF/OND PSTD sample relative to primary insomnia and controls showed the sleep disturbances in military-related PTSD were uniquely characterized by increased variability from night-to-night and person-to-person. Future research in this area may illuminate if this finding extends to nonmilitary PTSD samples, identify causes and consequences of this variability, develop new means for assessing sleep variability, and test how to tailor existing evidence-based treatment (i.e., CBT-I) so that practitioners are prepared to address the unique and specific needs of any patient with PTSD.
Acknowledgments
Funded by NINR #1RC1NR011728. Special thanks to Katherine Resovsky, Jennifer Salamat, and Rina Sobel Fox for logistical and technical support.
References
- American Psychiatric Association. (1994). Diagnostic and statistical manual of mental disorders (4th ed.). Washington, DC: Author. [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Klauminzer G, Charney DS, … Buckley TC (2000). Instruction manual for Clinician Administered PTSD Scale (CAPS). Washington, DC: National Center for Post-traumatic Stress Disorder. [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, & Keane TM (1995). The development of a clinician-administered PTSD scale. Journal of Traumatic Stress, 8, 75–90. doi: 10.1002/jts.2490080106 [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Cheng Y, Germain A, Moul DE, Franzen PL, Fletcher M, & Monk TH (2010). Night-to-night sleep variability in older adults with and without chronic insomnia. Sleep Medicine, 11, 56–64. doi: 10.1016/j.sleep.2009.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF III, Monk, TH, Berman SR, & Kupfer DJ (1989). The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research, 28, 193–213. doi: 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- Carney CE, Buysse DJ, Ancoli-Israel S, Edinger JD, Krystal AD, Lichstein KL, & Morin CM (2012). The consensus sleep diary: Standardizing prospective sleep self-monitoring. Sleep, 35, 287–302. doi: 10.5665/sleep.1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field A (2009). Discovering statistics using SPSS. Thousand Oaks, CA: Sage. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, & Williams JB (2012). Structured Clinical Interview for DSM-IV® Axis I Disorders (SCID-I), Clinician Version, Administration Booklet. Arlington, VA: American Psychiatric Publishing. [Google Scholar]
- Germain A, Hall M, Krakow B, Katherine Shear M, & Buysse DJ (2005). A brief sleep scale for posttraumatic stress disorder: Pittsburgh Sleep Quality Index Addendum for PTSD. Journal of Anxiety Disorders, 19, 233–244. doi: 10.1016/j.janxdis.2004.02.001 [DOI] [PubMed] [Google Scholar]
- Goldman SE, Stone KL, Ancoli-Israel S, Blackwell T, Ewing SK, Boudreau R, . . . Newman AB (2007). Poor sleep is associated with poorer physical performance and greater functional limitations in older women. Sleep, 30, 1317–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin RD, & Marusic A (2008). Association between short sleep and suicidal ideation and suicide attempt among adults in the general population. Sleep, 31, 1097–1101. [PMC free article] [PubMed] [Google Scholar]
- Hillman DR, Murphy AS, Antic R, & Pezzullo L (2006). The economic cost of sleep disorders. Sleep, 29, 299–305. [DOI] [PubMed] [Google Scholar]
- Hughes J, Jouldjian S, Washington DL, Alessi CA, & Martin JL (2013). Insomnia and symptoms of post-traumatic stress disorder among women veterans. Behavioral Sleep Medicine, 11, 258–274. doi: 10.1080/15402002.2012.683903 [DOI] [PubMed] [Google Scholar]
- Léger D, Guilleminault C, Bader G, Lévy E, & Paillard M (2002). Medical and socio-professional impact of insomnia. Sleep, 25, 625–629. [PubMed] [Google Scholar]
- McLay RN, Klam WP, & Volkert SL (2010). Insomnia is the most commonly reported symptom and predicts other symptoms of post-traumatic stress disorder in US service members returning from military deployments. Military Medicine, 175, 759–762. doi: 10.7205/MILmEd-D-10-00193 [DOI] [PubMed] [Google Scholar]
- Mellman T, Kulick-Bell R, Ashlock L, & Nolan B (1995). Sleep events among veterans with combat-related posttraumatic stress disorder. American Journal of Psychiatry, 152, 110–115. [DOI] [PubMed] [Google Scholar]
- Mendlowicz MV, & Stein MB (2000). Quality of life in individuals with anxiety disorders. American Journal of Psychiatry, 157, 669–682. doi: 10.1176/appi.ajp.157.5.669 [DOI] [PubMed] [Google Scholar]
- Morin CM, Belleville G, Bélanger L, & Ivers H (2011). The Insomnia Severity Index: Psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep, 34, 601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neylan TC, Marmar CR, Metzler TJ, Weiss DS, Zatzick DF, Delucchi KL, . . . Schoenfeld FB (1998). Sleep disturbances in the Vietnam generation: Findings from a nationally representative sample of male Vietnam veterans. American Journal of Psychiatry, 155, 929–933. [DOI] [PubMed] [Google Scholar]
- Ohayon MM, & Shapiro CM (2000). Sleep disturbances and psychiatric disorders associated with posttraumatic stress disorder in the general population. Comprehensive Psychiatry, 41, 469–478. doi: 10.1053/j.comp.2000.16568 [DOI] [PubMed] [Google Scholar]
- Peterson AL, Goodie JL, Satterfield WA, & Brim WL (2008). Sleep disturbance during military deployment. Military Medicine, 173, 230–235. [DOI] [PubMed] [Google Scholar]
- Sánchez-Ortuno MM, Carney CE, Edinger JD, Wyatt JK, & Harris A (2011). Moving beyond average values: Assessing the night-to-night instability of sleep and arousal in DSM-IV-TR insomnia subtypes. Sleep, 34, 531–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short MA, Gradisar M, Lack LC, Wright H, & Carskadon MA (2012). The discrepancy between actigraphic and sleep diary measures of sleep in adolescents. Sleep Medicine, 13, 378–384. doi: 10.1016/j.sleep.2011.11.005 [DOI] [PubMed] [Google Scholar]
- Spoormaker VI, & Montgomery P (2008). Disturbed sleep in post-traumatic stress disorder: Secondary symptom or core feature? Sleep Medicine Reviews, 12, 169–184. doi: 10.1016/j.smrv.2007.08.008 [DOI] [PubMed] [Google Scholar]
- Suh S, Nowakowski S, Bernert RA, Ong JC, Siebern AT, Dowdle CL, & Manber R (2012). Clinical significance of night-to-night sleep variability in insomnia. Sleep Medicine, 13, 469–475. doi: 10.1016/j.sleep.2011.10.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinkels CM, Ulmer CS, Beckham JC, Buse N, & Calhoun PS (2012). The association of sleep duration, mental health, and health risk behaviors among US Afghanistan/Iraq Era veterans. Sleep, 36, 1019–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot LS, Maguen S, Metzler TJ, Schmitz M, Posner DA, Weiss B, . . . Neylan TC (2014). Cognitive behavioral therapy for insomnia in posttraumatic stress disorder: A Randomized controlled trial. Sleep, 37, 327–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers FW, Keane TM, & Davidson JR (2001). Clinician-Administered PTSD Scale: A review of the first ten years of research. Depression and Anxiety, 13, 132–156. doi: 10.1002/da.1029 [DOI] [PubMed] [Google Scholar]
- Wilkins KC, Lang AJ, & Norman SB (2011). Synthesis of the psychometric properties of the PTSD checklist (PCL) military, civilian, and specific versions. Depression and Anxiety, 28, 596–606. doi: 10.1002/da.20837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit GK, Weiner J, Damato N, Sillup GP, & McMillan CA (1999). Quality of life in people with insomnia. Sleep, 22, S379–S385. [PubMed] [Google Scholar]


