Abstract
An emerging area of research has identified that an increased risk of musculoskeletal injury may exist upon returning to sports after a sport-related concussion. The mechanisms underlying this recently discovered phenomenon, however, remain unknown. One theorized reason for this increased injury risk includes residual neuromuscular control deficits that remain impaired despite clinical recovery. Thus, the objectives of this review were: (1) to summarize the literature examining the relationship between concussion and risk of subsequent injury and (2) to summarize the literature for one mechanism with a theorized association with this increased injury risk, i.e., neuromuscular control deficits observed during gait after concussion under dual-task conditions. Two separate reviews were conducted consistent with both specified objectives. Studies published before 9 December, 2016 were identified using PubMed, Web of Science, and Academic Search Premier (EBSCOhost). Inclusion for the objective 1 search included dependent variables of quantitative measurements of musculoskeletal injury after concussion. Inclusion criteria for the objective 2 search included dependent variables pertaining to gait, dynamic balance control, and dual-task function. A total of 32 studies were included in the two reviews (objective 1 n = 10, objective 2 n = 22). According to a variety of study designs, athletes appear to have an increased risk of sustaining a musculoskeletal injury following a concussion. Furthermore, dual-task neuromuscular control deficits may continue to exist after patients report resolution of concussion symptoms, or perform normally on other clinical concussion tests. Therefore, musculoskeletal injury risk appears to increase following a concussion and persistent motor system and attentional deficits also seem to exist after a concussion. While not yet experimentally tested, these motor system and attentional deficits may contribute to the risk of sustaining a musculoskeletal injury upon returning to full athletic participation.
1. Introduction
Sport-related concussion has been defined as a traumatically induced alteration of mental status that may or may not involve loss of consciousness [1, 2] and typically results in impaired mental status, balance, and delayed reaction time, as well as common symptoms (e.g., headache) [3]. To assess potential areas of dysfunction after concussion, clinicians use a variety of testing techniques [4–7]. Many of these techniques are highly sensitive acutely post-concussion (0.89–0.96), but demonstrate reduced sensitivity within the week after injury likely owing, in part, to a practice effect from repeat test administration [4, 5, 8, 9]. However, other assessment techniques that are not typically available to the clinician, such as laboratory or instrumented methods of testing, have identified deficits that persist beyond clinical recovery and self-report asymptomatic status [10–17]. Thus, even if an athlete can ‘pass’ the routine battery of clinical tests relative to their own baseline performance or normative values, true physiological deficits may continue to exist, and the athlete may return to athletic participation with residual neurological deficits [18].
One such example of a clinical test that may not be able to detect continued physiological deficits is the Balance Error Scoring System (BESS). It has been well established that impaired balance is one common result of a concussion [12, 19]. The BESS is the most frequently used balance test in collegiate athletic settings in USA during concussion baseline, diagnosis, and management protocols [4–6]. Although it was developed as a clinically feasible test instrument [20], the limitations of the BESS have been reported, most notably improved performance owing to a practice effect with repeat administration and test environment differences, resulting in poor test psychometrics [21–25]. Therefore, reliance on the BESS as a sole indicator of postural control system recovery after concussion may result in low sensitivity [3] and potentially clearance to return to athletic activities prior to full physiological restoration of the brain. Most athletes achieve baseline BESS values within 3–5 days post-concussion, often despite the continued presence of concussion-related symptoms [3, 9]. While concussion-related symptoms tend to persist for 5–7 days post-concussion, over 75% of athletes achieve baseline BESS values within 48 h, and over 90% demonstrate full BESS recovery within the first week of injury [3, 9]. Thus, while the BESS is a valuable diagnostic test, it may not provide a comprehensive method to assess postural stability when making return-to-play decisions.
Conversely, instrumented assessments of postural stability such as the Sensory Organization Test (SOT) may be more sensitive to continued deficits in the post-acute phases of concussion [19, 26]. Broadly speaking, the SOT has successfully identified impaired postural control acutely post-concussion with a higher sensitivity (0.62) than the BESS (0.34) [8, 9], supporting the notion that instrumented and objective measures of function are a better method to identify post-concussion deficits than clinically used subjective tests. Beyond the composite score outcome variables provided by the SOT, Cavanaugh and colleagues used the SOT center-of-pressure data to calculate non-linear metrics (e.g., approximate entropy), observing deficits at least 4 days post-injury [27]. Unfortunately, no followup studies have reported balance resolution using such methods, thus the duration of deficits using non-linear instrumented approaches remains unknown. Furthermore, use of the SOT in clinical settings is cost and space prohibitive, and very few National Collegiate Athletic Association Division I institutions routinely use the SOT [5].
Using force platform technology, several studies have identified deficits beyond clinical recovery [15, 17]. Powers et al. identified differences in COP velocity, but not displacement, at return to play (mean: 26 days post-concussion) when the athlete self-reported being symptom free [17]. Similarly, Gao et al. identified deficits using Shannon and Renyi entropy 10 days post-concussion despite symptom resolution and baseline performance on both the BESS and computerized neurocognitive testing [15]. Taken together, these results suggest that instrumented measures of postural control are more effective at identifying persistent deficits in postural control than currently used clinical post-concussion assessments (i.e., the BESS). Despite the advantages of such instrumented approaches, these tasks are limited by their reliance on a single task (i.e., only assessing motor function), are static (as opposed to dynamic) in nature, and are not feasible (e.g., cost, expertise) in most clinical environments.
More rigorous approaches to post-concussion deficit detection have continued to show sub-clinical deficits, even at the point of or after athletes are allowed to resume full athletic participation. Neuromuscular control refers to the many aspects that contribute to how the nervous system controls muscle activation and ultimately postural control [28], and has been one area that several researchers have investigated. The evaluation of neuromuscular control during various tasks, including gait, has allowed for the detection of deficits for a longer period of time after concussion than traditional postural control tests such as the BESS have been able to identify [29–31]. Gait measurements are a reliable method to quantify postural stability and changes in health status [32], and the neuromuscular control patterns responsible for gait are developed early in life [33], typically by age 7 years [34]. Thus, by the time an individual reaches adolescence, they are able to walk with little attentional focus on such an automated motor task [35, 36].
Consistent with this notion, recent work has reported acceptable test-retest consistency levels for gait tasks among adolescents and young adults [37–40]. In particular, dual-task gait tests (gait concurrently performed with a cognitive task) can contribute useful information in the assessment and management of a concussion [41]. Computerized attention tests probing abilities such as task switching and conflict resolution have been used to identify deficits after a concussion, indicating that concussion negatively affects attentional distribution abilities [42–44]. Similarly, attentional distribution is necessary during dual tasks [45]. Therefore, if attentional capacities are limited after injury, simultaneous task execution may create an observable deterioration in one or both tasks.
The Fifth Consensus Statement on Concussion in Sport indicates that gait and balance evaluations should be included within detailed neurological examinations of concussion [2]. However, instrumented assessments of gait or neuromuscular control are not used in a widespread fashion [6]. This may be because of a variety of factors, such as a lack of evidence regarding clinical meaningfulness of gait function, instrument cost, space, or personnel necessary to operate such protocols. As such, athletes who continue to have subtle deficits that are not detected by traditional clinical neurocognitive and static balance tests may be returned to play under standard clinical guidelines, perhaps prior to full physiological restoration of the brain [18]. These lingering deficits have the potential to be exacerbated in a more dynamic and cognitively challenging environment, such as during athletic competitions. To maximize goal attainment on the field, an athlete needs to properly distribute their attention across both internal and external stimuli, select appropriate motor responses in response to those stimuli, rapidly implement the response, and monitor and adjust the implementation [46]. Furthermore, these sequences need to be performed while allocating attentional focus among rapidly evolving aspects on the field during play. As with the effects of dual attention tasks in the laboratory environment, the cognitive challenges posed on the field may result in detrimental effects on an athlete’s neuromuscular control.
Deficits in neuromuscular control [47, 48] and attention [49] have been linked to an increased risk of musculoskeletal injury independent of concussion. These abilities are not routinely assessed in return-to-play evaluations [5, 6] and residual deficits have been frequently identified using instrumented measures after clinical recovery [30, 31, 42, 50–53]. It is therefore plausible that postconcussion neuromuscular and/or attention deficits may contribute to an increased risk of musculoskeletal injury upon resumption of athletic activities. Several studies have now observed an association between concussion and the risk of subsequent musculoskeletal injury [54–59]. Accordingly, these two areas of investigation (i.e., postconcussion deficits and subsequent musculoskeletal injury risk) appear to be linked and may be best understood when explored in conjunction with one another.
Neuromuscular control deficits after a concussion may go undetected by clinicians [11, 50, 52], likely in part owing to the need for sophisticated equipment to detect such deficits. Because neuromuscular control deficits can modulate the risk of experiencing a musculoskeletal injury among athletes independent of a concussion, neuromuscular control deficits after a concussion may be associated with an increased injury risk. To our knowledge, no literature review has directly addressed these areas of research in conjunction. Hence, the purpose of this scoping review was to investigate the evidence regarding musculoskeletal injury risk subsequent to a concussion, neuromuscular control deficits during divided attention assessments (i.e., dual task) that may persist for a prolonged period after concussion, and the potential associations between these research areas.
2. Methodological Considerations
2.1. Literature Search and Analysis
To investigate the literature on musculoskeletal injury risk after concussion and attentional-neuromuscular control deficits after concussion, two separate reviews were conducted in three stages: article retrieval, title and abstract review to assess for eligibility, and assessment for quality and assurance that inclusion criteria were met. Specifically, two topics were searched: (1) musculoskeletal injury risk after concussion (objective 1) and (2) dual-task gait deficits after concussion (objective 2). These two review topics were selected to identify the published literature existing in these areas and to describe the theoretical concepts that may be responsible for their relationship to one another.
2.2. Data Sources and Searches
Literature reviews were conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines [60]. Two separate searches were conducted, using the following search engines: PubMed, Web of Science, and Academic Search Premier (EBSCO-host) from the time of database inception to 9 December, 2016. Articles that were available online during the search period were included. Our search string for Objective 1 was: “(sports) AND (concussion OR mild traumatic brain injury) AND (musculoskeletal injury OR subsequent injury OR performance decrements).” Our search string for Objective 2 was: “(sports) AND (concussion OR mild traumatic brain injury) AND (gait OR locomotion) AND (dual task* OR secondary task* OR multi task* OR concurrent task* OR simultaneous task*).” Search results are described in Figs. 1 and 2. Reference lists from identified articles were also used as a supplemental search technique. All titles and associated abstracts were reviewed independently and publications that were not appropriate were excluded from the review.
Fig. 1.
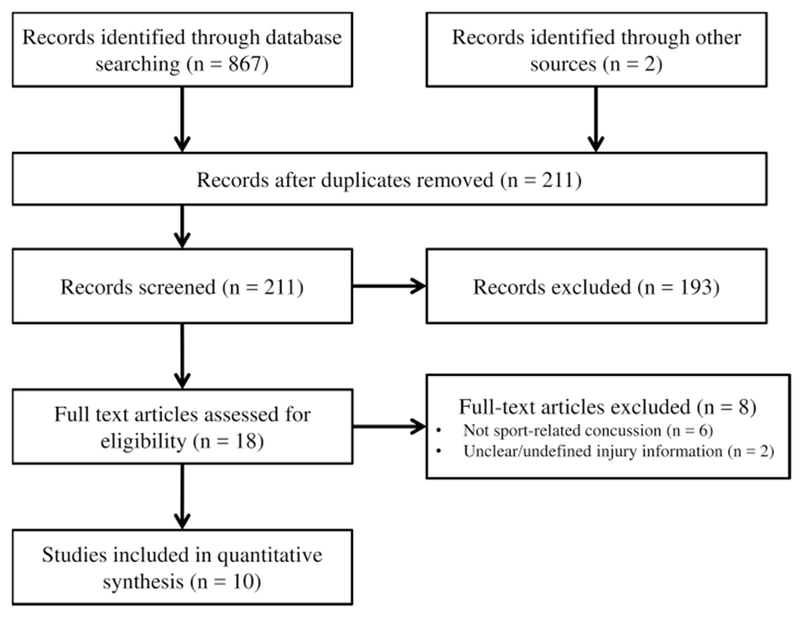
Objective 1: search for studies examining those who sustained a sport-related concussion, with dependent variables including quantitative measurements of musculoskeletal injury rates after a concussion
Fig. 2.
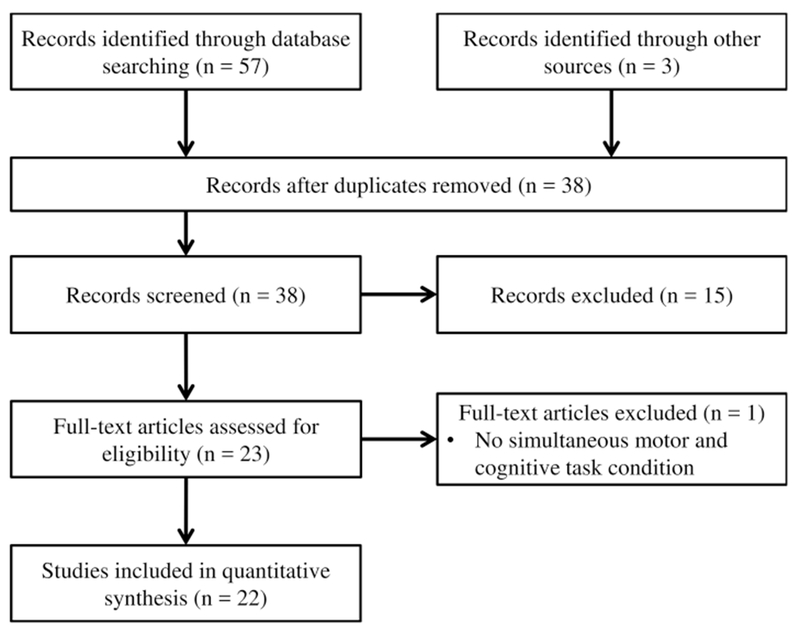
Objective 2: search for studies examining participants tested as a result of sport-related concussion on measurements of gait or dynamic balance control in dual-task conditions
Inclusion criteria for objective 1 included a sample consisting of those who sustained a sport-related concussion and dependent variables that included quantitative measurements of musculoskeletal injury after concussion. To review all relevant studies, we did not specify that if the data reported needed to include only ‘time-loss’ injuries or a specific type of injury or body location. Inclusion criteria for objective 2 were a sample consisting of participants diagnosed with a concussion by a sports medicine professional and/or based upon the definition provided by the International Consensus Statement on Concussion in Sport: “an injury caused by a direct blow to the head, face, neck, or elsewhere on the body with an impulsive force transmitted to the head, resulting in a graded set of clinical symptoms” [61] or a similar definition. Inclusion criteria for topic 2 also consisted of dependent variables that included measurements of gait or dynamic balance control, and independent variables that included a dual-task (simultaneous motor and cognitive performance) condition. Full-text articles were excluded if they were not original research or review articles, were abstracts, case studies, editorials, or if they included a sample of participants who had sustained a moderate-to-severe traumatic brain injury.
2.3. Quality Assessment
Two authors (DRH, RCL) independently identified studies for each literature review topic and discrepancies were resolved by consensus judgment among the authorship team. Owing to the lack of commonality of data elements among the studies, no data quality assessment, effect size calculations, or meta-analyses could be performed. Thus, the inclusion and exclusion criteria outlined constituted the elements required for identified studies to be included in our review.
3. Findings
3.1. Search Results
The combined literature searches led to 929 potential studies. Objective 1 search terms (musculoskeletal injury risk after concussion) identified 869 studies for potential inclusion through database searching and other sources. After duplicate removal, record screening, and full text assessment, ten studies were identified and included (Fig. 1). Objective 2 search terms (dual-task gait deficits after sport-related concussion) yielded 60 relevant studies through database searching and other sources. After duplicate removal, record screening, and full-text assessment, a total of 22 studies were included (Fig. 2). Therefore, a total of 32 studies were included for the two literature reviews.
3.2. Description of Included Studies: Concussion and Musculoskeletal Injury
Peer-reviewed scientific literature investigating musculoskeletal injury risk following concussion has recently emerged, with the first observation of this association published in 2009 [62], but nothing published again until 2014 [58]. Studies from our literature search included a variety of athlete cohorts of various ages and skill levels from several different countries (Table 1). Study design and analysis differences hindered interpretation of these findings as a collective. Most studies reported lower extremity injuries after a concussion [54–56, 59, 63]. However, the type of injuries included in other studies varied. Some did not explicitly state the type of injuries included [64, 65], included a small proportion of upper extremity injuries [62], or included any type of injury [58] or time-loss injuries only [57]. Despite the limitations and variation existing within this body of literature, interesting patterns appeared that suggested athletes are at an increased risk of musculoskeletal injury following concussion.
Table 1.
Summary of search 1 terms: studies investigating the association between concussion and risk of musculoskeletal injury
| Study | Cohort | Na (M/F) | Post-concussion time frame | Outcome (95% confidence interval) | Interpretation |
|---|---|---|---|---|---|
| Makdissi et al. [62] | Professional Australian football | 276 (276/0) | 1 competitive match |
Injury rate ratio 2.23 (0.93–5.04) |
Despite a large rate ratio, there were no statistical injury rate group differences. The authors only observed injuries in a single competitive match after concussion |
| Brooks et al. [54] | Mixed college | 257 (194/63) | 90 d |
OR 2.48 (1.04–5.91) |
Concussed athletes had higher odds of experiencing LE injuries than controls within a 90-day window after concussion |
| Cross et al. [57] | Professional rugby union | 810 (810/0) | 24 mo |
Injury rate ratio 1.6 (1.4–1.9) |
Professional rugby union players had a 60% greater risk of experiencing time-loss injuries than players with no concussion |
| Lynall et al. [56] | Mixed college | 102 (67/35) | 12 mo |
Injury rate ratio Pre- vs. post-concussionb = 1.97 (1.19–3.28) Concussion vs. control = 1.64 (1.07–2.51) |
Concussed athletes were 1.97 times more likely to experience an LE injury after concussion than before, and 1.64 times more likely compared with matched controls for up to 1-y post-concussion |
| Nordstrom et al. [58] | Professional soccer | 1665 (1665/0) | 12 mo |
HRc Post- to pre-concussion = 1.47 (1.05–2.05) Compared with controls: 0–3 mo: 1.56 (1.09–2.23) 3–6 mo: 2.78 (1.58–4.89) 6–12 mo: 4.07 (2.14–7.76) |
Professional soccer players were 47% more likely to experience an injury after concussion as compared with before concussion. Concussion increased acute injury risk (HR = 1.70; 1.20–2.41), but not gradual-onset injury risk (HR = 1.00; 0.60–1.66). The risk of injury was about 2.2 times greater in concussed players as compared with controls throughout the total follow-up period |
| Burman et al. [64] | Mixed athlete | 1540 (1091/449) | 24 mo |
OR Pre-concussiond = 1.98 (1.45–2.72) Post-concussione = 1.72 (1.26–2.37) |
Both pre- and post-concussion, concussed players were more likely than controls to experience a subsequent injury |
| Pietrosimone et al. [63] | Retired professional football | 2429 (2429/0) | Reported history |
ORf 1 concussion vs. 0 concussions = 1.59 (1.30–1.94) 2 concussions vs. 0 concussions = 2.29 (1.85–2.83) 3+ concussions vs. 0 concussions = 2.86 (2.36–3.48) |
Concussion and musculoskeletal injury histories were associated in retired professional football players |
| Nyberg et al. [65] | Professional ice hockey | 264 (264/0) | 42 d |
χ2 No statistical differences (p ≥ 0.12) Concussion group had more severe injuries (p ≥ 0.04) |
Compared with those who had a knee injury, concussed professional hockey players were not at an increased risk for injury, but did have more severe (absence >27 d) injuries within 21 d of return to play |
| Herman et al. [55] | Mixed college | 221 (158/63) | 90 d |
OR 3.39 (1.90–6.05) |
Concussed athletes had higher odds of experiencing LE injuries than controls within a 90-d window after concussion, but overall time loss owing to the subsequent injury was not different between groups |
| Gilbert et al. [59] | Mixed college | 335 (127/208) | Reported history |
ORg 1.61–2.87 |
Concussion and musculoskeletal injury histories were associated in college athletes, with specific associations between concussion and lateral ankle sprain (OR = 1.79), knee injury (OR = 2.13), and LE muscle strain (OR = 1.61) |
Unless otherwise noted, all studies compared a concussion group with a control group, and all outcome ratios are reported as concussion/control
F female, HR hazard ratio, LE lower extremity, M male, OR odds ratio
N indicates the total sample size (concussion and control groups), broken into total F and M participants
Compared injury rates in year post-concussion to year pre-concussion in the concussion group only
Reported results are when controlling for the number of injuries in the year preceding concussion
Analyzed injuries prior to concussion compared with the control group
Analyzed injuries after concussion compared with the control group
Reported OR for those who had a history of 1, 2, or 3+ concussions compared with those with 0 concussions
Reported multiple ORs for different LE injuries
3.2.1. Concussion and Musculoskeletal Injury in Collegiate Athletes
Four studies analyzed injury risk in college athlete cohorts, with the authors reporting very similar results (Table 1). Three of the four studies used historical data to prospectively compare musculoskeletal injury rates between concussed athletes and non-concussed control athletes over a period of time [54–56], while the fourth used a post-season questionnaire [59]. Collectively, these studies [54–56] indicated a significant elevated musculoskeletal injury risk during a time period up to a year after concussion. Researchers have also studied musculoskeletal injury risk after concussion among professional and high school athletes [57, 58, 62, 63, 65]. The findings among these different age groups and skill levels suggested a higher rate of musculoskeletal injury after a concussion [57, 58, 63], but this was not uniform across all investigations [62, 64, 65].
Two studies [54, 55] compared lower extremity injury rates between concussed and control athletes over a 90-day period following the point of return to play for the concussed athlete (Table 1). The reported odds of sustaining a lower extremity injury following concussion were 2.48 and 3.39 times that of the control athletes, respectively. The difference in the magnitude of the effect between these two studies may be owing to Brooks et al. [54] recording only non-contact injuries, whereas Herman et al. [55] recorded injuries from both contact and non-contact mechanisms. Additionally, the athletes in each study were drawn from different mixes of Division I collegiate sports. As such, differences in the overall number of competitive exposures, the inherent isk of injury associated with the different sports, and the number of athletes from each respective sport could have contributed to the difference in the magnitude of the reported odds. However, these studies had a similar number of cases, used similar criteria for matching concussed and control athletes, and used a similar followup period, thereby lending strength to the relative consistency of the findings between the studies.
Similar to these two studies, Lynall et al. [56] reported on differences in the risk of lower extremity musculoskeletal injury between concussed and matched non-concussed control athletes. While this study did not detect any significant differences between the two groups at 90 days, it did determine that concussed athletes had a risk of lower extremity injury of 1.64 times that of the matched control athletes over a 365-day follow-up period. Lynall et al. [56] also described an increased rate of musculoskeletal injury (1.97) in the year following concussion as compared to the year prior to concussion in the concussed group. The lack of a comparable finding at 90 days may have been owing to differences in sample size, with the number of concussed athletes in Lynall et al. [56] (n = 44) being approximately half that of Brooks et al. [54] (n = 87), and Herman et al. [55] (n = 90).
The fourth study in collegiate athletes, performed by Gilbert et al. [59], used survey methodology to investigate the association between concussion and musculoskeletal injury at the conclusion of an collegiate athletic career. The authors reported significant associations between concussion history, be it reported, unreported, or unrecognized, and a host of lower extremity injuries, including lateral ankle sprains, knee injuries, and muscle strains. Odds ratios for these associations ranged from 1.6 to 2.9. The study findings presented in this work were retrospective and relied on participant self-report rather than medical records, thus warranting caution regarding casuality.
3.2.2. Concussion and Musculoskeletal Injury in Professional Athletes
Several studies have also investigated the effect of concussion on musculoskeletal injury risk among professional athletes [57, 58, 62, 63, 65]. Cross et al. [57] and Nordstom et al. [58] investigated professional rugby and soccer athlete cohorts, respectively. Cross et al. [57] reported the incidence of musculoskeletal injury in the same 9-month season following concussion was 60% higher (incidence rate ratio = 1.6; 95% confidence interval 1.4—1.9) compared with those who had not sustained a concussion. Similarly, Nordstrom et al. [58] found an increased hazard of experiencing a musculoskeletal injury within a year after concussion compared with the athlete’s risk during the year prior to concussion (hazard ratio =1.47; 95% confidence interval 1.05–2.05). This study also identified a higher injury rate in the year preceding concussion among those who sustained a concussion compared with those who did not (mean number of injuries = 1.8 ± 1.6 vs. 0.9 ± 1.4; p < 0.001).
Pietrosimone et al. [63] investigated a cohort of 2429 retired professional Amercian Football players, using a cross-sectional survey-based approach [63]. Compared with National Football League players who did not sustain a concussion, retired National Football League players with one, two, or three or more concussions had an 18–63, 15–126, and 73–165% higher odds of reporting a serious musculoskeletal injury (i.e., not minor strains or sprains, which may have been more difficult for participants to remember), respectively. Similar to the study by Gilbert et al. [59], a measure of caution is appropriate with these study findings, as the data are subject to recall bias, could not be compared with medical records, and lack information regarding exposure rates and the temporal relationship between concussion and musculoskeletal injuries. Despite these limitations, taken together, these results suggest that similar to collegiate athletes, professional athletes are at an increased risk of lower extremity injury following concussion.
However, the results of additional studies among professional athletes do not uniformly indicate a relationship between concussion and subsequent increased risk of musculoskeletal injury. Nyberg et al. [65] compared injury rates between a concussed athlete cohort and a cohort of athletes returning to play after a medial collateral ligament or other ‘knee distortion’ injury (n = 104) using records over a 28-year period from a single elite-level ice hockey team. The authors reported no increased risk for musculoskeletal injury following concussion compared with those with knee injuries. Although not a significant finding, the authors reported that athletes who had sustained more than one concussion had an increased injury risk within 42 days of return to play (57.6 vs. 38.2%; p = 0.06). Makdissi et al. [62] investigated a professional Australian Football athlete cohort and compared the injury rate after concussion in 138 concussed athletes to that of 585 team-, position-, and game-matched non-concussed control athletes. Similar to Nyberg et al. [65], the authors reported more musculoskeletal injuries following return to activity after concussion, with an incidence rate ratio of 2.23 (95% confidence interval 0.93–5.04), but this finding was not statistically significant.
These two studies [62, 65] should be considered in the context of some notable limitations, which may serve to obscure any potential relationship between concussion and subsequent musculoskeletal injury risk. In the latter case, the study by Makdissi et al. [62] was powered to investigate on-field performance rather than injury rates, and used a follow-up period after concussion of a single match. This limited follow-up period resulted in only ten recorded injuries for the concussed athlete cohort and 19 in the control athlete cohort. In the former case, Nyberg et al. [65] compared concussed athletes with athletes who were recovering from a knee injury rather than uninjured non-concussed athletes. Given that several studies have demonstrated that prior musculoskeletal injury is a risk for future musculoskeletal injury [66–69], it is not unreasonable to assume that such an elevated injury risk in the injured-knee group could potentially obscure the presence of an increased injury risk among the concussed athletes that may have otherwise been noted should a non-injured comparison group been used. That being said, it is worth noting that Nordstrom et al. [58], using an alternative model for analysis, did note an increased hazard of injury after concussion compared with the risk of injury after other musculoskeletal injury types, with magnitudes ranging from 1.56 (within 3 months) to 4.07 (within 6–12 months).
3.2.3. Concussion and Musculoskeletal Injury in Recreational Athletes
One study compared injury rates in ice hockey, soccer, floorball, and handball athletes with concussion (n = 281) to that of athletes from these sports with an ankle injury (n = 1289) using records from the emergency department at a regional hospital over a 14-year period [64]. Each group was assessed for musculoskeletal injuries during a 2-year period: 1 year before and 1 year after the concussion or ankle injury. The authors noted that while athletes with a concussion were more likely to experience injuries both before and after concussion compared with before and after ankle injury in the comparison group, no difference in injury rates was observed when comparing concussed athletes before and after their concussion injury. Consistent with Nyberg et al. [65], an injured comparison group was used, thus similar limitations may have applied that may have served to obscure a potential difference in injury risk. Furthermore, this study did not report whether athletes elected to return to play after their injury or at what level, and relied on the assumption that athletes would be reporting all injuries to the emergency department. Further study is needed in younger age groups, and in younger athletes to further develop our understanding of musculoskeletal injury risk after concussion in these populations.
3.2.4. Summary
When taken as a whole, with increased consideration of those studies with the strongest designs, the current literature indicates there is likely an increased risk of musculoskeletal injury in athletes following return to play from a concussion. The mechanisms responsible for this risk may be multifaceted, but several authors [54–57] have posited that it at least partially relates to the continued presence of neuromuscular control deficits after a concussion.
3.3. Description of Included Studies: Concussion and Dual-Task Neuromuscular Control Deficits
Among the 22 studies identified that investigated neuromuscular control deficits after concussion through dualtask gait examinations, several different methodologies, age populations, time periods, and assessment methods were used (Table 2). Cognitive tests implemented during gait tasks included arithmetic, spelling, Stroop tests (visual and auditory), verbal fluency, or spatial memory tests (Table 2). Among the commonly reported gait performance variables, whole body center-of-mass movement, average walking speed, and obstacle clearance height were the most common (Table 2). Several studies reported that during dual-task conditions, gait performance variables continued to show significant deficits relative to controls for a longer period of time than other clinical measures, such as symptom resolution [13, 30, 31, 52, 53]. However, several null findings exist within this literature. Among children with a concussion, no dual-task gait deficits were found relative to controls despite the presence of singletask deficits [53]. Additionally, Martini et al. [70] found no differences in dual-task gait without an obstacle, Martini et al. [71] found no differences in any condition between adults who did and did not sustain an adolescent concussion, and Catena et al. [72] found no clear relationship between dynamic balance control and executive function deficits. Therefore, while several studies reported prolonged gait-related deficits following a concussion, this has not been consistent throughout the literature.
Table 2.
Summary of search 2 terms: studies investigating dual-task gait after concussion
| Study | Cohort | Na (M/F) | Testing time | Testing conditions | Outcome measures | Interpretation |
|---|---|---|---|---|---|---|
| Parker et al. [73] | Collegiate athletes | 20 (8/12) | Within 48 h post-concussion | Single- and dual-task gait (Q&A); tested using motion capture | Gait velocity, stride length, stride time, step width COM ROM and VEL; COM-COP distance |
Decreased dynamic stability in dual-task following concussion: greater ML sway |
| Parker et al. [31] | Collegiate athletes | 30 (18/12) | 2, 5, 14, and 28 d post-concussion | Single- and dual-task gait (Q&A); tested using motion capture | Gait velocity, stride length, step width COM ROM and VEL, COM-COP distance |
More conservative dual-task gait (slower and greater COM-COP distance) 28 d post-concussion compared with controls |
| Catena et al. [74] | College students | 28 (16/12) | Within 48 h post-concussion | Single- and dual-task gait (Q&A); tested using motion capture | Gait velocity, stride length, step width COM ROM and VEL, COM-COP distance |
Greater COM motion and velocity during single- and dual-task gait following concussion |
| Catena et al. [77] | College students | 28 (16/12) | Not stated | Single- and dual-task gait (Q&A), obstacle cross; tested using motion capture | Gait velocity, stride length, step width COM ROM and VEL, COM-COP distance |
Adaptation of conservative gait post-concussion, more in Q&A condition than obstacle cross or single-task gait |
| Parker et al. [79] | Collegiate athletes and non-athletes | 56 (N/A) | 2, 5, 14, and 28 d post-concussion | Single- and dual-task gait (Q&A); tested using motion capture | Gait velocity, COM ROM and VEL, COM-COP distance | Dual-task balance control impairments up to 1 mo following concussion. Athletes walked slower than non-athletes during single/dual-task gait |
| Catena et al. [76] | College students | 60 (32/28) | 2, 5, 14, and 28 d post-concussion | Single- and dual-task gait (Q&A), obstacle cross; tested using motion capture | COM ROM, COM VEL, COM-COP distance | Within 48 h of concussion, conservative single-task gait was observed. By 28 d post-injury, COM motion control during obstacle crossing was observed |
| Catena et al. [72] | College students | 20 (10/10) | 2, 6, 14, and 28 d post-concussion | Single- and dual-task gait (Stroop); tested using motion capture | COM ROM, COM VEL, Stroop reaction time | Individuals with concussion walked with slower sagittal plane COM motion and displayed attentional capacity deficits compared with controls |
| Martini et al. [70] | College students with and without a concussion history | 68 (37/31) | Average of 6.3 y post-concussion for those with a concussion history | Single- and dual-task gait (Brooks-Spatial Memory Task); tested using GaitRITE | Gait velocity, step length, stride width, stance time | Those with a history of concussion walked slower than those without during single-task gait only |
| Chiu et al. [78] | Collegiate athletes | 46 (28/18) | Within 48 h of concussion | Single- and dual-task gait (Q&A), obstacle cross; tested using motion capture | Obstacle clearance distance, cognitive task accuracy, inter-joint coordination (continuous relative phase) | Participants with concussion walked slower during Q&A and obstacle crossing compared with controls. These conditions also induced greater joint coordination pattern changes for those with a concussion |
| Fait et al. [52] | Elite athletes | 12 (8/4) | Average of 37 d post-concussion | Single- and dual-task gait (visual Stroop); tested using motion capture | Symptoms, neurocognitive function, maximum gait speed, minimal COM obstacle clearance, response errors, cognitive dual-task costs | No differences between healthy and concussion groups for symptoms or neurocognitive function. Those with a concussion had greater minimal COM obstacle clearance and greater dual-task costs than controls |
| Howell et al. [29] | Adolescent athletes | 40 (36/4) | 2, 8, 17, 30, and 59 d post-concussion | Single- and dual-task gait (auditory Stroop); tested using motion capture | Symptoms, mean gait velocity, step length, step width, COM ROM, COM VEL, dual-task costs | Symptom resolution occurred for all but 4 concussion subjects by 59 d post-injury. Dual-task COM ROM and VEL and dual-task costs were higher in concussion subjects at 59 d post-injury compared with controls |
| Howell et al. [50] | Adolescent athletes | 46 (40/6) | 2, 8, 17, 30, and 59 d post-concussion | Single- and dual-task gait (single/continuous auditory Stroop, and Q&A); tested using motion capture | Symptoms, COM ROM, COM VEL, average gait velocity | Symptoms resolved on average within 2 wk of concussion. As task complexity increased, gait stability decreased for the concussion group |
| Cossette et al. [98] | Young adult athletes | 21 (9/12) | Average of 158 d post-concussion | Single- and, dual-task gait (Stroop, verbal fluency, arithmetic), and obstacle crossing; tested using motion capture | Average gait velocity, dual-task costs | The concussion group exhibited greater dual-task costs, particularly during obstacle avoidance |
| Howell et al. [30] | Adolescent athletes | 38 (32/6) | Before and after return-to-activity clearance | Single- and dual-task gait (auditory Stroop); tested using motion capture | Change across time in COM ROM, COM VEL, average gait velocity, symptoms, attention | During dual-task conditions, frontal plane gait worsened after return to activity for concussion patients. No changes in single-task gait, symptoms, or attention for controls |
| Howell et al. [80] | Adolescent and young adult athletes | 76 (52/24) | 2, 8, 17, 30, and 59 d post-concussion | Dual-task gait (auditory Stroop); tested using motion capture | COM ROM, COM VEL, symptoms | Symptoms resolved by 2 wk and 1 mo post-concussion for young adults and adolescents, respectively. Gait balance control deficits were greater for adolescents post-concussion than young adults |
| Howell et al. [75] | Adolescent and young adult athletes | 17 (10/7) | 2, 8, 17, 30, and 59 d post-concussion | Dual-task gait (auditory Stroop); tested using an accelerometer | Average gait velocity, peak anterior acceleration, peak medial/lateral acceleration | During dual-task walking, participants with concussion displayed less peak medial–lateral acceleration than control participants during 55–75% of the gait cycle |
| Howell et al. [13] | Adolescent and young adult athletes | 29 (21/8) | Average of 58 d post-concussion | Single- and dual-task gait (auditory Stroop); tested using motion capture | Time to return to activity, COM ROM, COM VEL, symptoms | A strong correlation exists between time required to return to physical activity and dual-task COM motion at 2 mo post-concussion |
| Sambasivan et al. [53] | Physically active children and adolescents | 48 (28/20) | Average of 43 d post-concussion | Single- and dual-task gait (counting backwards by 3 s); tested using GaitRITE, BESS test | Stride width, step length, double support time, BESS errors | Children with concussion show single-task and obstacle cross gait deficits, even after self-reported symptom resolution. No dual-task deficits found |
| Fino [51] | Collegiate athletes | 9 (6/3) | Average: 7, 16, 23, 29, 37, 45, and 363 d post-injury | Single- and dual-task gait (Q&A); tested using body-worn accelerometers | Gait speed, stride time variability, Lyapunov exponents (local dynamic stability) | The addition of a cognitive dual task influenced stability in the concussed group more than in the control group, despite similar ST stability |
| Fino et al. [97] | Collegiate athletes | 8 (2/6) | Average: 7, 16, 23, 29, 37, and 45 d post-injury | Single- and dual-task gait (serial subtraction by 7 s); tested using motion capture | Kinematic turning characteristics | Altered turning kinematics were detected during gait despite absence of clinical deficits |
| Howell et al. [81] | Child and adolescent athletes | 68 (28/40) | Average of 9 d post-injury | Single- and dual-task gait (Q&A); tested using body-worn accelerometers | Average gait speed, cadence, double support time, gait cycle duration, stride length | Those with a history of multiple concussions prior to a subsequent concussion displayed smaller DT stride lengths than controls, no difference during ST walking |
| Martini et al. [71] | General community | 77 (44/33) | Average of 5.8–48.5 y post-injury | Single- and dual-task gait (Brooks-Spatial Memory Task), obstacle cross, obstacle cross with dual task; tested using motion capture | Gait velocity, step width, stride length, double support time, toe clearance over obstacle | There were no observable gait differences between those with and without a history of concussion occurring during adolescence |
All studies compared post-concussion participants with uninjured control participants, unless otherwise noted
BESS Balance Error Scoring System, COM whole-body center of mass, COP center of pressure, DT dual-task, F female, M male, ML medial-lateral, N/A not available, Q&A question and answer test (spelling words backwards, reciting the months in reverse order, or serial subtraction), ROM range of motion, ST single-task, VEL velocity
N indicates the total sample size (concussion and control groups), broken into total F and M participants
The first observations of lingering balance control deficits during gait were reported as early as 2005, when Parker and colleagues [73] identified that athletes with a concussion demonstrated deficits within 48 h of a concussion and throughout the subsequent month post-injury compared with matched controls. Similar studies that followed indicated similar shorter term but persistent locomotor dysfunction following a concussion [31, 72, 74]. Following the observation that measurable gait stability effects were present following concussion in this series of earlier studies, subsequent investigations followed up for a longer period of time after injury to determine the duration of gait-related impairments that exist after concussion [13, 29, 30, 50–53, 75] (Table 2).
3.3.1. Return to Play and Dual-Task Gait Deficits
Specific investigations into the presence of persistent gait deficits after clinical recovery have been conducted. Prior to returning to full athletic participation, adolescent athletes improved their dual-task gait balance control and this improvement was associated with symptom reduction [30]. Following clearance to return to play, however, worsened ability to maintain dynamic stability during dual-task gait was found despite no increase in symptoms [30]. Additionally, an association between returning to full sports participation after concussion and dual-task gait stability was found 2 months after the injury; those who returned to play sooner demonstrated a worse ability to maintain balance during gait than those who remained out longer [13]. Finally, among collegiate and elite athletes, athletes with a concussion continued to display gait deficits after being evaluated and determined by a physician to be fully recovered from the injury based on symptom report and neurocognitive testing [31, 52]. Although limited by relatively small sample sizes, these studies indicate that across several age groups, gait impairments persist after recovery has been determined from standard clinical measures and other testing methods, and may be reflective of incomplete recovery among athletes recovering from a concussion.
3.3.2. Post-Concussion Cognitive Task Completion during Gait
Six studies have investigated how cognitive task completion affects post-concussion gait performance relative to a single task (i.e., normal walking) and to control group performance at either single [70] or multiple time points after concussion [29, 31, 50, 51, 76]. Martini and colleagues [70] used a dual-task gait paradigm to test individuals on average 6 years after concussion, the longest follow-up time point identified in our literature search, while each of the other studies tested athletes acutely after concussion (i.e., with 48–72 h of injury) and again in a systematic testing timeline up to 1–2 months post-injury. The study performed by Martini et al. [70] reported altered gait patterns among those with a history of concussion compared with controls without a history of concussion. They reported slower gait velocity among those with a history of concussion in the single-task condition (no cognitive task), but not the dual-task condition where individuals walked and completed the Brooks visuospatial cognitive task. In more acute post-injury test paradigms (beginning 48–72 h post-injury), dual-task gait deficits appeared and continued to be detected even after singletask gait abilities recovered. Adolescents with a concussion demonstrated poorer gait balance control during a dual task that involved walking and completing an auditory Stroop task throughout the 2 months after injury, compared with their own single-task performance and the control group dual-task performance [29].
In a follow-up to this study, several different cognitive test forms were implemented during gait: the authors concluded that as cognitive task complexity increases, gait stability decreases throughout the 2 months post-concussion [50]. Furthermore, others have identified that singletask walking did not result in any significant differences between those with concussion and controls, while divided attention gait tasks resulted in balance control deficits that were detectable 14–28 days after injury [31, 76].
Finally, a recent study by Fino [51] indicated that those with concussion displayed decreased local dynamic stability and increased stride time variability during dual-task gait, but not single-task gait, relative to a group of matched control subjects. Collectively, these studies across different age populations, testing timelines after concussion, and measurement devices indicate dual-task assessments may identify deficits for a longer duration of time after concussion than single-task assessments.
3.3.3. Post-Concussion Gait Across the Age Spectrum
Several studies have investigated the effect of concussion on dual-task neuromuscular function within specific age groups as well using motion capture technology. Among collegiate athletes, there has been a consistent tendency for an adaptation of more conservative and less stable walking patterns (slower walking speed, greater and faster center-of-mass sway) from within 48 h of injury [73, 74, 77, 78] and throughout the subsequent month after concussion [31, 79]. Similarly, adolescent athletes display dual-task gait abnormalities relative to controls for up to 2 months on average [29, 50]. One study directly compared adolescent and young adult age groups, noting that adolescents demonstrated significantly greater dual-task gait deficits both within 72 h of a concussion and consistently across the ensuing 2 months, as well as longer symptom duration times, compared with young adults [80]. Despite these apparent acute effects of age on concussion, the long-term effects of concussion on dual-task gait are less clear. Martini and colleagues conducted two studies included in our scoping review that investigated how dual-task gait is affected by a concussion sustained years prior to testing. In the first study [70], they reported that college students who sustained a concussion 6.3 years prior to testing on average walked with slower gait velocity in the single-task condition but not the dual-task condition relative to those who reported no history of concussion. In the next study [71], they investigated how a concussion sustained during adolescence affected spatiotemporal gait patterns for young adults (aged 18–30 years), middle-aged adults (aged 40–50 years), and older adults (aged 60 years and older), noting no observable differences in single- and dual-task conditions.
3.3.4. Post-Concussion Dual-Task Gait with Portable Sensors
Finally, three studies have focused on the use of portable sensor technology (i.e., accelerometers and gyroscopes) to assess gait under dual-task conditions following a concussion [51, 75, 81]. Using a commercially available system, participants who were evaluated following their second or greater lifetime concussion demonstrated smaller stride lengths than healthy control participants during dualtask walking [81]. Further work has used body-worn accelerometers to measure gait stability after concussion, identifying greater peak medial-lateral trunk accelerations [75], decreased local dynamic stability (i.e., a measure of the ability of the neuromuscular control system to respond to disturbances) [51], and increased stride time variability [51] among participants with a concussion compared with controls (Table 2).
4. Discussion
4.1. Potential Neuromuscular Contributors to Injury Risk Following Concussion
Based on the results from our scoping reviews of the existing literature, there is a likely association between concussion and subsequent musculoskeletal injury. The mechanisms underlying this observation, however, remain unknown as direct experimental evidence does not yet exist. One leading theory for this association is that the ability to simultaneously complete motor and cognitive tasks may reflect an aspect of athletic competition that is not often tested during post-concussion evaluations, and may also play a role in preventing future injury. Dual-task neuromuscular deficits often go unrecognized as they are not a part of routine clinical care [6]. Because neuromuscular [47, 48] and attention [49] deficits are established risk factors for future injury, persistent neuromuscular control and/or attentional deficits that exist after a concussion may be modulating risk factors for sustaining a musculoskeletal injury upon returning to full athletic participation.
Different potential mechanisms for the observed increase in musculoskeletal injury risk after concussion have been proposed, many of which focus on neuromuscular deficits. Neuromuscular control deficits may be responsible for an increased musculoskeletal risk independent of whether an individual has sustained a concussion or not. Prior investigations have revealed that among healthy athletes, postural control [82–84] or neuromuscular control [85–87] deficits relate to the risk of sustaining a musculoskeletal injury. As noted by our review of the dualtask gait control literature, some studies demonstrate that neuromuscular function deficits exist acutely post-concussion and beyond the point of fulfillment of clinical return-to-play criteria. Although findings from longer term studies (i.e., years post-concussion) are inconclusive, acute effects are primarily found when the individual is asked to perform two different types of concurrent tasks.
It is notable that many of the observed deficits in the literature were during gait tasks imposing neuromuscular demands that are likely far less challenging than what is required during most athletic activities. Challenging and complex tasks may elicit greater impairments during sports, as athletes are required to generate motor patterns and distribute attention across multiple incoming sources of stimuli during gameplay. Assessing athletes during more challenging neuromuscular tasks that better reflect athletic demands may help identify those athletes who may continue to have deficits after concussion symptom resolution. One recent study by Dubose et al. [88] investigated the effect of concussion on neuromuscular performance during a jump-landing task. Compared with baseline pre-concussion jump-landing performance, concussed subjects demonstrated increased hip stiffness and reduced knee and leg stiffness. These changes were present even though the athletes who sustained a concussion were tested approximately 50 days post-injury and had been cleared to return to play. As such, neuromuscular control deficits may underlie the observed increased musculoskeletal injury risk after concussion, and more demanding neuromuscular challenges may help identify those who may be at elevated risk.
In a similar fashion, the use of cognitive challenges that help to simulate the dynamic nature of of the sports environment may help clinicians identify those who are at risk for another injury once they have returned to sport after a concussion. As testing under dual-task conditions may be more representative of the demands of sport than singletask tests, their inclusion during post-concussion examinations may help to identify those potentially at risk for another injury that may be missed using standard measures. From the time of injury, and throughout the ensuing recovery timeline, athletes consistently show greater deficits during dual-task walking relative to single-task walking [29–31, 50, 89]. As identified in our scoping review, the specific type of cognitive task that an individual performs during gait alters the amount of dynamic instability that athletes exhibit after a concussion, where more complex cognitive tasks induce greater deficits [50]. This may be owing to the inability of athletes who recently sustained a concussion to effectively divide their attention between different types of stimuli. Such attentional deficits may be reflective of executive dysfunction, and these abilities may also require a longer period of recovery than what symptom inventories or traditional computerized neurocognitive tests can identify [42–44]. Related to athletic abilities, one recent investigation noted that cognitive loading during a motor task reduces the ability to stiffen the knee joint during a rapid eccentric knee extension perturbation [90]. Specifically, slower muscle activation responses and lower muscle activity amplitudes were identified with the addition of a cognitive load, indicating altered motor abilities when attention is divided.
Similarly, several studies have noted that cognitive challenges can magnify neuromuscular deficits in athletic tasks, even in groups of participants who did not sustain a recent concussion [91–94]. This notion is further supported by recent transcranial magnetic stimulation studies that reported increased cortical inhibition during a dual task compared with a single task among healthy individuals [95, 96]. Such an observation may be owing to the inability of motor or attentional systems to properly allocate the necessary resources for optimal execution by motor and cognitive demands. As attentional deficits [42, 43] and dual-task gait impairments [29, 89, 97, 98] have been noted following concussion, it is reasonable to consider that the detrimental effect of a dual-task challenge on neuromuscular control would be magnified in concussed athletes.
The mechanism for these deficits in neuromuscular control and the inability to properly divide cognitive resources between different types of stimuli following concussion may be the result of lingering cortical impairments. Subtle cortical disruptions have been noted following concussion, leading to an overall ‘global hypoexcitability’ after concussion. Results obtained using transcranial magnetic stimulation indicate reduced intracortical facilitation and maximal voluntary muscle activation [99], increased intracortical inhibition [100], increased motor-evoked potential latency, and increased intracortical inhibition [101–103] following concussion. Furthermore, electroencephalographic evidence indicates that participants who sustained a concussion a week prior to testing performed at the same level as controls on traditional neurocognitive tests, but continue to exhibit dysfunctional brain activity [104]. Thus, neurophysiological measurement techniques may be able to identify the mechanistic underpinnings of neuromuscular deficits that exist after a concussion. As these deficits are not readily detectable, however, an athlete may be able to ‘pass’ the traditional battery of clinical concussion tests (i.e., symptom inventory, computerized neurocognitive testing, BESS), yet still experience physiological dysfunction [18]. Persistent cortical hypoexcitability could negatively affect functional movement in a dynamic sport environment as well. Athletes, especially those competing at high levels, rely on rapid identification and processing of their athletic environment, as well as rapid implementation of elite movement strategies.
These concepts have implications for clinical practice, as many currently used testing methods rely primarily on relatively simple single-task tests. However, standard clinical tests may not reflect the time course of physiological healing of the brain after concussion [18]. Identifying feasible methods to reflect physiological healing remains a challenge. Dual-task deficits after concussion have been identified using the clinically feasible tandem gait test for a longer post-injury period than single-task deficits [89], and dual-task tandem gait measures may be beneficial as a proxy measurements for instrumented average walking speed [105]. Thus, the combination of easily implemented combined motor-cognitive tests may allow clinicians to monitor recovery in a manner that reflects functional recovery, and perhaps allow clinicians to identify future risk of injury. Future research may benefit from studies that include measures of functional movement and musculoskeletal injury risk following a concussion.
4.2. Other Potential Contributors to Increased Musculoskeletal Injury Risk Following Concussion
The studies included in our review suggest an increased musculoskeletal injury risk following concussion. Although the neuromuscular control deficits observed during dual tasks after concussion identified in our review represent one theorized reason underlying the increased musculoskeletal injury risk, other potential contributing factors may exist. Several studies [106–112] have evaluated factors that may contribute to an increased injury incidence among individuals who have not sustained a concussion.
Conflicting evidence in the reviewed literature exists regarding musculoskeletal injury risk prior to concussion. Lynall et al. [56] observed no between-group musculoskeletal injury rate differences in the year preceding concussion, while musculoskeletal injury rates increased following concussion compared with both the control group and the pre-concussion time period in the concussed group. This is in contrast to authors who report athletes that sustain a concussion have higher musculoskeletal injury rates both before and after concussion. Nordstrom et al. [58] reported athletes who had concussion experienced more musculoskeletal injuries in the year preceding the concussion than the control group. To account for such between-group differences, the authors controlled for preconcussion musculoskeletal injury rates in their statistical analyses, reporting increased musculoskeletal injury risk after concussion.
Conversely, Burman et al. [64] reported increased musculoskeletal injury rates both before and after concussion as compared with a control group. Unlike Nordstrom et al. [58] the authors did not observe an increased musculoskeletal injury frequency following concussion. Although these studies did not match groups based on the number and type of athlete exposures, which may have contributed to these findings, the observed rates of injury prior to concussion suggest certain athletes may display greater risk-taking behavior, are more injury prone, or perhaps play a position with a higher injury risk. This notion may also contribute to the higher musculoskeletal injury rates of musculoskeletal injury both before and after a concussion.
Factors that place athletes at a higher risk of both concussion and musculoskeletal injury may include pre-existing attentional deficits. Individuals with attention-deficit/hyperactivity disorder (ADHD) have particularly well-known and more easily observed differences in baseline (non-concussed) neurocognitive performance compared with individuals without ADHD [106, 107]. Furthermore, one study reported that children with ADHD are at increased risk for a variety of traumatic injuries [108]. Recent studies have indicated that athletes with ADHD may also be at an increased risk for sport-related injury [109]. Furthermore, previous studies have indicated that musculoskeletal injury risk [46, 113, 114] and neuromuscular deficits [115] are each associated with ADHD.
Different personality traits may also contribute to the risk of concussion and musculoskeletal injuries. Although the literature examining this specific topic remains limited, it has been indicated that personality traits, such as sensation seeking or lack of caution, are perhaps associated with acute musculoskeletal injury risk [110–112]. However, the effect of personality traits on concussion risk remains poorly understood. One study suggested that aggression and hostile personality characteristics may be associated with a history of past concussions; however, the temporal relationship of these factors to each other has not yet been established [116].
Overall, these studies highlight the need to consider factors that may bias any potential relationship between concussion and subsequent musculoskeletal injury risk. Accounting for variables such as athlete exposure and risktaking behaviors in future investigations of musculoskeletal injury following concussion is needed to develop a better understanding of the link between concussion and musculoskeletal injury risk.
4.3. Limitations
It is important to consider limitations of this body of literature. We sought to investigate the theorized reasons that may, at least partially, explain the increased musculoskeletal injury risk after concussion in this review, but they remain hypotheses. Researchers must design prospective studies that account for athlete risk behavior, cortical excitability, and functional movement while also longitudinally assessing musculoskeletal injury risk before and after concussion. Including these outcomes together will greatly enhance our understanding of potential lingering deficits associated with sustaining a concussion that are not included in standard clinical testing batteries.
It is also important to note that at the time of this review, most investigations in the area of the association of concussion and subsequent musculoskeletal injury risk were in elite-level athletes (i.e., professional or collegiate athletes), and not among high school or youth athletes. Although no studies identified through our literature review investigated musculoskeletal injury risk after concussion among high school athletes, prolonged dual-task gait deficits after return to play have been identified among this population [28, 29], suggesting that a similar phenomenon may exist among this age group. Furthermore, only one study reported specifically on a female population, which was a sub-analysis of the overall studied population and comprised only 25 cases [55]. Future investigations should investigate athlete samples that include participants from across a diverse population sample to identify musculoskeletal injury outcomes following concussion.
Although our scoping review identified many dual-task gait studies conducted by several research groups across North America, the majority of the studies on dual-task gait emanated from a single research laboratory, which may have created a location bias. Furthermore, different outcome measures and inclusion criteria for patients with a concussion throughout the literature made it difficult to directly compare studies or to conduct meta-analytic analyses and draw subsequent conclusions. Additionally, the sports that were under investigation varied highly from study to study; thus, the generalizability of the findings may be limited. Finally, the majority of dual-task gait studies identified through our scoping review included relatively low sample sizes (mean = 38 ± 22 subjects, range = 8–77), limiting the overall generalizability.
5. Conclusions and Recommendations
Our scoping literature review suggests that following a sport-related concussion, athletes are more likely to sustain a subsequent musculoskeletal injury. Although the mechanism for this increased injury risk is not yet known, neuromuscular control deficits under divided attention conditions may be one contributing factor. Dual-task methodologies may provide an indication of the brain’s ability to integrate cognitive and motor information, which may be more reflective of sport participation than completing single-task balance and cognitive assessments. Therefore, clinicians may return athletes to play prior to complete recovery, and these continued sub-clinical deficits may partially explain, or at least relate to, the elevated subsequent musculoskeletal injury risk observed following a concussion. Continued development of clinically viable tests aimed at identifying subtle movement deficits may assist with the detection of an increased musculoskeletal injury risk, while the implementation of movement-based activities during concussion recovery may aid athletes in safely returning to play.
Key Points.
Based on existing literature, athletes appear more likely to sustain a subsequent musculoskeletal injury in the year after sustaining a concussion
The mechanism for this is unknown, but one contributing factor may be that continued neuromuscular control deficits exist for a longer period than standard clinical tests are equipped to identify
Acknowledgments
Funding David R. Howell has received research support through a research contract between Boston Children’s Hospital, Cincinnati Children’s Hospital Medical Center, and ElMindA Ltd. Thomas A. Buckley is funded, in part, by a grant from the National Collegiate Athletic Association and the Department of Defense. Daniel C. Herman is supported in part by National Institutes of Health Grant No. 5K12HD001097–17 (Rehabilitation Medical Scientist Training Program), and grants through the Foundation for Physical Medicine and Rehabilitation, American Medical Society for Sports Medicine Foundation, and American College of Sports Medicine Foundation.
Footnotes
Compliance with Ethical Standards
Conflict of interest David R. Howell, Robert C. Lynall, Thomas A. Buckley, and Daniel C. Herman have no conflicts of interest directly relevant to the content of this review.
References
- 1.Broglio SP, Cantu RC, Gioia GA, et al. National Athletic Trainers’ Association position statement: management of sport concussion. J Athl Train. 2014;49:245–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCrory P, Meeuwisse W, Dvorak J, et al. Consensus statement on concussion in sport: the 5th International Conference on Concussion in Sport held in Berlin, October 2016. Br J Sports Med. 2017;51:838–47. [DOI] [PubMed] [Google Scholar]
- 3.McCrea M, Guskiewicz KM, Marshall SW, et al. Acute effects and recovery time following concussion in collegiate football players: the NCAA concussion study. JAMA. 2003;290:2556–63. [DOI] [PubMed] [Google Scholar]
- 4.Buckley TA, Burdette G, Kelly K. Concussion-management practice patterns of National Collegiate Athletic Association Division II and III athletic trainers: how the other half lives. J Athl Train. 2015;50:879–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelly KC, Jordan EM, Joyner AB, et al. National Collegiate Athletic Association Division I athletic trainers’ concussion-management practice patterns. J Athl Train. 2014;49:665–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baugh CM, Kroshus E, Stamm JM, et al. Clinical practices in collegiate concussion management. Am J Sports Med. 2016;44:1391–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stache S, Howell D, Meehan WP. Concussion management practice patterns among sports medicine physicians. Clin J Sport Med. 2016;26:381–5. [DOI] [PubMed] [Google Scholar]
- 8.Broglio SP, Macciocchi SN, Ferrara MS. Sensitivity of the concussion assessment battery. Neurosurgery. 2007;60:1050–7 (discussion 1057–8). [DOI] [PubMed] [Google Scholar]
- 9.McCrea M, Barr WB, Guskiewicz K, et al. Standard regression-based methods for measuring recovery after sport-related concussion. J Int Neuropsychol Soc. 2005;11:58–69. [DOI] [PubMed] [Google Scholar]
- 10.Broglio SP, Macciocchi SN, Ferrara MS. Neurocognitive performance of concussed athletes when symptom free. J Athl Train. 2007;42:504–8. [PMC free article] [PubMed] [Google Scholar]
- 11.Buckley TA, Munkasy BA, Tapia-Lovler TG, et al. Altered gait termination strategies following a concussion. Gait Posture. 2013;38:549–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buckley TA, Oldham JR, Caccese JB. Postural control deficits identify lingering post-concussion neurological deficits. J Sport Health Sci. 2016;5:61–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Howell DR, Osternig LR, Christie AD, et al. Return to physical activity timing and dual-task gait stability are associated 2 months following concussion. J Head Trauma Rehabil. 2016;31:262–8. [DOI] [PubMed] [Google Scholar]
- 14.Slobounov S, Sebastianelli W, Hallett M. Residual brain dysfunction observed one year post-mild traumatic brain injury: combined EEG and balance study. Clin Neurophysiol. 2012;123:1755–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao J, Hu J, Buckley T, et al. Shannon and Renyi entropies to classify effects of mild traumatic brain injury on postural sway. PloS One. 2011;6:e24446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Slobounov S, Cao C, Sebastianelli W. Differential effect of first versus second concussive episodes on wavelet information quality of EEG. Clin Neurophysiol. 2009;120:862–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Powers KC, Kalmar JM, Cinelli ME. Recovery of static stability following a concussion. Gait Posture. 2014;39:611–4. [DOI] [PubMed] [Google Scholar]
- 18.Kamins J, Bigler E, Covassin T, et al. What is the physiological time to recovery after concussion? A systematic review. Br J Sports Med. 2017;51:935–40. [DOI] [PubMed] [Google Scholar]
- 19.Guskiewicz KM. Balance assessment in the management of sport-related concussion. Clin Sports Med. 2011;30:89–102. [DOI] [PubMed] [Google Scholar]
- 20.Riemann BL, Guskiewicz KM, Shields EW. Relationship between clinical and forceplate measures of postural stability. Hum Kinet J. 1999;8:71–2. [Google Scholar]
- 21.Burk JM, Munkasy BA, Joyner AB, et al. Balance error scoring system performance changes after a competitive athletic season. Clin J Sport Med. 2013;23:312–7. [DOI] [PubMed] [Google Scholar]
- 22.Rahn C, Munkasy BA, Joyner AB, et al. Sideline performance of the balance error scoring system during a live sporting event. Clin J Sport Med. 2015;25:248–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bell DR, Guskiewicz KM, Clark MA, et al. Systematic review of the balance error scoring system. Sports Health. 2011;3:287–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valovich McLeod TC, Perrin DH, et al. Serial administration of clinical concussion assessments and learning effects in healthy young athletes. Clin J Sport Med. 2004;14:287–95. [DOI] [PubMed] [Google Scholar]
- 25.Onate JA, Beck BC, Van Lunen BL. On-field testing environment and balance error scoring system performance during preseason screening of healthy collegiate baseball players. J Athl Train. 2007;42:446–51. [PMC free article] [PubMed] [Google Scholar]
- 26.Broglio SP, Ferrara MS, Sopiarz K, et al. Reliable change of the sensory organization test. Clin J Sport Med. 2008;18:148–54. [DOI] [PubMed] [Google Scholar]
- 27.Cavanaugh JT, Guskiewicz KM, Giuliani C, et al. Recovery of postural control after cerebral concussion: new insights using approximate entropy. J Athl Train. 2006;41:305–13. [PMC free article] [PubMed] [Google Scholar]
- 28.Riemann BL, Lephart SM. The sensorimotor system, part I: the physiologic basis of functional joint stability. J Athl Train. 2002;37:71–9. [PMC free article] [PubMed] [Google Scholar]
- 29.Howell DR, Osternig LR, Chou L-S. Dual-task effect on gait balance control in adolescents with concussion. Arch Phys Med Rehabil. 2013;94:1513–20. [DOI] [PubMed] [Google Scholar]
- 30.Howell DR, Osternig LR, Chou L-S. Return to activity after concussion affects dual-task gait balance control recovery. Med Sci Sports Exerc. 2015;47:673–80. [DOI] [PubMed] [Google Scholar]
- 31.Parker TM, Osternig LR, Van Donkelaar P, et al. Gait stability following concussion. Med Sci Sports Exerc. 2006;38:1032–40. [DOI] [PubMed] [Google Scholar]
- 32.Studenski S, Perera S, Wallace D, et al. Physical performance measures in the clinical setting. J Am Geriatr Soc. 2003;51:314–22. [DOI] [PubMed] [Google Scholar]
- 33.Sutherland DH, Olshen R, Cooper L, et al. The development of mature gait. J Bone Joint Surg Am. 1980;62:336–53. [PubMed] [Google Scholar]
- 34.Adolph KE, Vereijken B, Shrout PE. What changes in infant walking and why. Child Dev. 2003;74:475–97. [DOI] [PubMed] [Google Scholar]
- 35.Wang J, Wai Y, Weng Y, et al. Functional MRI in the assessment of cortical activation during gait-related imaginary tasks. J Neural Transm. 2009;116:1087–92. [DOI] [PubMed] [Google Scholar]
- 36.Godde B, Voelcker-Rehage C. More automation and less cognitive control of imagined walking movements in high- versus low-fit older adults. Front Aging Neurosci. 2010;2:pii: 139. https://doi.org/10.3389/fnagi.2010.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Howell DR, Osternig LR, Chou L-S. Consistency and cost of dual-task gait balance measure in healthy adolescents and young adults. Gait Posture. 2016;49:176–80. [DOI] [PubMed] [Google Scholar]
- 38.Howell DR, Oldham JR, DiFabio M, et al. Single-task and dualtask gait among collegiate athletes of different sport classifications: implications for concussion management. J Appl Biomech. 2017;33:24–31. [DOI] [PubMed] [Google Scholar]
- 39.Oldham JR, Munkasy BA, Evans KM, et al. Altered dynamic postural control during gait termination following concussion. Gait Posture. 2016;49:437–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buckley TA, Oldham JR, Munkasy BA, et al. Decreased anticipatory postural adjustments during gait initiation acutely post-concussion. Arch Phys Med Rehabil. 2017;98(10):1962–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Register-Mihalik JK, Littleton AC, Guskiewicz KM. Are divided attention tasks useful in the assessment and management of sport-related concussion? Neuropsychol Rev. 2013;23:300–13. [DOI] [PubMed] [Google Scholar]
- 42.Howell DR, Osternig L, van Donkelaar P, et al. Effects of concussion on attention and executive function in adolescents. Med Sci Sports Exerc. 2013;45:1030–7. [DOI] [PubMed] [Google Scholar]
- 43.Halterman CI, Langan J, Drew A, et al. Tracking the recovery of visuospatial attention deficits in mild traumatic brain injury. Brain. 2006;129:747–53. [DOI] [PubMed] [Google Scholar]
- 44.Mayr U, Laroux C, Rolheiser T, et al. Executive dysfunction assessed with a task-switching task following concussion. PloS One. 2014;9:e91379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yogev-Seligmann G, Hausdorff JM, Giladi N. The role of executive function and attention in gait. Mov Disord. 2008;23:329–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Herman D, Zaremski JL, Vincent HK, et al. Effect of neurocognition and concussion on musculoskeletal injury risk. Curr Sports Med Rep. 2015;14:194–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weiss K, Whatman C. Biomechanics associated with patellofemoral pain and ACL injuries in sports. Sports Med. 2015;45:1325–37. [DOI] [PubMed] [Google Scholar]
- 48.Read PJ, Oliver JL, De Ste Croix MBA, et al. Neuromuscular risk factors for knee and ankle ligament injuries in male youth soccer players. Sports Med. 2016;46:1059–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guy JA, Knight LM, Wang Y, et al. Factors associated with musculoskeletal injuries in children and adolescents with attention-deficit/hyperactivity disorder. Prim Care Companion CNS Disord. 2016. June 23;18(3). https://doi.org/10.4088/pcc.16m01937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Howell DR, Osternig LR, Koester MC, et al. The effect of cognitive task complexity on gait stability in adolescents following concussion. Exp Brain Res. 2014;232:1773–82. [DOI] [PubMed] [Google Scholar]
- 51. Fino PC. A preliminary study of longitudinal differences in local dynamic stability between recently concussed and healthy athletes during single and dual-task gait. J Biomech. 2016;49:1983–8. [DOI] [PubMed] [Google Scholar]
- 52.Fait P, Swaine B, Cantin J-F, et al. Altered integrated locomotor and cognitive function in elite athletes 30 days postconcussion: a preliminary study. J Head Trauma Rehabil. 2013;28:293–301. [DOI] [PubMed] [Google Scholar]
- 53.Sambasivan K, Grilli L, Gagnon I. Balance and mobility in clinically recovered children and adolescents after a mild traumatic brain injury. J Pediatr Rehabil Med. 2015;8:335–44. [DOI] [PubMed] [Google Scholar]
- 54.Brooks MA, Peterson K, Biese K, et al. Concussion increases odds of sustaining a lower extremity musculoskeletal injury after return to play among collegiate athletes. Am J Sports Med. 2016;44:742–7. [DOI] [PubMed] [Google Scholar]
- 55.Herman DC, Jones D, Harrison A, et al. Concussion may increase the risk of subsequent lower extremity musculoskeletal injury in collegiate athletes. Sports Med. 2017;47:1003–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lynall RC, Mauntel TC, Padua DA, et al. Acute lower extremity injury rates increase after concussion in college athletes. Med Sci Sports Exerc. 2015;47:2487–92. [DOI] [PubMed] [Google Scholar]
- 57.Cross M, Kemp S, Smith A, et al. Professional Rugby Union players have a 60% greater risk of time loss injury after concussion: a 2-season prospective study of clinical outcomes. Br J Sports Med. 2016;50:926–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nordström A, Nordström P, Ekstrand J. Sports-related concussion increases the risk of subsequent injury by about 50% in elite male football players. Br J Sports Med. 2014;48:1447–50. [DOI] [PubMed] [Google Scholar]
- 59.Gilbert FC, Burdette GT, Joyner AB, et al. Association between concussion and lower extremity injuries in collegiate athletes. Sports Health. 2016;8:561–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(264–9):W64. [DOI] [PubMed] [Google Scholar]
- 61.McCrory P, Meeuwisse WH, Aubry M, et al. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Br J Sports Med. 2013;47:250–8. [DOI] [PubMed] [Google Scholar]
- 62.Makdissi M, McCrory P, Ugoni A, et al. A prospective study of postconcussive outcomes after return to play in Australian football. Am J Sports Med. 2009;37:877–83. [DOI] [PubMed] [Google Scholar]
- 63.Pietrosimone B, Golightly YM, Mihalik JP, et al. Concussion frequency associates with musculoskeletal injury in retired NFL players. Med Sci Sports Exerc. 2015;47:2366–72. [DOI] [PubMed] [Google Scholar]
- 64.Burman E, Lysholm J, Shahim P, et al. Concussed athletes are more prone to injury both before and after their index concussion: a data base analysis of 699 concussed contact sports athletes. BMJ Open Sport Exerc Med. 2016;2:e000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nyberg G, Mossberg KH, Lysholm J, et al. Subsequent traumatic injuries after a concussion in elite ice hockey: a study over 28 years. Curr Res Concussion. 2015;2:109–12. [Google Scholar]
- 66.Hägglund M, Waldén M, Ekstrand J. Previous injury as a risk factor for injury in elite football: a prospective study over two consecutive seasons. Br J Sports Med. 2006;40:767–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Arnason A, Sigurdsson SB, Gudmundsson A, et al. Risk factors for injuries in football. Am J Sports Med. 2004;32(1 Suppl.):5S–16S. [DOI] [PubMed] [Google Scholar]
- 68.Beynnon BD, Murphy DF, Alosa DM. Predictive factors for lateral ankle sprains: a literature review. J Athl Train. 2002;37:376–80. [PMC free article] [PubMed] [Google Scholar]
- 69.Hewett TE, Myer GD, Ford KR. Anterior cruciate ligament injuries in female athletes: part 1, mechanisms and risk factors. Am J Sports Med. 2006;34:299–311. [DOI] [PubMed] [Google Scholar]
- 70.Martini DN, Sabin MJ, DePesa SA, et al. The chronic effects of concussion on gait. Arch Phys Med Rehabil. 2011;92:585–9. [DOI] [PubMed] [Google Scholar]
- 71.Martini DN, Goulet GC, Gates DH, et al. Long-term effects of adolescent concussion history on gait, across age. Gait Posture. 2016;49:264–70. [DOI] [PubMed] [Google Scholar]
- 72.Catena RD, van Donkelaar P, Chou L-S. The effects of attention capacity on dynamic balance control following concussion. J Neuroeng Rehabil. 2011;8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Parker TM, Osternig LR, Lee H-J, et al. The effect of divided attention on gait stability following concussion. Clin Biomech. 2005;20:389–95. [DOI] [PubMed] [Google Scholar]
- 74.Catena RD, Donkelaar P, Chou L-S. Cognitive task effects on gait stability following concussion. Exp Brain Res. 2006;176:23–31. [DOI] [PubMed] [Google Scholar]
- 75.Howell DR, Osternig LR, Chou L-S. Monitoring recovery of gait balance control following concussion using an accelerometer. J Biomech. 2015;48:3364–8. [DOI] [PubMed] [Google Scholar]
- 76.Catena R, van Donkelaar P, Chou LS. Different gait tasks distinguish immediate vs. long-term effects of concussion on balance control. J Neuroeng Rehabil. 2009;6:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Catena RD, van Donkelaar P, Chou L-S. Altered balance control following concussion is better detected with an attention test during gait. Gait Posture. 2007;25:406–11. [DOI] [PubMed] [Google Scholar]
- 78.Chiu S-L, Osternig L, Chou L-S. Concussion induces gait interjoint coordination variability under conditions of divided attention and obstacle crossing. Gait Posture. 2013;38:717–22. [DOI] [PubMed] [Google Scholar]
- 79.Parker TM, Osternig LR, van Donkelaar P, et al. Balance control during gait in athletes and non-athletes following concussion. Med Eng Phys. 2008;30:959–67. [DOI] [PubMed] [Google Scholar]
- 80.Howell DR, Osternig LR, Chou L-S. Adolescents demonstrate greater gait balance control deficits after concussion than young adults. Am J Sports Med. 2015;43:625–32. [DOI] [PubMed] [Google Scholar]
- 81.Howell DR, Beasley M, Vopat L, et al. The effect of prior concussion history on dual-task gait following a concussion. J Neurotrauma. 2017;34:838–44. [DOI] [PubMed] [Google Scholar]
- 82.Plisky PJ, Rauh MJ, Kaminski TW, et al. Star Excursion Balance Test as a predictor of lower extremity injury in high school basketball players. J Orthop Sports Phys Ther. 2006;36:911–9. [DOI] [PubMed] [Google Scholar]
- 83.McGuine TA, Greene JJ, Best T, et al. Balance as a predictor of ankle injuries in high school basketball players. Clin J Sport Med. 2000;10:239–44. [DOI] [PubMed] [Google Scholar]
- 84.Smith CA, Chimera NJ, Warren M. Association of Y balance test reach asymmetry and injury in Division I athletes. Med Sci Sports Exerc. 2015;47:136–41. [DOI] [PubMed] [Google Scholar]
- 85.Zazulak BT, Hewett TE, Reeves NP, et al. Deficits in neuromuscular control of the trunk predict knee injury risk: a prospective biomechanical-epidemiologic study. Am J Sports Med. 2007;35:1123–30. [DOI] [PubMed] [Google Scholar]
- 86.Hewett TE, Myer GD, Ford KR, et al. Biomechanical measures of neuromuscular control and valgus loading of the knee predict anterior cruciate ligament injury risk in female athletes: a prospective study. Am J Sports Med. 2005;33:492–501. [DOI] [PubMed] [Google Scholar]
- 87.Padua DA, DiStefano LJ, Beutler AI, et al. The landing error scoring system as a screening tool for an anterior cruciate ligament injury-prevention program in elite-youth soccer athletes. J Athl Train. 2015;50:589–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dubose DF, Herman DC, Jones DL, et al. Lower extremity stiffness changes after concussion in collegiate football players. Med Sci Sports Exerc. 2017;49:167–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Howell DR, Osternig LR, Chou L-S. Single-task and dual-task tandem gait test performance after concussion. J Sci Med Sport. 2017;20:622–6. [DOI] [PubMed] [Google Scholar]
- 90.Kim AS, Needle AR, Thomas SJ, et al. A sex comparison of reactive knee stiffness regulation strategies under cognitive loads. Clin Biomech (Bristol, Avon). 2016;35:86–92. [DOI] [PubMed] [Google Scholar]
- 91.Kipp K, Brown TN, McLean SG, et al. Decision making and experience level influence frontal plane knee joint biomechanics during a cutting maneuver. J Apl Biomech. 2013;29:756–62. [DOI] [PubMed] [Google Scholar]
- 92.Brown TN, O’Donovan M, Hasselquist L, et al. Soldier-relevant loads impact lower limb biomechanics during anticipated and unanticipated single-leg cutting movements. J Biomech. 2014;47:3494–501. [DOI] [PubMed] [Google Scholar]
- 93.Kim JH, Lee K-K, Ahn KO, et al. Evaluation of the interaction between contact force and decision making on lower extremity biomechanics during a side-cutting maneuver. Arch Orthop Trauma Surg. 2016;136:821–8. [DOI] [PubMed] [Google Scholar]
- 94.Collins JD, Almonroeder TG, Ebersole KT, et al. The effects of fatigue and anticipation on the mechanics of the knee during cutting in female athletes. Clin Biomech (Bristol, Avon). 2016;35:62–7. [DOI] [PubMed] [Google Scholar]
- 95.Holste KG, Yasen AL, Hill MJ, et al. Motor cortex inhibition is increased during a secondary cognitive task. Motor Control. 2016;20:380–94. [DOI] [PubMed] [Google Scholar]
- 96.Corp DT, Rogers MA, Youssef GJ, et al. The effect of dual-task difficulty on the inhibition of the motor cortex. Exp Brain Res. 2016;234:443–52. [DOI] [PubMed] [Google Scholar]
- 97.Fino PC, Nussbaum MA, Brolinson PG. Locomotor deficits in recently concussed athletes and matched controls during single and dual-task turning gait: preliminary results. J Neuroeng Rehabil. 2016;13:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cossette I, Ouellet M-C, McFadyen BJ. A preliminary study to identify locomotor-cognitive dual tasks that reveal persistent executive dysfunction after mild traumatic brain injury. Arch Phys Med Rehabil. 2014;95:1594–7. [DOI] [PubMed] [Google Scholar]
- 99.Powers KC, Cinelli ME, Kalmar JM. Cortical hypoexcitability persists beyond the symptomatic phase of a concussion. Brain Inj. 2014;28:465–71. [DOI] [PubMed] [Google Scholar]
- 100.De Beaumont L, Mongeon D, Tremblay S, et al. Persistent motor system abnormalities in formerly concussed athletes. J Athl Train. 2011;46:234–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Livingston SC, Goodkin HP, Hertel JN, et al. Differential rates of recovery after acute sport-related concussion. J Clin Neurophysiol. 2012;29:23–32. [DOI] [PubMed] [Google Scholar]
- 102.Livingston SC, Saliba EN, Goodkin HP, et al. A preliminary investigation of motor evoked potential abnormalities following sport-related concussion. Brain Inj. 2010;24:904–13. [DOI] [PubMed] [Google Scholar]
- 103.Miller NR, Yasen AL, Maynard LF, et al. Acute and longitudinal changes in motor cortex function following mild traumatic brain injury. Brain Inj. 2014;28:1270–6. [DOI] [PubMed] [Google Scholar]
- 104.Teel EF, Ray WJ, Geronimo AM, et al. Residual alterations of brain electrical activity in clinically asymptomatic concussed individuals: an EEG study. Clin Neurophysiol. 2014;125:703–7. [DOI] [PubMed] [Google Scholar]
- 105.Howell DR, Oldham JR, Meehan WP, et al. Dual-task tandem gait and average walking speed in healthy collegiate athletes. Clin J Sport Med. 2017. https://doi.org/10.1097/jsm.0000000000000509 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 106.Gardner RM, Yengo-Kahn A, Bonfield CM, et al. Comparison of baseline and post-concussion ImPACT test scores in young athletes with stimulant-treated and untreated ADHD. Phys Sportsmed. 2017;45:1–10. [DOI] [PubMed] [Google Scholar]
- 107.Elbin RJ, Kontos AP, Kegel N, et al. Individual and combined effects of LD and ADHD on computerized neurocognitive concussion test performance: evidence for separate norms. Arch Clin Neuropsychol. 2013;28:476–84. [DOI] [PubMed] [Google Scholar]
- 108.DiScala C, Lescohier I, Barthel M, et al. Injuries to children with attention deficit hyperactivity disorder. Pediatrics. 1998;102:1415–21. [DOI] [PubMed] [Google Scholar]
- 109.Clendenin AA, Businelle MS, Kelley ML. Screening ADHD problems in the sports behavior checklist: factor structure, convergent and divergent validity, and group differences. J Atten Disord. 2005;8:79–87. [DOI] [PubMed] [Google Scholar]
- 110.Osborn ZH, Blanton PD, Schwebel DC. Personality and injury risk among professional hockey players. J Inj Violence Res. 2009;1:15–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lysens RJ, Ostyn MS, Vanden Auweele Y, et al. The accident-prone and overuse-prone profiles of the young athlete. Am J Sports Med. 1989;17:612–9. [DOI] [PubMed] [Google Scholar]
- 112.Patel DR, Luckstead EF. Sport participation, risk taking, and health risk behaviors. Adolesc Med. 2000;11:141–55. [PubMed] [Google Scholar]
- 113.Swanik CB, Covassin T, Stearne DJ, et al. The relationship between neurocognitive function and noncontact anterior cruciate ligament injuries. Am J Sports Med. 2007;35:943–8. [DOI] [PubMed] [Google Scholar]
- 114.Wilkerson GB. Neurocognitive reaction time predicts lower extremity sprains and strains. Int J Athl Ther Train. 2012;17:4–9. [Google Scholar]
- 115.Herman DC, Barth JT. Drop-jump landing varies with baseline neurocognition: implications for anterior cruciate ligament injury risk and prevention. Am J Sports Med. 2016;44:2347–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dretsch MN, Silverberg N, Gardner AJ, et al. Genetics and other risk factors for past concussions in active-duty soldiers. J Neurotrauma. 2017;34:869–75. [DOI] [PubMed] [Google Scholar]


