Abstract
Secreted proteins in the Wnt family regulate gene expression in target cells by causing the accumulation of the transcriptional activator β-catenin. In the absence of Wnt, a protein complex assembled around the scaffold protein Axin targets β-catenin for destruction, thereby preventing it from transducing inappropriate signals. Loss of Axin or its binding partners APC and GSK3 results in aberrant activation of the Wnt signaling response. We have analyzed the effects of mutant forms of Drosophila Axin with large internal deletions when expressed at physiological levels in vivo, either in the presence or absence of wild type Axin. Surprisingly, even deletions that completely remove the binding sites for fly APC, GSK3 or β-catenin, though they fail to rescue to viability, these mutant forms of Axin cause only mild developmental defects, indicating largely retained Axin function. Furthermore, two lethal Axin deletion constructs, AxinΔRGS and AxinΔβcat(ΔArm), can complement each other and restore viability. Our findings support a model in which the Axin complex is assembled through cooperative tripartite interactions among the binding partners, making the assembly of functional complexes surprisingly robust.
Keywords: Wnt signaling, Axin, mutant analysis, scaffold, destruction complex, complex assembly, in vivo, Drosophila, APC, GSK3, beta-catenin
Introduction
Canonical Wnt signaling is critically important for many aspects of embryonic development and for the maintenance of functional stem cells in adults. Besides causing severe developmental defects, dysregulation of Wnt signaling also results in a variety of disorders associated with misregulation of cell proliferation and differentiation, including abnormal changes in bone mass and osteoporosis, colorectal cancer, and medulloblastoma (reviewed in Logan & Nusse (2004) and Reya & Clevers (2005)). Wnts are secreted glycoproteins that function as morphogens during embryogenesis. Activation of the Wnt signaling cascade in target cells requires the binding of Wnt ligands to receptor complexes that consist of Frizzleds plus members of the LDL-receptor-related protein (LRP) family of transmembrane receptors (Arrow in Drosophila; LRP5 and LRP6 in vertebrates). This receptor complex can apparently activate a number of signaling components, the most critical of which is Dishevelled. In turn, Dishevelled inhibits a constitutively active protein complex, termed the destruction complex, which is responsible for maintaining the ‘off’ state of the Wnt signaling pathway by targeting β-catenin for degradation. This destruction complex consists of the scaffold protein Axin, which binds two other key components, Adenomatous Polyposis Coli (APC) and Glycogen Synthase Kinase-3 (GSK-3). While active, the Axin complex is thought to promote the phosphorylation of β-catenin by GSK-3, triggering the subsequent ubiquitination and degradation of β-catenin by the proteasome. Thus, despite the constitutive production of β-catenin, its levels in the cytoplasm are kept low by the destruction complex. When the destruction complex is inhibited by Dishevelled, however, β-catenin accumulates in the cytoplasm and enters the nucleus, where it binds TCF/Lef transcription factors and activates the expression of genes required for the Wnt signaling response.
In vertebrates, two genes each encode Axin, APC and GSK3 (Polakis, 2007), while only a single Axin gene and one GSK-3 orthologue Shaggy (Sgg)/Zeste-white3 (Zw3) are present in Drosophila, although two partially redundant APC genes have been identified. Complete loss of function of Axin or Shaggy, or loss of both APC genes in flies, results in identical phenotypes that mimic a fully activated Wnt pathway. These results demonstrate that a functional Axin destruction complex is critically important for the normal control of Wnt signaling. However, our understanding of the Axin destruction complex derives largely from in vitro biochemical analyses, which have shown that each combination of Axin, APC, GSK3 and β-catenin can be co-immunoprecipitated (reviewed in Luo and Lin (2004)); Axin itself is also capable of forming homodimers (Hedgepeth et al., 1999; Sakanaka and Williams, 1999; Julius et al., 2000; Luo et al., 2005). In several instances, overexpression of mutated forms of Axin lacking the binding sites for these other components was shown to disrupt the function of the complex (Nakamura et al., 1998; Fagotto et al., 1999; Hedgepeth et al., 1999; Hinoi et al., 2000; Kishida et al., 1999; Sakanaka et al., 1998; Willert et al., 1999a; Zeng et al., 1997; Zeng et al., 1997; Yanagawa et al., 2000) although this strategy has also produced contradictory results. For example, overexpressing a mutant Axin lacking its APC binding (RGS) domain produced the same effects as wild type Axin in the developing Drosophila wing, while overexpressing a similar mutant form of the Axin in Xenopus embryos or tissue culture dominantly interfered with the function of the destruction complex (Cliffe et al., 2003; Nakamura et al., 1998; Willert et al., 1999a; Fagotto et al., 1999; Hedgepeth et al., 1999; Yanagawa et al., 2000; Cliffe et al., 2003; Tolwinski et al., 2003). Such paradoxical effects raise concerns about cell-type and species-specific differences in the dynamics of the destruction complex, negative interference with endogenous wild type protein, and artifacts associated with the overexpression of proteins involved in signal transduction.
An important issue that remains unknown concerns the stochiometric composition of the destruction complex. In particular, it is still not known which complement of Axin binding partners is essential, whether dimeric Axin is required in vivo for fully functional destruction complexes, and whether an Axin protein complex can still function without full occupancy of its binding sites. Given the fact that 44 different configurations of the Axin complex are formally possible, an in vivo analysis of its functional stochiometry would provide critical information about how physiological interactions among its different components that may modulate Wnt signaling. To date, an analysis of how binding site-deficient isoforms of Axin affect the function of the destruction complex has yet to be performed with physiological concentrations of the proteins in their normal cellular context. To address this issue in this study, we expressed either wild type Axin or mutant forms of the protein (lacking the specific binding sites for different components of the destruction complex) at near-physiological levels in flies lacking endogenous Axin. Contrary to our expectation that many of these mutant proteins would result in axinnull phenotypes, all of the deletion constructs were able to rescue the axinnull phenotype, to varying degrees. This in vivo analysis indicates a highly cooperative assembly of the destruction complex, involving the recruitment of the different components via indirect interactions with other bound partners. As a result, the Axin-based destruction complex behaves in a surprisingly robust manner, ensuring the proper regulation of β-catenin levels in the context of Wnt signaling.
RESULTS
Full rescue of the axinnull mutant by ubiquitously expressed Axin
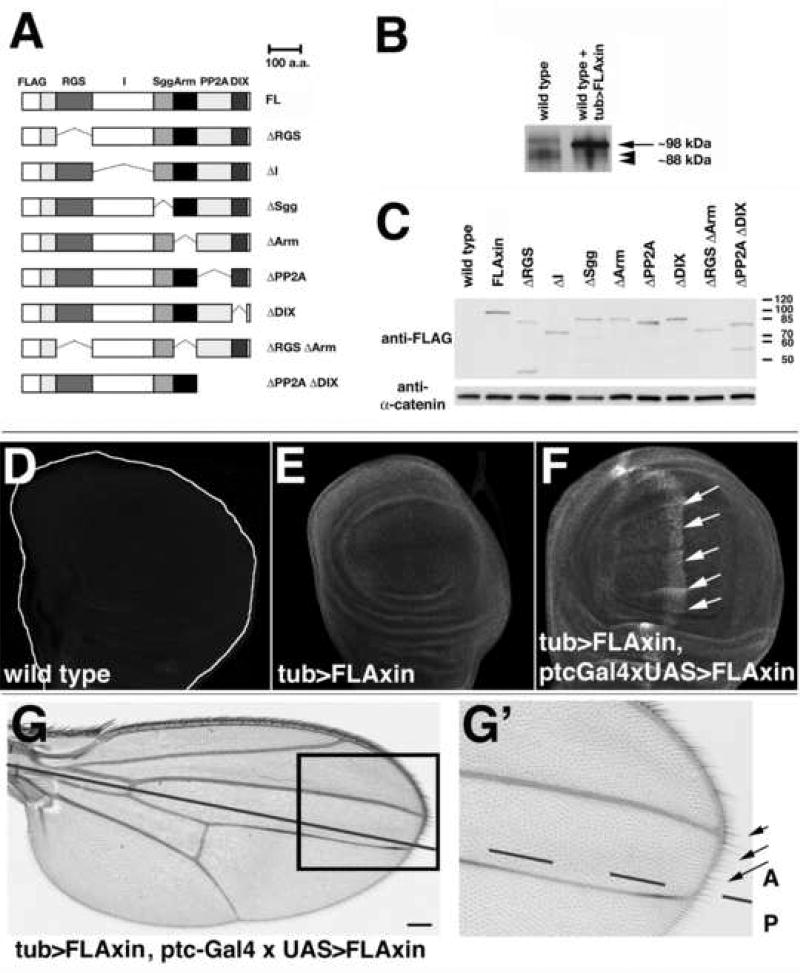
To validate our strategy for analyzing the structure-function relationships of Axin in vivo, we first designed a construct used the tubulin promoter to drive ubiquitous expression of full-length (FL) FLAG epitope-tagged Axin (here referred to as FLAxin or tub>FLAxin; Fig. 1. All constructs in this study are FLAG-tagged). Transgenic flies expressing tub>FLAxin in addition to endogenous wild type Axin showed no visible defects (see Fig. 1E–G, and data not shown). In Western blots, ectopic Axin could be readily distinguished from endogenous protein by its 6xFLAG tag and higher mobility: 98 kDa versus ~88 kDa for the native protein consistent with the slower mobility of Axin observed previously (Behrens et al., 1998; Itoh et al., 1998; Willert et al., 1999a). When we examined the relative levels of endogenous and ectopic Axin in immunoprecipitates of extracts from wild type and FLAxin expressing wild type embryos, we found that the expression levels of the FLAG-tagged FLAxin were ~4.3 fold higher than endogenous Axin (Fig. 1B, C; Suppl. Fig. 1). As described below, tub>FLAxin expression rescued both the viability and fertility of axinnull mutant flies, demonstrating that this FLAG-tagged construct of full-length Axin is functional and expressed at sufficient levels to support all stages of development.
Figure 1.
tub>FLAxin is expressed at near-physiological levels, while Axin expression at ~8.6 fold higher than endogenous Axin induces no obvious defects.
(A) FLAG-tagged Axin constructs used in this study (see Material and methods for precise position of deletions). (B) Immunoprecipitation with an anti-Axin serum from 0–12 h embryo lysates followed by Western blot analysis (probed with anti-Axin serum; after Willert et al. (1999a), revealed endogenous Axin in the wild type lane (arrowheads) and FLAG-tagged Axin (arrow) in flies expressing tub>FLAxin in addition to wild type Axin (right lane). tub>FLAxin was expressed ~4.3 fold higher than endogenous Axin. Equal amounts of lysate were immunoprecipitated, as indicated by the equivalent levels of endogenous Axin in both lanes. (C) Western blot analysis of 0–12 h embryonic lysates with anti-FLAG antibody using maternal expression of the Axin mutant proteins shown in (A). All constructs were expressed within a 2.5 fold range. The blot was also probed with an α-catenin antibody, a protein not regulated by Wnt signaling (Yanagawa et al., 2000), as a loading control. (D–F) FLAxin was visualized in third instar wing imaginal discs using the anti-FLAG antibody. (D) A wild type wing disc not expressing FLAxin is shown to illustrate levels of background staining. (E) Ubiquitous expression of FLAxin using the tub>FLAxin construct (tub>FLAxin). (F) Expression tub>FLAxin was further increased by co-expression of ptc-Gal4 × UAS> FLAxin (using the ptc-Gal4 driver), stained with an anti-FLAG antibody (in wild type). The stripe of ptc-Gal4 driven expression in the wing pouch is indicated with arrows. The fluorescence intensity profile was plotted relative to a ubiquitously expressed protein (α-catenin), as shown in Suppl. Fig. 3 and averaged for nine imaginal discs. Maximal expression in (F) was ~2 fold above the ubiquitous levels of expression provided by tub>FLAxin. (G,G’) Adult wing of a fly expressing ptc-Gal4 × UAS>FLAxin in addition to endogenous Axin and plus tub>FLAxin. No defects were detected in the bristle pattern or at the wing margin (arrows), where maximal levels of Axin would have been present during development (anterior to the anteroposterior compartment boundary; continuous black line in G, dashed in G’ for better visibility of the wing margin). A, anterior; P, posterior. Bar = 20µm in D–F, 30 µm in G and 15 µm in G’.
Importantly, no defects were observed in adult structures, including the wing (Suppl. Fig. 2). Wing margin bristles are particularly sensitive indicators for proper levels of Wg signaling, since they only form where maximal levels of Wg signal are present. Excess signaling allows ectopic bristles to differentiate; conversely, lower levels of signaling results in the loss of bristles, while severe reductions in Wg signaling causes the loss of wing tissue, manifested by notching in the wing (Couso et al., 1994; Axelrod et al., 1996; Cadigan et al., 1998; Baig-Lewis et al., 2007). Notably, when we examined the wing margin in tub>FLAxin axinnull flies, we observed none of these defects (Fig. 1G,G’, and data not shown). To determine whether with our constructs higher levels of Axin could also induce wing defects, we created a UAS construct of Axin and drove its expression with the ptc-Gal4 promoter construct. Previous studies have shown that Ptc-Gal4 drives high levels of expression in a stripe anterior to the anteroposterior compartment boundary within the developing wing, a pattern that intersects the future wing margin (Fig. 1F–G). However, the wings of these flies showed no defects (not shown). Therefore, to increase the expression of FLAG-Axin still further and also provide an internal reference to distinguish ectopic from endogenous Axin, we expressed ptc-Gal4/UAS-Axin in the tub>FLAxin flies. Whereas our data indicated that tub>FLAxin levels are ~4× higher than endogenous Axin (assuming a linear increase in fluorescence associated with anti-FLAG(Axin) immunoreactivity (Fig. 1B), we estimated that maximal levels of Axin in the ptc-Gal4 stripe of these flies were ~2 fold higher than induced by tub>FLAxin alone (Fig. 1F, Suppl. Fig. 3D), ~8.6 fold above endogenous Axin levels. Nevertheless, adult wings showed no defects (Fig. 1G,G’). Similarly, we detected no visible defects in the embryos of any of these lines, although the survival rate of the tub>FLAxin animals was ~20% lower than wild type (Fig. 2A). Nevertheless, despite this modest reduction in viability, these results indicate that the level of Axin induced by tub>FLAxin expression is within the physiological range required for normal development. Moreover, these studies also show that Axin-dependent signaling is well-regulated in vivo, allowing cells to functionally compensate for fluctuations in Axin protein concentration.
Figure 2.
The impact of mutant Axin protein expression differs, depending on the developmental stage
(A) Maternally expressed mutant Axin proteins (introduced alongside wild type Axin protein) can kill embryos. (B) When AxinΔX proteins were only expressed zygotically (in wild type background), no significant effect on embryonic hatch rate was observed. (C) Eclosion rates of adults expressing only zygotic AxinΔX proteins (relative to non-expressing siblings) are shown for embryos from (B) that were allowed develop further. tub>FLAxin flies survive slightly better than non-tub>FLAxin siblings marked by the slightly detrimental dominant mutation Sp, which results in survival rates greater than 100% (Material and methods). Error bars represent standard deviation.
Only some mutant Axin proteins interfere with wild type Axin function
Having demonstrated that full-length FLAG-tagged Axin is capable of rescuing axin null mutants, we next generated a series of constructs lacking each of the binding sites for other proteins in the destruction complex, as previously determined in immunoprecipitation assays (Behrens et al., 1998; Ikeda et al., 1998; Nakamura et al., 1998; Kishida et al., 1998; Sakanaka et al., 1998; Yamamoto et al., 1998; Fagotto, 1999; Hamada, 1999). Axin domains that would be considered most critical for Axin function are the binding sites for APC (the Axin RGS domain), Sgg kinase (fly GSK3), Armadillo (fly β-catenin), and the DIX domain. The DIX domain is thought to bind Dsh and also is part of an Axin homodimerization domain that extends into the adjacent PP2A domain (Sakanaka et al., 1998; Hedgepeth et al., 1999; Julius et al., 2000). In addition, we also designed constructs with deletions of large intervening sequence between the RGS and Sgg-binding domains, termed the ‘I’ domain, and the large domain between Arm-binding domain and DIX domain; this latter domain also contains a binding site for the catalytic subunit of protein phosphatase 2A (PP2A). Transgenic flies were generated that expressed each construct under control of the tubulin promoter, plus an inducible flip-out cassette to prevent uncontrolled or leaky expression (see Material and methods for details). As shown in Figure 1C, Western blot analysis revealed that all of these constructs could be readily detected in embryonic lysates at similar levels of expression, indicating that even large internal deletions of the Axin coding domain did not greatly increase turnover of the resultant proteins.
Next, we asked whether any of the mutant Axin proteins dominantly interfered with Axin-dependent signaling when co-expressed at near-physiological levels with endogenous Axin protein. A number of previous studies have reported that overexpressing Axin constructs lacking one or more of its protein interaction domains can cause developmental defects, presumably due to interference with normal Wnt signaling (Fagotto et al., (1999) and Hedgepeth et al. (1999) used the strong CMV IE94 enhancer/promoter; Willert et al., (1999a) used the Da-Gal4 and 69B-Gal4 drivers). Since not all developmental stages may be equally sensitive to this type of effect by such constructs, we compared the effects of expressing AxinΔX protein as maternally deposited or zygotically expressed proteins, and examined their effects on embryonic hatching and adult emergence (Fig. 2).
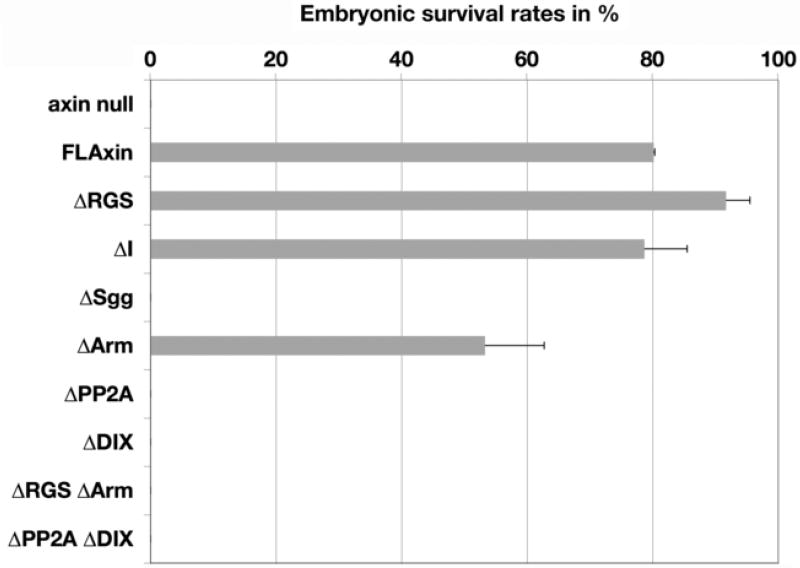
In wild type embryos, maternally deposited Axin ensures that the OFF state of Wg signaling is maintained from the time the egg is fertilized until Wg signaling is initiated by zygotic gene expression at about 3.5 hours of development. This regulation requires that maternally deposited Axin is sufficiently active to ensure proper development. Our tub>FLAxin constructs also induced maternal expression of the protein at physiologically relevant levels, as was evident from tub>FLAxin rescue of the axinnull mutant. We therefore induced the expression of our Axin mutant (tub>AxinΔX) constructs in females by removal of the flip-out cassette (Suppl. Fig. 4; Material and methods), resulting in the co-deposition of wild type and AxinΔX protein into their developing eggs. We then assessed the frequency at which embryos completed development by assessing their ability to hatch as larvae (Fig. 2A). Expression of FLAxin has a modest effect, reducing embryonic survival by 20%, compared to wild type animals (Fig. 2A). Surprisingly, when we expressed each of the Axin mutant proteins, some embryos managed to hatch in each case, though the rates differed significantly. Expression of AxinΔDIX and AxinΔPP2A had the strongest effect, reducing the hatch rate to 6–10 %; the presence of AxinΔRGS, AxinΔArm and AxinΔSgg reduced embryonic survival to 24–46%, while expression of AxinΔI has only a modest effect, reducing the survival rate to 63% (Fig. 2A). Thus, the dominant-negative effect of these constructs increases in the order FLAG-Axin < ΔI < ΔSgg ≤ ΔArm ≤ ΔRGS < ΔDIX ≤ ΔPP2A. These results demonstrate that Axin mutant proteins expressed at physiological levels (Fig. 1B,C) can nevertheless dominantly interfere with the regulation of Wnt signaling.
However, even in the presence of the most potent Axin mutant proteins, some animals still could complete embryonic development and hatch normally. To assess the potential developmental problems caused by AxinΔX proteins, we also examined larval cuticles from embryos that contained maternally expressed AxinΔX protein as well as wild type Axin. The larval cuticle provides a record of defective Wnt signaling within the embryonic ectoderm, manifested as a loss of denticle fate when signaling is increased or the lack of smooth cuticle if Wnt signaling is reduced. We scored the cuticles on a scale of 1–10, where 1 represents a complete loss of denticles (as seen in the axin mutant); 5 represents a wild type phenotype consisting of 8 abdominal denticle bands intervened by 7 domains of smooth cuticle; and 10 represents a complete loss of smooth cuticle (as seen in the wingless mutant). No significant effect was observed when we expressed AxinΔSgg (5.1; n=186), AxinΔI (5.2; n=147), AxinΔDIX (5.2; n=249) or AxinΔArm (4.1; n=141). Expression of AxinΔRGS had a modest dominant effect (3.7; n=134), inducing loss of some denticles and occasionally of an entire denticle band. This result is consistent with a modest increase in Wnt signaling, due to the less efficient destruction of Armadillo by the Axin destruction complex. In contrast, AxinΔPP2A was the only AxinΔX protein that produced a strong dominant phenotype (consistent with the loss of normal Axin inhibition by Wnt), resulting in the loss of 1–2 smooth cuticle bands (7.5; n=164). The modest effect of other Axin mutant proteins (expressed at physiological levels) contrasts with dramatic effects on development previously observed when Axin constructs were over-expressed (Yamamoto et al., 1998; Fagotto, 1999; Hedgepeth, 1999; Willert et al., 1999a).
In the foregoing experiment, AxinΔX protein was provided maternally to manipulate the repression of Wg signaling prior to the onset of zygotic transcription. However, this strategy might also compromise the subsequent control of normal, zygotic Wg signaling. To address this issue, we introduced an activated copy of tub>AxinΔX in sperm (Material and methods). Surprisingly, we found that the hatch rates of embryos expressing any of the AxinΔX constructs were not significantly different from embryos expressing wild type Axin (FLAxin), and the majority of embryos in all cases completed development (Fig. 2B). In general, hatched embryos also displayed wild type cuticles (not shown). These findings indicate that expression of Axin mutant proteins in the presence of wild type protein can have significant effects on development, but the timing and level of their expression is critical, as exemplified by the more pronounced effect of maternally expressed AxinΔX.
Since zygotically expressed mutant protein did not reduce embryonic hatch rates significantly, we next examined whether continued expression from the onset of zygotic expression throughout development would affect survival to adulthood. When we expressed the different AxinΔX constructs (tub>AxinΔX) with the tubulin promoter in this way, we found the same trend in relative potencies seen during embryonic development (Fig. 2C): The AxinΔI and AxinΔArm constructs induced relatively minor reductions in adult eclosion without defects in escapers, while AxinΔPP2A and AxinΔDIX were dominantly lethal. AxinΔRGS also caused a partial reduction in adult viability but was better tolerated when expressed zygotically instead of maternally. The most striking difference was seen with AxinΔSgg, which had a moderate effect when expressed maternally but was dominantly lethal when expressed throughout development.
These studies also showed that, the ability of animals expressing mutant Axin protein to complete embryonic development (Fig. 2B) did not ensure that they would continue to survive: zygotic expression of several of the constructs were well tolerated with respect to embryonic hatching but subsequently proved to be dominantly lethal during post-embryonic stages. The dominant effects of AxinΔSgg, AxinΔPP2A or AxinΔDIX are consistent with previous reports describing severe effects associated with similar constructs (Fagotto et al., 1999; Hedgepeth et al., 1999; Hinoi et al., 2000). However, we also observed occasional escapers expressing AxinΔArm, AxinΔRGS and AxinΔI Axin mutant proteins, although more rarely in the case of AxinΔRGS and AxinΔI. When expressed during later phases of post-embryonic development (during the pupal and adult stages), none of our Axin constructs caused obvious behavioral defects or acute lethality.
Thus, when expression of the different Axin mutant proteins was maintained at roughly physiological levels and limited to the zygotic period of gene activity, embryos could typically complete development without acquiring gross morphological defects, although all lines showed increased post-natal mortality. We propose that our results reflect the modest ability of these proteins to interfere with endogenous Axin complex function, due to the ability of the mutant proteins to form functional heterodimeric complexes with wild type Axin.
A subset of Axin mutant proteins retain functional activity
As summarized above, expression of the different mutant Axin proteins at levels similar to that of the wild type protein interfered with Wnt signaling to varying degrees; however, these studies did not distinguish between the ability of the mutant proteins to interfere with the function of the endogenous destruction complex versus the potential activity of the mutant proteins themselves. Importantly, overexpressed Axin would be expected to titrate its binding partners away from the endogenous destruction complexes, thereby disrupting the normal regulation of Wnt signaling. Therefore, to examine the residual function of the different Axin mutant proteins, we expressed each protein in axinnull mutant embryos. tub>AxinΔX expression was induced in females during metamorphosis, simultaneously with the induction of axinnull germ line clones, thereby removing the maternal contribution of wild type Axin during oogenesis. This strategy also circumvented the developmental lethality caused by several of the constructs (AxinΔSgg, AxinΔPP2A, AxinΔDIX; see Fig. 2), allowing us to generate the adult females needed for this analysis (Material and methods).
As a stringent test for residual Axin activity, we first examined whether any of the mutant constructs could restore viability to the lethal axinnull mutant, thereby allowing embryos to develop sufficiently well to hatch as larvae. Indeed, the majority of axinnull embryos expressing maternally derived AxinΔRGS or AxinΔI and about half of the embryos expressing AxinΔArm successfully hatched, although none of the other mutant constructs proved sufficient to support the completion of embryonic development (Fig. 3). However, all of the embryos expressing any of our mutant Axin proteins subsequently died at later stages (not shown), indicating that the restoration of Axin function was incomplete. This persistent lethality indicates that the residual function of these mutant proteins falls short of wild type protein activity.
Figure 3.
Some Axin mutant proteins retain enough function to allow embryos to hatch axinnull embryos expressing tub>AxinΔX were scored for their ability to hatch, to provide a qualitative measure of AxinΔX function. By these criteria, AxinΔRGS and AxinΔI functioned as well as the positive control FLAxin. AxinΔArm provided a substantial rescue (~50%) while embryos expressing any of the other AxinΔX proteins died before hatching.
The Wg target Engrailed is regulated normally by several Axin mutant proteins in axinnull embryos
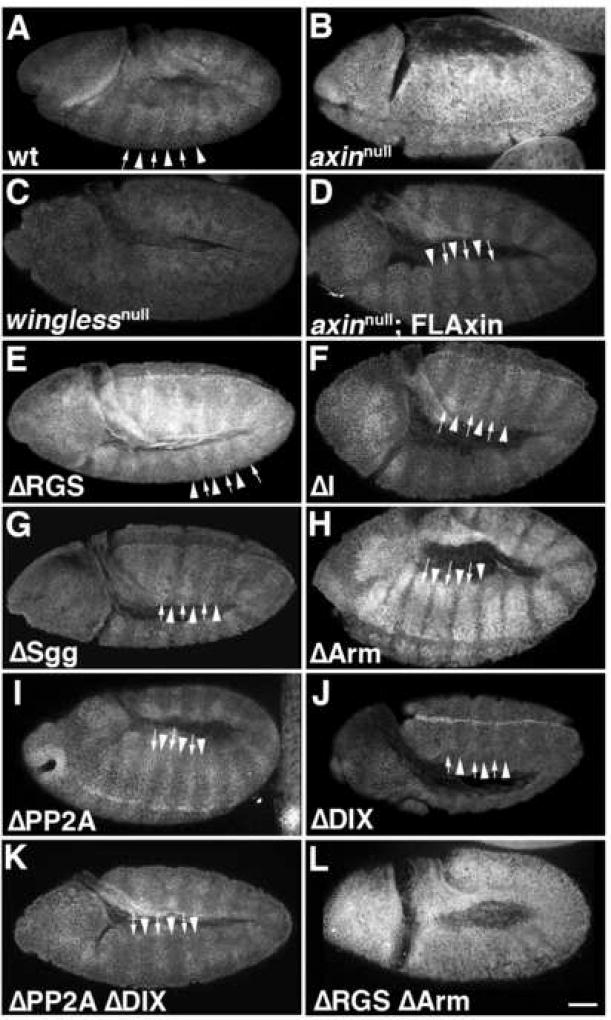
For a more detailed analysis of the residual Axin activity exhibited by our mutant constructs, we also examined molecular markers of Wg signaling in embryos. Embryonic segmentation depends on the establishment of a compartment boundary in each segment through the juxtaposition of Wg and Engrailed (En)/Hedgehog (Hh) expressing cells. Initiation of Wg and En expression in each segment is initially controlled by pair-rule genes, but subsequently, Wg and En/Hh expression become mutually dependent: absence of either protein results in the subsequent loss of expression of the other. Thus, in winglessnull embryos, En expression also subsequently disappears from the ectoderm as the embryos develop (Bejsovec and Martinez Arias, 1991; DiNardo et al., 1988; Dougan and DiNardo, 1992). In wild type embryos, the first phase of Wg signaling can therefore be monitored by examining the pattern of En in the ectoderm, which is normally limited by the range of Wg diffusion to ~2 rows of cells (2.1 ±0.48; n=281; Fig. 4A,M).
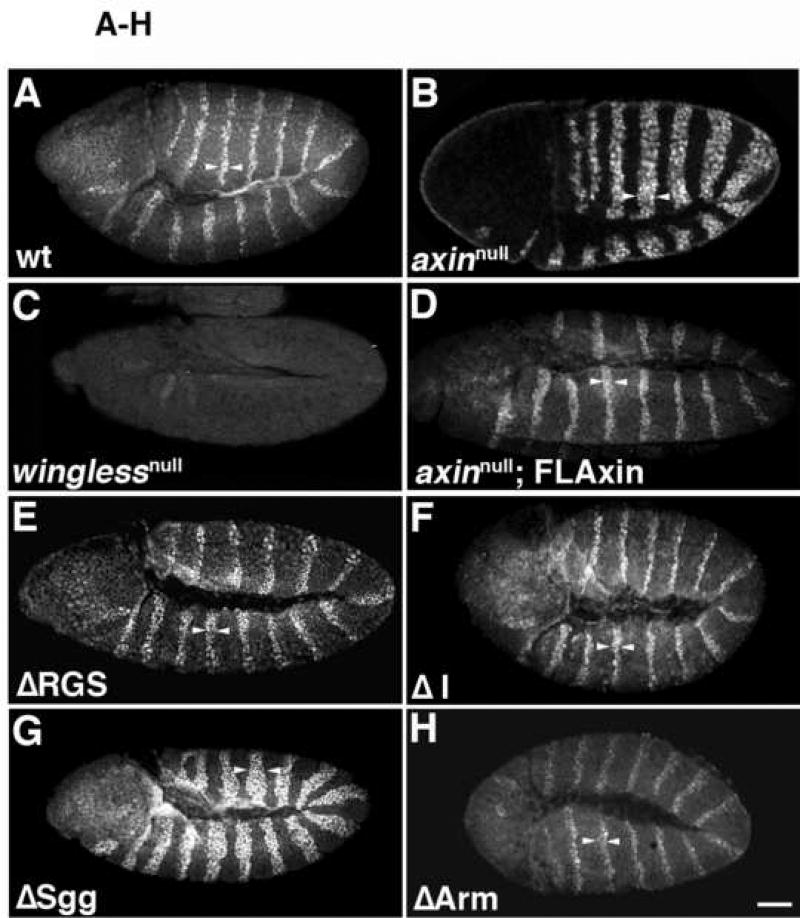
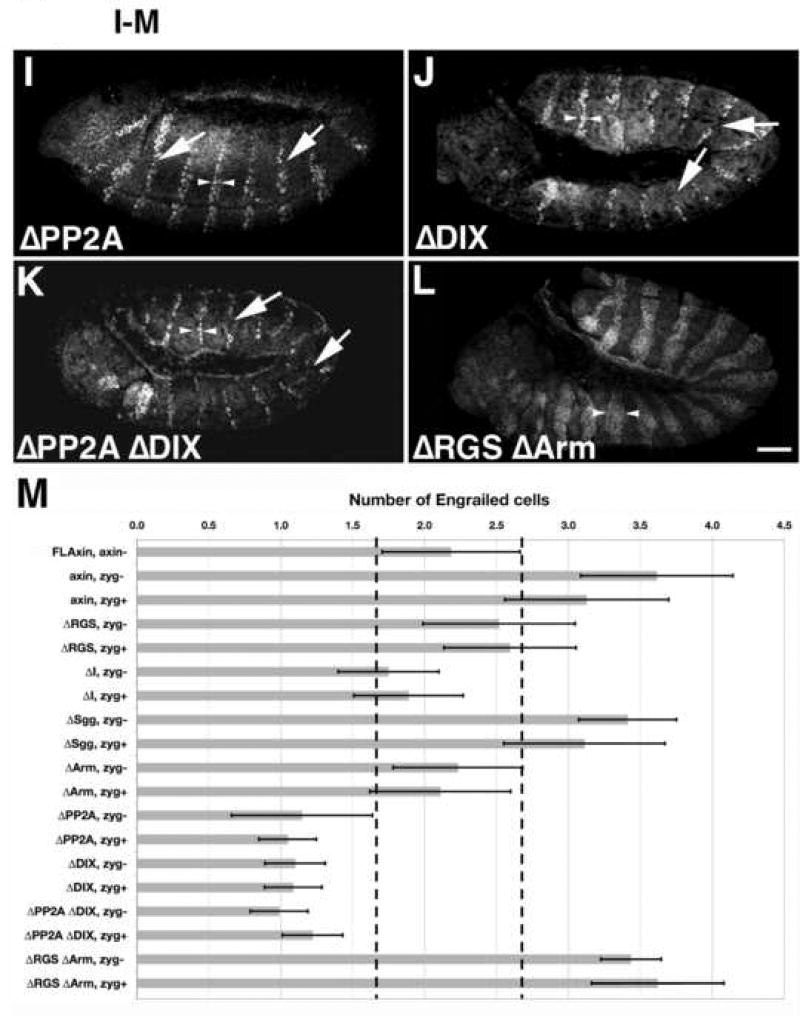
Figure 4.
Most Axin mutant proteins containing internal deletions retained substantial activity, with the exception of AxinΔSgg. Stage 9–10 embryos were immunostained with anti-Engrailed (En) antibodies as an assay for altered Wnt signaling (preparations are shown in lateral or ventrolateral view). Arrowheads indicate the width of the Engrailed stripes. (A) A wild type (wt) embryo exhibiting the normal pattern of En stripes; each stripe is ~2 cells wide. (B) In axinnull embryos, the width of the stripes increases to 3–6 cells (arrowheads). (C) Example of a winglessnull embryo, in which the normal pattern of En expression was completely lost, (homozygous winglessnull embryos were identified by the absence of the eve-lacZ marked balancer chromosome; see methods). (D) An axinnull embryo expressing tub>FLAxin exhibited the wild type pattern of En stripes (compare with A), demonstrating that this construct rescued normal Wg signaling. (E–L) axinnull embryos that expressed maternally deposited tub>AxinΔX protein. (E) An AxinΔRGS embryo, in which the En stripes were enlarged to ~2–3 cells. (F) An AxinΔI embryo, which also showed an intermediate effect on En stripe width. (G) An AxinΔSgg embryo, in which En stripe width was most similar to axinnull embryos (compare with in B). (H) An AxinΔArm embryo, with En stripes of normal width. (I) An AxinΔPP2A in which the En stripes were abnormally narrow and occasionally interrupted (arrows). (J) AxinΔDIX embryos also exhibited similar defects. (K) An AxinΔPP2AΔDIX exhibited the same pattern of narrow and interrupted En stripes as seen in the single deletions mutant proteins (compare with I, J). (L) Example of an AxinΔRGSΔArm double deletion mutant, which had abnormally widened En stripes (similar to B, G). (M) Quantification of the width of Engrailed stripes as a measure of Axin activity. The number of Engrailed-positive cells was quantified for both axinnull; tub>AxinΔX embryos (referred to as ‘zygotic−‘), and for axinmaternal−/zygotic+; tub>AxinΔX embryos that expressed a wild type copy of Axin zygotically (referred to as zygotic+). No zygotic rescue was apparent (see Suppl. Fig. 5) as the embryos look indistinguishable from axin null mutants; the range of variation in wild type embryos is indicated by dashed vertical lines. Examples of embryos expressing AxinΔX constructs zygotically (axinmaternal−/zygotic+) are also shown in Suppl. Fig. 5. Bar = 40 µm
By contrast, ~4–5 rows of cells are actually competent to express En, but the Axin destruction complex prevents En expression in the more posterior rows where the level of Wg activation falls below a certain threshold. This effect could be demonstrated in maternal−/zygotic− axin mutants (axinnull) that mimicked maximal Wg signaling (Fig. 4B, M; embryos exhibited 3.6 ±0.48 rows of En+ cells; n=179). The number of rows of En-positive cells thus provides a measure for how well different Axin constructs can maintain the OFF state of Wg signaling by assembling functional destruction complexes (catalytic activity), and how well Axin mutants can be inactivated within competent rows of cells that are exposed to sufficient levels of Wg (Axin regulation/inactivation). This second aspect of Wg signaling is exemplified in winglessnull mutant embryos, where Axin is not inactivated and En expression is lost (Fig. 4C; Bejsovec and Martinez Arias, 1991; DiNardo et al., 1988; Dougan and DiNardo, 1992). We therefore examined the patterns of En expression in the embryonic ectoderm to gauge how well our different tub>FLAxin constructs could rescue normal Wg signaling in axinnull embryos.
First, as an additional means of testing whether our Axin constructs were expressed at physiologically relevant levels, we examined whether expression of wild type Axin (via tub>FLAxin) could rescue normal patterns of En in the embryonic ectoderm. As shown in Figure 4D & M, the number of En-positive rows of cells in these animals was indistinguishable from wild type (2.18 ± 0.48; n=454). Next, we examined the ability of our AxinΔX proteins to regulate Engrailed expression in axinnull embryos. As summarized in Figure 4M, the constructs could be segregated into three groups, based on their effectiveness. The first group exhibited activities similar to wild type Axin, including AxinΔRGS (2.5 ± 0.5 rows of En-positive cells), AxinΔI (1.8 ± 0.4), and AxinΔArm (2.3 ± 0.4; Fig. 4E,F,H). In contrast, expression of the AxinΔSgg construct resulted in widened Engrailed stripes (3.4 ± 0.6 rows), indicating a loss of catalytic activity (Figs. 4G,M) that was similar to the effect seen in axinnull embryos (compare with Fig. 4B). However, the third group resulted in significantly reduced numbers of Engrailed cells, including AxinΔPP2A (1.2 ± 0.2 rows; Fig. 4I) and AxinΔDIX (1.1 ± 0.2; Fig. 4J, M). This effect indicates a loss of normal Wg signaling, which could result either from ‘hyperactive’ forms of Axin or forms that are not properly inactivated by Wnt signaling. However, unlike winglessnull embryos, a substantial portion of normal En expression was still maintained in flies expressing AxinΔPP2A and AxinΔDIX, indicating that destruction complexes formed with these constructs that could still be partially regulated by Wnt signaling. We also noted that besides being narrower, the En stripes in these embryos were often interrupted (arrows in Fig. 4I,J). Discontinuities of this type would be expected to result in the local loss of the parasegment border and subsequent segmental fusions, which we did indeed observe at later stages (described below).
Since AxinΔPP2A and AxinΔDIX generated very similar phenotypes, we also generated AxinAxinΔPP2AΔDIX embryos to test whether these two deletions in the C-terminal half of the protein might induce additive effects. However, the combined mutation produced an indistinguishable number of Engrailed cells (1.22±0.21; Fig. 4K,M) compared to single deletions of the PP2A or the DIX domain. This result indicates that a shared regulatory process was affected by the two deletions, either because both domains are essential for normal function or because the deletion of the PP2A domain disrupts the function of the DIX domain.
Considering previous reports on the importance of the APC and β-catenin binding sites for Axin function, we were surprised that neither the AxinΔRGS nor the AxinΔArm construct produced detectable defects in the foregoing experiments. To further investigate this issue, we deleted both domains simultaneously (AxinΔRGSΔArm). In contrast to the single deletions, embryos expressing this construct showed a significant increase in the number of Engrailed cells (3.17±0.42 rows per segment; Fig. 4L,M), which was indistinguishable from the effects seen in axinnull embryos (Fig. 4B,M). This finding lends additional support to our previous results with post-embryonic stages (Fig. 2–3), indicating that deletion of either the APC binding domain (AxinΔRGS) or the β-catenin binding domain (AxinΔArm) reduced but did not abolish Axin function in vivo, while simultaneous deletion of both domains resulted in a non-functional protein. As noted above, maternally expressed Axin is essential for embryonic survival, in that the presence of a zygotically expressed Axin alone could not rescue the viability of axinnull embryos (Fig. 3). Likewise, when we examined En expression in embryos expressing only zygotic Axin (see Material and methods), we observed no significant difference compared to axinnull mutants (Fig. 4M, Suppl. Fig. 5), underscoring the critical requirement of maternally expressed Axin.
In summary, with the notable exception of AxinΔSgg embryos (which resembled axinnull), all of the other deletion constructs tested in this analysis produced phenotypes that were intermediate between winglessnull mutants and axinnull animals. Thus, despite previous studies suggesting that the deleted domains play essential roles in the Axin destruction complex, our data indicate that all of these constructs retained substantial catalytic activity and were still subject to regulation by Wnt signaling. This finding was quite surprising and raised the question whether AxinΔX activity would also be sufficient to modulate Armadillo levels into a striped pattern.
Axin mutant proteins modulate Armadillo protein levels indicating retained function
Concomitant with the regulation of En expression, Wg-dependent inactivation of Axin also results in the accumulation of Armadillo (Arm; fly β-catenin) throughout the cytoplasm and nucleus within segmental stripes of the developing ectoderm during embryonic stages 9–10 (Fig. 5A; Peifer et al., 1994). This striped pattern of Arm accumulation can be abolished by the loss of Axin (resulting in the stabilization of Arm in all cells; Fig. 5B) or through loss of Wg/Wnt, which precludes Arm accumulation in the normal striped pattern (Fig. 5C; Peifer et al., 1994). Thus, the pattern of Arm striping provides an in vivo assay for directly testing whether mutant Axin constructs can assemble catalytically active destruction complexes, and whether these constructs are subject to normal inhibitory regulation by Wg signaling.
Figure 5.
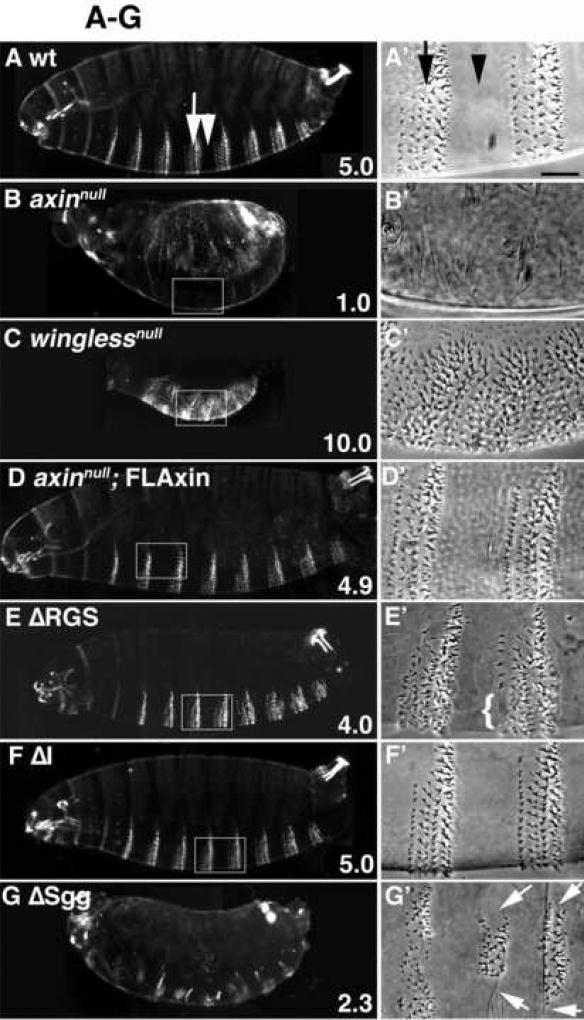
Armadillo stability is modulated by all of the different Axin proteins carrying internal deletions. Stage 9–10 embryos were immunostained with anti-Armadillo (Arm) antibodies; each preparation is shown in lateral or ventrolateral view. (A) A wild type (wt) embryo, exhibiting the normal pattern of alternating stripes of Armadillo accumulation (arrows) and reduced levels of Armadillo (arrowheads). (B) In an axinnull embryo, Armadillo was uniformly present at much higher levels than in wt embryos, so that no Armadillo striping was apparent. (C) In winglessnull embryos, Armadillo expression was substantially reduced and Armadillo striping was lost (homozygous winglessnull embryos were identified by the absence of the eve-lacZ marked balancer chromosome). (E–L) axinnull embryos expressing maternally deposited tub>AxinΔX protein. In (E–K), Armadillo striping was restored, indicating a significant level of normal Axin activity was provided by these AxinΔX proteins. (D) Example of an axinnull embryo rescued with wild type FLAxin. (E) Embryo rescued with AxinΔRGS. (F) Embryo rescued with AxinΔI. (G) Embryo rescued with AxinΔSgg. (H) Embryo rescued with AxinΔArm. (I) Embryo rescued with AxinΔPP2A. (J) Embryo rescued with AxinΔDIX. (K) Embryo rescued with AxinΔPP2AΔDIX. (L) An axinnull embryo expressing AxinΔRGSΔArm failed to show Armadillo striping and was indistinguishable from axinnull embryos (compare with B). Because the confocal settings used to image these preparations were optimized to show relative variations in Armadillo levels, absolute levels cannot be compared. All of these embryos were axinmaternal−/zygotic−, but the same phenotypes were also seen when a zygotic wild type copy of Axin was present (compare with Suppl. Fig. 6). Bar = 40 µm
When we expressed our tub>FLAxin construct in axinnull embryos, we found that the subsequent pattern of Arm striping was indistinguishable from wild type (compare Figs. 5A,D), indicating that tub>FLAxin functions like wild type Axin with respect to the regulation of Arm levels. Unexpectedly, when we examined the effects of our different AxinΔX proteins in this assay, we found that all of the single deletion constructs could also rescue the normal pattern of Arm striping in axinnull mutants (Fig. 5E–J), indicating that these mutant forms of Axin retained significant activity. Moreover, even the double deletion mutant AxinΔPP2AΔDIX produced Armadillo striping (Fig. 5K). Again consistent with the analysis of Engrailed regulation, both AxinΔRGS and AxinΔArm regulate Armadillo levels (Fig. 5E, H), while the double deletion mutant AxinΔRGSΔArm is indistinguishable from Axin loss of function (Fig. 5B,L). Absolute Armadillo levels appeared higher in axinnull, ΔRGS, ΔSgg and ΔArm causing the confocal microscope’s detector to be saturated if the same settings were used as to image the wild type embryo (not shown). It should be noted that each preparation shown in Fig. 5 was immunolabeled using identical conditions but the lack of an internal reference precluded a quantitative comparison of Arm levels based on relative fluorescent intensity. Lastly, the presence of zygotically expressed wild type Axin also did not detectably affect Armadillo striping (Supplemental Fig. 6), consistent with its lack of effect on Engrailed expression in axinnull embryos (as noted above). Thus, the modulation of Armadillo expression into stripes of high and low expression is a direct reflection of the ability of Axin single deletion proteins (AxinΔX) to be catalytically active in the destruction complex, yet be inactivated in cells receiving Wnt signal.
Axin mutant proteins provide enough function for segmented cuticles
As embryogenesis proceeds (at stage 11), the expression of Wg and En become independent from each other, when in a second phase of signaling, Wg directs the cell fate determination in the anterior compartment of each segment. These cells are induced to adopt smooth cuticle fate, which becomes apparent as the cuticle is laid down in preparation for hatching (Bejsovec and Martinez Arias, 1991; Dougan and DiNardo, 1992; Willert et al., 1999a). Posteriorly, rapid endocytosis and degradation of Wg in the En-expressing cells of each segment allows these cells to adopt a denticle fate (Dubois et al., 2001). The denticles are arranged as trapezoidal bands consisting of six rows of cells with characteristic shape and orientation (Fig. 6A, A’). During the specification of denticle fate, Axin activity prevents inappropriate activation of the Wg signaling pathway (in the absence of Wg ligand): diminished Axin function should therefore either reduce the number of denticles (or abolish them altogether, as in the axin mutant; Fig. 6B,B’), while hyperactive Axin or Axin that cannot be inactivated by the Wg signal should cause the normally smooth anterior cells to generate denticles instead (as in a wingless mutant, Fig. 6C,C’). Thus, the formation of alternating denticle bands and smooth cuticle provides an in vivo assay for distinguishing two opposing aspects of Axin function: catalytic activity (whereby it promotes Armadillo degradation), and the regulation of Axin (its inactivation) by the Wnt signal.
Figure 6.
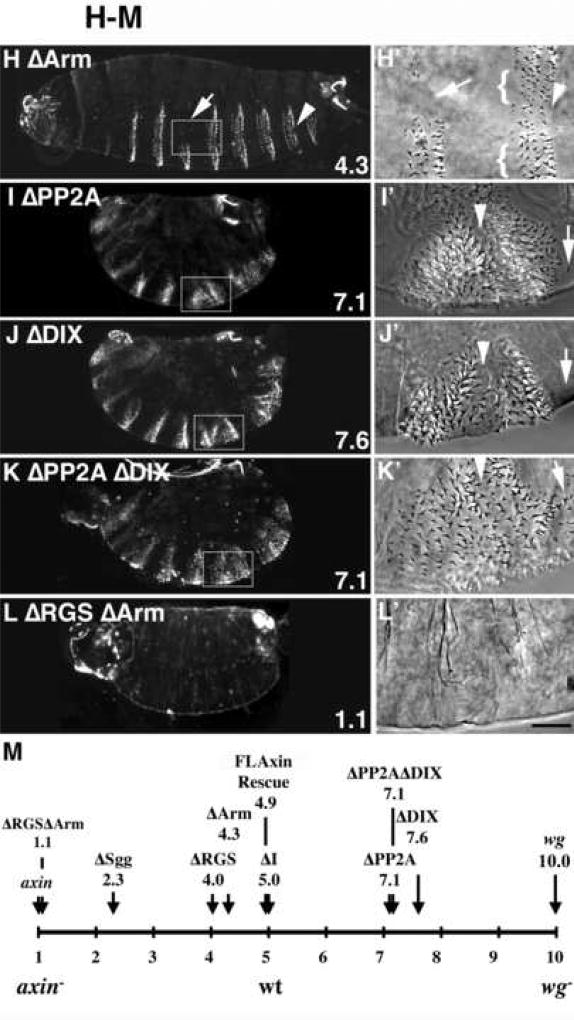
AxinΔX proteins with internal deletions all retain varying degrees of functional activity in vivo. Embryos expressing each of the tub>FLAG-AxinΔX constructs were scored for axin activity based on their ability to restore the wild type pattern of segmental denticle belts in the abdominal region, consisting of six rows of denticles (A, A’, arrow) alternating with smooth cuticle (arrowhead); in (A’) smooth cuticle and denticle band are indicated by a black arrowhead and arrow, respectively. The average phenotypic score is indicated in the lower right corner (criteria for the scores are described in Material and methods). (B, B’) An axinnull mutant embryo lacked all denticles. (C, C’) A winglessnull embryo lacked smooth cuticle. (D, D’) Expression of FLAxin in axinnull embryos restored the wild type pattern of denticle belts. No ectopic denticles were present. (E) Expression of AxinΔRGS also almost completely restored the wild type pattern, although a few row 1 denticles were frequently missing (E’, bracket). (F, F’) Expression of AxinΔI produced a wild type pattern of denticle bands. (G) Embryos expressing AxinΔSgg cuticles showed a marked loss of denticles. (G’) A magnified ventral view of another embryo, showing that significant portions of the abdominal belts did not form (arrows). (H, H’) Embryos expressing AxinΔArm also showed a loss of denticles, including a frequent loss of row1 denticles (brackets in H’), loss of posterior denticles (arrowhead) and replacement of part of the belt by smooth cuticle. (I) Embryo expressing AxinΔPP2A exhibited deletions in its smooth cuticle bands (I’, arrowhead) and the appearance of ectopic denticles (arrow). In dark field illumination, ‘ghost’ images of denticle belts that are below the plane of focus can be also seen (I; also visible in J–K). (J) Embryo expressing AxinΔDIX showed a similar deletion of smooth cuticle bands (J’, arrowhead) and the appearance of ectopic denticles (J’, arrow). (K) An AxinΔPP2AΔDIX double deletion mutant showed the loss of smooth cuticle, similar to the single deletions (compare with I, J). (L) An axinnull embryo expressing AxinΔRGSΔArm lacked all denticles and appeared similar to axinnull embryos (compare with B). (M) Schematic representation of the average cuticle score in each of the AxinΔX embryos, compared to embryos with complete loss of Axin function (score = 1.0), wild type embryos (score = 5.0) and (at the opposite end of the spectrum) wingless (wg) mutants (with complete loss of signaling; score = 10). Note that the single domain deletions failed to produce dramatic phenotypes, while only the double AxinΔRGSΔArm deletion resulted in complete loss of function. In contrast, AxinΔPP2A and AxinΔDIX both retained substantial regulation by Wg signaling, apparent as the presence of smooth cuticle, which clearly distinguishes them from wingless embryos (compare I–K to C). See Material and methods for scoring criteria. Bar = 100 µm in A–L; 25 µm in A’–L’.
To assess cuticle phenotypes produced by our different Axin constructs, we scored embryos on a 1–10 point scale, where a score of 5 represents wild type (Fig. 6A,M), axinnull embryos that lack all denticles were scored as 1.0 (n=239; Fig. 6B,M) and winglessnull embryos lacking all smooth cuticle were scored as 10.0 (n=564; Fig. 6C,M). When we examined cuticles from axinnull embryos expressing tub>FLAxin, we found them to be virtually wild type (score = 4.93, n=290), with only minor defects that reflected an occasional loss of row 1 denticles (Fig. 6D, D’, M; and not shown). This result is consistent with our other assays in which tub>FLAxin provided an effective rescue of the axinnull phenotype. Strikingly, expression of any of our single deletion AxinΔX constructs also rescued the cuticle pattern of axinnull mutant embryos to varying degrees (Fig. 6E–L), resulting in relatively normal patterns of alternating smooth cuticle and denticle bands. In addition, all of these embryos were clearly segmented, indicating that both the catalytic function and Wnt-dependent regulation of Axin remained intact. Three of the constructs (AxinΔRGS, AxinΔI and AxinΔArm) were able to substitute almost completely for endogenous Axin (Fig. 6E, F, H), producing cuticle scores of between 4 and 5 (Fig. 6M). AxinΔI cuticles were indistinguishable from wild type (compare Figs. 6A & F), while AxinΔRGS animals displayed an occasional loss of row1 denticles (Fig. 6E; arrow indicates row1 and bracket shows denticle loss). Similar minor defects were seen with AxinΔArm, although larger parts of the denticle bands were also occasionally lost in these animals (Fig. 6H,H’). Thus, AxinΔRGS and AxinΔArm exhibit moderate defects in their ability to target Armadillo for degradation, resulting in a partial increase in Wnt signaling above normal levels and the occasional loss of denticles.
This effect was considerably more pronounced in embryos expressing AxinΔSgg (Fig. 6G), which typically exhibited the loss of 4–6 of the 8 abdominal denticle bands (average cuticle score of 2.3; Fig. 6M), while the remaining denticle bands were reduced in size (Fig. 6G,G’). This phenotype indicates a substantial loss of Axin catalytic activity (required for targeting Armadillo for degradation), consistent with the effects of this construct in earlier assays (see Fig. 4). However, the presence of partial denticles bands in these animals revealed that even the AxinΔSgg construct retained sufficient residual activity to block signaling in these regions. This conclusion is also consistent with our observation that the AxinΔSgg construct can still modulate Armadillo striping at earlier stages, as shown in Fig. 5.
More dramatic effects were seen in embryos expressing AxinΔPP2A, AxinΔDIX or the double deletion mutant AxinΔPP2AΔDIX (cuticle scores of 7.1–7.6), all of which displayed the loss of normal smooth cuticle and concomitant fusion of adjacent denticle bands (Fig. 6I–K, M). Ectopic denticles were also observed within residual regions of smooth cuticle, anterior and posterior to denticle bands (Fig. 6I–J, and not shown). The loss of entire stripes of smooth cuticle in these embryos can be attributed to the discontinuities that we detected in Engrailed expression during the initial phase of Wg signaling in these genotypes (Fig. 4I–K), indicating that these constructs to are substantially less sensitive to inhibition by Wnt signaling than wild type Axin. The initial loss of normal Engrailed at stage 10 would then result in local but permanent loss of Wg expression in these regions, precluding the specification of smooth cuticle during the second phase of Wg signaling. Nevertheless, it should be noted that despite the severity of these phenotypes, neither AxinΔPP2A, AxinΔDIX nor the double deletion ΔPP2A ΔDIX completely lost their responsiveness to Wg signaling, which would have led to a wingless phenotype in which the embryos would lack all smooth cuticle (Fig. 6C). Lastly, while the single deletion mutants AxinΔRGS and AxinΔArm both retained substantial activity (Fig. 6E,H), the double deletion mutant AxinΔRGSΔArm completely lacked denticles (score of 1.1), identical to the axinnull phenotype (compare Fig. 6B and L). This result is also consistent with our assays at earlier stages, which demonstrated that this construct lacks functional Axin activity (Fig. 4–5).
Cooperative Axin complex assembly explains robustness of function
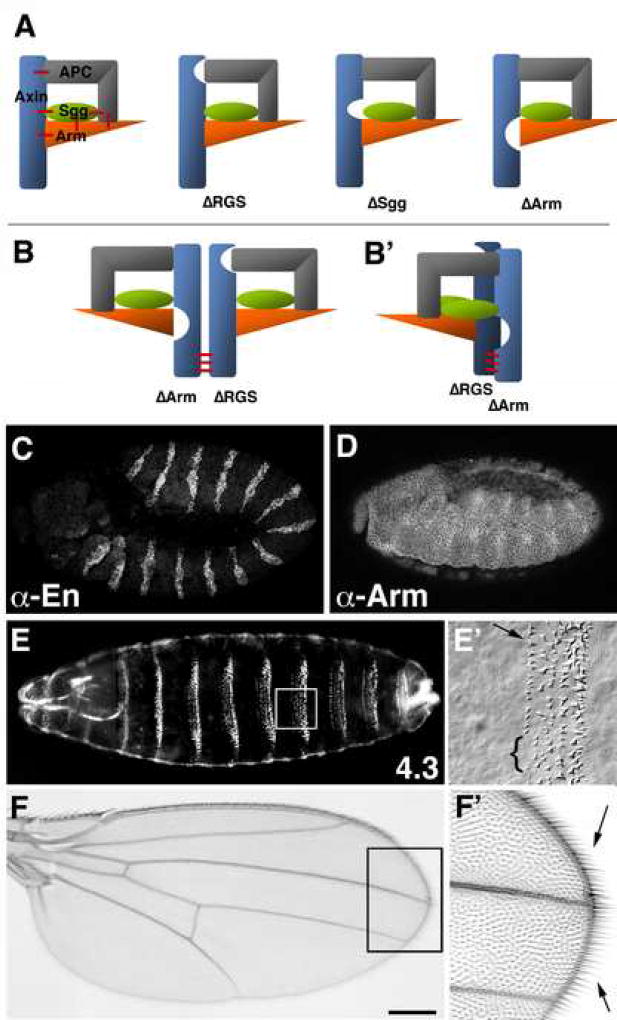
Taken together, our in vivo analyses of Axin mutant proteins lacking single domains demonstrated that each of the single domain deletion constructs retained considerable catalytic activity and was regulated by Wnt signaling. Given previous evidence that each of the deleted domains mediate important interactions with other proteins, these results posed a paradox: if the destruction complex model is correct, then APC, GSK3/Shaggy and β-catenin/Armadillo must be coordinately assembled by Axin to form a functional unit. Any complex involving one of our mutant Axin proteins would also need to contain these components (despite the absence of a particular binding domain) to remain at least partly functional. If for example the Armadillo binding domain in Axin mediates its interaction with Armadillo, how could our AxinΔArm construct still retain the ability to regulate Armadillo levels? One solution to this paradox comes from evidence that APC, GSK3/Sgg and β-catenin/Armadillo can interact which each other, independent of their individual interactions with Axin itself (Fig. 7A; Hart et al., 1998; Kishida et al., 1998). In addition, Axin also can homodimerize (Hsu et al., 1999; Hedgepeth et al., 1999; Kishida et al., 1999), raising the possibility that two different mutant Axins with distinct deletions might functionally complement each other (Fig. 7B). Thus, an appropriate combination of indirect interactions between mutant Axins and the other components of the destruction complex would permit the complex to retain some degree of activity.
Figure 7.
Tripartite interactions among the components of the Axin complex explain the robust nature of its assembly and function
(A) Model: Tripartite interactions within the Axin complex result in highly cooperative assembly of the different components, which allows recruitment of components despite the loss of their primary binding sites in Axin mutants. Schematic illustration of an Axin complex containing APC, Shaggy/GSK-3 kinase and Armadillo/β-catenin. In addition to direct interactions between Axin and each of these binding partners, additional interactions occur between APC, Shaggy and Armadillo (red bars). These additional interactions allow each of the components to be recruited into the complex and retain substantial function, even when one of the primary binding sites in Axin has been deleted (e.g. AxinΔRGS, AxinΔSgg and AxinΔArm). (B) Model of a heterodimeric Complex consisting of two mutant forms of Axin (with complementary deletions; AxinΔRGS and AxinΔArm), that interact through dimerization domains (red bars). By this model, each Axin monomeric complex may still recruit the full complement of APC, Shaggy and Armadillo (as illustrated in (A), resulting in complexes that retain most or all of their functionality. (B’) Alternative model in which a single molecule of APC and Armadillo are recruited into the dimeric complex, where they interact in trans. (C–F) axinnull embryos expressing a heteroallelic combination of tub>AxinΔRGS and tub>AxinΔArm, which rescues the axinnull mutant to viability. (C) The pattern of Engrailed stripes (revealed with anti-En immunostaining) is identical to that of wild type embryos (compare with Fig. 4A). (D) The pattern of Armadillo stripes (revealed with anti-Arm immunostaining) is identical to that of wild type embryos (compare with Fig. 5A). (E, E’) Cuticle preparations of tub>AxinΔRGS and tub>AxinΔArm embryos reveal a wild type pattern of denticle band segmentation (E), with only the occasional loss of denticles in row1 (E’ arrow); in this particular example, 4–5 denticles are missing (bracket in E’). (F) Axin signaling in adult axinnull flies is fully rescued by the combined expression of AxinΔRGS and AxinΔArm, as indicated by normal wing formation without any abnormal loss or gain of margin bristles (F’, arrows). Bar = 60 µm in C,D; 100 µm in E; 25µm in E’; 30 µm in F and 15 µm in F’.
Since several of our Axin deletion constructs produced phenotypes that resembled wild type embryos but all were lethal in an axinnull mutant background, we tested whether pair-wise combinations of the single deletion constructs could complement each other to restore viability. Among the 15 different potential combinations that we assayed, only the combination of AxinΔRGS and AxinΔArm restored viability and fertility in an axinnull background (Fig. 7B–F; and not shown). These embryos displayed both normal Engrailed stripes (2.25 ± 0.43 Engrailed cells; Fig. 7C) and Armadillo stripes (Fig. 7D) that were indistinguishable from wild type controls. Cuticle preparations from these animals also exhibited only minor defects, such as the occasional loss of some row-1 denticles (bracket in Fig. 7E’), with an average cuticle score of 4.3 ± 0.7 (Fig. 7E,E’; wild type is 5.0). Wnt signaling also appeared normal during wing development, including the normal differentiation wing margins with appropriate bristle patterns that were indistinguishable from wild type animals (Fig. 7F,F’). In contrast, none of the other pair-wise combinations of single deletion constructs restored normal levels of Axin activity. As discussed below, these results support a revised model for how the different components of the Axin destruction complex can be assembled into functional units, and raise questions about the interpretation of previously published studies involving overexpression of the different components.
Discussion
Tripartite interactions result in robust Axin complex assembly in vivo
Our in vivo analyses have revealed several important aspects of the Axin destruction complex that were previously unrecognized. The first is that ubiquitous expression of Axin using the tubulin promoter can fully rescue the axinnull mutant (Figs. 1, 3–6), demonstrating that general expression of Axin can fully restore function and viability. Moreover, since constitutive expression with this promoter will effectively bypass transcriptional regulation of Axin levels by Wnt signaling, these studies also demonstrate that Axin activity is well modulated at the post-transcriptional level. This is apparent from two aspects of Axin function: by acting as a scaffold for the assembly of the destruction complex, Axin serves a catalytic function in targeting β-catenin for degradation; and secondly, Axin is itself subject to negative regulation when a Wnt signal is transduced.
The second important finding is that both of aspects of Axin function are largely retained even when a variety of its domains are deleted (Figs. 3–6). This result is particularly striking since loss of binding sites for key binding partners of the destruction complex (such as APC, GSK3/Sgg and the β-catenin/Arm) would be expected to severely compromise Axin’s function as a scaffold. Based on these results, we postulate that the components of the destruction complex can still be assembled into a functional unit by their indirect recruitment to Axin via interactions with other binding partners within the complex (Fig. 7A). This model would explain why assembly of the destruction complex is so robust. Indeed, investigations into pair-wise interactions among the different components of the complex have shown that APC, GSK-3/Sgg and β-catenin/Arm not only bind to Axin but also directly interact with each other (Kishida et al., 1998; Rubinfeld et al., 1996; Hart et al., 1998).
Thirdly, our results demonstrate that the AxinΔRGSΔArm double deletion construct does not function, while the AxinΔRGS + AxinΔArm heteroallelic combination functions similar to wild type Axin. The restoration of Axin activity by this combination of single deletion constructs could support two alternate models. It is possible that the AxinΔRGS and AxinΔArm fortuitously balanced each other in the overall regulation of Armadillo, while functioning independent of each other. However, both mutant proteins showed a similar loss of catalytic activity when expressed individually, (Figs. 6E,H,M, 5E,H; and not shown), and the double deletion mutant AxinΔRGSΔArm produced phenotypes that were indistinguishable from axinnull mutants (Fig. 4L, 5L, 6L). We therefore think it is unlikely that the complementary effect the two single deletion constructs when expressed together was due to residual Axin activity in the absence of the APC (RGS) and Armadillo binding domains. Instead, we favor the explanation that the two mutant proteins complement each other by functioning in a single protein complex which acts like wild type Axin (Fig. 7B). By this model, dimerization of AxinΔRGS and AxinΔArm provide a complement of two copies each of APC and Armadillo through indirect binding of APC and Armadillo to other subunits within the complex (Fig. 7B, left panel). Alternatively, only a single molecule of APC and/or Armadillo might be present within the heteroallelic complex, so that APC-Armadillo interactions occur in trans between the two dimerized Axin molecules (Fig. 7B, right panel). Whether each monomeric complex has the full complement of binding partners (due to their indirect recruitment) or whether only a single molecule of APC and/or β-catenin/Armadillo may be assembled into these heteroallelic complexes remains to be determined. An important functional conclusion from this model is that Axin can function as a dimer, even though our experiments with AxinΔPP2AΔDIX suggest that direct Axin dimerization is not required for its catalytic activity in all circumstances (Figs. 4K,5K,6K). We note that Hedgepeth et al. (1999) observed some degree of complementation between AxinΔRGS and AxinΔGSK3 in injection experiments with Xenopus embryos, which also provides evidence for robust complex assembly in vivo. However, we did not observe complementation with the combination of AxinΔRGS + AxinΔSgg, which failed to restore axinnull flies to viability and suggested that under physiological expression levels in flies such possible complementation remains incomplete (data not shown).
Although it is widely assumed that a single destruction complex containing all of its component proteins must be assembled for the normal transduction of Wnt signaling (Hart et al., 1998; Ikeda et al., 1998; Rubinfeld et al., 1996), an unambiguous demonstration of this process has been lacking. Our data provide new support for this model, consistent with assembly of the destruction complex in a highly cooperative manner through tripartite interactions between Axin, APC, GSK3/Sgg and β-catenin/Armadillo (Fig. 7A). This model may also explain previous studies by overexpression in flies and tissue culture suggesting that the RGS domain of Axin (believed necessary for the recruitment of APC to the complex) might be dispensable, in that Axin constructs lacking this domain could still promote the normal degradation of β-catenin (Hart et al., 1998; Willert et al., 1999a).
Our findings may also shed light on the mechanism of complex assembly via interactions among the different components of the destruction complex as well as with Axin itself. Since pair-wise combinations of APC, GSK3/Shaggy and β-catenin/Armadillo can apparently interact, and complexes in which Axin does not directly contact all partners still retain partial activity does this mean that Axin in fact dispensable? Our results indicate that this is not the case, as axinnull flies were unable to regulate Armadillo levels and are consistent with published data (Hamada et al., 1999). Moreover, if only one of the three proteins could bind to Axin, such as in the double deletion mutant AxinΔRGSΔArm, no activity was observed (Fig. 4L,5L,6L). Thus, Shaggy binding to Axin is not sufficient to recruit APC and Armadillo into the complex or target Armadillo for normal destruction. These results indicate that in solution, no pre-assembled complex exists for APC, Shaggy and Armadillo in the absence of the Axin scaffold. At least two of the three components (APC, GSK3/Shaggy, β-catenin/Armadillo) must therefore be bound to Axin, which then allows the third component to bind, possibly by inducing a conformational change or other modification in Axin to promote this process. In this context, the varying degrees of severity in the phenotypes produced by our different deletion constructs suggests that binding of Shaggy to Axin may be somewhat more critical than that of APC or β-catenin/Armadillo (Fig. 3–6).
Our model also differs from previous suggestions that the assembly of the destruction complex involves interactions within the complex that may regulate the activity of the other components. For example, it has been proposed that APC binding to Axin de-represses Axin activity, and that APC binding to GSK3 releases the GSK3 inhibition by Axin (Hart et al., 1998; Hedgepeth et al., 1999). In contrast, we favor a model of unordered complex assembly, based on in vitro experiments (Lee et al., 2003). The importance of interactions between APC, GSK3/Shaggy and β-catenin/Armadillo to ensure cooperative complex assembly suggest that these interactions may be targets for inhibition and modulation by Wnt or other pathways. Indeed, mutations or truncations in the mutation-cluster-region (MCR) of APC disrupt interactions with Armadillo/β-catenin, resulting in an increase of Wg signaling levels corresponding to the loss of interaction sequences (McCartney et al., 2006), an aspect of APC function that is important both during normal development and in the progression tumorigenesis (reviewed in Logan and Nusse (2004)). Mutations in the MCR region of APC identified in tumor cells have led to the view that this results in complete loss of β-catenin regulation and mimicking maximal activation of Wnt signaling, which then might promote tumor development. However, recent studies have shown that a moderate increase in the constitutive activation of Wnt signaling will promote the development of cancer rather than mutations that cause maximal activation (‘just right’ hypothesis; Smits et al., 2000; Albuquerque et al., 2002). Peifer, McCartney and collaborators provided strong evidence for this ‘just right’ hypothesis by examining the activity of APC mutations since they find significant retention of fly β-catenin under physiological conditions in Drosophila (McCartney et al., 2006). This finding further supports our conclusion that Axin complex assembly occurs very robustly and that secondary interaction within the complex may partly compensate for diminished APC-β-catenin interaction. A comparison of these fly APC mutant phenotypes (McCartney et al., 2006) with our Axin mutants suggest that only AxinΔSgg sufficiently increases signaling to be a candidate for the ‘just-right’ model, while other Axin mutants retain a substantial degree of normal functionality. This result may explain why relatively few mutations in Axin are associated with cancer (Logan and Nusse, 2004). It also raises the prospect that the relatively weak effects produced by other deletions or mutations in Axin on Axin’s scaffold function might be compounded by the synergistic effects of mutations in the sites where APC, GSK3 and β-catenin interact with each other, while each individual mutation alone might produce insignificant effects.
In marked contrast, the opposite effect was observed with AxinΔPP2A, AxinΔDIX and the AxinΔPP2AΔDIX double mutants, all of which displayed a ‘hyperactive’ Axin phenotype (Fig. 4I–K, 5I–K, 6I–K) indicative of a partial loss of regulation by the Wg/Wnt signal. It is intriguing, however, that all of these mutants still resembled wild type embryos more than wingless mutants, indicating that they still retained a substantial degree of Wnt-dependence. Our in vivo experiments could not distinguish between the possibility that Wnt signaling may interact with other sites within these mutant Axin proteins or whether other components of the destruction complex may be independently inhibited by the Wnt signal. Although removing either the PP2A domain or the DIX domain did not completely eliminate Wnt-dependent inactivation of the destruction complex (Fig. 4I–K, 5I–K, 6I–K), our data still support the model that that the primary function of these C-terminal domains in vivo is to render Axin susceptible to inhibition by Wnt signaling. As noted in previous studies, the PP2A and DIX domains are thought to provide two Dishevelled binding sites that allow Dishevelled to inhibit Axin catalytic function (Kishida et al., 1999; Itoh et al., 1998; Julius et al., 2000; Penton et al., 2002). Thus, the removal of these domains should result in the loss of Dishevelled-dependent regulation of Axin activity, consistent with the wingless-like phenotypes that we observed in these mutants. The fact that these mutants did not completely recapitulate the wingless phenotype might reflect the presence of a putative third Dishevelled binding within the N-terminus of Axin (Julius et al., 2000) or, more likely, may be mediated via Dishevelled-dependent inhibition of other components of the destruction complex, such as APC or GSK3/Shaggy. Such possible mechanisms include Axin phosphorylation (Willert et al., 1999b; Yamamoto et al., 1999) or, in vertebrates, regulation through the GSK3 inhibiting protein GBP/RRAT (Farr et al., 2000; Thomas et al., 1999). Therefore, the inactivation of the destruction complex in response to Wnt/Wg signaling might result from the cumulative effect of separate regulatory steps involving several of its components. This hypothesis is consistent with the prevailing view that regulation of destruction complex activity is mediated through Dishevelled but also involves Arrow/LRP (Wehrli et al., 2000; Mao et al., 2001; Semenov et al., 2001; Tolwinski et al., 2003; Davidson et al., 2005; Zeng et al., 2005; Baig-Lewis et al., 2007).
Previous studies employing overexpression strategies indicated that Axin constructs lacking the β-catenin binding site nevertheless retain significant function (Fagotto et al., 1999; Hinoi et al., 2000), and our current data based on more physiological expression levels support this conclusion, In other respects, however, our experiments produced markedly different results from what has been previously reported. For example, it was previously reported that over-expression of an Axin construct lacking part of its RGS domain induced an increase in β-catenin levels in Xenopus (Fagotto et al., 1999), while over-expression of a similar construct reduced β-catenin signaling in Drosophila (Willert et al., 1999a). In contrast, we found that AxinΔRGS expressed in the absence of endogenous Axin functioned similar to wild type (Fig. 3, 4E, 5E, 6E), underlining the need to examine components of the Wnt signaling pathway under physiological conditions and in the absence of endogenous protein. It should also be noted that frequently the constructs in previous work used terminal deletions of Axin, instead of the internal deletions that we designed for the current studies.
Axin mutant proteins and their effects on animal development
Given the importance of the Wnt signaling pathway for multiple aspects of differentiation, it is not surprising that even modest defects in its regulation would result in cumulative abnormalities that were non-viable. However, other studies have shown that if only some cells in a particular tissue are defective in Wnt signaling (as in the case of axinnull or shaggynull mutant clones), the loss of axin activity in these local regions was well tolerated by the animal (Baig-Lewis et al., 2007; Hamada et al., 1999; see also Ripoll et al., 1988). Likewise, we would predict that animals expressing our deletion constructs in local clones of cells would survive. Therefore the lethal effect of Axin mutant proteins when expressed throughout the entire organism differs substantially from a situation where only particular tissues express mutated components of the Wnt signaling pathway, as occurs in a variety of cancers.
By far the most surprising outcome of our current studies was the discovery that Axin proteins with deleted binding sites nevertheless retained a considerable degree of functional activity during embryonic development, even when expressed in the absence of endogenous Axin (Figs. 2–7). Conversely, animals expressing many of these constructs subsequently died, even if wild type Axin was present. In this context, the delayed lethality caused by the presence of the mutant constructs most likely is due to their cumulative interference with Wnt signaling over the course of development, or it might result from stage-specific sensitivity to the mutant proteins. Thus, maternally deposited Axin mutant protein (which was present from the beginning of embryonic development) was more detrimental to embryonic survival (Fig. 2A) than zygotically expressed mutant protein (Fig. 2B), which allowed virtually all embryos expressing AxinΔX proteins to hatch. However, many of these animals subsequently died during post-embryonic stages, indicating that the continued expression of most Axin mutant proteins in wild type animals (with the exception of expressed AxinΔArm) proved to be dominantly lethal.
To the best of our knowledge, our studies represent the first systematic analysis of a scaffold protein in metazoa, in which the components of the signaling complex assembled by the scaffold protein were manipulated in vivo and at physiological expression levels. This functional analysis revealed an unexpected robustness in the functional activity of the complex that can best be explained by a model of cooperative assembly derived from tripartite interactions among the binding partners, consistent with a variety of biochemical studies that were performed in vitro. The results of our analysis also provide important insight into how mutant forms of Axin may alter normal Wnt signaling activity in the context of tumorigenesis, which in turn might help identify new targets for therapeutic intervention.
Material and Methods
Axin constructs
Axin and Axin deletion constructs with N-terminal 6x FLAG tags were generated using a combination of polymerase chain reaction (PCR) cloning, standard restriction digests and homologous recombination in yeast (Erdeniz et al., 1997; Sambrook and Russell, 2001). The following domains were deleted in Axin: ΔRGS, R53-I172; ΔI, C182–C384; ΔSgg/Zw3, D387-R452; ΔArm, E462-S538; ΔPP2, K568-P684; ΔDIX, G685–G734; ΔPP2AΔDIX was truncated at K568. Fly transformation plasmids were of the type tubulin(α1)promoter>w+>FLAG-Axin-tubulin3’UTR (tub>w+>AxinΔX) and UAS>w+>FLAG-Axin-tub3’UTR, where tubulin(α1) or the yeast UAS promoter control expression, if the “>w+>” flipout-cassette has been removed through Flip-recombinase-mediated recombination (construct design and induction as in Wehrli and Tomlinson (1998). The presence of the “>w+>” flipout-cassette allowed us to generate viable transgenic flies expressing potentially lethal constructs by preventing leaky transcription. A hsp70>flipase (hs-flip) construct was used as an inducible source of flipase (Struhl and Basler, 1993). Transgenic flies were generated and constructs mapped using standard techniques.
a) Axin construct expression in wild type
Maternal induction of construct expression was achieved through heat-shocking adult female flies of the genotype y w hs-flip; tub>w+>FLAG-AxinΔX (heat shock was applied twice for 2h at 37°C). As a result, these flies expressed the constructs during oogenesis and deposited the product into the egg, in addition to endogenous Axin. Eggs were then collected and used for Western blot analysis (Fig. 1) and to determine embryonic hatch rates (Fig. 2). Zygotic expression of the constructs was induced by removing the “>w+>” flip-out cassette during spermatogenesis, using a sperm-specific flipase (β2-tubulin>flipase (Struhl and Basler, 1993).
b) Induction of Axin constructs in the absence of endogenous Axin
To induce maximal number of axinnull mutant female germ line stem cells using the ovoD technique (Chou and Perrimon, 1996), three heat shocks were administered during pupation (1 hour at 38.5 °C, each). The resultant pulsed expression of flipase simultaneously removed the “>w+>” flipout-cassette and induced constitutive expression of the tub>AxinΔX constructs in the genotype y w hs-flip / + ; tub>w+>AxinΔX / + ; FRT82B ovoD / FRT82B axinS044230(“+” denotes wild type). Virgin females of this genotype were crossed to males of y w ; + ; FRT82B axinS044230 / TM3 ftz-lacZ; all embryos from this cross were maternally mutant for axin, and if they lacked the ftz-lacZ marker, then they were axinmaternal−/zygotic−, making them axinnull embryos. To distinguish maternal−/zygotic− from maternal−/zygotic+ embryos in cuticle analysis, males carrying a Dfd-EYFP balancer chromosome (G.J. Beitel, unpublished; Bloomington Stock Center) over the axin mutant chromosomes were crossed with females producing germ line clones producing female; embryos were then selected for or against Dfd-YFP expression, aged for 24 hours and analyzed as cuticle preparations. The axin allele used here is axinS044230, which is a transcript null allele (Hamada et al., 1999). We verified the data obtained with axinS044230 by examining the activity of FLAxin, AxinΔRGS and AxinΔSgg using the axinE77 allele of Lee and Treisman (2001) (Suppl. Fig. 7). The axinE77 allele truncates the protein at Q406 (this study), which deletes the Sgg, Arm, PP2A and DIX domain; axinE77 is therefore a null allele. tub>FLAG-Axin fully rescued axinE77 mutant animals after a second lethal mutation, present on the original chromosome, was recombined off (data not shown).
Scoring of cuticles
Cuticles were analyzed using phase contrast and blindly quantified. Scoring criteria: The abdominal region, consisting of 8 denticle belts and 7 bands of smooth cuticle, was scored on 1–10 scale. Embryos were scored as follows: 1 = loss of 7–8 denticle belts (=axin phenotype); 2 = loss of 4–7 denticle belts; 3 = loss of 1–3 denticle belts; 4 = no loss of denticle belts, but minor loss of denticle within the belts; 5 = wild type; 6 = the presence of some ectopic denticles in smooth cuticle bands; 7 = loss of 1–2 smooth cuticle bands; 8 = loss of 3–4 smooth cuticle bands; 9 = loss of 5–6 smooth bands; 10 = no smooth cuticle (wingless phenotype). Bands were scored as missing if more than 50% of the denticle band was deleted.
Hatch rates
embryonic. Embryos were collected in cages, incubated at 25 °C for an additional 30 hours and counted, as in McCartney et al. (2006). At least 650 embryos were analyzed for each cross.
adult. Zygotic expression of tub>w+>AxinΔX was induced using sperm-specific flipase (which functions in trans; see above) in males of the genotype y w; tub>w+>AxinΔX / Sp; β2-tubulin>flipase / MKRS; these flies were then crossed to y w virgin females, and their survival rates were determined by comparing survival of tub>AxinΔX offspring to the population of Sp siblings.
Immunohistochemistry
Discs were dissected and stained using our published methods (Wehrli et al., 2000). Antibodies used were mouse anti-FLAG M2 (Sigma), mouse anti-En/Inv 4D9 (Developmental Studies Hybridoma Bank, DSHB), mouse anti-Armadillo N2 7A1 (DSHB) and rat anti-α-catenin DCAT-1 (DSHB) and rabbit anti-lacZ (1:2000, Abcam); secondary antibodies were Alexa488 and Alexa546 (Molecular Probes). Images were collected in multi-track scanning mode on a Zeiss Axiovert LSM5 Pascal laser-scanning microscope. Relevant laser lines were 488 nm (Argon), 543 nm (HeNe1) and filters used were BP505-530 and LP560.
Photography
Cuticles and mounted wings were photographed on a Zeiss Axioplan2 microscope and AxioCam MRm Zeiss digital camera. Pictures were manipulated and assembled using Adobe Photoshop 7.0.
Supplementary Material
Quantification of tub>FLAxin protein levels reveals ~4.3 fold higher levels of FLAG-Axin compared to endogenous Axin. (A) Shown is a lighter exposure of the Western blot from Fig. 1B and the segments used for quantification are indicated by rectangles and numbered. (B) Quantification of mean pixel intensity is shown prior to (‘mean’ column) and after subtraction of average background values of segments 11–13, as shown in the right column. NIH Image was used in the quantification. Approximately 4.3× higher levels of FLAG-Axin are present, as is apparent from the ratio of combined FLAG-Axin intensities (segments #1+#2) to the combined segments of the two endogenous Axin bands (#3+#4+#5+#6). (C) The net values (right column of panel(B)) are shown as a graphic representation.
Ubiquitously expressed FLAxin rescues axin mutant wings and legs. (A, B) wild type, (C, D) tub>FLAxin/+ ; axinnull/axinnull. (A) wild type wing, magnified in A’ to indicate margin bristles (arrows). (B) a normal male first leg is shown with claws on left (the second claw is outside the focal plane) and five tarsal segments; the tibia is incompletely visible on the right. Maximal Wg signaling is required in the ventral regions indicated by the landmark apical bristle at the distal end of the tibia (arrow) and the sex comb (large arrowhead). The sex comb derives from a specialized row of transverse bristles, which forms in the anterior compartment (for illustrations see Struhl and Basler, 1993). The transverse bristles found between rows 5 and 6 on the tibia (between arrowheads) are characteristic bristles for posterior cell fates. (C, C’) In the wing of an axinnull mutant expressing FLAxin, no margin defects are observed. Ectopic margin bristles would have been indicative of loss of Axin catalytic activity, whereas loss of margin or margin bristles would have indicated loss of Axin complex inhibition by Wg. (D) male first leg of an axinnull mutant expressing FLAxin displays the relevant land marks, such as the apical bristle (arrow), sex combs (arrowhead) and the posterior transverse bristles (between arrowheads). Insufficient Axin function in the dorsal compartment would be expected to lead to malformation of leg segments and leg duplications (Hamada et al. 1999), but was not observed.
Ratio-imaging revealed local doubling of FLAG-Axin expression when ptc-Gal4 driven expression of UAS>FLAxin was present in tub>FLAxin expressing wing discs. The wing disc shown in (A–C) is identical to the one shown in Fig. 1F; it is stained to visualize α-catenin as a ubiquitously expressed protein (panel A, magenta; α-catenin is not regulated by Wnt signaling (Yanagawa et al., 2000), FLAxin (panel B, green) and merged in (C). (D) Shows the plotted ratio of average FLAG-Axin fluorescence intensity to α-catenin fluorescence intensity, along the white box shown in A–C. The blue profile is from a single disc (the one shown in A–C, and Fig. 1F), whereas the red profile is the average of nine imaginal discs; the brown line represents the profile of its standard deviation. As indicated on the right of panel D, peak levels of expression in the ptc-Gal4 driven stripe are ~2× that compared to tub>FLAG-Axin alone. The asterix indicates peak expression levels achieved through the combination of ptc-Gal4 mediated and tub>FLAxin expression.
Method: Non-FLAG-Axin expressing wing discs were used to calibrate the background level (such a disc is shown in Fig. 1D) and the confocal setting was adjusted to prevent saturation of the detector. Collected Z-stacks visualizing α-catenin and FLAG staining, respectively, were projected and exported to ImageJ (as described in Baig-Lewis et al. (2007)). A region of interest (intersecting the ptc-Gal4 driven stripe of FLAG-Axin expression) was selected (white box in A–C), the profile projected and exported to Microsoft Excel (version 11.3.5). The ratio of fluorescence intensity of FLAG-Axin to α-catenin was plotted, as shown in (D). Bar = 20µm
Substitution of endogenous Axin expression by that of tub>AxinΔX constructs. (A) The white+ flip-out cassette prevents expression of the AxinΔX construct by disrupting the transcriptional activity of the tubulin alpha1 promoter (tub prom). The white+ cassette is flanked by two Flipase recombination target (FRT) sequences (white arrowheads), which in cis are recombined by flp-recombinase (flipase) and the white+ cassette is removed, resulting in the expression of AxinΔX under control of the tubulin promoter. The proximity of the two FRT sequences makes this recombination a high-frequency event. (B) Induction of axinnull germ line clones, which removes maternal Axin. The dominant female sterile ovoD prevents germ cells from generating fertile eggs (Chou and Perrimon, 1996). The ovoD chromosome carries a wild type Axin gene and thus complements the axinnull chromosome (top). FRT sequences present near the centromere of both chromosomes can undergo trans recombination in the presence of flipase, which after DNA replication can result in the exchange of the ovoD and axinnull carrying arm of the third chromosome. After completion of mitosis, a cell clone homozygous mutant for axinnull that is now fertile and a twin clone wild type for Axin but homozygous for ovoD is generated. Therefore all eggs laid will lack maternal Axin. Trans-recombination between the chromosome arms is much less frequent than the cis-recombination removing the white+ cassette within a construct. Since we induced events described in (A) and (B) in the same flies, germ cells homozygous for axinnull also will have removed the white+ cassette in the constructs and induced maternal (and zygotic) expression of AxinΔX. Recombination was induced during pupal stages, which resulted in viable adults even though the expressed constructs may have been dominant lethal if induced earlier during development.
Embryos receiving a zygotic wild type copy of Axin were indistinguishable from axinnull embryos expressing tub>AxinΔX protein (compare to Fig. 4). Zygotic+ embryos were identified by a ftz-lacZ construct carried by the axin+ balancer chromosome (ftz-lacZ stain is not shown). Each panel shows a Stage 9–10 embryo immunostained with anti-En antibodies. Panels A and B are repeated from figure 4 for comparison.
(A) wild type embryo. (B) An axinnull embryo. (C–J) axinmaternal−/zygotic+ embryos with tub>AxinΔX (maternally deposited AxinΔX protein), as follows: (C) AxinΔRGS, (D) AxinΔI, (E) AxinΔSgg; note that the En stripe width in this embryo is most similar to axinnull (compare with B); (F) AxinΔArm, (G) AxinΔPP2A, (H) AxinΔDIX, (I) AxinΔPP2AΔDIX, (J) AxinΔRGSΔArm. One Engrailed stripe is indicated between arrowheads for each embryo. Interrupted Engrailed stripes are indicated by arrows. Bar = 40 µm
Armadillo striping is not affected by the presence of a zygotic wild type copy of Axin. Each panel shows a Stage 9–10 embryo immunostained with anti-Armdillo. Panel A and B are from figure 5, for comparison. ftz-lacZ staining used to identify the presence of the axin+ balancer chromosome is not shown. (A) wild type embryo. (B) An axinnull embryo; (C–J) axinmaternal−/zygotic+ embryos with tub>AxinΔX (maternally deposited AxinΔX protein), as follows: (C) AxinΔRGS, (D) AxinΔI, (E) AxinΔSgg, (F) AxinΔArm, (G) AxinΔPP2A, (H) AxinΔDIX, (I) AxinΔPP2AΔDIX, (J) AxinΔRGSΔArm. Arrows indicate stripes of Armadillo accumulation, while stripes of diminished Armadillo are indicated by arrowheads. Bar = 40 µm
Cuticles of axinE77 mutant embryos expressing FLAxin, AxinΔRGS or AxinΔSgg look indistinguishable from axinS044230 embryos expressing these same Axin constructs. Embryonic cuticles are shown with dark field illumination in A, B, C and using phase contrast in A’, B’, C’. (A,A’) FLAxin; axinE77 shows no detectable defects in denticle bands. (B, B’) AxinΔRGS; axinE77 shows occasional missing denticles (arrowheads). (C,C’) AxinΔSgg; axinE77 embryonic cuticles display loss of several denticles or entire denticle belts (arrows), though segmentation remains still apparent. Phenotypes appear indistinguishable from embryos shown in Figure 6. The average phenotypic score is indicated in the lower right corner (see Materials and methods for details). Bar = 100 µm in A–C; 25 µm in A’–C’.
Acknowledgments
We are very grateful to Drs. Jan Christian and Philip Copenhaver for many helpful and detailed comments on the manuscript, Drs. Roel Nusse and Tetsu Akiyama for providing critical reagents for this work. We also acknowledge the Bloomington Stock Center and the Developmental Studies Hybridoma Bank (DSHB) for providing us with fly stocks and antibodies, respectively. We thank Drs. Jan Christian, Philip Copenhaver, Steve DiNardo, Peter Klein, Richard Maurer, Caroline Enns, Mike Forte, Raphael Kopan, David Kimelman, Keith Wharton, Victor Hatini, Alan Spradling and Bruce Schnapp for discussions and advice. Kaitlin Leonard and Jocelyn Early provided excellent technical assistance. The confocal microscope used in this study is part of the Metal Ion Core Facility, supported by P01-GM067166. This work was supported by NIH grant GM67029 to M.W.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albuquerque C, et al. The 'just-right' signaling model: APC somatic mutations are selected based on a specific level of activation of the beta-catenin signaling cascade. Hum Mol Genet. 2002;11:1549–60. doi: 10.1093/hmg/11.13.1549. [DOI] [PubMed] [Google Scholar]
- Axelrod JD, et al. Interaction between Wingless and Notch signaling pathways mediated by dishevelled [see comments] Science. 1996;271:1826–32. doi: 10.1126/science.271.5257.1826. [DOI] [PubMed] [Google Scholar]
- Baig-Lewis S, et al. Wingless/Wnt signal transduction requires distinct initiation and amplification steps that both depend on Arrow/LRP. Dev Biol. 2007;306:94–111. doi: 10.1016/j.ydbio.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens J, et al. Functional interaction of an axin homolog, conductin, with beta-catenin, APC, and GSK3beta. Science. 1998;280:596–9. doi: 10.1126/science.280.5363.596. [DOI] [PubMed] [Google Scholar]
- Bejsovec A, Martinez Arias A. Roles of wingless in patterning the larval epidermis of Drosophila. Development. 1991;113:471–85. doi: 10.1242/dev.113.2.471. [DOI] [PubMed] [Google Scholar]
- Cadigan KM, et al. Wingless repression of Drosophila frizzled 2 expression shapes the Wingless morphogen gradient in the wing. Cell. 1998;93:767–77. doi: 10.1016/s0092-8674(00)81438-5. [DOI] [PubMed] [Google Scholar]
- Chou TB, Perrimon N. The autosomal FLP-DFS technique for generating germline mosaics in Drosophila melanogaster. Genetics. 1996;144:1673–9. doi: 10.1093/genetics/144.4.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cliffe A, et al. A role of Dishevelled in relocating Axin to the plasma membrane during wingless signaling. Curr Biol. 2003;13:960–6. doi: 10.1016/s0960-9822(03)00370-1. [DOI] [PubMed] [Google Scholar]
- Couso JP, et al. The wingless signalling pathway and the patterning of the wing margin in Drosophila. Development. 1994;120:621–636. doi: 10.1242/dev.120.3.621. [DOI] [PubMed] [Google Scholar]
- Davidson G, et al. Casein kinase 1 gamma couples Wnt receptor activation to cytoplasmic signal transduction. Nature. 2005;438:867–72. doi: 10.1038/nature04170. [DOI] [PubMed] [Google Scholar]
- DiNardo S, et al. Two-tiered regulation of spatially patterned engrailed gene expression during drosophila embryogenesis. Nature. 1988;332:604–609. doi: 10.1038/332604a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougan ST, DiNardo S. wingless generates cell type diversity among engrailed expressing cells. Nature. 1992;360:347–350. doi: 10.1038/360347a0. [DOI] [PubMed] [Google Scholar]
- Dubois L, et al. Regulated endocytic routing modulates wingless signaling in Drosophila embryos. Cell. 2001;105:613–24. doi: 10.1016/s0092-8674(01)00375-0. [DOI] [PubMed] [Google Scholar]
- Erdeniz N, et al. Cloning-free PCR-based allele replacement methods. Genome Res. 1997;7:1174–83. doi: 10.1101/gr.7.12.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagotto F, et al. Domains of axin involved in protein-protein interactions, Wnt pathway inhibition, and intracellular localization. J Cell Biol. 1999;145:741–56. doi: 10.1083/jcb.145.4.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr GH, 3rd, et al. Interaction among GSK-3, GBP, axin, and APC in Xenopus axis specification. J Cell Biol. 2000;148:691–702. doi: 10.1083/jcb.148.4.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada F, et al. Negative regulation of Wingless signaling by D-axin, a Drosophila homolog of axin. Science. 1999;283:1739–42. doi: 10.1126/science.283.5408.1739. [DOI] [PubMed] [Google Scholar]
- Hart MJ, et al. Downregulation of beta-catenin by human Axin and its association with the APC tumor suppressor, beta-catenin and GSK3 beta. Curr Biol. 1998;8:573–81. doi: 10.1016/s0960-9822(98)70226-x. [DOI] [PubMed] [Google Scholar]
- Hedgepeth CM, et al. Regulation of glycogen synthase kinase 3beta and downstream Wnt signaling by axin. Mol Cell Biol. 1999;19:7147–57. doi: 10.1128/mcb.19.10.7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinoi T, et al. Complex formation of adenomatous polyposis coli gene product and axin facilitates glycogen synthase kinase-3 beta-dependent phosphorylation of beta-catenin and down-regulates beta-catenin. J Biol Chem. 2000;275:34399–406. doi: 10.1074/jbc.M003997200. [DOI] [PubMed] [Google Scholar]
- Hsu W, et al. Identification of a domain of Axin that binds to the serine/threonine protein phosphatase 2A and a self-binding domain. J Biol Chem. 1999;274:3439–45. doi: 10.1074/jbc.274.6.3439. [DOI] [PubMed] [Google Scholar]
- Ikeda S, et al. Axin, a negative regulator of the Wnt signaling pathway, forms a complex with GSK-3beta and beta-catenin and promotes GSK-3beta-dependent phosphorylation of beta-catenin. Embo J. 1998;17:1371–84. doi: 10.1093/emboj/17.5.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K, et al. Axis determination in Xenopus involves biochemical interactions of axin, glycogen synthase kinase 3 and beta-catenin. Curr Biol. 1998;8:591–4. doi: 10.1016/s0960-9822(98)70229-5. [DOI] [PubMed] [Google Scholar]
- Julius MA, et al. Domains of axin and disheveled required for interaction and function in wnt signaling. Biochem Biophys Res Commun. 2000;276:1162–9. doi: 10.1006/bbrc.2000.3607. [DOI] [PubMed] [Google Scholar]
- Kishida S, et al. Axin, a negative regulator of the wnt signaling pathway, directly interacts with adenomatous polyposis coli and regulates the stabilization of beta-catenin. J Biol Chem. 1998;273:10823–6. doi: 10.1074/jbc.273.18.10823. [DOI] [PubMed] [Google Scholar]
- Lee JD, Treisman JE. The role of Wingless signaling in establishing the anteroposterior and dorsoventral axes of the eye disc. Development. 2001;128:1519–29. doi: 10.1242/dev.128.9.1519. [DOI] [PubMed] [Google Scholar]
- Lee E, et al. The roles of APC and Axin derived from experimental and theoretical analysis of the Wnt pathway. PLoS Biol. 2003;1:E10. doi: 10.1371/journal.pbio.0000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- Luo W, Lin SC. Axin: a master scaffold for multiple signaling pathways. Neurosignals. 2004;13:99–113. doi: 10.1159/000076563. [DOI] [PubMed] [Google Scholar]
- Luo W, et al. Axin contains three separable domains that confer intramolecular, homodimeric, and heterodimeric interactions involved in distinct functions. J Biol Chem. 2005;280:5054–60. doi: 10.1074/jbc.M412340200. [DOI] [PubMed] [Google Scholar]
- Mao J, et al. Low-density lipoprotein receptor-related protein-5 binds to Axin and regulates the canonical Wnt signaling pathway. Mol Cell. 2001;7:801–9. doi: 10.1016/s1097-2765(01)00224-6. [DOI] [PubMed] [Google Scholar]
- McCartney BM, et al. Testing hypotheses for the functions of APC family proteins using null and truncation alleles in Drosophila. Development. 2006;133:2407–18. doi: 10.1242/dev.02398. [DOI] [PubMed] [Google Scholar]
- Nakamura T, et al. Axin, an inhibitor of the Wnt signalling pathway, interacts with beta-catenin, GSK-3beta and APC and reduces the beta-catenin level. Genes Cells. 1998;3:395–403. doi: 10.1046/j.1365-2443.1998.00198.x. [DOI] [PubMed] [Google Scholar]
- Peifer M, et al. wingless signal and Zeste-white 3 kinase trigger opposing changes in the intracellular distribution of Armadillo. Development. 1994;120:369–380. doi: 10.1242/dev.120.2.369. [DOI] [PubMed] [Google Scholar]
- Polakis P. The many ways of Wnt in cancer. Curr Opin Genet Dev. 2007;17:45–51. doi: 10.1016/j.gde.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–50. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- Ripoll P, et al. A gradient of affinities for sensory bristles across the wing blade of Drosophila melanogaster. Development. 1988;103:757–767. [Google Scholar]
- Rubinfeld B, et al. Binding of GSK3beta to the APC-beta-catenin complex and regulation of complex assembly. Science. 1996;272:1023–1026. doi: 10.1126/science.272.5264.1023. [DOI] [PubMed] [Google Scholar]
- Sakanaka C, Williams LT. Functional domains of axin. Importance of the C terminus as an oligomerization domain. J Biol Chem. 1999;274:14090–3. doi: 10.1074/jbc.274.20.14090. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Russell D. Molecular Cloning: A Laboratory Manual. Third. Cold Spring Harbor Lab. Cold Spring Harbor; New York: 2001. [Google Scholar]
- Semenov MV, et al. Head inducer Dickkopf-1 is a ligand for Wnt coreceptor LRP6. Curr Biol. 2001;11:951–61. doi: 10.1016/s0960-9822(01)00290-1. [DOI] [PubMed] [Google Scholar]
- Smits R, et al. Somatic Apc mutations are selected upon their capacity to inactivate the beta-catenin downregulating activity. Genes Chromosomes Cancer. 2000;29:229–39. doi: 10.1002/1098-2264(2000)9999:9999<::aid-gcc1033>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Struhl G, Basler K. Organizing activity of wingless protein in Drosophila. Cell. 1993;72:527–540. doi: 10.1016/0092-8674(93)90072-x. [DOI] [PubMed] [Google Scholar]
- Thomas GM, et al. A GSK3-binding peptide from FRAT1 selectively inhibits the GSK3-catalysed phosphorylation of axin and beta-catenin. FEBS Lett. 1999;458:247–51. doi: 10.1016/s0014-5793(99)01161-8. [DOI] [PubMed] [Google Scholar]
- Tolwinski NS, et al. Wg/Wnt signal can be transmitted through arrow/LRP5,6 and Axin independently of Zw3/Gsk3beta activity. Dev Cell. 2003;4:407–18. doi: 10.1016/s1534-5807(03)00063-7. [DOI] [PubMed] [Google Scholar]
- Wehrli M, et al. arrow encodes an LDL-receptor-related protein essential for Wingless signalling. Nature. 2000;407:527–30. doi: 10.1038/35035110. [DOI] [PubMed] [Google Scholar]
- Willert K, et al. A Drosophila Axin homolog, Daxin, inhibits Wnt signaling. Development. 1999a;126:4165–73. doi: 10.1242/dev.126.18.4165. [DOI] [PubMed] [Google Scholar]
- Willert K, et al. Wnt-induced dephosphorylation of axin releases beta-catenin from the axin complex. Genes Dev. 1999b;13:1768–73. doi: 10.1101/gad.13.14.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H, et al. Phosphorylation of axin, a Wnt signal negative regulator, by glycogen synthase kinase-3beta regulates its stability. J Biol Chem. 1999;274:10681–4. doi: 10.1074/jbc.274.16.10681. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, et al. Axil, a member of the Axin family, interacts with both glycogen synthase kinase 3beta and beta-catenin and inhibits axis formation of Xenopus embryos. Mol Cell Biol. 1998;18:2867–75. doi: 10.1128/mcb.18.5.2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagawa S, et al. Biochemical characterization of the Drosophila axin protein. FEBS Lett. 2000;474:189–94. doi: 10.1016/s0014-5793(00)01601-x. [DOI] [PubMed] [Google Scholar]
- Zeng L, et al. The mouse Fused locus encodes Axin, an inhibitor of the Wnt signaling pathway that regulates embryonic axis formation. Cell. 1997;90:181–92. doi: 10.1016/s0092-8674(00)80324-4. [DOI] [PubMed] [Google Scholar]
- Zeng X, et al. A dual-kinase mechanism for Wnt co-receptor phosphorylation and activation. Nature. 2005;438:873–7. doi: 10.1038/nature04185. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Quantification of tub>FLAxin protein levels reveals ~4.3 fold higher levels of FLAG-Axin compared to endogenous Axin. (A) Shown is a lighter exposure of the Western blot from Fig. 1B and the segments used for quantification are indicated by rectangles and numbered. (B) Quantification of mean pixel intensity is shown prior to (‘mean’ column) and after subtraction of average background values of segments 11–13, as shown in the right column. NIH Image was used in the quantification. Approximately 4.3× higher levels of FLAG-Axin are present, as is apparent from the ratio of combined FLAG-Axin intensities (segments #1+#2) to the combined segments of the two endogenous Axin bands (#3+#4+#5+#6). (C) The net values (right column of panel(B)) are shown as a graphic representation.
Ubiquitously expressed FLAxin rescues axin mutant wings and legs. (A, B) wild type, (C, D) tub>FLAxin/+ ; axinnull/axinnull. (A) wild type wing, magnified in A’ to indicate margin bristles (arrows). (B) a normal male first leg is shown with claws on left (the second claw is outside the focal plane) and five tarsal segments; the tibia is incompletely visible on the right. Maximal Wg signaling is required in the ventral regions indicated by the landmark apical bristle at the distal end of the tibia (arrow) and the sex comb (large arrowhead). The sex comb derives from a specialized row of transverse bristles, which forms in the anterior compartment (for illustrations see Struhl and Basler, 1993). The transverse bristles found between rows 5 and 6 on the tibia (between arrowheads) are characteristic bristles for posterior cell fates. (C, C’) In the wing of an axinnull mutant expressing FLAxin, no margin defects are observed. Ectopic margin bristles would have been indicative of loss of Axin catalytic activity, whereas loss of margin or margin bristles would have indicated loss of Axin complex inhibition by Wg. (D) male first leg of an axinnull mutant expressing FLAxin displays the relevant land marks, such as the apical bristle (arrow), sex combs (arrowhead) and the posterior transverse bristles (between arrowheads). Insufficient Axin function in the dorsal compartment would be expected to lead to malformation of leg segments and leg duplications (Hamada et al. 1999), but was not observed.
Ratio-imaging revealed local doubling of FLAG-Axin expression when ptc-Gal4 driven expression of UAS>FLAxin was present in tub>FLAxin expressing wing discs. The wing disc shown in (A–C) is identical to the one shown in Fig. 1F; it is stained to visualize α-catenin as a ubiquitously expressed protein (panel A, magenta; α-catenin is not regulated by Wnt signaling (Yanagawa et al., 2000), FLAxin (panel B, green) and merged in (C). (D) Shows the plotted ratio of average FLAG-Axin fluorescence intensity to α-catenin fluorescence intensity, along the white box shown in A–C. The blue profile is from a single disc (the one shown in A–C, and Fig. 1F), whereas the red profile is the average of nine imaginal discs; the brown line represents the profile of its standard deviation. As indicated on the right of panel D, peak levels of expression in the ptc-Gal4 driven stripe are ~2× that compared to tub>FLAG-Axin alone. The asterix indicates peak expression levels achieved through the combination of ptc-Gal4 mediated and tub>FLAxin expression.
Method: Non-FLAG-Axin expressing wing discs were used to calibrate the background level (such a disc is shown in Fig. 1D) and the confocal setting was adjusted to prevent saturation of the detector. Collected Z-stacks visualizing α-catenin and FLAG staining, respectively, were projected and exported to ImageJ (as described in Baig-Lewis et al. (2007)). A region of interest (intersecting the ptc-Gal4 driven stripe of FLAG-Axin expression) was selected (white box in A–C), the profile projected and exported to Microsoft Excel (version 11.3.5). The ratio of fluorescence intensity of FLAG-Axin to α-catenin was plotted, as shown in (D). Bar = 20µm
Substitution of endogenous Axin expression by that of tub>AxinΔX constructs. (A) The white+ flip-out cassette prevents expression of the AxinΔX construct by disrupting the transcriptional activity of the tubulin alpha1 promoter (tub prom). The white+ cassette is flanked by two Flipase recombination target (FRT) sequences (white arrowheads), which in cis are recombined by flp-recombinase (flipase) and the white+ cassette is removed, resulting in the expression of AxinΔX under control of the tubulin promoter. The proximity of the two FRT sequences makes this recombination a high-frequency event. (B) Induction of axinnull germ line clones, which removes maternal Axin. The dominant female sterile ovoD prevents germ cells from generating fertile eggs (Chou and Perrimon, 1996). The ovoD chromosome carries a wild type Axin gene and thus complements the axinnull chromosome (top). FRT sequences present near the centromere of both chromosomes can undergo trans recombination in the presence of flipase, which after DNA replication can result in the exchange of the ovoD and axinnull carrying arm of the third chromosome. After completion of mitosis, a cell clone homozygous mutant for axinnull that is now fertile and a twin clone wild type for Axin but homozygous for ovoD is generated. Therefore all eggs laid will lack maternal Axin. Trans-recombination between the chromosome arms is much less frequent than the cis-recombination removing the white+ cassette within a construct. Since we induced events described in (A) and (B) in the same flies, germ cells homozygous for axinnull also will have removed the white+ cassette in the constructs and induced maternal (and zygotic) expression of AxinΔX. Recombination was induced during pupal stages, which resulted in viable adults even though the expressed constructs may have been dominant lethal if induced earlier during development.
Embryos receiving a zygotic wild type copy of Axin were indistinguishable from axinnull embryos expressing tub>AxinΔX protein (compare to Fig. 4). Zygotic+ embryos were identified by a ftz-lacZ construct carried by the axin+ balancer chromosome (ftz-lacZ stain is not shown). Each panel shows a Stage 9–10 embryo immunostained with anti-En antibodies. Panels A and B are repeated from figure 4 for comparison.
(A) wild type embryo. (B) An axinnull embryo. (C–J) axinmaternal−/zygotic+ embryos with tub>AxinΔX (maternally deposited AxinΔX protein), as follows: (C) AxinΔRGS, (D) AxinΔI, (E) AxinΔSgg; note that the En stripe width in this embryo is most similar to axinnull (compare with B); (F) AxinΔArm, (G) AxinΔPP2A, (H) AxinΔDIX, (I) AxinΔPP2AΔDIX, (J) AxinΔRGSΔArm. One Engrailed stripe is indicated between arrowheads for each embryo. Interrupted Engrailed stripes are indicated by arrows. Bar = 40 µm
Armadillo striping is not affected by the presence of a zygotic wild type copy of Axin. Each panel shows a Stage 9–10 embryo immunostained with anti-Armdillo. Panel A and B are from figure 5, for comparison. ftz-lacZ staining used to identify the presence of the axin+ balancer chromosome is not shown. (A) wild type embryo. (B) An axinnull embryo; (C–J) axinmaternal−/zygotic+ embryos with tub>AxinΔX (maternally deposited AxinΔX protein), as follows: (C) AxinΔRGS, (D) AxinΔI, (E) AxinΔSgg, (F) AxinΔArm, (G) AxinΔPP2A, (H) AxinΔDIX, (I) AxinΔPP2AΔDIX, (J) AxinΔRGSΔArm. Arrows indicate stripes of Armadillo accumulation, while stripes of diminished Armadillo are indicated by arrowheads. Bar = 40 µm
Cuticles of axinE77 mutant embryos expressing FLAxin, AxinΔRGS or AxinΔSgg look indistinguishable from axinS044230 embryos expressing these same Axin constructs. Embryonic cuticles are shown with dark field illumination in A, B, C and using phase contrast in A’, B’, C’. (A,A’) FLAxin; axinE77 shows no detectable defects in denticle bands. (B, B’) AxinΔRGS; axinE77 shows occasional missing denticles (arrowheads). (C,C’) AxinΔSgg; axinE77 embryonic cuticles display loss of several denticles or entire denticle belts (arrows), though segmentation remains still apparent. Phenotypes appear indistinguishable from embryos shown in Figure 6. The average phenotypic score is indicated in the lower right corner (see Materials and methods for details). Bar = 100 µm in A–C; 25 µm in A’–C’.