Abstract
Background: The Capabilities of Upper Extremity Test (CUE-T) and the Graded Redefined Assessment of Strength, Sensibility and Prehension (GRASSP) were both developed to detect change in upper extremity (UE) function in persons with tetraplegia. Objective: To compare the responsiveness and minimal clinically important difference (MCID) of the CUE-T and the quantitative prehension (QtP) scale of the GRASSP. Methods: Subjects included 69 persons with tetraplegia: 60 with acute and 9 with chronic injuries. Subjects were assessed twice 3 months apart using the CUE-T, QtP-GRASSP, and upper extremity motor scores (UEMS). Subjects rated their impression of change in overall and right/left UE function from −7 to +7. The standardized response mean (SRM) was determined for acute subjects. MCID was estimated using a small subjective change (2–3 points) and change in UEMS. Results: Subjects were 41.9 ± 18.1 years old, neurological levels C1–C7; 25 were motor complete. For acute subjects, the SRMs for total/side CUE-T scores were 1.07/0.96, and for the QtP-GRASSP they were 0.88/0.78. MCIDs based on subjective change for total/side CUE-T scores were 11.7/6.1 points and for QtP-GRASSP were 6.4/3.0 points. Based on change in UEMS, MCIDs for total/side were 11.9/6.3 points for CUE-T and 6.0/3.3 points for QtP-GRASSP. Some subjects had changes in the CUE-T due to its arm items that were not seen with the QtP-GRASSP. Conclusion: Both the CUE-T and QtP-GRASSP are responsive to change in persons with acute cervical spinal cord injury with large SRMs. The CUE-T detects some changes in UE function not seen with the QtP-GRASSP.
Keywords: outcome assessment, tetraplegia, upper extremity
Tetraplegia due to traumatic cervical spinal cord injury (SCI) accounts for 54% of persons admitted to the SCI Model Systems of Care, a group of centers funded by the National Institute on Disability, Independent Living, and Rehabilitation Research, Department of Health and Human Services, to study outcomes related to traumatic SCI in the United States. Incomplete tetraplegia at 41.4% is the most common category of neurologic injury admitted to these centers.1 Despite an overall good prognosis for walking, persons with incomplete tetraplegia have significant residual motor impairment in the upper extremities2 and require assistance in many areas of self-care.3 In a survey of 681 persons with SCI, 48.7% of persons with tetraplegia indicated that their quality of life would be impacted most by improvements in arm/hand function, followed by 13% for sexual function and only 7.8% for walking.4
Until recently there were few assessments of upper extremity (UE) function for persons with cervical SCI. The Grasp and Release Test5 was developed to assess performance in persons with C5-6 tetraplegia using a neuroprosthesis and was not meant to be a comprehensive assessment of function for persons of all cervical spinal cord levels. The Capabilities of Upper Extremity Questionnaire (CUE-Q) is a patient-reported assessment of UE functional capacity. Patients are asked to rate level of difficulty performing 32 upper limb actions on a scale of 0 (unable to do) to 4 (no difficulty).6 There may be differences in patient-reported performance and observed performance of function, particularly soon after injury when the individual has limited lived experience with a disability6 making an objective measure desirable.
In the past several years, two assessments designed specifically for persons with tetraplegia due to cervical SCI have been developed: the Graded Redefined Assessment of Strength, Sensibility and Prehension (GRASSP)7 and the Capabilities of Upper Extremity Test (CUE-T).8 Both are reported to have good test-retest reliability and validity.9,10 These assessments were developed primarily for clinical research, in order to be able to detect small but clinically meaningful changes in function.9,10
The GRASSP is a multidimensional assessment that includes strength, sensation, qualitative prehension, and quantitative prehension (QtP). The strength and sensibility components are impairment measures. The QtP scale is a capacity measure in the Activities domain, using the International Classification of Functioning, Disability and Health (ICF) framework – assessing what the individual can do in a standardized setting without equipment or assistance.11 The QtP-GRASSP evaluates different grasp patterns, but it allows item completion using an alternative grasp pattern, albeit with lower scores than if the expected grasp pattern is used.12 The GRASSP is being used as a secondary outcome measure in several clinical trials, such as the SPRING trial,13 and is being collected by the European Multicenter Study about Spinal Cord Injury (EM-SCI) researchers.14
The CUE-T was developed using the Institute of Medicine model of disablement.15 It was designed to evaluate “functional limitation”, which is a “restriction or lack of ability to perform an action or activity in the manner or within the range considered normal.”15(p6) Functional limitations operate at the level of the individual and reflect the combined impact of impairments on the actions a person can do without assistance or equipment. This domain is most closely aligned with the Activities-Capacity domain of the ICF.16 The CUE-T evaluates UE actions such as reaching, lifting, pulling, and pushing in addition to various grasp patterns. Test procedures are designed to minimize the impact of functional limitations not involved in a particular test item. For example, a chest strap or trunk support can be used for the “lift up” item, leaning forward is not permitted for reaching, and items for grasp patterns are placed close to the subject to minimize the need to reach. In the CUE-T, grasping tasks must be completed using a designated grasp pattern; alternative grasp patterns are not allowed.17 The intent is to assess the action, for example “pick up something using tripod grasp pattern” not the task “pick up a pencil.” The CUE-T is also being used in the SPRING trial13 and is being collected by the NeuroRecovery Network to evaluate changes in UE function.
In addition to reliability and validity, important characteristics of an evaluative instrument are the minimal detectable difference (MDD), responsiveness, and minimal clinically important difference(MCID).The MDD is based on variability in test-retest scores in stable patients; differences greater than the MDD are likely to reflect a true change rather than measurement error.18 Typically a 95% confidence interval is used, and the MDD is determined by multiplying the standard error of measurement by 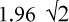 .19,20 The MDD was referred to as repeatability by Bland and Altman19 and the smallest real difference by Beckerman et al.20 The MDD for the QtP-GRASSP has been reported as 9.7 points for the combined score and 6.0/5.3 points for the right/left side scores.21 The MDD for the CUE-T has been reported as 10.8 points for the total score and 6.3/6/1 points for right/left side scores.10 Recalculation of MDD after dropping pronation and supination items gives values of 9.6 for total score and 5.2/4.8 for right/left side scores (unpublished data).
.19,20 The MDD was referred to as repeatability by Bland and Altman19 and the smallest real difference by Beckerman et al.20 The MDD for the QtP-GRASSP has been reported as 9.7 points for the combined score and 6.0/5.3 points for the right/left side scores.21 The MDD for the CUE-T has been reported as 10.8 points for the total score and 6.3/6/1 points for right/left side scores.10 Recalculation of MDD after dropping pronation and supination items gives values of 9.6 for total score and 5.2/4.8 for right/left side scores (unpublished data).
Responsiveness, or sensitivity to change, is the ability of an instrument to detect small but important clinical changes.22 There are several methods for assessing responsiveness; commonly used indices look at change over time compared to variability in baseline scores (eg, the effect size) or compared to variability in the amount of change (eg, the standardized response mean [SRM]).23,24 The SRM is the average change from time 1 to time 2 divided by the standard deviation of the change.24 There is not uniform agreement of the interpretation of values for the SRM, but it has been proposed that values of 0.2 represent small, 0.5 moderate, and 0.8 large responsiveness.24 Responsiveness of the GRASSP has been reported using the SRM. This value varies depending on the population and time interval. For a sample of cervical SCI of all American Spinal Injury Association Impairment Scale (AIS) grades, SRM from 1 to 3 months post injury has been reported as 0.81 and from 1 to 6 months post injury as 0.94.25 Responsiveness of the CUE-T has not previously been reported.
The development of more sensitive measures has raised the question of the clinical relevance of small changes. In clinical trials, it is important to show that a statistically significant difference between treatment and control groups is also clinically important.18,26 The MCID has been proposed as an indication of a clinically meaningful change. Jaeschke defined the MCID as “the smallest difference in score in the domain of interest which patients perceive as beneficial and which would mandate, in the absence of troublesome side effects and excessive cost, a change in the patient's management.”27(p408) Jaeschke et al27 estimated the MCID of the chronic Respiratory Questionnaire by asking patients enrolled in a trial whether their shortness of breath during daily activities since the prior visit was better, the same, or worse and, if different, to rate how much better or worse on a 7-point scale. They then compared the average change scores for people who said they did not change with those who changed a small, medium, or large amount. The average change in those who report a small change represents the MCID. The criteria used to define a small change using this method has been either a change of 1 to 3 points27 or 2 to 3 points.28,29 We chose to use the more conservative 2 to 3 point change definition in this study.
There are difficulties using subjective impressions of change to define the MCID. For one, patients vary in the value they place on a particular improvement.30 The same patient may change the value placed on a given change as time passes and they have more experience living with the limitations imposed by the injury.18 Patients and clinicians may differ in their impression of the significance of a change in function.31 For these reasons we also estimated the MCID based on an objective anchor – change in the upper extremity motor score (UEMS) of the International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI).32 The MCID for QtP-GRASSP (range, 0–60) is reported to be 6 points for AIS A-B and 12 points for AIS C-D,33 although it was not clear how these numbers were determined. The MCID has not previously been reported for the CUE-T.
The purpose of this study is to compare the responsiveness and MCID of the CUE-T and the QtP-GRASSP in persons with cervical SCI.
Methods
Subjects with traumatic cervical SCI who were (1) at least 15 years old at time of injury, (2) motor levels C1–C8, (3) AIS grades A-D, (4) an UEMS > 0 at the first testing session, and (5) enrolled within 3 months of injury were recruited by five centers. We excluded subjects who had fractures of the UE or shoulder, peripheral nerve injury involving the UE, medical conditions that prevent them from being upright in a chair/wheelchair for testing, or other conditions that prevent them from following the testing protocol. One site enrolled subjects greater than 3 months post injury who were receiving outpatient therapy. These subjects are included here as they changed less and their data are useful in determining the MCID, as this is based on change in subjects who feel that they improved by only a small amount.
Examiners were trained on all assessments at a workshop at the start of the project. Test instruction manuals and training videos were developed and posted on a password-protected server as a reference for examiners. In total there were 17 assessors involved in testing across the four centers. Fourteen assessors were OTs, one was a PT, and two were research assistants with BA degrees. In most cases, two assessors were present for CUE-T and QtP-GRASSP testing.
Subjects were assessed twice approximately 3 months apart. At session 1, strength and sensation were assessed at C4-T1 using the standardized methods of the ISNCSCI.34 We also collected the CUE-Q, the CUE-T, and GRASSP. At session 2, we performed the same assessments as at session 1 plus the subjective global impression of change (see below for details). The subjective change questions were obtained after the CUE-Q to give subjects a frame of reference for the type of actions being assessed for change, but before the CUE-T and GRASSP so that results on the objective testing would not influence self-report of improvement or worsening. We did not perform the full ISNCSCI examination to reduce subject burden and because the CUE-T and GRASSP evaluate upper limb function. Although some test items may be influenced by trunk control, the ISNCSCI examination does not include trunk muscles and lower extremity motor scores would not be relevant. The sensory scores were used to determine sensory levels; sensory scores were not used in other analyses.
This research was conducted in accord with the ethical standards of the responsible conduct of research and was approved by the institutional review boards of the participating institutions.
Measures
Upper extremity motor score
The UEMS is the sum of the manual muscle test (MMT) scores of the upper limb in the ISNCSCI.32 There are five muscles in each extremity scored on a 0 to 5 scale. Scores for each extremity can range from 0 to 25, with the UEMS from 0 to 50.
Capabilities of Upper Extremity Questionnaire
The CUE-Q is a 32-item self-report measure. Persons are asked to rate the level of difficulty performing specific actions with the upper limb on a 5-point scale from 0 = unable to do to 4 = no difficulty.6 There are 15 actions involving the right or left arm or hand and two involving both upper limbs. Responsiveness from admission to discharge of acute inpatient rehabilitation was found to be 0.92 using the SRM.6 The CUE-Q was administered at both time points, but results are not reported here. The CUE-Q was administered just before the Subjective Global Impression of Change (see below) and before the CUE-T.
Capabilities of Upper Extremity Test
The CUE-T as originally described consisted of 17 items tested on the right and left sides and two bilateral items, for a total of 36 items.8 The pronation and supination items had poor test-retest reliability, and subsequent analyses found that they contributed little information to the total score (unpublished data). Therefore, these items were dropped. The final CUE-T reported here has 32 items, each scored on a 0 to 4 point scale. The total score can range from 0 to 128. The scale is meant to have subscales of right/left side (15 items each) and right/left hand (9 items each), with scores ranging from 0 to 60 and 0 to 36, respectively.
Graded Redefined Assessment of Strength, Sensibility and Prehension
The GRASSP is a multidimensional measure assessing strength, sensibility, and qualitative and quantitative prehension.9 The quantitative prehension (QtP) scale, reported here, consists of six tasks tested on each side and scored from 0 to 5, for a possible score of 0 to 30 per side or 0 to 60 total. Test-retest reliability of GRASSP component scores ranges from 0.86 to 0.98, with reliability of QtP values 0.93 to 0.96.9 Validity has been evaluated by examining correlation with similar measures; Pearson correlation of QtP with the self-care subscale of the Spinal Cord Independence Measure is 0.79 and with the CUE questionnaire it is 0.83.9
Subjective global impression of change
At the time of the second testing session, participants were asked if their ability to perform actions with their upper limbs, such as those asked about in the CUE-Q, had improved, become worse, or stayed the same since the first test session. If they indicated that function had changed, then they were asked to rate the amount of change on a 7-point scale where 1 = almost the same, hardly any better (or worse) at all to 7 = a very great deal better (or worse). They were asked to rate their upper limbs globally and the right and left limbs separately.
Statistical analysis
The SRM24 was determined in acute subjects for the total and right/left side scores of the CUE-T, QtP-GRASSP, and UEMS. There were no significant differences between right and left side scores for any of the measures, so right and left side scores were combined for the analyses by side. Values of 0.2, 0.5, and 0.8 represent small, moderate, and large responsiveness, respectively.24
The method of Jaeschke27 was used to estimate the MCID using the subjective global impression of change ratings. There were too few subjects who reported subjective worsening (more than 1 point worse) to determine MCID for loss of function, so only improvement was evaluated. For determining the MCID, subjects were grouped according to self-reported change as follows: “no change” for those who reported no change or a change of 1 point (−1 to +1), “small change” if improvement was 2 to 3 points, “moderate change” if 4 to 5 points, or “large change” if 6 to 7 points.29 The MCID is the average change in the “small change” group.
We used changes in UEMS as the objective anchor for MCID. For the total UEMS, we defined no change as 0 to 2 points, small as 3 to 6 points, moderate as 7 to 10 points, and large as >10 points. For right or left UEMS, we defined no change as 0 to 1 point, small as 2 to 3 points, moderate as 4 to 5 points, and large as >5 points. These criteria are estimates, as there is no standard for what constitutes an MCID for the UEMS. The average change in CUE-T and QtP-GRASSP scores by subjective and objective change groups was calculated for the total scores and the side scores. The MCID is the average change in the “small change” group.
Correlations among UEMS, CUE-T, and QtP-GRASSP scores and change scores were evaluated using the Spearman correlation coefficient. We used the following interpretation of the size of the correlations: <0.26 indicates little to no association, .26 to .50 fair, .51 to .75 moderate to good, and .76 to 1.0 very good to excellent.35
Results
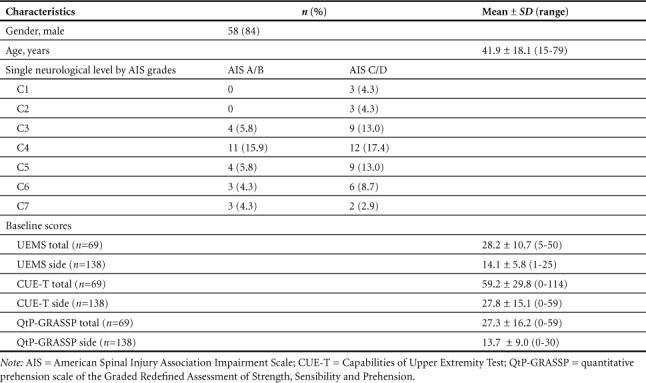
There were 85 subjects enrolled in the study: 74 with acute injuries (<3 months post injury) and 11 with chronic injuries (>3 months post injury). Second assessments were completed on 69 of these subjects: 60 with acute injuries and 9 with chronic injuries. Sixteen subjects did not return for the second assessment. We did not collect reasons for dropout. There were no statistically significant differences in age, gender, AIS grade, neurologic level of injury or baseline UEMS, total CUE-T or total QtP-GRASSP scores between those with and without a second assessment. The final sample consisted of 58 men and 11 women, average age 41.9 ± 18.1 years (Table 1). Neurologic levels of injury ranged from C1 to C7. Distribution of AIS grades at initial evaluation were A = 8, B = 17, C = 22, D = 22.
Table 1.
Baseline characteristics of subjects
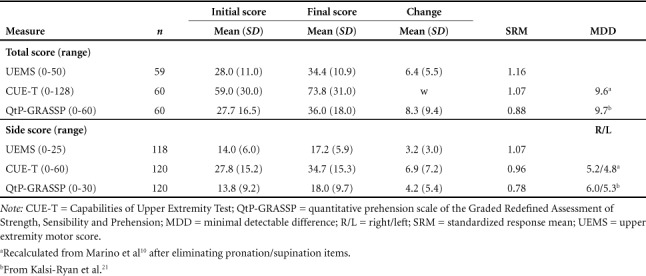
Initial, final, and change scores for UEMS, CUE-T, and QtP-GRASSP in acute subjects are found in Table 2. The SRM for all measures (UEMS = 1.16, CUE-T = 1.07, and QtP-GRASSP = 0.88) indicated large responsiveness in the subacute period after SCI.
Table 2.
Total and side change scores, standardized response mean, and minimum detectable difference
Change in QtP-GRASSP and CUE-T scores by subjective and objective change groups are found in Table 3 and Figure 1. Both measures demonstrated an increased change in scores with increasing subjective and objective change ratings. The MCID for the total CUE-T score was 12 points (out of 128) based on a small change in the subjective and objective anchors. For right/left sides there was a similar pattern, with an MCID of 6 points (out of 60). Corresponding values for the QtP-GRASSP were 6 points (out of 60) for the MCID of the total score and 3 points (out of 30) for the side score.
Table 3.
Change by subjective and objective change for total and side scores of CUE-T and QtP-GRASSP
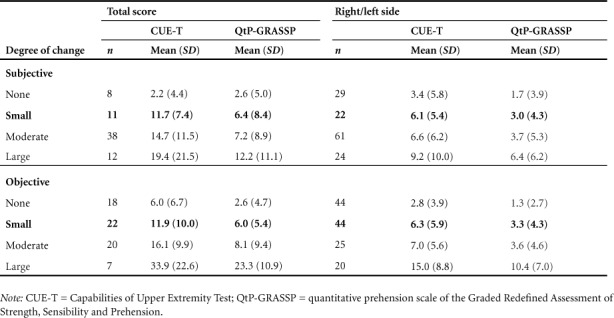
Figure 1.
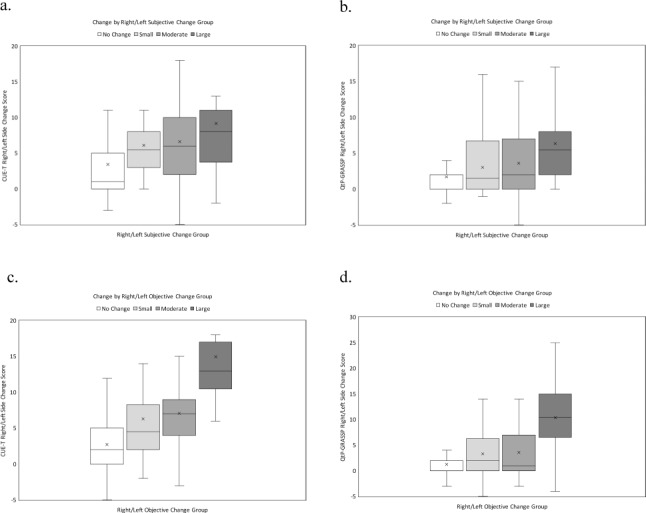
Boxplots of change in right/left side scores for Capabilities of Upper Extremity Test (CUE-T) and quantitative prehension scale of the Graded Redefined Assessment of Strength, Sensibility and Prehension (QtP-GRASSP) based on subjective impression of change group: (a) CUE-T, (b) QtP-GRASSP and objective change group, (c) CUE-T, and (d) QtP-GRASSP. Outliers are excluded from figures. See text for definition of change groups.
Comparison of CUE-T and GRASSP scores by side indicate that there is a subset of subjects who score zero on the GRASSP who have positive scores on the CUE-T. There were no subjects with zero on the CUE-T who had a positive QtP-GRASSP score. There were 14 limbs in 10 subjects where this pattern occurred. Six of these subjects were motor complete, with the relevant side motor level ranging from C4 to C6, single side UEMS from 1 to 13, and CUE-T score from 1 to 20. The motor incomplete subjects had motor levels from C1–C5, single side UEMS from 4 to 11, and CUE-T scores from 1 to 14. Two subjects had large increases in CUE-T scores while remaining zero on the GRASSP; one classified as C1-D went from 0 to 12 on the side CUE-T and the other classified as C4-C went from 3 to 14 points. A scatterplot of QtP-GRASSP by CUE-T scores for side at baseline is shown in Figure 2a. The rectangle indicates positive CUE-T scores with zero QtP-GRASSP scores. Breaking the CUE-T score into ARM and HAND portions (Figures 2 b, c) reveals that the ARM items account for the majority of these cases.
Figure 2.
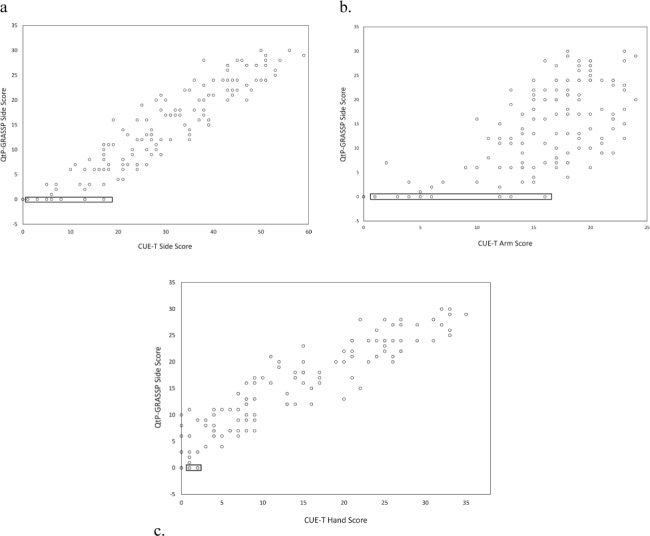
Plot of baseline scores of right/left sides for Capabilities of Upper Extremity Test (CUE-T) and quantitative prehension scale of the Graded Redefined Assessment of Strength, Sensibility and Prehension (QtP-GRASSP): (a) CUE-T total side score; (b) CUE-T arm score; (c) CUE-T hand score, demonstrating that nonzero scores in CUE-T measure, where QtP-GRASSP scores are at or near 0 (2a), are due primarily to CUE-T ARM portion (2b) and not HAND portion (2c). Rectangles highlight values with zero QtP-GRASSP and positive CUE-T scores.
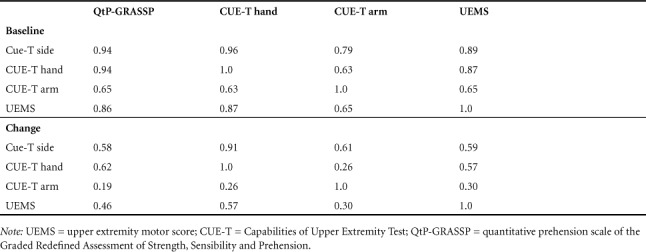
Spearman correlations among the CUE-T and GRASSP side scores (Table 4) show that there is an excellent correlation between CUE-T hand scores and GRASSP scores, but a moderate correlation between CUE-T arm scores and GRASSP scores. When looking at change scores, there is a moderate correlation between CUE-T hand scores and GRASSP scores, but little correlation between change in GRASSP and CUE-T arm scores.
Table 4.
Spearman correlation coefficients among baseline and change CUE-T and QtP-GRASSP side scores
Discussion
Both the CUE-T and QtP-GRASSP demonstrate large responsiveness in persons with acute tetraplegia. Responsiveness was similar for both measures and equivalent to that of the UEMS over the same time period. The amount of change and SRM of the QtP-GRASSP in this study (8.3 points, SRM of 0.88) is similar to that reported by Velstra et al (9.12 points, SRM of 0.81) when looking at change from 1 to 3 months post injury.25 Change in UEMS was also similar: 6.4 points, SRM = 1.16 in this study; 5.14 points, SRM = 0.92 in Velstra et al.25
Differences were seen between the two measures when assessing subjects with little hand function. The CUE-T identified function not identified with the QtP-GRASSP, suggesting that there is a floor effect for the QtP-GRASSP. This is consistent with prior findings, where a person with a C4 AIS A injury had a right-sided GRASSP MMT score of 5 but a QtP-GRASSP score of 0.9 The QtP-GRASSP focuses on prehension so it is not surprising that scores reflect hand more than other upper limb actions such as reaching, lifting, pulling, and pushing, which depend on more proximal muscles. The MMT scale of the GRASSP is more comprehensive than the AIS UEMS and may detect changes not observed using the QtP scale, but it is an impairment measure. Changes in GRASSP MMT scores do not necessarily result in improved functional use of the upper limb any more than would changes in UEMS.18 The inclusion of both arm and hand items in the CUE-T makes it a more comprehensive functional capacity assessment of the upper limb.
The correlations among scores and change scores illustrate the differences in the UEMS, CUE-T, and QtP-GRASSP. There is a high correlation between the functional measures, and both are highly correlated to impairment. A different picture emerges when looking at the associations among change scores. Change in CUE-T and QtP-GRASSP are only moderately correlated. As expected, there is a low correlation between changes in the ARM items of the CUE-T and the QtP-GRASSP, indicating that these items are assessing different aspects of upper limb function. The moderate correlation between change in hand items in the CUE-T and the QtP-GRASSP indicate that the two measures are assessing change differently. This may be due to additional items in the CUE-T assessing hand function or to the differences in scoring. In addition to tests of grasp patterns, the CUE-T assesses pressing with the tip of the index finger and using the thumb to press buttons on a handheld device. It also evaluates the ability to acquire and release a large and small item. The CUE-T does not give credit for alternative methods of completing an item, for example, one cannot use an alternative grasp pattern, or use a knuckle instead of the tip of the index finger to push buttons on a calculator. The QtP-GRASSP does permit grasp patterns other than the expected pattern to be used, but scores these items lower than if the expected pattern is used.
Similar results for the MCID were obtained using the subjective global impression of change and an objective anchor based on UEMS change. As a proportion of scale score range, the MCID was similar for both measures. The CUE-T total score (range, 0–128) had a MCID of 12 points, compared to the QtP-GRASSP (score range, 0–60) where the MCID was 6.0 to 6.4 points. Although our sample included persons with AIS grades A-D, the MCID for QtP-GRASSP was similar to that reported for persons with motor complete SCI (6 points) and smaller than for motor incomplete SCI (12 points).33 We used a conservative definition for the subjective change, requiring a score of 2 or 3 out of 7 for improvement.29 The MCID would have been slightly smaller had a score of 1 also been accepted.27 Defining the MCID for SCI is challenging; persons in the first few months after SCI may not have the experience and perspective of what amount of change in function is meaningful.18 While objective anchors can support subjective impressions of change, there is not agreement on what constitutes the MCID of related outcomes, such as the UEMS or the Spinal Cord Independence Measure. In addition, there is an imperfect correlation between changes in impairment, such as the UEMS, and functional capacity, such as the CUE-T or QtP-GRASSP. Whether a change in UEMS results in a functional improvement or not depends upon the distribution of score changes.18 Nevertheless, the similarity in MCID obtained using subjective and objective anchors is reassuring.
Responsiveness of a measure must be related to its reliability. The MCID should be larger than the MDD, defined as the smallest change that is beyond measurement error.18 The MDD is determined by multiplying the standard error of measurement by 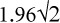 .19,20 The CUE-T satisfies this criterion; the MDDs of 9.5 for the total score and 5.2 for a side score are less than the MCIDs of 12 for the total score and 6 for the side score. The reported MDD for the QtP-GRASSP total and side scores (9.7 and 6.0)21 are larger than the MCIDs of 6.4 and 3.0. Only large subjective and objective changes exceed the MDD in our study.
.19,20 The CUE-T satisfies this criterion; the MDDs of 9.5 for the total score and 5.2 for a side score are less than the MCIDs of 12 for the total score and 6 for the side score. The reported MDD for the QtP-GRASSP total and side scores (9.7 and 6.0)21 are larger than the MCIDs of 6.4 and 3.0. Only large subjective and objective changes exceed the MDD in our study.
There are differences between the GRASSP and the CUE-T that may influence the choice of one or the other for a research or clinical purpose. The GRASSP is a composite measure with separate scores for strength, sensation, and prehension. There is no total score. The CUE-T assesses upper limb functional limitations. If information on sensation and strength is desired then another measure must be used, such as the upper limb sensory and motor portion of the ISNCSCI examination. The GRASSP and CUE-T take a different approach to item scoring. In the CUE-T, the action or grasp pattern is specified and alternative patterns are not allowed. QtP-GRASSP items have an expected grasp pattern, but tasks can be completed using an alternative pattern except for scores of 4 or 5.12 At the lower task scores, the QtP-GRASSP may show improvements due to improved compensatory strategies rather than improvement in the expected grasp pattern, which is not the case with the CUE-T. Depending on the purpose of the assessment, one or the other test may be more appropriate.
The CUE-T takes longer to perform than the QtP-GRASSP. In most cases it takes 40 to 60 minutes to complete the CUE-T, which is about twice as long as the QtP-GRASSP. Further testing is needed to determine if a short version of the CUE-T would have acceptable responsiveness. It is recommended that two assessors be present for testing the CUE-T because many test items require counting the number of repetitions in 30 seconds and monitoring and correcting technique, both of which are difficult for one person to do simultaneously. The GRASSP test item scoring uses time to complete a set task with technique and number of drops recorded. This can be accomplished with one assessor.
The GRASSP has been available for a number of years and has been used more often than the CUE-T. It must be purchased from Neural Outcomes, Inc., which in May 2018 was priced at $1,250.36 The CUE-T has been assembled on request, as for the SPRING trial. A price for the kit has not been finalized, but would be approximately $1,500. The most expensive items are the grasp and pinch dynamometers, which many facilities may have on hand. Eliminating the need to provide these would reduce the cost of the test kit. A list of test items and instructions on how to make those items not available for purchase have been provided to some researchers. Participating centers in the NeuroRecovery Network produced their own kits in this way. Groups that require only one kit or that are not concerned about kits being identical may want to put together kits themselves.
This study was led by the developers of the CUE-T, who were involved in training of assessors but not in recruitment or testing. Most subjects were persons with recent injuries who would be expected to improve between test sessions, which may have increased the amount of responsiveness seen for all assessments. The study sample had relatively few motor complete subjects, limiting the ability to compare responsiveness by level of injury and AIS grade. Additional data with larger numbers of motor complete subjects across cervical levels of injury would be useful to identify subgroups where responsiveness is better for one measure or the other.
A second version of the GRASSP has recently been reported with fewer locations for sensibility testing (3 per hand instead of 6) and quantitative prehension (4 tasks instead of 6).37 Detailed analysis of the CUE-T items should be performed to see if some items can be eliminated in order to reduce the burden of testing. Results of ongoing clinical trials using both the CUE-T and GRASSP will provide a comparison of responsiveness in the setting that these measures were meant to be used.
Conclusion
In this study involving primarily persons with acute complete and incomplete tetraplegia, the CUE-T and QtP-GRASSP both displayed large responsiveness. As a percent of total and subscale scores, the MCID of both measures was similar. The reported MDD was similar to the MCID for the CUE-T, but larger than the MCID for the QtP-GRASSP. The CUE-T detected changes in some subjects that the QtP-GRASSP did not, primarily due to the arm items in the CUE-T. Results from clinical trials using both measures will provide information on how these assessments perform in the setting for which they were developed.
Footnotes
Conflicts of Interest
Contact the corresponding author for information on how to obtain the CUE-T kit and testing instructions. Thomas Jefferson University and the Department of Rehabilitation Medicine receive payment for preparation and supply of the CUE-T kits if these are provided by the Department. Dr. Marino does not receive payment for the kits. The remaining authors have nothing to disclose.
Funding
Craig H. Neilsen Foundation grant #260597
REFERENCES
- 1.National Spinal Cord Injury Statistical Center Complete Public Version of the 2016 Annual Statistical Report for the Spinal Cord Injury Model Systems. Birmingham, AL: University of Alabama at Birmingham; 2016. [Google Scholar]
- 2.Marino RJ, Burns S, Graves DE, Leiby BE, Kirshblum S, Lammertse DP. Upper- and lower-extremity motor recovery after traumatic cervical spinal cord injury: An update from the national spinal cord injury database. Arch Phys Med Rehabil. 2011;92(3):369–375. doi: 10.1016/j.apmr.2010.09.027. [DOI] [PubMed] [Google Scholar]
- 3.Penrod LE, Hegde SK, Ditunno JF., Jr. The effect of age on prognosis for functional recovery in acute traumatic central cord syndrome (CCS) Arch Phys Med Rehabil. 1990;71(12):963–968. [PubMed] [Google Scholar]
- 4.Anderson KD. Targeting recovery: Priorities of the spinal cord-injured population. J Neurotrauma. 2004;21(10):1371–1383. doi: 10.1089/neu.2004.21.1371. [DOI] [PubMed] [Google Scholar]
- 5.Wuolle KS, Van Doren CL, Thrope GB, Keith MW, Peckham PH. Development of a quantitative hand grasp and release test for patients with tetraplegia using a hand neuroprosthesis. J Hand Surg Am. 1994;19(2):209–218. doi: 10.1016/0363-5023(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 6.Oleson CV, Marino RJ. Responsiveness and concurrent validity of the revised capabilities of upper extremity-questionnaire (CUE-Q) in patients with acute tetraplegia. Spinal Cord. 2014;52(8):625–628. doi: 10.1038/sc.2014.77. [DOI] [PubMed] [Google Scholar]
- 7.Kalsi-Ryan S, Curt A, Verrier MC, Fehlings MG. Development of the Graded Redefined Assessment of Strength, Sensibility and Prehension (GRASSP): Reviewing measurement specific to the upper limb in tetraplegia. J Neurosurg Spine. 2012;17(1 suppl):65–76. doi: 10.3171/2012.6.AOSPINE1258. [DOI] [PubMed] [Google Scholar]
- 8.Marino RJ, Patrick M, Albright W et al. Development of an objective test of upper-limb function in tetraplegia: The Capabilities of Upper Extremity Test. Am J Phys Med Rehabil. 2012;91(6):478–486. doi: 10.1097/PHM.0b013e31824fa6cc. [DOI] [PubMed] [Google Scholar]
- 9.Kalsi-Ryan S, Beaton D, Curt A et al. The Graded Redefined Assessment of Strength Sensibility and Prehension: Reliability and validity. J Neurotrauma. 2012;29(5):905–914. doi: 10.1089/neu.2010.1504. [DOI] [PubMed] [Google Scholar]
- 10.Marino RJ, Kern SB, Leiby B, Schmidt-Read M, Mulcahey MJ. Reliability and validity of the Capabilities of Upper Extremity Test (CUE-T) in subjects with chronic spinal cord injury. J Spinal Cord Med. 2015;38(4):498–504. doi: 10.1179/2045772314Y.0000000272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization International Classification of Functioning, Disability and Health: ICF. Geneva: Author; 2001. [Google Scholar]
- 12.Kalsi-Ryan S. Graded Redefined Assessment of Strength, Sensibility and Prehension Version 1.0. International GRASSP Research and Design Team; 2008. [Google Scholar]
- 13.Fehlings MG, Kim KD, Aarabi B et al. Rho inhibitor VX-210 in acute traumatic subaxial cervical spinal cord injury: Design of the SPinal Cord Injury Rho INhibition InvestiGation (SPRING) clinical trial. J Neurotrauma. 2018;35(9):1049–1056. doi: 10.1089/neu.2017.5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.European Multicenter Study about Spinal Cord Injury: The assessments. https://www.emsci.org/index.php/project/the-assessments Accessed May 1, 2018.
- 15.Institute of Medicine Enabling America: Assessing the Role of Rehabilitation Science and Engineering. Washington, DC: National Academies Press; 1997. [PubMed] [Google Scholar]
- 16.Marino RJ. Domains of outcomes in spinal cord injury for clinical trials to improve neurological function. J Rehabil Res Dev. 2007;44(1):113–122. doi: 10.1682/jrrd.2005.08.0138. [DOI] [PubMed] [Google Scholar]
- 17.Marino RJ. Capabilities of Upper Extremity Test Manual of Administration, Version 1.1. Philadelphia: Thomas Jefferson University; 2016. [Google Scholar]
- 18.Wu X, Liu J, Tanadini LG et al. Challenges for defining minimal clinically important difference (MCID) after spinal cord injury. Spinal Cord. 2015;53(2):84–91. doi: 10.1038/sc.2014.232. [DOI] [PubMed] [Google Scholar]
- 19.Bland JM, Altman DG. Measurement error. BMJ. 1996;313(7059):744. doi: 10.1136/bmj.313.7059.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beckerman H, Roebroeck ME, Lankhorst GJ, Becher JG, Bezemer PD, Verbeek ALM. Smallest real difference, a link between reproducibility and responsiveness. Qual Life Res. 2001;10(7):571–578. doi: 10.1023/a:1013138911638. [DOI] [PubMed] [Google Scholar]
- 21.Kalsi-Ryan S, Beaton D, Ahn H et al. Responsiveness, sensitivity, and minimally detectable difference of the Graded and Redefined Assessment of Strength, Sensibility, and Prehension, Version 1.0. J Neurotrauma. 2016;33(3):307–314. doi: 10.1089/neu.2015.4217. [DOI] [PubMed] [Google Scholar]
- 22.Deyo RA, Diehr P, Patrick DL. Reproducibility and responsiveness of health status measures. Control Clin Trials. 1991;12:142S–158S. doi: 10.1016/s0197-2456(05)80019-4. [DOI] [PubMed] [Google Scholar]
- 23.Norman GR, Wyrwich KW, Patrick DL. The mathematical relationship among different forms of responsiveness coefficients. Qual Life Res. 2007;16(5):815–822. doi: 10.1007/s11136-007-9180-x. [DOI] [PubMed] [Google Scholar]
- 24.Husted JA, Cook RJ, Farewell VT, Gladman DD. Methods for assessing responsiveness: A critical review and recommendations. J Clin Epidemiol. 2000;53(5):459–468. doi: 10.1016/s0895-4356(99)00206-1. [DOI] [PubMed] [Google Scholar]
- 25.Velstra IM, Curt A, Frotzler A et al. Changes in strength, sensation, and prehension in acute cervical spinal cord injury: European Multicenter Responsiveness Study of the GRASSP. Neurorehabil Neural Repair. 2015;29(8):755–766. doi: 10.1177/1545968314565466. [DOI] [PubMed] [Google Scholar]
- 26.Walker MD. Acute spinal-cord injury. N Eng J Med. 1991;324(26):1885–1887. doi: 10.1056/NEJM199106273242608. [DOI] [PubMed] [Google Scholar]
- 27.Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Control Clin Trials. 1989;10(4):407–415. doi: 10.1016/0197-2456(89)90005-6. [DOI] [PubMed] [Google Scholar]
- 28.Juniper EF, Guyatt GH, Willan A, Griffith LE. Determining a minimal important change in a disease-specific quality of life questionnaire. J Clin Epidemiol. 1994;47(1):81–84. doi: 10.1016/0895-4356(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 29.Corallo V, Torre M, Ferrara G et al. What do spinal cord injury patients think of their improvement? A study of the minimal clinically important difference of the Spinal Cord Independence Measure III. Eur J Phys Rehabil Med. 2017;53(4):508–515. doi: 10.23736/S1973-9087.17.04240-X. [DOI] [PubMed] [Google Scholar]
- 30.Guyatt GH, Osoba D, Wu AW et al. Methods to explain the clinical significance of health status measures. Mayo Clin Proceed. 2002;77(4):371–383. doi: 10.4065/77.4.371. [DOI] [PubMed] [Google Scholar]
- 31.Harvey LA, Folpp H, Denis S et al. Clinicians' and patients' impressions of change in motor performance as potential outcome measures for clinical trials. Spinal Cord. 2011;49(1):30–35. doi: 10.1038/sc.2010.105. [DOI] [PubMed] [Google Scholar]
- 32.Kirshblum SC, Burns SP, Biering-Sorensen F et al. International Standards for Neurological Classification of Spinal Cord Injury (revised 2011) J Spinal Cord Med. 2011;34(6):535–546. doi: 10.1179/204577211X13207446293695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.GRASSP Version 1.0: Psychometric Properties. http://www.grassptest.com/psychometric-properties/ Accessed May 1, 2018.
- 34.Kirshblum SC, Waring W, Biering-Sorensen F et al. Reference for the 2011 revision of the International Standards for Neurological Classification of Spinal Cord Injury. J Spinal Cord Med. 2011;34(6):547–554. doi: 10.1179/107902611X13186000420242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dawson B, Trapp RG. Basic and Clinical Biostatistics. 4th ed. New York: McGraw-Hill; 2004. [Google Scholar]
- 36.Neural outcomes products. http://neuraloutcomes.com/shop/ Accessed May 1, 2018.
- 37.Kalsi-Ryan S, Albisser U, Fehlings M GRASSP version 2: A comprehensive SCI upper limb outcome measure. Paper presented at: ASIA 44th Annual Scientific Meeting; May 2–4, 2018; Rochester, Minnesota. [Google Scholar]


