Key Points
Question
How effective are mobile health interventions for improving health outcomes in youth 18 years or younger?
Findings
Thirty-seven studies evaluating the use of mobile health interventions in approximately 30 000 participants were included in the meta-analysis. Mobile health interventions had a small but significant positive effect on health outcomes in youth.
Meaning
Mobile health interventions appear to be a viable health behavior change intervention modality for youth.
Abstract
Importance
Mobile health interventions are increasingly popular in pediatrics; however, it is unclear how effective these interventions are in changing health outcomes.
Objective
To determine the effectiveness of mobile health interventions for improving health outcomes in youth 18 years or younger.
Data Sources
Studies published through November 30, 2016, were collected through PubMed, Cumulative Index to Nursing and Allied Health Literature, Educational Resources Information Center, and PsychINFO. Backward and forward literature searches were conducted on articles meeting study inclusion criteria. Search terms included telemedicine, eHealth, mobile health, mHealth, app, and mobile application.
Study Selection
Search results were limited to infants, children, adolescents, or young adults when possible. Studies were included if quantitative methods were used to evaluate an application of mobile intervention technology in a primary or secondary capacity to promote or modify health behavior in youth 18 years or younger. Studies were excluded if the article was an unpublished dissertation or thesis, the mean age of participants was older than 18 years, the study did not assess a health behavior and disease outcome, or the article did not include sufficient statistics. Inclusion and exclusion criteria were applied by 2 independent coders with 20% overlap. Of 9773 unique articles, 36 articles (containing 37 unique studies with a total of 29 822 participants) met the inclusion criteria.
Data Extraction and Synthesis
Of 9773 unique articles, 36 articles (containing 37 unique studies) with a total of 29 822 participants met the inclusion criteria. Effect sizes were calculated from statistical tests that could be converted to standardized mean differences. All aggregate effect sizes and moderator variables were tested using random-effects models.
Main Outcomes and Measures
Change in health behavior or disease control.
Results
A total of 29 822 participants were included in the studies. In studies that reported sex, the total number of females was 11 226 (53.2%). Of those reporting age, the average was 11.35 years. The random effects aggregate effect size of mobile health interventions was significant (n = 37; Cohen d = 0.22; 95% CI, 0.14-0.29). The random effects model indicated that providing mobile health intervention to a caregiver increased the strength of the intervention effect. Studies that involved caregivers in the intervention produced effect sizes (n = 16; Cohen d = 0.28; 95% CI, 0.18-0.39) larger than those that did not include caregivers (n = 21; Cohen d = 0.13; 95% CI, 0.02-0.25). Other coded variables did not moderate study effect size.
Conclusions and Relevance
Mobile health interventions appear to be a viable health behavior change intervention modality for youth. Given the ubiquity of mobile phones, mobile health interventions offer promise in improving public health.
This meta-analysis examines the use of mobile phones by health professionals as an intervention to achieve improved health outcomes in individuals 18 years or younger.
Introduction
Mobile phones are commonly used by health professionals as a platform to deploy interventions to elicit health behavior change and improve health outcomes.1 In the United States, more than 75% of adults 49 years or younger own a smartphone and an estimated 73% of adolescents own or have access to a smartphone.2,3 Moreover, on an average day, adolescents spend more than 2.5 hours using a smartphone,4 with their device always in close proximity. The ubiquitous nature of mobile phone use coupled with continual technological advances have given rise to a proliferation in mobile health (mHealth) interventions in which mobile devices target a range of health promotion and disease management foci.5,6
There are several intuitive factors that have led to the expeditious growth of mHealth interventions. First, mHealth programs can collect dynamic health-related data and deliver intervention content to individuals in their natural environment, outside of a clinical encounter, at key times that have a higher likelihood of modifying behavior. Second, the kinds of high-throughput data that are available from sensors and smartphones, including activity trackers and digital pillboxes, increase the ability for researchers to tailor interventions to participants in real time to encourage engagement in positive health behaviors (eg, prompt exercise when an activity tracker has been stationary). Third, the app marketplace has quickly become saturated with a wide range of mHealth-related platforms because businesses have recognized the value of the wealth of data (eg, data on populations can be leveraged for health management and marketing) that these apps can provide.
To date, however, the mHealth ecosystem has not met its potential to disseminate evidence-based interventions focused on health promotion.7 A critical initial step toward facilitating the maturation of the mHealth ecosystem is for the scientific community to increase our understanding of what existing mHealth interventions are most effective and elucidate ways that the interventions can be improved. Understanding the aggregate effectiveness of mHealth interventions in the empirical literature may help to accelerate the rate of scientific discovery and then improve the incorporation of evidence-based practices in the marketplace.
Reviews of the effectiveness of mHealth interventions have predominantly focused on adult populations.5,8,9 These reviews suggest that mHealth interventions appear to be promising for a number of health-related areas, including chronic illness management, adherence, and appointment attendance. A small but growing number of studies have used mHealth technologies in pediatric populations. These mHealth interventions have targeted a range of outcomes (eg, improving diabetes control and physical activity promotion) and have produced mixed findings.10,11 To our knowledge, the only systematic review of mHealth intervention effectiveness focused exclusively on pediatric obesity.12 Given the burgeoning interest in mHealth and the increasing number of pediatric-focused mHealth interventions in recent years, it is an ideal time to more broadly assess how effective mHealth interventions are at eliciting meaningful change in health outcomes. The objectives of the present meta-analysis were to (1) determine the effectiveness of mHealth interventions at improving health outcomes in youth 18 years or younger, (2) assess study- and sample-level moderating factors that may be critical drivers of mHealth intervention effectiveness, and (3) characterize the risk of bias of the extant pediatric mHealth intervention literature.
Methods
Literature Search and Study Selection
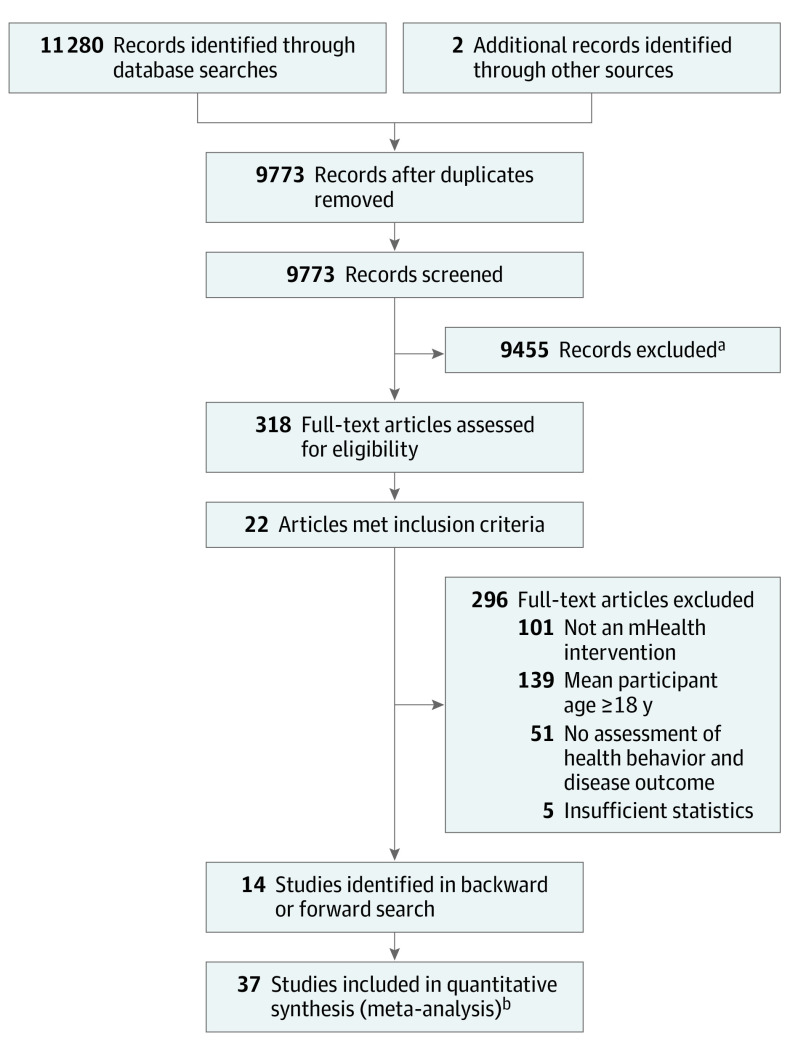
PubMed/MEDLINE, Cumulative Index to Nursing and Allied Health Literature, Educational Resources Information Center, and PsycINFO databases were searched on November 30, 2016, with the assistance of a research librarian. Search terms included telemedicine, eHealth, mobile health, mHealth, app, and mobile application. Search results were limited to infants, children, adolescents, or young adults when possible (eMethods in the Supplement). Only articles published in English were included.
The initial search produced a total of 11 280 articles. Two additional articles13,14 from a recent review of mHealth interventions for pediatric obesity12 were identified as relevant to the present study. After the removal of duplicate studies, the titles and abstracts of 9773 unique articles were screened by 2 authors (D.A.F. and A.O.). Review articles, unpublished theses or dissertations, and articles that were unrelated to pediatric mHealth were removed. Following this level of screening, 318 full-text articles were evaluated. Articles were included if quantitative methods were used to evaluate the use of mobile intervention technology in a primary or secondary capacity to promote or modify health behavior (eg, physical activity promotion) in youth 18 years or younger. Exclusion criteria were applied in the following order: (1) the article did not address the use of mobile intervention technology in a primary or secondary capacity to promote or modify health behavior, (2) the mean age of the participants was older than18 years, (3) the article did not assess a health behavior and disease outcome, or (4) the article did not include sufficient statistics to report effect sizes.
A random 20% of the full-text articles (n = 64) were assessed for inclusion and exclusion criteria by 2 independent coders (D.A.F. and C.C.C.) to evaluate interrater reliability. There was 100% agreement between the raters when assessing whether an article should be included or excluded in a dichotomous fashion. Agreement declined to 98% with regard to the specific reason for exclusion because each criterion was applied in order, making it possible that raters could exclude studies for different reasons. Twenty-two articles met the inclusion criteria. Backward and forward reference searches were conducted, resulting in the identification of 14 additional articles meeting the inclusion criteria. The final sample of 36 articles included 37 unique studies (Figure 1).
Figure 1. PRISMA Flow Diagram.
aReview articles, unpublished theses or dissertations, and articles that were unrelated to pediatric mHealth were removed.
bTwo unique studies were included in 1 article.
Data Extraction and Coding
Calculation of Effect Size
Any statistical information that could be converted into a standardized mean difference was used in the analysis. These included P values, sample sizes, t tests, F tests, odds ratios, and nonparametric tests. Study effect sizes were calculated using Comprehensive Meta-Analysis, version, 2.2.064 (Biostat Inc).
Data Extraction Procedures
Two authors (C.C.C. and C.M.A.) and a trained research assistant separately categorized all articles and extracted data using a standardized codebook. Disagreements were resolved through discussion until there was 100% agreement.
Extraction of Outcomes
Only variables measuring a health behavior or disease functioning associated with a health behavior were coded. For instance, outcomes could include laboratory values (eg, hemoglobin A1c level) or behaviors (eg, dietary intake). A total of 93 effect sizes were calculated from 37 studies. A given study could contribute more than 1 effect size as described below in the statistical approach to aggregation.
Aggregate Effect Sizes
Because study procedures allowed for the inclusion of multiple outcomes, it was necessary to aggregate effect sizes at the study level before computing an overall aggregate effect size. Including multiple outcomes for each study provides a more conservative estimate of the effect of the individual study and serves as a weak protection against “P hacking” (ie, conducting analyses on multiple dependent variables in the hopes that 1 will be significant). After aggregating effect sizes at the study level, a standardized mean difference was computed across each study.
Assessment of Risk of Bias in Included Studies
The Cochrane Collaboration risk of bias tool15 was used to categorize risk of bias in (1) random sequence generation, (2) blinding of participants and personnel, (3) incomplete outcome data, and (4) selective reporting. Categorical ratings of high, unclear, or low were assigned for each domain within the 37 individual studies. Specifically, a high risk of bias rating was given if the report made it clear that the method used introduced the potential that the findings could be biased. An unclear rating was assigned if the report made it unclear whether the study findings were likely to be biased. The risk of bias was rated as low if it was clear from the method and reporting that the issues assessed could not have biased the study.
Coding Moderator Variables
Several moderator variables were coded. Intervention length was coded as a statistically continuous moderator. A categorical moderator indicating whether caregivers were active recipients of the mHealth intervention was coded. Studies were also coded for the use of only text message interventions in comparison with interventions that included text messaging in addition to other components (eg, smartphone apps or online surveys). Another moderator examined whether studies used a theoretical framework for the intervention. Specifically, authors needed to indicate in the article that the mHealth intervention components were guided by a theoretical model (eg, health belief model). The type of outcome (ie, occurring once or behaviors that were repeated) was also coded. A separate categorical moderator variable was created for studies in which the mean age of the participants fell in the preadolescence (<13 years) or adolescence (13-18 years) range (eg, received a 0 if mean age was younger and/or if only the caregiver received the intervention). Finally, whether or not the study was developed with patient stakeholder input or used intent-to-treat analysis were coded as moderators.
Data Analysis Plan
Studies reporting multiple outcomes were aggregated as a single effect size in the analysis. This approach helps to ensure accurate SEs as well as to reduce bias that can occur by treating studies as independent when data were gathered from the same sample.16 Effect sizes were expressed as a standardized mean difference (ie, Cohen d). Cohen17 provided general guidelines for interpreting the magnitude of study effect sizes noting that 0.20 to 0.49 qualifies as a small effect, 0.50 to 0.79 constitutes a medium effect, and above 0.80 is a large effect. Potential moderator variables were tested using the random effects analog to the analysis of variance technique described by Lipsey and Wilson.18
Results
Participant and Study Characteristics
Of 36 unique articles, the final sample of 37 studies included a total of 29 822 participants. In studies that reported sex, the total number of females was 11 226 (53.2%) and the average age was 11.35 years. Other relevant demographic information, study characteristics, and mHealth components for all included studies are provided in eTable 1 in the Supplement. Reporting of demographic information was suboptimal across the study sample. Fifteen studies (40.5%) did not report mean participant age and 14 studies (37.8%) did not report race/ethnicity. Of participants with race/ethnicity reported, 4887 (43.7%) were Hispanic/Latino, 1616 (14.4%) were African American, and 341 (3.15%) were white; 4354 (38.8%) of the participants were of another, unknown, or preferred-not-to-state category of race/ethnicity.
Risk of Bias
A large proportion of studies was rated as having a high risk of bias regarding the blinding of participants and personnel (21 [56.8%]) and attrition (16 [43.2%]). Risk of selection bias was also relatively high (14 [37.8%]); however, 11 (29.7%) studies did not provide information to determine risk. All but 1 study19 was coded as having a low risk of bias for selective reporting. The Table provides the risk of bias at the individual study level for all articles.
Table. Risk of Bias at the Study Levela.
| Source | Selective Reportingb | Incomplete Outcome Datac | Blinding of Participants and Personneld | Random Sequence Generatione |
|---|---|---|---|---|
| Ahlers-Schmidt et al,20 2012 | Low | Low | Low | Low |
| Bauer et al,21 2010 | Low | High | High | High |
| Bin-Abbas et al,10 2014 | Low | Unclear | High | High |
| Burbank et al,22 2015 | Low | High | High | High |
| Cafazzo et al,23 2012 | Low | High | High | High |
| Cornelius et al,24 2013 | Low | High | High | High |
| de Niet et al,25 2012 | Low | Low | High | Low |
| Direito et al,26 2015 | Low | Low | High | Low |
| Domek et al,27 2016 | Low | Low | High | High |
| Fassnacht et al,28 2015 | Low | High | High | Unclear |
| Franklin et al,29 2006 | Low | Low | High | Low |
| Frøisland et al,19 2012 | Unclear | Low | High | High |
| Haji et al,30 2016 | Low | High | Low | Low |
| Hanauer et al,31 2009 | Low | Low | Low | Unclear |
| Hashemian et al,32 2015 | Low | Low | Unclear | Low |
| Herbert et al,11 2014 | Low | Unclear | High | High |
| Hingle et al,33 2014 | Low | High | High | High |
| Hofstetter et al,34 2015 | Low | Unclear | Low | Low |
| Kharbanda et al,35 2011 | Low | Low | High | High |
| Lu et al,14 2013 | Low | Low | High | High |
| Mason et al,36 2016 | Low | High | High | Unclear |
| Mulvaney et al,37 2012 | Low | High | High | High |
| Newton et al,38 2009 | Low | Low | High | Unclear |
| Nguyen et al,13 2012 | Low | Low | High | Low |
| Nollen et al,39 2014 | Low | High | Low | Unclear |
| Patrick et al,40 2013 | Low | Low | Low | Unclear |
| Rand et al,41 2015 | Low | High | Low | Low |
| Rodgers et al,42 2014 | Low | High | High | High |
| Shapiro et al,43 2008 | Low | High | Low | Unclear |
| Sharma et al,44 2011 | Low | High | Low | High |
| Shi et al,45 2013 | Low | Low | Low | Unclear |
| Stockwell et al, 201246 | Low | Low | Low | Low |
| Stockwell et al,47 2012 (adolescents) | Low | Low | Low | Unclear |
| Stockwell et al,47 2012 (pediatrics) | Low | Low | Low | Unclear |
| Stockwell et al,48 2015 | Low | Low | Low | Low |
| Thompson et al,49 2016 | Low | High | High | Low |
| Uddin et al,50 2016 | Low | High | Low | Unclear |
A high risk of bias rating was given if the report made it clear that the method used introduced the potential that the findings could be biased. An unclear rating was assigned if the report made it unclear whether the study findings were likely to be biased. The risk of bias was rated as low if it was clear from the method and reporting that the issues assessed could not have biased the study.
Reporting bias.
Attrition bias.
Performance bias.
Selection bias.
Aggregate Effect Size
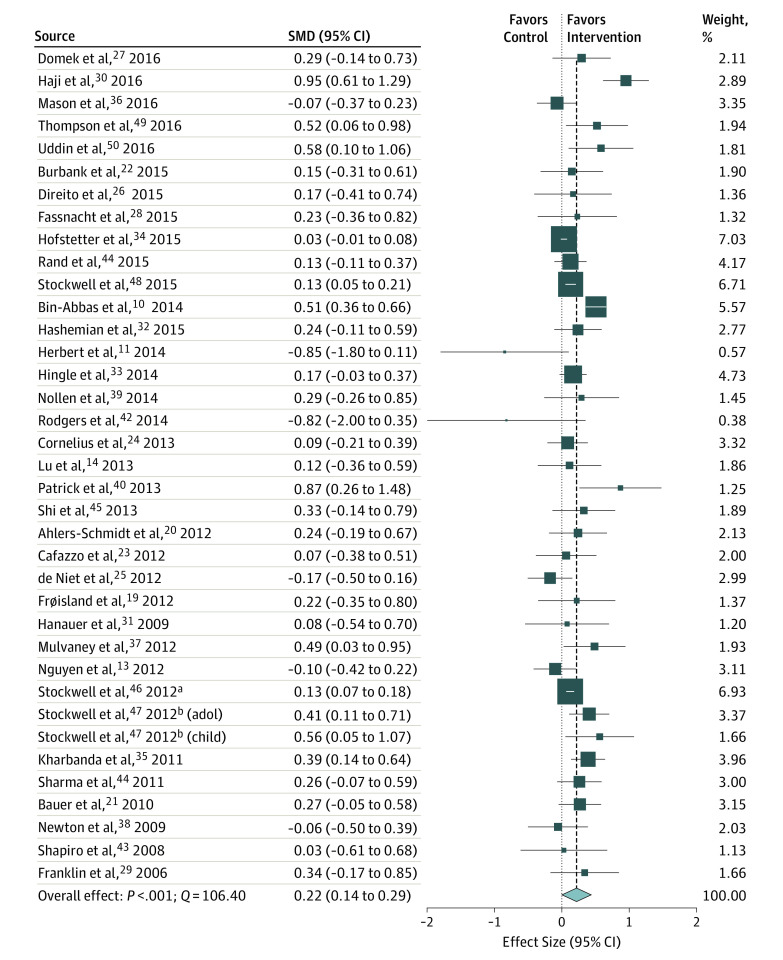
The aggregate effect size of mHealth interventions compared with the controls was small, but significant (n = 37; Cohen d = 0.22; 95% CI, 0.14-0.29) (Figure 2). However, significant heterogeneity was observed in the effect sizes (Q = 106.40; P < .001). The amount of systematic variability in study effect size was substantial (I2 = 66.17), suggesting that moderators of effect size could be used to better understand drivers of treatment effectiveness.
Figure 2. Summary of Point Estimates and Heterogeneity of Effect Sizes for Included mHealth Interventions.
The size of the data marker represents weighting of the study in the analyses. SMD indicates standardized mean difference.
Coded Moderators
Assigned coding of assessed moderator variables for each study is presented in eTable 2 in the Supplement. Caregiver involvement in the intervention emerged as a significant moderator in the random effects analysis (P = .05). Specifically, studies that involved caregivers in the intervention produced larger effect sizes (n = 16; Cohen d = 0.28; 95% CI, 0.18-0.39) compared with those that did not (n = 21; Cohen d = 0.13; 95% CI, 0.02-0.25). Moreover, the variability remaining in the study effect sizes was trivial beyond what could be explained by the moderator. Use of theory to guide intervention development (Q = 77; P = 0.38), use of text messaging (Q = 0.46; P = .50), preadolescent vs adolescent as the intervention target (Q = 3.03; P = .08), recurrence of the outcome (Q = 0.68; P = .41), length of study (Q = 0.49; P = .48), use of intent-to-treat analysis (Q = 0.62; P = .43), and involvement of stakeholders in intervention development (Q = 0.03; P = .84) did not moderate study effect sizes.
Fail-safe Number Calculation
A number of factors may lead to studies not appearing in the literature. Some unpublished studies are likely to contain null findings. Therefore, it is important to understand the number of null results that it would take to overturn the findings in the present study. Based on the effect size observed for the omnibus test, 634 null findings would be necessary to overturn the findings presented herein.51
Discussion
The primary objective of the present study was to determine the effectiveness of mHealth interventions in improving health-related outcomes in youth 18 years or younger. Results indicate that mHealth interventions can be effective in eliciting meaningful improvements in pediatric health behavior and associated pediatric health outcomes. The small but significant aggregate effect size of mHealth interventions (Cohen d = 0.22) aligns well with the findings from previous meta-analyses of adherence promotion52 and eHealth53 health behavior change interventions. These findings suggest that mHealth interventions may be a viable modality for health care professionals to effect health-related changes in pediatric populations.
Moderator analyses revealed that mHealth interventions with caregiver involvement produced larger changes in health outcomes, on average, than did those that exclusively targeted youth. Youth health behaviors and outcomes are multidetermined and influenced by a combination of factors within the individual, family, community, and health care system domains.54 Among these factors, caregiver involvement has been shown to be an integral component in both health promotion55,56 and chronic illness management in youth.57 Results from the present study indicate that mHealth interventions that include caregiver involvement may have an advantage in producing positive health outcome changes in youth. However, there are 2 important points regarding this finding that should be considered. First, 9 of the 16 mHealth interventions that included caregivers targeted children 5 years or younger—a developmental period during which caregivers are primarily responsible for their child’s health. Second, a number of these interventions focused on immunizations (68.8%) and were designed so that caregivers were the predominant recipient of mHealth intervention content (eg, caregiver received text message reminders for their child’s immunization). Given the early stage of the mHealth literature, it will be necessary to replicate and extend this finding to determine whether the extent to which caregivers are involved in a mHealth intervention is a driver of effectiveness.
Specifically, it remains an open question whether parental involvement increases the effect of an mHealth intervention beyond the technology alone when the child or adolescent is the predominant recipient of the intervention content. Of particular importance will be studies that distinguish the effect of caregiver involvement in adolescence against early childhood since developmental stage is likely to be an important moderator. These findings fit well with a recent meta-analysis of health promotion interventions for youth reporting that interventions that cut across multiple domains (eg, family and community) were superior in eliciting health behavior change.58 Future research is needed to determine whether mHealth interventions that incorporate additional domains beyond caregiver involvement may produce greater changes in health outcomes.
Use of theory to guide intervention development was not a moderator of mHealth intervention effectiveness. This finding runs counter to previous research indicating that interventions that incorporate health behavior change theory to guide development are often superior in web-based studies.1 In anticipation of a modest number of published mHealth intervention studies, we coded studies as using theory to guide intervention development if the authors broadly mentioned a theoretical framework during study development. This inclusive coding scheme may have attenuated study findings given that the degree of incorporation of theory into interventions can modulate effectiveness.1 Despite our coding scheme, most studies (n = 24) were coded as not using a guiding theoretical model. There have been calls for the incorporation of health behavior change theory into pediatric behavioral interventions to increase optimization and advance our understanding of which theoretical domains may be integral to effectiveness.59 Additional assessment of the effectiveness of mHealth interventions is needed as future studies incorporate established theoretical models of health behavior change.
Several other coded demographics and study characteristics did not moderate the effectiveness of mHealth interventions, including youth age, use of text messaging, type of health outcome (recurring vs nonrecurring), length of study, and patient stakeholder involvement in intervention development. These findings may suggest that mHealth interventions can be effective for a wide range of youth and for a variety of pediatric health outcomes. However, these conditional effects should be interpreted with caution in light of a rapidly developing mHealth intervention literature base. Subgroup sample sizes across moderator categories were sometimes small, thereby limiting the power to assess conditional differences in effectiveness. Reassessment of these moderators is needed as mHealth interventions continue to move beyond text messaging and use differing study designs.
The findings of the present meta-analysis should be interpreted in light of the quality of the pediatric mHealth intervention literature. The use of randomized controlled trial designs (n = 24) and intent-to-treat analyses (n = 19) was relatively modest. Furthermore, consistent with previous findings in the adult literature,5,9 we identified a risk of bias in some areas that is notable given the small but meaningful aggregate effect size of pediatric mHealth interventions. Our ratings revealed that the most frequent risks of bias among the reviewed studies were potential failure to blind participants and personnel, problems with attrition, and potential biased allocation of participants to treatment groups. We found infrequent instances of selective reporting. The use of less-stringent research designs (eg, pre-post) and the frequency of bias in the extant pediatric mHealth literature are limitations and introduce a degree of uncertainty regarding the efficacy of mHealth interventions and the possibility that the effect size will change as more studies are conducted.
These findings reflect a clear need for reducing bias in subsequent mHealth studies. First, participants should be randomized to reduce selection bias. Second, blinding of participants and study staff to intervention conditions should occur whenever possible. Although double-blinding is typically impractical in face-to-face behavioral interventions, a unique benefit of mHealth is the possibility to develop and deploy attention control conditions (eg, sham text messages about general health information vs messages focused on health behavior change techniques). Third, to reduce bias related to attrition, participant flow throughout a trial should be clearly reported and the use of intent-to-treat analyses is encouraged. Fourth, given that only 37.8% of the studies reported on the racial and ethnic composition of the sample, assessment and transparent reporting of demographic characteristics are imperative in future mHealth interventions. Implementation of these recommendations and thorough reporting of methodologic processes would improve confidence in the reported effects of mHealth interventions on pediatric health outcomes.
Limitations
Despite the strengths of the present study, there are several limitations. Due to the modestly sized pediatric mHealth literature, we were not able to examine the effectiveness of mHealth interventions for specific disease groups or health topics, health outcomes, or other intervention components (eg, personalized feedback). These are important issues to examine as the mHealth literature grows. mHealth interventions that did not report on changes in at least 1 youth health outcome were excluded. Although this exclusion resulted in a more stringent test of the effectiveness of mHealth interventions, studies that focused solely on changes in knowledge, self-efficacy, motivation, and other related outcomes were not included.
Inclusive definitions were used in the coding of moderator variables for this initial meta-analysis given the nascent state of pediatric mHealth intervention literature. As previously noted, broader coding may have reduced the ability to find meaningful associations between moderators and mHealth intervention effectiveness. Furthermore, some of the moderator categories included a small number of studies (eg, nonrecurring outcomes). Moderation effects may shift with additional studies and should therefore be interpreted with caution. mHealth intervention studies that had a sample of participants whose mean age was older than 18 years were excluded from the meta-analysis. In doing so, we acknowledge that studies that spanned a broader age range or focused on young adults (eg, 18-25 years) were excluded from analyses. Finally, the meta-analysis did not focus on the effectiveness of mHealth interventions to improve mental health outcomes; this is an important area for future investigation.
Conclusions
To our knowledge, the present study is the first to examine the aggregate effects of mHealth interventions on improving health outcomes in youth. The findings indicate that mHealth interventions are a promising and potentially effective route for pediatric health care professionals to use with patients and their caregivers. Given the ubiquity of mobile phone use and the willingness of youth to use their mobile devices for health-related activities, mHealth interventions appear poised to be a viable health behavior change intervention modality. To extend the promise of pediatric mHealth interventions, future work is needed to explicitly incorporate and test the effect of competing and complementary theoretical mechanisms of behavior change.59 Moreover, additional studies are needed to determine the optimal positioning of mHealth interventions within a larger ecologic context (ie, whether health systems, families, or schools should be included and the appropriate developmental levels). To increase confidence in the robustness of the findings, the full extent of methodologic rigor should be incorporated into mHealth studies. As the research community executes the agenda outlined herein, future work will be better positioned to provide policy recommendations for hospitals and governments seeking to use mHealth approaches to population-level pediatric health management.
eMethods. PubMed Electronic Search Strategy
eTable 1. Studies Included in the Meta-analysis
eTable 2. Assigned Coding of Moderator Variables by mHealth Intervention Study
References
- 1.Webb TL, Joseph J, Yardley L, Michie S. Using the internet to promote health behavior change: a systematic review and meta-analysis of the impact of theoretical basis, use of behavior change techniques, and mode of delivery on efficacy. J Med Internet Res. 2010;12(1):e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith A. Chapter one: a portrait of smartphone ownership. PewResearchCenter. http://www.pewinternet.org/2015/04/01/chapter-one-a-portrait-of-smartphone-ownership/. Published April 1, 2015. Accessed September 10, 2016.
- 3.Lenhart A. A majority of American teens report access to a computer, game console, smartphone, and a tablet. PewResearchCenter. http://www.pewinternet.org/2015/04/09/a-majority-of-american-teens-report-access-to-a-computer-game-console-smartphone-and-a-tablet/. Published April 9, 2015. Accessed September 10, 2016.
- 4.Rideout V. The common sense census: media use by tweens and teens. Common Sense Media. https://www.commonsensemedia.org/sites/default/files/uploads/research/census_researchreport.pdf. Published 2015. Accessed September 10, 2016.
- 5.Anglada-Martinez H, Riu-Viladoms G, Martin-Conde M, Rovira-Illamola M, Sotoca-Momblona JM, Codina-Jane C. Does mHealth increase adherence to medication? results of a systematic review. Int J Clin Pract. 2015;69(1):9-32. [DOI] [PubMed] [Google Scholar]
- 6.Hamine S, Gerth-Guyette E, Faulx D, Green BB, Ginsburg AS. Impact of mHealth chronic disease management on treatment adherence and patient outcomes: a systematic review. J Med Internet Res. 2015;17(2):e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brannon EE, Cushing CC. A systematic review: is there an app for that? translational science of pediatric behavior change for physical activity and dietary interventions. J Pediatr Psychol. 2015;40(4):373-384. [DOI] [PubMed] [Google Scholar]
- 8.Free C, Phillips G, Felix L, Galli L, Patel V, Edwards P. The effectiveness of M-health technologies for improving health and health services: a systematic review protocol. BMC Res Notes. 2010;3:250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Free C, Phillips G, Watson L, et al. The effectiveness of mobile-health technologies to improve health care service delivery processes: a systematic review and meta-analysis. PLoS Med. 2013;10(1):e1001363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bin-Abbas B, Jabbari M, Al-Fares A, El-Dali A, Al-Orifi F. Effect of mobile phone short text messages on glycaemic control in children with type 1 diabetes. J Telemed Telecare. 2014;20(3):153-156. [DOI] [PubMed] [Google Scholar]
- 11.Herbert LJ, Mehta P, Monaghan M, Cogen F, Streisand R. Feasibility of the SMART Project: a text message program for adolescents with type 1 diabetes. Diabetes Spectr. 2014;27(4):265-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turner T, Spruijt-Metz D, Wen CK, Hingle MD. Prevention and treatment of pediatric obesity using mobile and wireless technologies: a systematic review. Pediatr Obes. 2015;10(6):403-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nguyen B, Shrewsbury VA, O’Connor J, et al. Twelve-month outcomes of the Loozit randomized controlled trial: a community-based healthy lifestyle program for overweight and obese adolescents. Arch Pediatr Adolesc Med. 2012;166(2):170-177. [DOI] [PubMed] [Google Scholar]
- 14.Lu F, Turner KMB. Reducing adolescent obesity with a mobile fitness application: study results of youth age 15 to 17. Paper presented at: 2013 Institute of Electrical and Electronics Engineers 15th International Conference on e-Health Networking, Applications and Services (Healthcom 2013); Lisbon, Portugal. [Google Scholar]
- 15.Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Chichester, England: Wiley-Blackwel; 2011. [Google Scholar]
- 16.Gleser LJ, Olkin I. Stochastically dependent effect sizes. In: Cooper HHL, ed. The Handbook of Research Synthesis. New York, NY: Russell Sage Foundation; 1994:339-355. [Google Scholar]
- 17.Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: L Erlbaum Associates; 1988. [Google Scholar]
- 18.Lipsey M, Wilson D. Practical Meta-Analysis. Thousand Oaks, CA: Sage Publications; 2001. [Google Scholar]
- 19.Frøisland DH, Arsand E, Skårderud F. Improving diabetes care for young people with type 1 diabetes through visual learning on mobile phones: mixed-methods study. J Med Internet Res. 2012;14(4):e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahlers-Schmidt CR, Chesser AK, Nguyen T, et al. Feasibility of a randomized controlled trial to evaluate Text Reminders for Immunization Compliance in Kids (TRICKs). Vaccine. 2012;30(36):5305-5309. [DOI] [PubMed] [Google Scholar]
- 21.Bauer S, de Niet J, Timman R, Kordy H. Enhancement of care through self-monitoring and tailored feedback via text messaging and their use in the treatment of childhood overweight. Patient Educ Couns. 2010;79(3):315-319. [DOI] [PubMed] [Google Scholar]
- 22.Burbank AJ, Lewis SD, Hewes M, et al. Mobile-based asthma action plans for adolescents. J Asthma. 2015;52(6):583-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cafazzo JA, Casselman M, Hamming N, Katzman DK, Palmert MR. Design of an mHealth app for the self-management of adolescent type 1 diabetes: a pilot study. J Med Internet Res. 2012;14(3):e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cornelius JB, Dmochowski J, Boyer C, St Lawrence J, Lightfoot M, Moore M. Text-messaging–enhanced HIV intervention for African American adolescents: a feasibility study. J Assoc Nurses AIDS Care. 2013;24(3):256-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Niet J, Timman R, Bauer S, et al. The effect of a short message service maintenance treatment on body mass index and psychological well-being in overweight and obese children: a randomized controlled trial. Pediatr Obes. 2012;7(3):205-219. [DOI] [PubMed] [Google Scholar]
- 26.Direito A, Jiang Y, Whittaker R, Maddison R. Apps for improving fitness and increasing physical activity among young people: the AIMFIT pragmatic randomized controlled trial. J Med Internet Res. 2015;17(8):e210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Domek GJ, Contreras-Roldan IL, O’Leary ST, et al. SMS text message reminders to improve infant vaccination coverage in Guatemala: a pilot randomized controlled trial. Vaccine. 2016;34(21):2437-2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fassnacht DB, Ali K, Silva C, Gonçalves S, Machado PPP. Use of text messaging services to promote health behaviors in children. J Nutr Educ Behav. 2015;47(1):75-80. [DOI] [PubMed] [Google Scholar]
- 29.Franklin VL, Waller A, Pagliari C, Greene SA. A randomized controlled trial of Sweet Talk, a text-messaging system to support young people with diabetes. Diabet Med. 2006;23(12):1332-1338. [DOI] [PubMed] [Google Scholar]
- 30.Haji A, Lowther S, Ngan’ga Z, et al. Reducing routine vaccination dropout rates: evaluating two interventions in three Kenyan districts, 2014. BMC Public Health. 2016;16:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hanauer DA, Wentzell K, Laffel N, Laffel LM. Computerized Automated Reminder Diabetes System (CARDS): e-mail and SMS cell phone text messaging reminders to support diabetes management. Diabetes Technol Ther. 2009;11(2):99-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hashemian TS, Kritz-Silverstein D, Baker R. Text2Floss: the feasibility and acceptability of a text messaging intervention to improve oral health behavior and knowledge. J Public Health Dent. 2015;75(1):34-41. [DOI] [PubMed] [Google Scholar]
- 33.Hingle MDS, Snyder AL, McKenzie NE, et al. Effects of a short messaging service-based skin cancer prevention campaign in adolescents. Am J Prev Med. 2014;47(5):617-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hofstetter AM, DuRivage N, Vargas CY, et al. Text message reminders for timely routine MMR vaccination: A randomized controlled trial. Vaccine. 2015;33(43):5741-5746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kharbanda EO, Stockwell MS, Fox HW, Andres R, Lara M, Rickert VI. Text message reminders to promote human papillomavirus vaccination. Vaccine. 2011;29(14):2537-2541. [DOI] [PubMed] [Google Scholar]
- 36.Mason M, Mennis J, Way T, et al. Text message delivered peer network counseling for adolescent smokers: a randomized controlled trial. J Prim Prev. 2016;37(5):403-420. [DOI] [PubMed] [Google Scholar]
- 37.Mulvaney SA, Anders S, Smith AK, Pittel EJ, Johnson KB. A pilot test of a tailored mobile and web-based diabetes messaging system for adolescents. J Telemed Telecare. 2012;18(2):115-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Newton KH, Wiltshire EJ, Elley CR. Pedometers and text messaging to increase physical activity: randomized controlled trial of adolescents with type 1 diabetes. Diabetes Care. 2009;32(5):813-815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nollen NL, Mayo MS, Carlson SE, Rapoff MA, Goggin KJ, Ellerbeck EF. Mobile technology for obesity prevention: a randomized pilot study in racial- and ethnic-minority girls. Am J Prev Med. 2014;46(4):404-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patrick K, Norman GJ, Davila EP, et al. Outcomes of a 12-month technology-based intervention to promote weight loss in adolescents at risk for type 2 diabetes. J Diabetes Sci Technol. 2013;7(3):759-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rand CM, Brill H, Albertin C, et al. Effectiveness of centralized text message reminders on human papillomavirus immunization coverage for publicly insured adolescents. J Adolesc Health. 2015;56(5)(suppl):S17-S20. [DOI] [PubMed] [Google Scholar]
- 42.Rodgers CC, Krance R, Street RL, Hockenberry MJ. Symptom prevalence and physiologic biomarkers among adolescents using a mobile phone intervention following hematopoietic stem cell transplantation. Oncol Nurs Forum. 2014;41(3):229-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shapiro JR, Bauer S, Hamer RM, Kordy H, Ward D, Bulik CM. Use of text messaging for monitoring sugar-sweetened beverages, physical activity, and screen time in children: a pilot study. J Nutr Educ Behav. 2008;40(6):385-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sharma R, Hebbal M, Ankola AV, Murugabupathy V. Mobile-phone text messaging (SMS) for providing oral health education to mothers of preschool children in Belgaum City. J Telemed Telecare. 2011;17(8):432-436. [DOI] [PubMed] [Google Scholar]
- 45.Shi HJ, Jiang XX, Yu CY, Zhang Y. Use of mobile phone text messaging to deliver an individualized smoking behaviour intervention in Chinese adolescents. J Telemed Telecare. 2013;19(5):282-287. [DOI] [PubMed] [Google Scholar]
- 46.Stockwell MS, Kharbanda EO, Martinez RA, Vargas CY, Vawdrey DK, Camargo S. Effect of a text messaging intervention on influenza vaccination in an urban, low-income pediatric and adolescent population: a randomized controlled trial. JAMA. 2012;307(16):1702-1708. [DOI] [PubMed] [Google Scholar]
- 47.Stockwell MS, Kharbanda EO, Martinez RA, et al. Text4Health: impact of text message reminder-recalls for pediatric and adolescent immunizations. Am J Public Health. 2012;102(2):e15-e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stockwell MSH, Hofstetter AM, DuRivage N, et al. Text message reminders for second dose of influenza vaccine: a randomized controlled trial. Pediatrics. 2015;135(1):e83-e91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thompson D, Cantu D, Ramirez B, et al. Texting to increase adolescent physical activity: feasibility assessment. Am J Health Behav. 2016;40(4):472-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Uddin MJS, Shamsuzzaman M, Horng L, et al. Use of mobile phones for improving vaccination coverage among children living in rural hard-to-reach areas and urban streets of Bangladesh. Vaccine. 2016;34(2):276-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rosenthal R. The file drawer problem and tolerance for null results. Psychol Bull. 1979;86(3):638-641. [Google Scholar]
- 52.Kahana S, Drotar D, Frazier T. Meta-analysis of psychological interventions to promote adherence to treatment in pediatric chronic health conditions. J Pediatr Psychol. 2008;33(6):590-611. [DOI] [PubMed] [Google Scholar]
- 53.Cushing CC, Steele RG. A meta-analytic review of eHealth interventions for pediatric health promoting and maintaining behaviors. J Pediatr Psychol. 2010;35(9):937-949. [DOI] [PubMed] [Google Scholar]
- 54.Modi AC, Crosby LE, Hines J, Drotar D, Mitchell MJ. Feasibility of web-based technology to assess adherence to clinic appointments in youth with sickle cell disease. J Pediatr Hematol Oncol. 2012;34(3):e93-e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beets MW, Cardinal BJ, Alderman BL. Parental social support and the physical activity-related behaviors of youth: a review. Health Educ Behav. 2010;37(5):621-644. [DOI] [PubMed] [Google Scholar]
- 56.O’Connor TM, Jago R, Baranowski T. Engaging parents to increase youth physical activity a systematic review. Am J Prev Med. 2009;37(2):141-149. [DOI] [PubMed] [Google Scholar]
- 57.Ellis DA, Podolski CL, Frey M, Naar-King S, Wang B, Moltz K. The role of parental monitoring in adolescent health outcomes: impact on regimen adherence in youth with type 1 diabetes. J Pediatr Psychol. 2007;32(8):907-917. [DOI] [PubMed] [Google Scholar]
- 58.Cushing CC, Brannon EE, Suorsa KI, Wilson DK. Systematic review and meta-analysis of health promotion interventions for children and adolescents using an ecological framework. J Pediatr Psychol. 2014;39(8):949-962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McGrady ME, Hommel KA. Targeting health behaviors to reduce health care costs in pediatric psychology: descriptive review and recommendations. J Pediatr Psychol. 2016;41(8):835-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. PubMed Electronic Search Strategy
eTable 1. Studies Included in the Meta-analysis
eTable 2. Assigned Coding of Moderator Variables by mHealth Intervention Study