Abstract
The objective of this study was to determine the impact of postnatal dexamethasone treatment on the neonatal unit on the school age lung function of very prematurely born children. Children born prior to 29 weeks of gestational age had been entered into a randomised trial of two methods of neonatal ventilation (United Kingdom Oscillation Study). They had comprehensive lung function measurements at 11 to 14 years of age. One hundred and seventy-nine children born at a mean gestational age of 26.9 (range 23–28) weeks were assessed at 11 to 14 years; 50 had received postnatal dexamethasone. Forced expiratory flow at 75% (FEF75), 50%, 25% and 25–75% of the expired vital capacity, forced expiratory volume in one second, peak expiratory flow and forced vital capacity and lung volumes including total lung capacity and residual volume were assessed. Lung function outcomes were compared between children who had and had not been exposed to dexamethasone after adjustment for neonatal factors using linear mixed effects regression. After adjustment for confounders all the mean spirometry results were between 0.38 and 0.87 standard deviations lower in those exposed to dexamethasone compared to the unexposed. For example, the mean FEF75 z-score was 0.53 lower (95% CI 0.21 to 0.85). The mean lung function was lower as the number of courses of dexamethasone increased. In conclusion, postnatal dexamethasone exposure was associated with lower mean lung function at school age in children born extremely prematurely. Our results suggest the larger the cumulative dose the greater the adverse effect on lung function at follow-up.
Introduction
Corticosteroids administered to prematurely born infants can facilitate early extubation and reduce the rate of bronchopulmonary dysplasia (BPD) [1, 2]. Postnatally administered corticosteroids have, however, been shown to adversely affect lung growth in rats resulting in emphysematous lungs with fewer airspaces and delayed alveolarization [3]. There are limited and conflicting data regarding the long term pulmonary effects of postnatal corticosteroids on prematurely born children. In a study of 16 children born between 24 to 29 weeks of gestation, who had been entered into a randomised controlled trial (RCT) at 10 days of age, no significant differences were found in respiratory morbidity between 7.8 and 9.2 years [4]. In a follow up of another RCT, no statistically significant differences were found in lung function results (forced expiratory volume in one second (FEV1), forced vital capacity (FVC), FEV1/FVC ratio, peak expiratory flow (PEF), forced expiratory flow between 25% and 75% of VC (FEF25-75%)) at 13 to 17 years between the 68 prematurely born children who had received corticosteroids and the 74 who had received placebo at a median age of four weeks [5]. There were also no significant differences in inhaler use (relative risk (RR) 0.67, 95% CI 0.37 to 1.22), wheezing (RR 0.80, 95% CI 0.53 to 1.20) or cough (RR 1.19, 95% CI 0.67 to 2.23) between the two groups [5]. One study reported a positive effect in that only 48% of children who had received a 42 day tapering course of postnatal corticosteroids had an FEV1 below the normal range at 8 to 11 years of age in comparison to 68% of the controls (p = 0.03) [6]. The parent reported incidence of asthma, however, was similar in the two groups [6] and a further study of those children reported no significant differences in impaired aerobic fitness between the two groups [7]. In contrast, in a cross-sectional study, 105 prematurely born children who had been exposed to postnatal corticosteroids, had lower FEV1 (p = 0.01), FEF25-75% (p = 0.003), and PEF (p = 0.02) at age 9–11 years [8]. It is important to note that none of those study populations [5–8] had been exposed to postnatal surfactant and few to antenatal corticosteroids.
The United Kingdom Oscillation Study (UKOS) was a randomised trial of two modes of neonatal ventilation involving 797 infants born prior to 29 weeks of gestational age. More than 90% of the infants were exposed both to antenatal corticosteroids and postnatal surfactant [9]. We previously demonstrated that administration of postnatal dexamethasone was associated with significantly increased proportions of children with respiratory hospital readmission: (0.35 vs 0.15, difference = 0.20; 95% CI: 0.08 to 0.31) and neurodevelopmental impairment (0.59 vs 0.45, difference = 0.14; 95% CI: 0.02 to 0.26) after adjustment for confounding [10]. The aim of this present study was to analyse the lung function results of those entered into the UKOS trial at 11 to 14 years [11] to test the hypothesis that those exposed to postnatal dexamethasone had lower mean lung function results.
Methods
Children from UKOS were invited to attend for follow-up at 11 to 14 years of age at King’s College Hospital NHS Foundation Trust. Ethical approval was granted by the South Thames Multicentre Research Ethics Committee and by the South West London National Research Ethics Service Committee for the follow up study [11]. Parents gave informed, written consent.
Lung function testing was carried out as previously described [11]. FEF75, FEF50, FEF25 and FEF25-75, FEV1, PEF and FVC were assessed by spirometry. Lung volumes were assessed using a helium-dilution technique (functional residual capacity, FRCHe) and plethysmography ((FRCpleth), total lung capacity (TLC) and residual volume (RV)). Respiratory resistance was measured by impulse oscillometry at 5Hz and 20Hz. The diffusing capacity of the lungs for carbon monoxide was measured using a single breath technique. All tests were done in accordance to guidance from the American Thoracic Society and the European Respiratory Society. The results were converted into z-scores to adjust for age, sex, and height [11], except for PEF and respiratory resistance which were expressed as the percentage predicted for height and sex [12, 13].
Data for postnatal corticosteroid exposure was captured when the original UKOS study was undertaken in 1998–2001. Correspondence confirmed that dexamethasone was the corticosteroid used in the postnatal period in all units. An average course of dexamethasone would be 0.25 mg twice daily for three days, followed by 0.15 mg twice daily for three days, followed by 0.05 mg twice daily for three days.
Statistical analysis
To examine the associations between postnatal dexamethasone use and lung function at follow-up, linear mixed effects regression models were used to allow for clustering of data arising from multiple births [14]. All other predictor variables were forced into the model and treated as fixed effects. Measures of dexamethasone use considered were: a yes/no indicator, the number of courses of dexamethasone (none, one, two or three) and the length of exposure to dexamethasone (0, 1–6, 7–12 or >12 days) [10]. Initially associations were explored in univariable models and then in a multivariable model. To adjust for potential confounders, the following factors were included in the multivariable models: sex, birth weight z-score, gestational age, maternal smoking during pregnancy, oxygen dependency at 36 weeks postmenstrual age (PMA), major cranial ultrasound abnormality, air leak, patent ductus arteriosus (PDA), pulmonary haemorrhage, mode of ventilation and age at the time of follow-up assessment. Sensitivity analyses were conducted to also adjust for antenatal steroids and postnatal surfactant. Differences in mean lung function results by dexamethasone exposure were also shown as the difference in the proportions with abnormal lung function using the fifth centile in healthy children to define abnormal. The distributional approach was used for these calculations to retain the same power as the comparison of means [15, 16] and followed the approach we used in previous work [11]. (Table D in S1 File).
A further set of sensitivity analyses were conducted to explore the robustness of the adjustment for confounders. This used propensity score matching to compare outcomes in children who had and had not been exposed to dexamethasone. Full details are given in Table C in S1 File).
Subjects with complete data in the variables required for adjustment in the multivariable models or matching in the propensity score analysis were included in the analysis. Where one, but not all, lung function assessment results were available, cases were excluded only from analyses of the measures for which the data were missing. Analysis was conducted using Stata 13MP.
Results
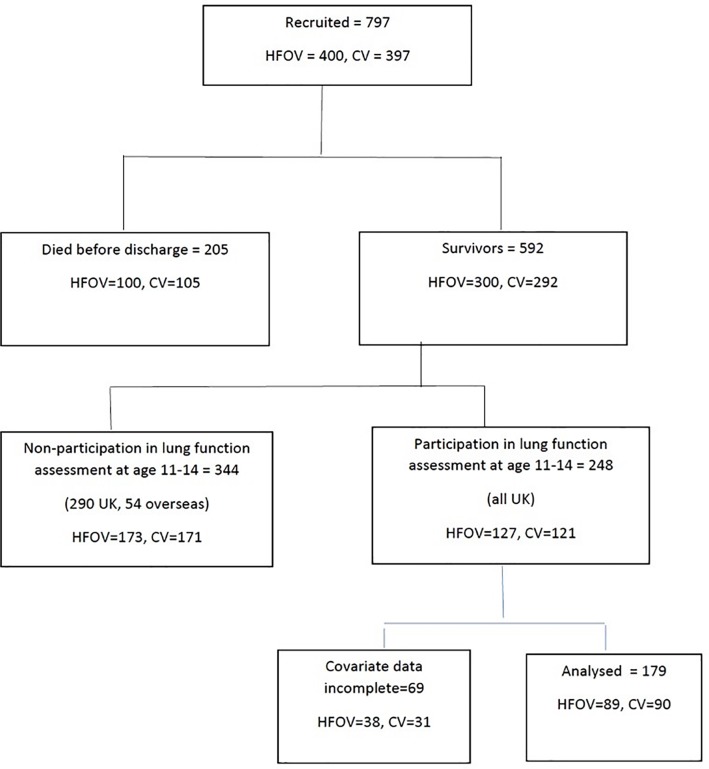
Two hundred and forty-eight children attended for lung function assessment; 179 had complete data at baseline and were included in these analyses (Fig 1). There were no significant differences between those with and without complete data for the majority of the lung function results, except for RV and FRCpleth z scores (Table A in S1 File). Similarly, there were no significant differences in baseline characteristics comparing those included (n = 179) and those with incomplete data (n = 223) (Table B in S1 File).
Fig 1. Study flow chart.
Fifty children (28%) had received at least one course of dexamethasone in the neonatal period and 129 were not exposed to dexamethasone (Table 1). The children exposed to dexamethasone had significantly lower mean birth weight and gestational age, were less likely to be multiples, a greater proportion had an air leak and have lower Apgar five minute scores and a greater proportion were oxygen dependent at 36 weeks PMA (Table 1).
Table 1. Characteristics of children who were or were not exposed to dexamethasone.
The data are presented as the mean (SD) or n (%) unless otherwise shown.
| No dexamethasone exposure | Dexamethasone exposure | p-value | |
|---|---|---|---|
| N | 129 | 50 | |
| Birth weight (grams) | 939 (205) | 810 (175) | <0.001 |
| Birthweight SDS | -0.63 (0.98) | -0.90 (1.12) | 0.11 |
| Gestational age (weeks) | 26.7 (1.2) | 25.6 (1.3) | <0.001 |
| Male sex | 60 (47%) | 31 (62%) | 0.063 |
| Multiple birth | 37 (29%) | 7 (14%) | 0.041 |
| Oxygen dependency at 36 weeks PMA | 62 (48%) | 43 (86%) | <0.001 |
| Major ultrasound abnormality in neonatal period | 16 (12%) | 7 (14%) | 0.775 |
| Airleak | 9 (7%) | 12 (24%) | 0.001 |
| Patent ductus arteriosus | 34 (26%) | 20 (40%) | 0.074 |
| Pulmonary haemorrhage | 5 (3.9%) | 5 (10%) | 0.109 |
| HFOV | 60 (47%) | 29 (58%) | 0.168 |
| Apgar score at 5 mins (mean, range) | 9 (8–9) | 8 (7–9) | <0.001 |
| Maternal smoking in pregnancy | 32 (25%) | 9 (18%) | 0.331 |
| Antenatal steroids | 14 (88%) | 47 (96%) | 0.160 |
| Postnatal surfactant | 127 (98%) | 49 (98%) | >0.999 |
Comparison of the lung function results of those exposed and not exposed to dexamethasone demonstrated significant differences in favour of those unexposed in all the results using linear mixed model adjustment, with differences in the means of the spirometry results ranging from 0.38 to 0.87 standard deviations (Table 2). The differences in mean z-scores for the spirometry, measures are expressed as the equivalent percentage of children with abnormal function. For example, the percentage of children with an abnormal FEF75 is estimated as 21% in the non-exposed and 43% in the exposed group. The other spirometry measures show differences between the exposed and unexposed children ranging from 7.6 to 34 percentage points (Table 3).
Table 2. Lung function and postnatal dexamethasone exposure.
| No dexamethasone exposure | Dexamethasone exposure | Unadjusted | Adjusted using multiple regression | ||||
|---|---|---|---|---|---|---|---|
| Lung Function | N | Mean (SD) |
Mean (SD) |
Difference (95% CI) |
p-value | Difference (95% CI) |
p-value |
| FEF75 z score | 179 | -0.95 (0.91) |
-1.45 (0.71) |
-0.46 (0.74 to -0.18) |
0.001 | -0.53* (-0.85 to -0.21) |
0.002 |
| FEF50 z score | 179 | -1.04 (0.89) |
-1.71 (0.81) |
-0.66 (0.94 to -0.38) |
<0.001 | -0.74 (-1.05 to -0.43) |
<0.001 |
| FEF25 z score | 179 | -0.82 (0.91) |
-1.53 (0.86) |
-0.70 (-0.99 to -0.41) |
<0.001 | -0.75 (-1.07 to -0.44) |
<0.001 |
| FEF25-75 z score | 169 | -1.24 (1.07) |
-1.98 (1.05) |
-0.70 (-1.06 to -0.35) |
<0.001 | -0.70 (-1.08 to -0.33) |
<0.001 |
| FEV1 z score | 179 | -0.55 (1.03) |
-1.44 (1.03) |
-0.86 (-1.20 to—0.53) |
<0.001 | -0.87 (-1.24 to -0.51) |
<0.001 |
| FVC z score | 179 | -0.24 (0.96) |
-0.73 (1.11) |
-0.46 (-0.79 to -0.13) |
0.006 | -0.38 (-0.75 to -0.01) |
0.043 |
| FEV1:FVC z score | 179 | -1.17 (1.69) |
-2.32 (2.11) |
-1.14 (-1.74 to -0.55) |
<0.001 | -1.43 (-2.09 to -0.78) |
<0.001 |
| PEF % pred* | 178 | 86.07 (14.64) |
77.36 (13.98) |
-8.34 (-13.06 to -3.62) |
0.001 | -10.74 (-16.06 to -5.41) |
<0.001 |
| RV z score | 152 | 0.26 (1.09) |
1.29 (1.67) |
0.99 (0.53 to 1.45) |
<0.001 | 0.86 (0.36 to 1.36) |
0.001 |
| FRCpleth z | 157 | -0.11 (1.25) |
0.39 (1.39) |
0.49 (0.04 to 0.94) |
0.031 | 0.39 (-0.11 to 0.90) |
0.128 |
| FRChe z score | 168 | -0.73 (1.09) |
-0.42 (1.00) |
0.32 (-0.04 to 0.68) |
0.083 | 0.27 (-0.13 to 0.66) |
0.186 |
| DLCO z score | 149 | -0.93 (1.11) |
-1.04 (1.02) |
-0.06 (-0.46 to 0.34) |
0.764 | 0.09 (-0.33 to 0.52) |
0.658 |
| At 5 Hz | 170 |
96.06 (21.38) |
100.11 (27.03) |
4.60 (-3.22 to 12.42) |
0.249 | 9.57 (1.13 to 18.02) |
0.026 |
| At 20 Hz | 170 | 93.94 (19.82) |
91.28 (4.46) |
-2.28 (-10.13 to 5.58) |
0.570 | 2.49 (-6.27 to 11.25) |
0.578 |
*expressed as percentage predicted for height and sex
Table 3. Adjusted differences in mean lung function with equivalent differences in the proportions (as percentage) with abnormal lung function (below/above 5th centile for healthy population*).
| Adjusted mean lung function (Table 2 in paper) |
Percentage with abnormal lung function (rounded to 2 significant figures) | Adjusted difference in percentage with abnormal lung function | |||
|---|---|---|---|---|---|
| Lung Function measure |
N | Difference (exposed-unexpo) (95% CI) | No dexamethasone exposure | Dexamethasone exposure | Difference (exposed-unexpo) (95% CI) |
| FEF75 z score+ | 179 | -0.53 (-0.85 to -0.21) |
21% | 43% | 22% (12 to 33%) |
| FEF50 z score | 179 | -0.74 (-1.05 to -0.43) |
22% | 57% | 34% (24 to 44%) |
| FEF25 z score | 179 | -0.75 (-1.07 to -0.44) |
15% | 47% | 31% (21 to 41%) |
| FEF25-75 z score | 169 | -0.70 (-1.08 to -0.33) |
35% | 64% | 28% (18 to 39%) |
| FEV1 z score | 179 | -0.87 (-1.24 to -0.51) |
13% | 42% | 29% (20 to 39%) |
| FVC z score | 179 | -0.38 (-0.75 to -0.01) |
8% | 15% | 7.6% (1.7 to 14%) |
| FEV1:FVC z | 179 | -1.43 (-2.09 to -0.78) |
38% | 70% | 32% (22 to 42%) |
| PEF % pred | 178 | -10.74 (-16.06 to -5.41) |
- | - | - |
| RV z score | 152 | 0.86 (0.36 to 1.36) |
13% | 35% | 22% (12 to 32%) |
| FRCpleth z | 157 | 0.39 (-0.11 to 0.90) |
- | - | - |
| FRChe z score | 168 | 0.27 (-0.13 to 0.66) |
- | - | - |
| DLCO z score | 149 | 0.09 (-0.33 to 0.52) |
- | - | - |
| At 5 Hz | 170 |
9.57 (1.13 to 18.02) |
- | - | - |
| At 20 Hz | 170 | 2.49 (-6.27 to 11.25) |
- | - | - |
Sensitivity analyses adjusting for confounding using propensity score matching gave very similar results to the linear mixed models (Table D in S1 File). Further sensitivity analysis adjusting for antenatal steroids and postnatal surfactant made no appreciable difference (Table E in S1 File). Similarly, adjustment for tobacco smoke exposure at age 11–14 [17] did not affect the estimates or statistical significance (data not shown).
The mean lung function was lower in children who had received more courses of dexamethasone compared to one course and this remained significant after adjustment for most measures (Table 4). Similar trends were seen with the length of exposure with longer exposure being associated with poorer mean lung function which remained significant after adjustment (Table 5).
Table 4. Lung function and number of postnatal dexamethasone courses.
| Unadjusted | Adjusted | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| None | One course difference (95% CI) p-value |
Two courses difference (95% CI) p-value |
Three courses difference (95% CI) p-value |
Overall p-value |
None | One course difference (95% CI) p-value |
Two courses difference (95% CI) p-value |
Three courses difference (95% CI) p-value |
Test for trend p-value |
|
| N | 129 | 35 | 11 | 4 | 129 | 35 | 11 | 4 | ||
| FEF75 z score | ref | -0.37 (-0.68 to -0.06) 0.021 |
-0.59 (-1.11 to -0.08) 0.024 |
-0.92 (-1.76 to -0.08) 0.032 |
0.006 | ref | -0.48 (-0.81 to -0.14) 0.006 |
-0.59 (-1.16 to -0.03) 0.040 |
-1.02 (-1.88 to -0.15) 0.021 |
0.001 |
| FEF50 z score | ref | -0.52 (-0.84 to -0.21) 0.001 |
-1.01 (-1.53 to -0.49) <0.001 |
-0.95 (-1.80 to -0.10) 0.029 |
<0.001 | ref | -0.65 (-0.98 to -0.33) <0.001 |
-0.99 (-1.53 to -0.44) <0.001 |
-1.15 (-1.99 to -0.31) 0.007 |
<0.001 |
| FEF25 z score | ref | -0.61 (-0.94 to -0.28) <0.001 |
-0.85 (-1.39 to -0.30) 0.002 |
-1.13 (-2.01 to -0.24) 0.012 |
<0.001 | ref | -0.71 (-1.05 to -0.38) <0.001 |
-0.78 (-1.34 to -0.22) 0.006 |
-1.22 (-2.08 to -0.37) 0.005 |
<0.001 |
| FEF25-75 z score* | ref | -0.49 (-0.88 to -0.10) 0.014 |
-1.22 (-1.85 to -0.58) <0.001 |
1.19 (-2.23 to -0.15) 0.024 |
<0.001 | ref | -0.60 (-1.00 to -0.20) 0.003 |
-0.96 (-1.62 to -0.31) 0.004 |
-1.29 (-2.31 to -0.28) 0.013 |
<0.001 |
| FEV1 z score | ref | -0.64 (-1.02 to -0.27) 0.001 |
-1.32 (-1.93 to -0.70) <0.001 |
-1.60 (-2.60 to -0.60) 0.002 |
<0.001 | ref | -0.74 (-1.13 to -0.36) <0.001 |
-1.16 (-1.80 to -0.52) <0.001 |
-1.65 (-2.63 to -0.67) 0.001 |
<0.001 |
| FVC z score | ref | -0.26 (-0.63 to 0.10) 0.158 |
-0.82 (-1.43 to -0.22) 0.007 |
-1.19 (-2.18 to -0.21) 0.017 |
0.005 | ref | -0.27 (-0.67 to 0.12) 0.174 |
-0.59 (-1.25 to 0.06) 0.077 |
-1.06 (-2.06 to -0.05) 0.039 |
0.009 |
| FEV1:FVC z score | ref | -1.00 (-1.67 to -0.33) 0.003 |
-1.48 (-2.57 to -0.38) 0.008 |
-1.56 (-3.35 to 0.23) 0.088 |
0.002 | ref | -1.30 (-2.00 to -0.59) <0.001 |
-1.82 (-2.99 to -0.64) 0.002 |
-1.98 (-3.77 to -0.18) 0.031 |
<0.001 |
| PEF % of predicted** | ref | -8.11 (-13.35 to -2.87) 0.002 |
-3.51 (-12.10 to 5.07) 0.423 |
-23.27 (-37.33 to -9.22) <0.001 |
<0.001 | ref | -10.27 (-15.85 to -4.70) <0.001 |
-6.55 (-15.88 to 2.77) 0.168 |
-26.51 (-40.91 to -12.11) <0.001 |
<0.001 |
| RV z score*** | ref | 0.74 (0.23 to 1.25) 0.004 |
1.66 (0.81 to 2.52) <0.001 |
1.73 (-0.03 to 3.48) 0.053 |
<0.001 | ref | 0.68 (0.15 to 1.22) 0.011 |
1.47 (0.58 to 2.35) 0.001 |
1.27 (-0.45 to 2.99) 0.147 |
<0.0010.002 |
| FRCpleth z score**** | ref | 0.33 (-0.17 to 0.82) 0.194 |
1.10 (0.23 to 1.97) 0.013 |
0.45 (-1.34 to 2.24) 0.621 |
0.065 | ref | 0.25 (-0.29 to 0.78) 0.363 |
1.11 (0.18 to 2.04) 0.020 |
0.14 (-0.17 to 1.96) 0.877 |
0.063 |
| FRChe z score | ref | 0.36 (-0.04 to 0.77) 0.080 |
0.11 (-0.54 to 0.76) 0.751 |
0.50 (-0.56 to 1.56) 0.353 |
0.299 | ref | 0.29 (-0.13 to 0.71) 0.178 |
0.18 (-0.52 to 0.88) 0.616 |
0.22 (-0.84 to 1.28) 0.689 |
0.338 |
| DLCO z score | ref | 0.07 (-0.37 to 0.51) 0.762 |
-0.13 (-0.90 to 0.64) 0.735 |
-1.11 (-2.33 to 0.12) 0.077 |
0.332 | ref | 0.19 (-0.26 to 0.64) 0.412 |
0.11 (-0.68 to 0.89) 0.785 |
-1.00 (-2.21 to 0.21) 0.106 |
0.680 |
| Respiratory resistance (% predicted) | ||||||||||
| At 5 Hz | ref | 4.03 (-4.65 to 12.72) 0.362 |
10.06 (-4.43 to 24.55) 0.174 |
-6.96 (-33.17 to 19.25) 0.603 |
0.436 | ref | 9.55 (0.60 to 18.50) 0.036 |
12.86 (-2.31 to 28.04) 0.097 |
-1.77 (-27.87 to 24.32) 0.894 |
0.093 |
| At 20Hz | ref | 0.82 (-7.91 to 9.56) 0.854 |
-5.54 (-20.16 to 9.07) 0.457 |
-25.71 (-51.84 to 0.41) 0.054 |
0.227 | ref | 4.63 (-4.63 to 13.89) 0.327 |
-0.59 (-16.24 to 15.06) 0.941 |
-20.74 (-47.49 to 6.02) 0.129 |
0.669 |
Footnote: differences are exposed–unexposed.
The children who received no dexamethasone are the reference group for all comparisons.
Some lung function results were only available for certain children
*169
**178
*** 152
**** 157
Table 5. Lung function and length of exposure to postnatal dexamethasone (days).
| Unadjusted | Adjusted | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1–6, Difference (95% CI) p-value |
7–12, Difference (95% CI) p-value |
>12, Difference (95% CI) p-value |
Overall p-value | 0 | 1–6 days, Difference (95% CI) p-value |
7–12 days, Difference (95% CI) p-value |
>12 days, Difference (95% CI) p-value |
Test for trend p-value | |
| N | 129 | 6 | 21 | 22 | 129 | 6 | 21 | 22 | ||
| FEF75 z score |
ref | -0.23 (-0.89 to 0.43) 0.490 |
-0.34 (-0.74 to 0.05) 0.086 |
-0.63 (-1.01 to -0.25) 0.001 |
0.008 | ref | -0.36 (-1.01 to 0.30) 0.286 |
-0.45 (-0.86 to -0.03) 0.034 |
-0.68 (-1.10 to -0.26) 0.002 |
<0.001 |
| FEF50 z score |
ref | -0.51 (-1.16 to 0.14) 0.126 |
-0.46 (-0.85 to -0.06) 0.025 |
-0.89 (-1.28 to -0.50) <0.001 |
<0.001 | ref | -0.61 (-1.22 to 0.01) 0.054 |
-0.60 (-1.00 to -0.19) 0.004 |
-0.95 (-0.14 to -0.54) <0.001 |
<0.001 |
| FEF25 z score |
ref | -0.80 (-1.49 to -0.10) 0.025 |
-0.55 (-0.96 to -0.14) 0.009 |
-0.83 (-1.23 to -0.43) <0.001 |
<0.001 | ref | -0.80 (-1.45 to -0.15) 0.017 |
-0.67 (-1.08 to -0.26) 0.001 |
-0.84 (-1.26 to -0.42) <0.001 |
<0.001 |
| FEF25-75 z score* |
ref | -0.67 (-1.46 to 0.13) 0.099 |
-0.55 (-1.04 to -0.06) 0.028 |
-0.84 (-1.33 to -0.35) 0.001 |
0.002 | ref | -0.73 (-1.43 to -0.02) 0.043 |
-0.64 (-1.13 to -0.14) 0.001 |
-0.76 (-1.27 to -0.25) 0.003 |
0.008 |
| FEV1 z score |
ref | -0.87 (-1.77 to -0.07) 0.033 |
-0.53 (-1.00 to -0.06) 0.027 |
-1.17 (-1.63 to -0.71) <0.001 |
<0.001 | ref | -0.88 (-1.63 to -0.14) 0.020 |
-0.63 (-1.10 to -0.16) 0.009 |
-1.13 (1.61 to -0.65) <0.001 |
<0.001 |
| FVC z score |
ref | -0.80 (-1.58 to -0.02) 0.045 |
-0.08 (-0.54 to -0.38) 0.731 |
-0.73 (-1.18 to -0.28) 0.001 |
0.005 | ref | -0.78 (-1.55 to -0.01) 0.047 |
-0.07 (-0.55 to 0.40) 0.765 |
-0.57 (-1.06 to -0.09) 0.020 |
0.049 |
| FEV1:FVC z score |
ref | -0.45 (-1.84 to 0.94) 0.523 |
-0.98 (-1.81 to -0.15) 0.021 |
-1.51 (-2.32 to -0.69) <0.001 |
0.001 | ref | -0.67 (-2.02 to 0.68) 0.329 |
-1.30 (-2.16 to -0.45) 0.003 |
-1.83 (-2.70 to -0.96) <0.001 |
<0.001 |
| PEF % predicted** | ref | -8.99 (-19.85 to 1.87) 0.105 |
-9.55 (-16.21 to -2.59) 0.005 |
-7.61 (-14.10 to 1.13) 0.021 |
0.005 | ref | -9.96 (-20.27 to 0.34) 0.058 |
-11.79 (-18.81 to -4.77) 0.001 |
-10.08 (-17.12 to -2.98) 0.005 |
<0.001 |
| RV z score*** |
ref | 1.58 (0.48 to2.67) 0.005 |
0.21 (-0.41 to 0.82) 0.510 |
1.59 (0.97 to 2.22) <0.001 |
<0.001 | ref | 1.37 (0.31 to 2.44) 0.012 |
0.21 (-0.41 to 0.82) 0.516 |
1.41 (0.77 to 2.05) <0.001 |
<0.001 |
| FRCpleth z score**** |
ref | 0.78 (-0.25 to 1.80) 0.138 |
0.04 (-0.58 to 0.66) 0.899 |
0.83 (0.1 to 1.47) 0.011 |
0.040 | ref | 0.61 (-0.43 to 1.65) 0.250 |
-0.01 (-0.65 to 0.64) 0.980 |
0.77 (0.08 to 1.45) 0.028 |
0.083 |
| FRChe
z score |
ref | 0.36 (-0.47 to 1.19) 0.392 |
0.28 (-0.22 to 0.77) 0.273 |
0.35 (-0.15 to 0.86) 0.170 |
0.380 | ref | 0.26 (-0.56 to 1.07) 0.538 |
0.22 (-0.29 to 0.73) 0.403 |
0.33 (-0.20 to 0.86) 0.223 |
0.184 |
| DLCO
z score |
ref | 0.34 (-0.69 to 1.37) 0.517 |
-0.07 (-0.61 to 0.47) 0.790 |
-0.07 (-0.64 to 0.50) 0.803 |
0.902 | ref | 0.35 (-0.65 to 1.34) 0.493 |
0.07 (-0.48 to 0.62) 0.795 |
0.07 (-5.06 to 0.65) 0.803 |
0.7162 |
| Respiratory resistance % predicted | ||||||||||
| At 5 Hz | ref | 17.35 (0.51 to 34.20) 0.043 |
-2.15 (-12.74 to 8.44) 0.690 |
8.85 (-2.33 to 20.03) 0.121 |
0.101 | ref | 20.41 (4.34 to 36.47) 0.013 |
3.30 (-7.56 to 14.16) 0.552 |
12.68 (0.90 to 24.47) 0.035 |
0.054 |
| At 20 Hz | ref | 11.41 (-6.57 to 29.39) 0.214 |
-4.36 (-15.06 to 6.35) 0.425 |
-4.35 (-15.73 to 7.03) 0.454 |
0.395 | ref | 12.24 (-5.30 to 29.79) 0.171 |
-0.05 (-11.33 to 11.23) 0.993 |
1.62 (-10.66 to 13.90) 0.796 |
0.828 |
Footnote: Diff: difference in means exposed—unexposed
The children who received no dexamethasone are the reference group for all comparisons
Some lung function results were only available for certain children
*169
**178
*** 152
**** 157
Discussion
Our results from a secondary analysis suggest that postnatal dexamethasone exposure was associated with lower mean lung function at school age in children born very prematurely after adjusting for neonatal factors using careful adjustment for confounding with linear mixed effects modelling. In particular, we have shown significant differences in airway function and increased gas trapping as indicated by a higher residual volume. These results were replicated using a different form of adjustment, propensity score matching. The difference observed is substantial being over one half of a standard deviation for FEF75, equivalent to a difference of 22 percentage points in children with abnormal lung function. In addition, our results suggest that the longer the course or the greater the number of courses the greater the observed reduction in lung function. These results were consistent with our earlier study that showed an association between dexamethasone exposure and poor respiratory and neurological outcomes in infancy in the same population and using the same rigorous statistical approach to control for confounding [10]. In contrast, results of an earlier study had suggested that a longer duration of the corticosteroid might improve respiratory outcomes. Mean lung function at 15 years of age was significantly better in those who had received a 42 day course compared to those who had received an 18 day course [18]. There were, however no significant differences between those who received the 42 day course or the placebo and only 22 children in total were included in the study. Our analysis was of a larger cohort and we adjusted carefully for possible confounders.
Our results of impaired lung function following postnatal dexamethasone exposure are consistent with findings in animal models showing abnormal lung function. Although, we highlight that the significant reduction in airway function was more than the reduction in lung volume. In an early animal study [3], postnatal corticosteroids resulted in emphysematous lungs with fewer air spaces and delayed alveoarisation. A more recent study has highlighted that dexamethasone treatment of newborn rats resulted in delayed expression of elastin and smooth muscle actin and the parenchymal expression of tenascin-C (TNC) was delayed [19]. The authors postulated that neonatal corticosteroids impaired the first phase of alveolarisation by altering TNC and elastin expression [19]. In mice treated with dexamethasone, the mRNA expression level of fibrillin-1, which is a key protein reinforcing elastic fibres, were lower than in controls. Fibrillin-1 consists of microfibrils as a scaffold to form elastic fibres and fibulin-5. On the other hand, the peak mRNA expression of tropelastin, the main component in elastic fibres, occurred earlier in the dexamethasone group [20]. The authors postulated this imbalance in the expression of tropelastin and microfibril might interfere with the efficient formation of elastic fibres, resulting in thinning of the alveolar walls [20]. Indeed, in the dexamethasone group there were fewer and larger alveoli [20]. Furthermore, we did not find any significant difference in diffusing capacity results between the two groups.
Our study has strengths and limitations. We report a wide range of lung function measures at 11 to 14 years of age in large number of extremely prematurely born children. Ninety per cent of the children had been exposed to antenatal corticosteroids and nearly all received postnatal surfactant and hence our population is similar to those currently receiving intensive care. We included in our modelling oxygen dependency at 36 weeks PMA as this has been associated with poorer lung function at follow up. We do not report the results of a randomised trial, but analysed our data using rigorous statistical modelling to control for confounding due to differences in neonatal factors and clustering due to multiple births (25% in our sample). The analyses for dexamethasone exposure as a yes/no outcome were also undertaken using propensity score matching. It was not possible to use propensity score matching for the three measures of dexamethasone exposure, that is timing of administration, number of courses and days of exposure due to the small numbers in the different dexamethasone-use categories and so no sensitivity analyses were possible for those outcomes. We do not report whether the lung function abnormalities were associated with an increased need for medications, excess symptoms or exercise intolerance. We intend to investigate this when we assess the UKOS population when they are older.
There were no significant differences in the acute outcomes in the children who had been entered into our RCT of two neonatal ventilation modes [9], but at 11–14 years mean lung function results were superior in the HFOV group [11]. Adjustment for mode of ventilation was made in this present analysis and did not explain the difference in outcomes by dexamethasone exposure. We found no evidence of differences in those with complete or incomplete data and so we consider that our results are generalizable to extremely prematurely born children. These data are observational and so we cannot exclude the possibility that the associations observed are due to unmeasured residual confounding, but we made every effort to control for confounders.
In conclusion, our results suggest postnatal dexamethasone exposure was associated with lower lung function at 11–14 years of age in children born extremely prematurely. In addition, our results suggest the larger the cumulative dose the worse the lung function at follow-up.
Supporting information
Table A: Lung function at follow up of children with and without complete data.
Table B: Baseline characteristics of the infants included and not included due to missing lung function data.
Table C: Mean FEF75 z-score by neonatal factors (n = 179).
Table D: Lung function and postnatal dexamethasone exposure: sensitivity analyses adjusted for confounding using propensity score matching.
Table E: Sensitivity analyses adjusting for antenatal steroids and postnatal surfactant.
Table F: Random effects estimates from adjusted models presented in Table 2 main paper.
(DOC)
Acknowledgments
Funding: This is a secondary analysis of a study originally funded by National Institute for Health Research (NIHR) Health Technology Assessment Programme. The current research was supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy's and St Thomas' NHS Foundation Trust and King's College London (Professors Greenough and Peacock).
The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health. JLP is an NIHR Senior Investigator.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This is a secondary analysis of a study originally funded by National Institute for Health Research (NIHR) Health Technology Assessment Programme. The current research was supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy's and St Thomas' NHS Foundation Trust and King's College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health. JLP is an NIHR Senior Investigator.
References
- 1.Doyle LW, Cheong JL, Ehrenkranz RA, Halliday HL. Early (< 8 days) systemic postnatal corticosteroids for prevention of bronchopulmonary dysplasia in preterm infants. Cochrane Database Syst Rev. 2017;10: CD001146 doi: 10.1002/14651858.CD001146.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doyle LW, Cheong JL, Ehrenkranz RA, Halliday HL. Late (> 7 days) systemic postnatal corticosteroids for prevention of bronchopulmonary dysplasia in preterm infants. Cochrane Database Syst Rev. 2017;10: CD001145 doi: 10.1002/14651858.CD001145.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tschanz SA, Damke BM, Burri PH. Influence of postnatally administered glucocorticoids on rat lung growth. Biol Neonate. 1995;68: 229–245. doi: 10.1159/000244241 [DOI] [PubMed] [Google Scholar]
- 4.Mieskonen S, Eronen M, Malmbeg LP, Turpenen M, Kari MA, Hallman M. Controlled trial of dexamethasone in neonatal chronic lung disease: an 8 year follow up of cardiopulmonary function and growth. Acta Paediatr. 2003;92: 896–904. [PubMed] [Google Scholar]
- 5.Jones RA, Collaborative Dexamethasone Trial Follow-up Group. Randomized, controlled trial of dexamethasone in neonatal chronic lung disease: 13- to 17-year follow-up study: II. Respiratory status, growth, and blood pressure. Pediatrics. 2005;116: 379–384. doi: 10.1542/peds.2004-1819 [DOI] [PubMed] [Google Scholar]
- 6.Nixon PA, Washburn LK, Schechter MS, O’Shea TM. Follow-up study of a randomized controlled trial of postnatal dexamethasone therapy in very low birth weight infants: effects on pulmonary outcomes at age 8 to 11 years. J Pediatr. 2007;150: 345–350. doi: 10.1016/j.jpeds.2006.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nixon PA, Washburn LK, Mudd LM, Webb HH, O’Shea TM. Aerobic fitness and physical activity levels of children born prematurely following randomization to postnatal dexamethasone. J Pediatr. 2011;158: 65–70. doi: 10.1016/j.jpeds.2010.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith LJ, van Asperen PP, McKay KO, Selvadurai H, Fitzgerald DA. Post-natal corticosteroids are associated with reduced expiratory flows in children born very preterm. J Paediatr Child Health. 2011;47: 448–454. doi: 10.1111/j.1440-1754.2010.01992.x [DOI] [PubMed] [Google Scholar]
- 9.Johnson AH, Peacock JL, Greenough A, Marlow N, Limb ES, Marston L, et al. ; United Kingdom Oscillation Study Group. High-frequency oscillatory ventilation for the prevention of chronic lung disease of prematurity. N Engl J Med. 2002;347: 633–642. doi: 10.1056/NEJMoa020432 [DOI] [PubMed] [Google Scholar]
- 10.Qin G, Lo JW, Marlow N, Calvert SA, Greenough A, Peacock JL. Postnatal steroids and neurodevelopmental and respiratory outcomes at two years in babies born extremely preterm. PLoS ONE. 2017;12: e0181176 doi: 10.1371/journal.pone.0181176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zivanovic S, Peacock J, Alcazar-Paris M, Lo JW, Marlow N, Calvert S; United Kingdom Oscillation Study Group. Late outcomes of a randomized trial of high-frequency oscillation in neonates. N Engl J Med. 2014;370: 1121–1130. doi: 10.1056/NEJMoa1309220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenthal M, Bain SH, Cramer D, Helms P, Denison D, Bush A, et al. Lung function in which children aged 4 to 19 years: 1 –Spirometry. Thorax. 1993;48: 794–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nowowiejska B, Tomalak W, Radlinski J, Siergiejko G, Latawiec W, Kaczmarski M. Transient reference values for impulse oscillometry for children aged 3–18 years. Pediatr Pulmonol. 2008;43: 1193–1197. doi: 10.1002/ppul.20926 [DOI] [PubMed] [Google Scholar]
- 14.Sauzet O, Wright K, Marston L, Brocklehurst P, Peacock JL. Modelling the hierarchical structure in data sets with very small clusters: a simulation study to explore the effect of the proportion of clusters when the outcome is continuous. Stat Med. 2013;32: 1429–1439. doi: 10.1002/sim.5638 [DOI] [PubMed] [Google Scholar]
- 15.Peacock JL, Sauzet O, Ewings SM, Kerry SM. Dichotomizing continuous data while retaining statistical power using a distributional approach. Stat Med. 2012;31: 3089–3103. doi: 10.1002/sim.5354 [DOI] [PubMed] [Google Scholar]
- 16.Sauzet O, Breckenkamp J, Borde T, Brenne S, David M, Razum O, et al. A distributional approach to obtaining adjusted comparisons of proportions of a population at risk. Emerg Themes Epidemiol. 2016;13: 8 doi: 10.1186/s12982-016-0050-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lo J, Zivanovic S, Lunt A, Alcazar-Paris M, Andradi G, Thomas M, Marlow N, Calvert S, Peacock J, Greenough A. Longitudian assessment of lung function in extremely prematurely born children. Pediatr Pulmonol. 2018;53: 324–331. doi: 10.1002/ppul.23933 [DOI] [PubMed] [Google Scholar]
- 18.Gross SJ, Anbar RD, Mettelman BB. Follow up at 15 year of preterm infants from a controlled trial of moderately early dexamethasone for the prevention of chronic lung disease. Pediatrics. 2005;115: 681–687. doi: 10.1542/peds.2004-0956 [DOI] [PubMed] [Google Scholar]
- 19.Roth-Kleiner M, Berger TM, Gremlich S, Tschanz SA, Mund SI, Post M, et al. Neonatal steroids induce a down-regulation of tenascin-C and elastin and cause a deceleration of the first phase and an acceleration of the second phase of lung alveolarization. Histochem Cell Biol. 2014;14: 75–84. [DOI] [PubMed] [Google Scholar]
- 20.Kamei M, Miyajima A, Fujisawa M, Matsuoka Y, Hirota T. Effects of postnatal dexamethasone treatment on mRNA expression profiles of genes related to alveolar development in an emphysema model in mice. J Toxicol Sci. 2014;39: 665–670. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table A: Lung function at follow up of children with and without complete data.
Table B: Baseline characteristics of the infants included and not included due to missing lung function data.
Table C: Mean FEF75 z-score by neonatal factors (n = 179).
Table D: Lung function and postnatal dexamethasone exposure: sensitivity analyses adjusted for confounding using propensity score matching.
Table E: Sensitivity analyses adjusting for antenatal steroids and postnatal surfactant.
Table F: Random effects estimates from adjusted models presented in Table 2 main paper.
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.