Abstract
Polycomb Group (PcG) genes are transcriptional repressors that are described to be important during development and differentiation. There is significant interest in PcGs proteins because of their role in stem cell biology and tumorigenesis. In this study we characterize the expression of a selection of PcG genes in the adult germline of zebrafish and during embryogenesis. In adults, expression of selected PcG genes is found to be enriched in germ line over somatic tissues. Therefore, the germ line of adult zebrafish was analyzed for the expression pattern of a selection of PcG genes by whole mount in situ hybridization. We detected presence of the tested PcG gene transcripts at early stages of both oogenesis and spermatogenesis. This enriched expression for early stages of gametogenesis is also observed in developing gonads at 4 and 5 weeks post fertilization. Additionally, zebrafish embryos were used to study the spatio-temporal expression patterns of a selection of PcG genes during development. The PcG genes that we tested are maternally loaded and ubiquitously expressed at early developmental stages, except of ezh1. The expression of the PcG genes that were assessed becomes enriched anteriorly and is more defined during tissue specification. The data shown here is an important resource for functional PcG gene studies in vivo.
Introduction
Polycomb group (PcG) proteins are important negative epigenetic regulators of transcription by modifying histone tails [1]. PcG proteins were first identified for their role in maintaining cell identity, thereby determining patterning of Drosophila embryos [2]. In addition, PcG proteins are described to be involved in a plethora of other biological processes, which include differentiation, cell cycle control, X-chromosome inactivation, and tumorigenesis [3]. PcG proteins can assemble in so-called Polycomb Repressive Complexes (PRCs): PRC1 and PRC2. PRC2 consists of the core components Eed, Suz12, and Ezh1/2. The PcG proteins Ezh1 and Ezh2 are mutually exclusive and tri-methylate lysine 27 of histone H3 (H3K27me3). The core of PRC1 consists of Ring1A/B, a member of the Pcgf family, a Phc protein, and a Cbx protein. PRC1 is recruited to H3K27me3 and places the histone H2A lysine 119 ubiquitination mark (H2AK119Ub), which in turn stabilizes the repressive H3K27me3 mark [4–6]. PcG proteins and their associated molecular mechanisms by which they regulate transcription are evolutionarily conserved. The zebrafish genome has undergone genome duplication, however, over time some duplicated genes are lost or have taken a different function [5,7,8]. The high number of duplicates and even splice-variants of the different homologs can make gene function analysis a complex manner [5].
Regulation of gene transcription by PcG proteins and epigenetic gene regulation in general are dynamic processes that are shown to be important during development of multiple vertebrate systems. For example, in mice Ezh2 and Rnf2 were both found to be essential for embryonic development, since knock-outs were shown to be embryonic lethal [9,10]. This early lethality makes it challenging to study the function of Ezh2 and Rnf2 in the murine system [9,10]. In contrast, zebrafish PcG mutants are reported to survive gastrulation and serve as a very suitable model to study PcG function development [4,11–13]. Zygotic rnf2 zebrafish mutants die around 4–5 days post fertilization (dpf), showing a range of phenotypes, including craniofacial defects, the absence of pectoral fins, and motility problems [13,14]. The zygotic pcgf1 zebrafish mutant fish survive until adulthood, but display growth defects and premature aging [4]. Recently, ezh2 zygotic zebrafish mutants were described to harbor intestinal problems and show lethality around 11 dpf [12]. The ezh2 mRNA is maternally loaded and maternal zygotic ezh2 (MZezh2) mutants show defects in maintenance of cellular identity and tissue integrity and die around 2 dpf [11]. The different timing of lethality of zygotic ezh2 mutants and MZezh2 mutants indicates the importance of the maternal load for proper development.
Though PcG genes are known for their role in embryonic development, less is known about their role during gametogenesis. In germ cells, PcG genes are crucial for preservation of genetic integrity, generation of genetic diversity, and for transmission of genetic information to the progeny. In invertebrates such as C.elegans and Drosphila, PRC2 function has been studied during gametogenesis. In C.elegans, mutants for orthologues of PRC2, which are maternal sterile effect-2 and -6 (mes-2 and mes-6), show that loss of maternal MES function leads to germ line degeneration and sterility [15,16]. Drosophila oocytes lacking E(z) (orthologue of Ezh2) were found to transdifferentiate into nurse cells [17]. This has led to the conclusion that cell fate relies on PRC2 functioning, and on the presence of the H3K27me3 mark in the germ line [17]. Recently, it was described that PRC2 plays a role in murine germ line development, as PRC2 aggregates in the nucleus of mouse fetal germ cells [18]. During murine germ line development DNA methylation is reduced, and H3K27me3 is the crucial repressive mechanism responsible for epigenetic reprogramming, which relies on Ezh2 functioning [18]. In addition, PcG proteins were suggested to be involved in vertebrate oogenesis by establishing developmental competence, since double knockout Ring1/Rnf2 murine oocytes show aberrant gene expression detected by changes in the maternal load [19].
Both male and female gametes are produced during the lifetime of zebrafish. This unique characteristic facilitates studying the different stages of oogenesis and spermatogenesis during adulthood [20]. During zebrafish development germ plasm is already present at the cleavage furrows of the 2-cell stage embryo. As from the 32-cell stage, germ plasm is found in distinct primordial germ cells, which have migrated to the start of the yolk extension by 1 dpf [21]. One of the genes that is highly expressed in these cells is the germ cell marker vasa [22]. Around 4 weeks post fertilization zebrafish sex is determined. Zebrafish oocytes contain maternal mRNA and are externally fertilized and the zygote undergoes rapid cell divisions. Around 3.3 hours post fertilization (hpf) mid-blastula transition (MBT) takes place. At MBT the zygotic genome is activated (ZGA) and the maternal mRNA load is degraded. Already before MBT epigenetic marks are reported to be present in zebrafish, albeit at very low levels [23]. Especially at ZGA epigenetic marks, including H3K27me3, show a dramatic increase at developmental regulatory genes [23].
To understand the role of the epigenome during embryonic and gonad development, a crucial first step is to enhance the knowledge about PcG gene expression. Current data available on PcG expression during zebrafish embryogenesis and gametogenesis is limited. A recent publication shows the expression levels measured by RNA-sequencing at 18 developmental time points from 1 cell to 5 dpf [24]. This data serves as a useful database, however lacks spatial information. In addition, the spatial information on the expression of PRC core-components is incomplete, and often not available of the same stages of development. The gene expression of pcgf family members (pcgf genes, and bmi1a/-b), phc2, and of ezh1 and ezh2 during early zebrafish development has already been partly reported [4,6,11,25]. However, spatio-temporal data on the remaining PcG genes during embryogenesis is lacking. Also, PcG gene expression in the germ line of zebrafish is not studied. Importantly, the presence of a specific RNA transcript can serve as a proxy for the presence in the maternal load. Nevertheless, this does not give information about the expression and potential role during gametogenesis. In this study, we give an overview of the spatio-temporal expression of some of the main PcG genes, which assemble in PRC1 and PRC2. Since zebrafish serve as a unique vertebrate model for studying PcG genes in early development, the expression patterns of PcG genes profiles described in this paper are very useful for follow-up studies using PcG mutants. Moreover, our results can be used to get a better insight in the potential role of PcG proteins in germ line development and embryogenesis.
Materials and methods
Zebrafish maintenance
Zebrafish (wildtype strains: AB/Albino/TL) were maintained in water of 27.5°C in a 14/10h light/dark cycle. The evening before spawning, one male and one female were placed into a tank with a divider and placed together the following morning. Spontaneous spawning occurred when the male and female were put together at the moment the light turned on. Embryos were collected and staged according to Kimmel et al. [26]. The zebrafish experiments described in this study were conducted according to the Dutch and European Union guidelines for the handling of laboratory animals. The experimental procedures carried out on zebrafish were reviewed and approved by the Utrecht University and Radboud University Animal Experiments Committee.
RNA extraction and RT-PCR
Adult fish were euthanized with 2-phenoxyethanol (0.1% v/v). Three biological replicates of ovary, testes, tail muscle, and eye were obtained by dissection [27] and homogenized in TriZol (Ambion). The ZYMO RNA microprep kit was used to isolate RNA and treat the samples with DNAseI. cDNA was generated using oligod(T) primers and Superscript reverse transcriptase (Promega). RT-qPCR was run on a BioRad machine in three technical replicates and the housekeeping gene rsp18 was used as reference gene. The genes and primers used for RT-qPCR are shown in Table 1. As a control a minus reverse transcriptase sample for each condition was tested.
Table 1. RT-qPCR primer sequences.
| sequence forward primer (5’– 3’) | sequence reverse primer (5’– 3’) | |
|---|---|---|
| ezh2 | AAATCGGAGAAGGGTCCTGT | TCTGTTGGAGCTGAACATGC |
| eed | ATCTGGTACATGCGCTTCTC | GGTGGTGCACTTTGCTTTAT |
| suz12a | GCATGACCACCAGAGATACC | CAGGGCTCTCCTCTATCTCC |
| rnf2 | GGATGGAGTCAGCGAGATTG | TCTACATTGATGGGGCTTGC |
| bmi1a | GTTGATGCTGCAAATGGGTC | TTGCGCCCTATGATCGAAAA |
| pcgf6 | ACTGAGAGGGCTTGAAGTTCC | CCCACAAACTCCAACATCAG |
| ezh1 | ACGGCGATTTGACTGGAACA | AGGAAGCGTCTAGTGAGGTCT |
| suz12b | GTCAGCAAGAAGAGGGCTAC | CGGGTTGAGAGAGGTTTAGC |
| pcgf5a | TCTCTCCGGTTCTCACTACC | GCGCCAGCGATTTCATTATC |
| pcgf5b | AATAATGGGCAAAGACCACA | GACTTGCCATTCAATTTGCT |
| rbbp4 | CACACTGCAGAGGTCAACTG | TTTCATCTTTGTGCGACTCA |
| rsp18 | CATCCCAGAGAAGTTTCAGCACATC | CGCCTTCCAACACCCTTAATAGC |
Probe synthesis for whole mount in situ hybridization (WISH)
Antisense RNA probes were in vitro transcribed as described before [4,11]. Plasmids containing PcG genes bmi1a, eed, suz12a, phc2a, ezh2, and ezh1 were ordered at Imagen and if needed subcloned in pCS2+, followed by linearization and in vitro transcription using T3, SP6, or T7 RNA polymerase (details in Table 2). Antisense rbbp4 riboprobe was amplified from cDNA using a forward rbbp4 primer 5’-CAGGCCCTTTAGTGAGGGTTAATTcacactgcagaggtcaactg-3’ with a T7 tag (T7 sequence in capitals), and a reverse primer for rbbp4 5’-CAGG TAATACGACTCACTATAGGG-cctgaacctcagtgtctgct-3’ tagged with a T7 polymerase sequence (T7 sequence in capitals). Template cDNA was generated by reverse-transcribing RNA which was isolated from adult whole fish tissue. After PCR clean-up T7 RNA polymerase was used to prepare the RNA probe. The probe is used for WISH shown in Figs 1B, 2, 3, and 4.
Table 2. Whole mount in situ hybridization probes from plasmids.
| Gene name | Clone number | Plasmid | Accession number | restriction enzyme for linearization |
polymerase for IVT |
Used in Figure |
|---|---|---|---|---|---|---|
| ezh2 | IRAKp961B17283Q | pME18S-FL3 | AW175260, BC124588, AW170898 | NotI, NheI | SP6 | 1, 2, 3, 4, 5 |
| eed | IRBOp991D1166D | pME18S-FL3 | CO935796, BC093351 | KpnI, XbaI | SP6 | 1, 2, 3, 4, 5 |
| suz12a | IRALp962D0460Q | pDNR-LIB | BC078293, CK679253 | EcoRI, SpeI | T7 | 1, 2, 3, 4, 5 |
| phc2a | IRAKp961K13101Q | pME18S-FL3 | AW422704, BC044345, AW343587 | BamHI | T3 | 1, 2, 3, 4, 5 |
| rnf2 | IRBOp991A1115D | pME18S-FL3 | BF157635, BC044472, BG302967 | HindIII | T7 | 1 |
| bmi1a | IRBOp991C0922D | pME18S-FL3 | BC049423, BM957934, BM958248 | BamHI, EcoRV | T3 | 1, 4, 5 |
| pcfg6 | IRAKp961I23304Q | pExpress-1 | EE326287, BC139554 | EcoRI | T7 | 4 |
| ezh1 | IRBOp991F1276D | pExpress-1 | CN509434, BC114282 | XhoI, BamHI | SP6 | 1, 2, 3, 4 |
| pcgf1 | n.a. | SPT18 | BC134012.1 | NarI | T7 | 1 |
| pcgf5a | n.a. | SPT19 | BC163687.1 | HindIII | T7 | 1 |
| pcgf5b | n.a. | SPT20 | BC116619.1 | NarI | T7 | 1 |
IVT: in vitro transcription, n.a: not applicable
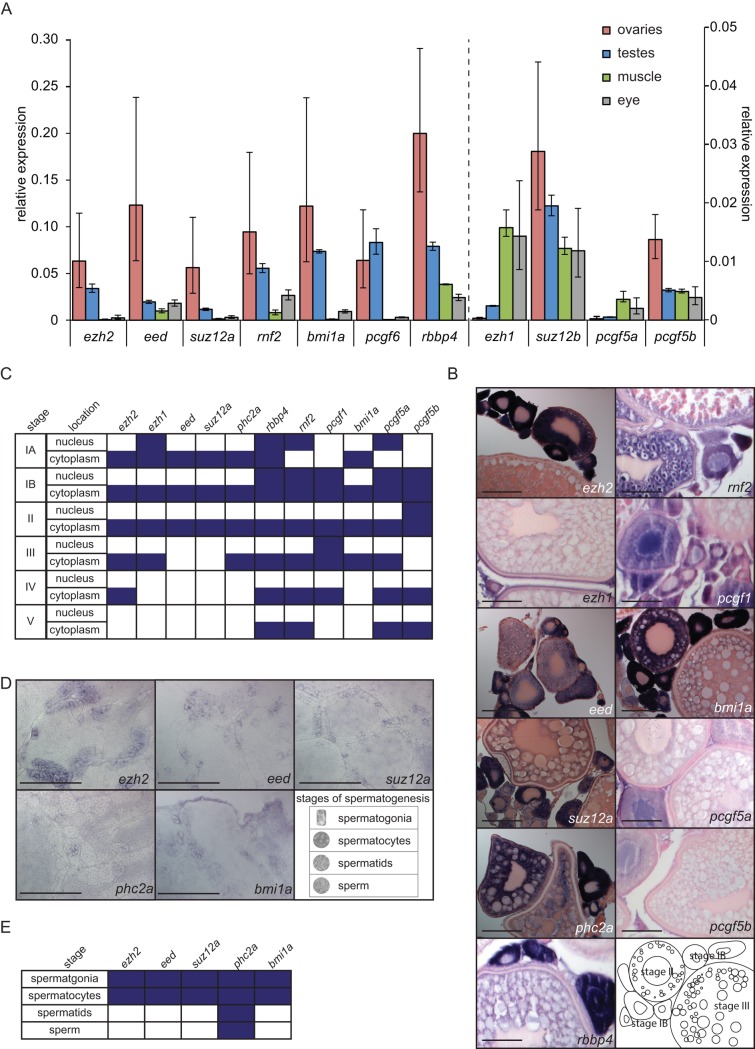
Fig 1. Expression of the majority of the PcG genes that were tested is enriched in the adult germ line.
(A) Relative expression assessed by RT-qPCR for ezh2, eed, suz12a, rnf2, bmi1a, pcgf6, and rbbp4 and for the lower expressed genes ezh1, suz12b, pcgf5a, and pcgf5b in the adult ovary and testis and two somatic tissues: muscle and eye. Relative expression to the reference gene rsp18 is shown. Data based on three biological replicates and three technical replicates. Error bars indicate standard deviation. (B) Spatio-temporal expression of ezh2, ehz1, eed, suz12a, phc2a, rbbp4, rnf2, pcgf1, bmi1a, pcgf5a, pcgf5b, and pcgf6 in adult ovaries. Lower right panel shows a schematic representation of stage IB, II, and III of oogenesis. Stages of oogenesis are assessed according to Selman et al. [32]. Scale bar: 100 μm. (C) Expression of the PcG genes that were tested in different stages of oogenesis. Purple boxes indicate expression of the corresponding gene at that stage of oogenesis. Stages of oogenesis are assessed according to Selman et al. [32]. (D) Spatio-temporal expression of ezh2, eed, suz12a, phc2a, and bmi1a in adult testes. Lower right panel shows examples of the four stages of spermatogenesis. Scale bar: 100 μm. (E) Expression of the PcG genes that were tested in different stages of spermatogenesis. Purple boxes indicate positive expression of the corresponding gene at that stage of spermatogenesis. Stages of spermatogenesis are determined according to Leal et al. [33].
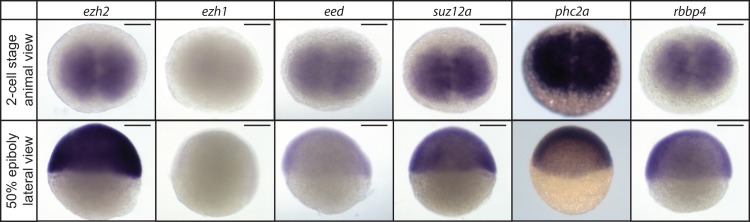
Fig 2. Expression of PRC2 members at early stages of embryonic development.
Spatio-temporal expression assessed by whole mount in situ hybridization of ezh2, ezh1, eed, suz12a, phc2a and rbbp4, at the 2-cell stage (0.75 hpf) and 50% epiboly (5.3 hpf). Scale bar: 200 μm.
Fig 3. Expression of PRC2 members at 1 dpf.
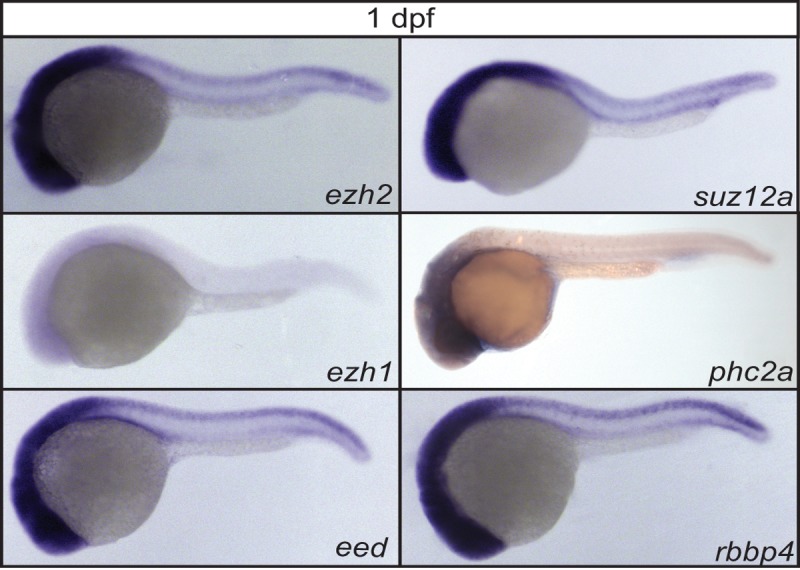
Lateral view of the spatio-temporal expression assessed by whole mount in situ hybridization of the PRC2 members: ezh2, ezh1, eed, suz12a, phc2a, and rbbp4, at 1 dpf zebrafish embryos.
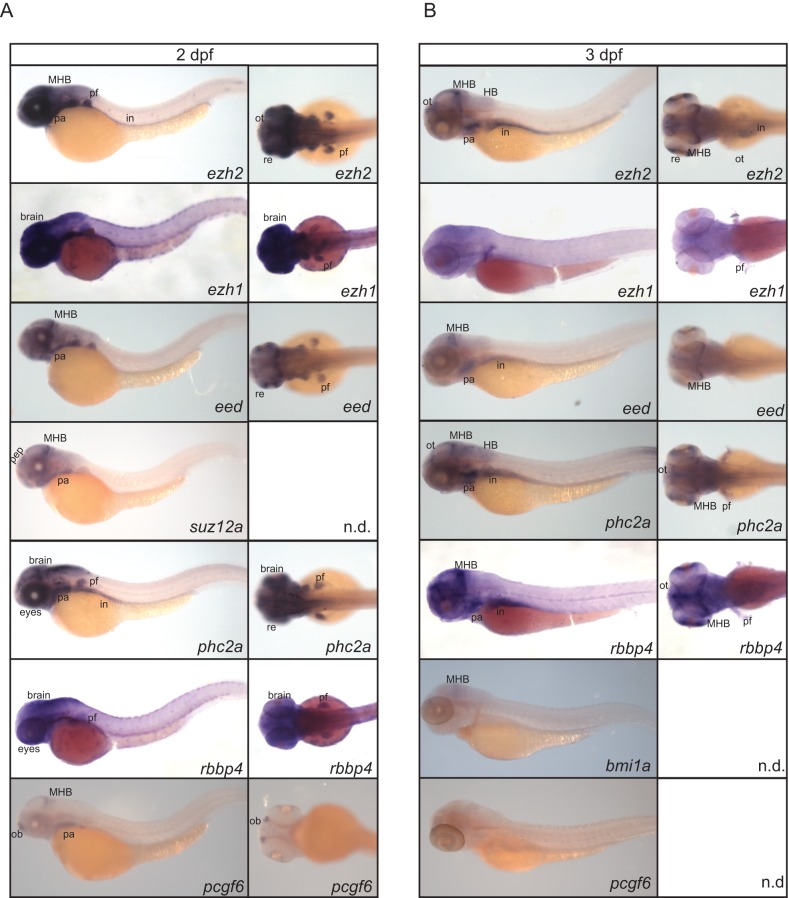
Fig 4. Expression of PcG genes at 2 and 3 dpf.
(A) Spatio-temporal expression assessed by whole mount in situ hybridization of ezh2, ezh1, eed, suz12a, phc2a, rbbp4, and pcgf6 at 2 dpf. Lateral views are shown for all genes. Dorsal views of ezh2, ehz1, eed, phc2a, and rbbp4. Ventral view of pcgf6. (B) Spatio-temporal expression assessed by whole mount in situ hybridization of ezh2, ezh1, eed, phc2a, rbbp4, bmi1a, and pcgf6 at 3 dpf. Lateral views are shown for all genes. Dorsal views of ezh2, ezh1, eed, phc2a, and rbbp4. in: intestine, pf: pectoral fin (buds), HB: hindbrain, MHB: mid-hind brain boundary, pep: presumptive epiphysis, pa: pharyngeal arches 3–7, ot: optic tectum, re: retina, ob: olfactory bulb, n.d. = no data.
Whole mount In Situ hybridization (WISH)
Embryos (2-cell stage or 50% epiboly) were fixed overnight at 4°C in 4% PFA (Aurion, 151710) in PBST (PBS with 0.1% Tween-20), after which they were washed with PBST, dechorionated, and gradually transferred to 100% methanol. Embryos of 1, 2, and 3 dpf were dechorionated when needed and subsequently fixed overnight at 4°C in 4% PFA in PBST, after which they were washed with PBST and gradually transferred to 100% methanol. Prior to WISH, embryos were transferred back to PBST. WISH was performed as described previously [28,29]. The embryos were mounted in methylcellulose (4%) and imaged by light microscopy on a Leica MZFLIII, with a Leica DFC450 camera or on a Leica DM2500 microscope with a Leica DFC7000T camera.
WISH on gonads
Adult gonads were dissected from euthanized adult zebrafish [27] and fixed overnight at 4°C in 4% PFA in PBST, after which they were gradually transferred to 100% methanol in steps of 1 hour. Gonads were transferred to PBST before starting WISH. WISH on gonads was performed as described previously [28,30]. After stopping the staining reaction, gonads were stored in 100% methanol.
Histology
After WISH, gonads (ovaries and testis) were dehydrated stepwise with ethanol and xylene. Subsequently, gonads were transferred to plastic or paraffin overnight. The following day tissues were embedded in plastic or paraffin and sectioned (5 μm for testis and 10 μm for ovaries). Gonads on which WISH for ezh2, eed, suz12a, phc2a, or bmi1a was performed, were embedded in plastic and ovaries on which WISH for ezh1, rbbp4, rnf2, pcgf1, pcgf5a, or pcgf5b was performed, were embedded in paraffin. For counterstaining of ovaries neutral red (Sigma-Aldrich, 0.1%) (plastic sections) or nuclear fast red (N3020, Sigma-Aldrich) (paraffin sections) was used. Slides were dewaxed (3x5 minutes xylene, 2x1 minute 100% alcohol, 2x1 min. 95% alcohol, 1 min. 70% alcohol, 1 min. 50% alcohol, 1 minute H2O) and incubated for 10 minutes in 0.1% neutral red or 0.1% nuclear fast red [30,31]. Sections were washed briefly with water, dehydrated with xylene, and covered with a cover slip using permount or Depex for future imaging. Images of plastic embedded tissue were made using a Zeiss Axioplan microscope equipped with a Zeiss Axiocam digital camera. Paraffin sections were imaged using a Leica DM2500 microscope with a Leica DFC7000T camera.
Results
PcG expression is enriched in the adult germ cells over somatic tissue
The expression level of the PcG genes ezh2, ezh1, eed, suz12a, suz12b, rbbp4, bmi1a, pcgf5a, pcgf5b, pcgf6, and rnf2 was assessed by RT-qPCR from adult male and female gonads and two somatic tissues: muscle and eye. The expression of the reference gene rsp18 was used for normalization. Enrichment in gene expression of ezh2, eed, suz12a, rnf2, bmi1a, pcgf6, rbbp4, suz12b, and pcgf5b was detected in the germ line over somatic tissue (Fig 1A, S1 Table). As a next step, we investigated the expression of a selection of PcG genes in adult germ cells in more detail using whole mount in situ hybridization (WISH, Fig 1B–1E).
Oogenesis encompasses 6 different stages of oocytes [32]. Assessment of the different stages was performed based on morphology [32]. Eleven different PcG genes were tested in the adult ovary (Fig 1B). mRNA expression in the cytoplasm and nucleus throughout the different stages of oogenesis is summarized in Fig 1C. In stage IA oocytes, which are cells in the pre-follicle phase, we detected ezh2, ezh1, eed, suz12a, phc2a, rbbp4, and bmi1a in the cytoplasm. Only ezh1, rbbp4, rnf2, and pcgf5a were detected in the nucleus at stage IA. In stage IB oocytes the expression of rbbp4, rnf2, pcgf1, pcgf5a, and pcgf5b was detected in both the nucleus and cytoplasm. The expression of ezh2, ezh1, eed, suz12a, phc2a, and bmi1a was only visible in the cytoplasm. Stage II oocytes showed expression of all PcG genes tested in their cytoplasm. Additionally, pcgf5b was also detected in the nuclei of stage II oocytes. In stage III-V expression of the majority of the PcG genes we tested is not detected in the nucleus. Only pcgf1 mRNA was detected in stage III oocytes. The PcG genes ezh2, ezh1, phc2a, rbbp4, rnf2, pcgf1, bmi1a, and pcgf5a show expression in the cytoplasm of stage III oocytes. In stage IV expression of ezh2, rbbp4, rnf2, pcgf1, pcgf5a, and pcgf5b was detected in the cytoplasm. Lastly, rbbp4, rnf2, pcgf5a, and pcgf5b expression was detected in the cytoplasm of stage V oocytes.
Additionally, we tested the expression of ezh2, eed, suz12a, phc2a, and bmi1a in adult testes. We discriminated between four different stages of spermatogenesis according to the morphology of the cells, as described by Leal et al. [33] Early stages of spermatogenesis, the spermatogonia and spermatids showed expression of ezh2, eed, suz12a, phc2a, and bmi1a (Fig 1D and 1E). Mature stages of spermatogenesis: spermatids and sperm showed expression of phc2a (Fig 1D and 1E).
The majority of PRC2 genes is maternally provided
To determine whether components of PRC2 are maternally provided and therefore present before zygotic genome activation (ZGA) we performed whole mount in situ hybridization on 2-cell stage embryos (0.75 hpf; Fig 2). We observed that ezh2, eed, suz12a, phc2a and rbbp4, are maternally provided to the embryo, but there was no detectable presence of ezh1 mRNA. In addition, we also studied the gene expression of these PRC2 components after ZGA, at 50% epiboly, and observed ubiquitous expression for ezh2, eed, suz12a, phc2a, and rbbp4 (5.3 hpf; Fig 2). We did not detect expression of ezh1 at 50% epiboly.
Expression of a selection of PcG genes during tissue specification
To investigate the expression patterns of a selection of PcG genes during tissue specification, we next performed whole mount in situ hybridization at 1, 2, and 3 dpf. Whereas the expression of the majority of the PcG genes we tested, except for ezh1, was ubiquitously present at early stages (2-cell and 50% epiboly), the expression becomes more anteriorly enriched at 1 dpf (Fig 3). The expression of ezh2, eed, suz12a, phc2a, and rbbp4 was clearly visible. However, mRNA expression of ezh1 was detected at a very low level at the anterior side of the embryo.
Spatial restriction of the expression of this selection of PcG genes becomes more apparent at 2 and 3 dpf (Fig 4). At 2 dpf the transcripts of ezh2, ezh1, eed, suz12a, phc2a, rbbp4, and pcgf6 were enriched in brain tissue, such as the mid-hindbrain barrier, and expressed in the pharyncheal arches (Fig 4A). Furthermore, ezh2, ezh1, eed, rbbp4, and phc2a are expressed in the pectoral fin buds and both ezh2 and phc2a were detected in the intestine at 2 dpf (Fig 4A). The PRC2-components ezh2, eed, rbbp4, and phc2a are expressed in the intestine at 3 dpf. At 3 dpf ezh2, eed, phc2a, rbbp4, and bmi1a were expressed in the mid-hindbrain barrier. Overall, enrichment of anterior expression was observed for ezh2, eed, phc2a, and rbbp4 at 3 dpf (Fig 4B).
The expression of a selection of PcG genes during germ cell development is detectable as from 4 weeks post fertilization
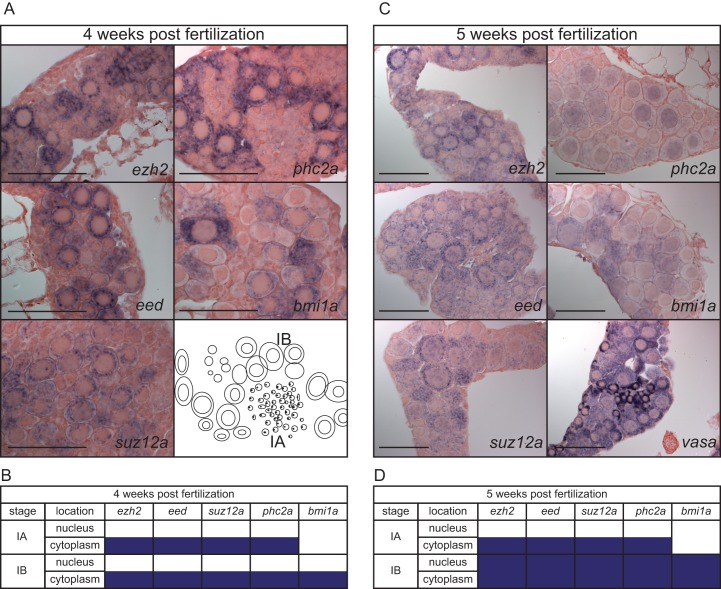
In adult gametes expression of the tested PcG genes was detected, but we did not observe them in primordial germ cells at 1, 2, or 3 dpf (Figs 3 and 4). To determine when these PcG genes start to be expressed in the developing germ line, we performed whole mount in situ hybridization at gonads from 3 to 5 weeks post fertilization for a subset of PcG genes. We were not able to detect the expression of these PcG genes at 3 weeks post fertilization in the developing gonads. At 4 weeks post fertilization we detected the expression of ezh2, eed, suz12a, and phc2a in the cytoplasm of stage IA oocytes. Furthermore, all genes tested were detected in the cytoplasm of stage IB oocytes (Fig 5A and 5B). We additionally tested expression of ezh2, eed, suz12a, phc2a, and bmi1a, in presumptive ovaries at 5 weeks post fertilization, and expression was observed for all, except bmi1a, in the cytoplasm of the stage IA oocytes. At 5 weeks post fertilization, all PcG genes tested were detected in both the nucleus and the cytoplasm of stage IB oocytes. As a control we used vasa, a germ cell marker (Fig 5C).
Fig 5. Expression of PcG genes in developing gonads.
(A) Spatio-temporal expression assessed by whole mount in situ hybridization of ezh2, eed, suz12a, phc2a, and bmi1a at 4 weeks post fertilization. (B) Expression patterns of ezh2, eed, suz12a, phc2a, and bmi1a, in gonads at 4 weeks post fertilization. Purple boxes indicate expression of the corresponding gene at that stage of oogenesis. (C) Spatio-temporal expression assessed by whole mount in situ hybridization at 5 weeks post fertilization of ezh2, eed, suz12a, phc2a, bmi1a, and the germ cell marker vasa. (D). Expression of ezh2, eed, suz12a, phc2a, and bmi1a, at 5 weeks post fertilization. Purple boxes indicate expression of the corresponding gene at that stage of oogenesis. Stages of oogenesis are assessed according to Selman et al. [32].
Discussion
PcG genes are expressed in the germ line of adult zebrafish
Safeguarding germ cell fate is extremely important since germ cells form the basis for the existence and propagation of a species. Therefore, correct gene expression must be maintained in germ cells. Amongst the mechanisms that are reported for this maintenance are transcriptional repression, chromatin state protection, and protection of genome integrity [34]. Transcriptional repression is described to be an important event and therefore investigating the expression of PcG genes in adult gametes as well as in developing gonads will give us insight in the potential role of PcG-mediated gene repression in zebrafish germ cells. In this study we found that expression of nine out of eleven PcG genes that were tested is enriched in the germ line over somatic tissue in adult zebrafish. The mRNA levels of ezh1 and pcgf5a are higher in eye and muscle tissue, compared to gonad tissue. Zygotes are reported to not have maternal load of ezh1 and pcgf5a [24]. The lack of maternal load for these genes could be a correlated to our observation of low expression of these genes in adult gonads [24].
Because germ cells have the capacity to form a totipotent zygote upon fertilization, it is expected that germ cells remain in a relatively quiescent state compared to somatic cells [34]. Germ cells can be regarded as a stem cell-like model. They both have the potential to give rise to all different cell types. PcG gene expression is associated to stem cell maintenance [35]. Detailed spatio-temporal expression analysis of eleven different PcG genes in ovaries indicates that stage IB oocytes show mRNA expression of PRC1 members in both the nucleus and the cytoplasm which is in contrast to the mature stages of the oogenesis, where only rbbp4, rnf2, pcgf5a, and pcgf5b mRNAs are detected in the cytoplasm. The other genes tested were not detected in stage V oocytes. PcG proteins act as transcriptional repressors and since we mainly observe their expression in stage I-II oocytes, we hypothesize that transcription is actively repressed by PcG proteins in early stage oocytes. These early stage oocytes can be considered as the germ line stem cells. The presence of PcG genes in these early stages of oogenesis suggests that especially these cells undergo chromatin remodeling and changes in the epigenome.
In later stages of oogenesis we did not detect the mRNA of the tested PcG genes in the nucleus. In these mature oocytes we are often also not able to detect PcG gene expression in the cytoplasm. One can regard the content of the cytoplasm as the maternal load that is transmitted to the zygote. We analyzed the expression of the PRC2 members by whole mount in situ hybridization at the 2-cell stage. We found ezh2, eed, suz12a, phc2a, and rbbp4 to be present, and did not detect ezh1. Since zygotic genome activation occurs after the 2-cell stage, this means that the PRC2 members tested, except ezh1, are maternally loaded. The RT-qPCR results also indicate that ezh1 is expressed at low levels in the gonads. The transcripts of PRC1 components rnf2, pcgf1, bmi1a, bmi1b, pcgf5a, and pcgf6 were also reported to be maternally loaded [4,13]. The PRC1 component pcgf5b was reported not to be present at the 2-cell stage [4]. Stage V oocytes are the basis for the zygote and therefore the PcG transcripts that are maternally provided are also expected to be present in the cytoplasm of stage V oocytes. However, this is not what we consistently observed. A potential explanation for why ezh2, eed, suz12a, phc2a, pcgf1, and bmi1a are not observed in stage V oocytes could be that the mRNA has been diluted, and is therefore not detectable by WISH, in these large stage V oocytes.
Ezh2 is maternally loaded and we find this mRNA also to be present in the germ line. Likely, during germ line development, Ezh2 plays a role in regulating transcription. Therefore, it was unexpected that zebrafish with an ezh2 mutant germ line are fertile [11]. Ezh2 inhibitors are sometimes used as anti-cancer drugs and pharmacological inhibition of Ezh2 in the murine germ line resulted in depletion of H3K27me3 [18,36]. What the effects are on fertility is an important remaining question.
The testis, especially the early stages of spermatogenesis, the spermatogonia and spermatocytes, showed expression of ezh2, eed, suz12a, phc2a, and bmi1a. The analogy of presence of PcG genes in the early stages of gametogenesis, in both the female and male germ line, hints towards a conserved mechanism that requires PcG protein functioning in early gametogenesis.
Role of PcG genes in embryogenesis
Once the stage V oocyte is fertilized, a zygote develops with maternal load consisting of a number of mRNA transcripts. Based on published whole mount in situ hybridization experiments, PRC1 members rnf2 and pcgf1-6, except pcgf5b, are maternally loaded [4,13]. This contradicts the results of pcgf5a and pcgf5b expression during development as was found by a high-resolution mRNA expression time course of embryonic development in zebrafish [24]. The expression of pcgf5a is reported to be absent until 75% epiboly and expression is first observed at the 1–4 somite [24]. Before ZGA expression of pcgf5b is already observed, when analyzed by RNA-seq. A potential explanation for this finding is the high similarity between these two transcripts, which could make distinguishing them problematic, both by RNA-sequencing as well as by whole mount in situ hybridization.
At early stages of embryogenesis the PRC2 members were ubiquitously expressed, except ezh1, which was not detected. The expression of ezh2 is similar to previously published [11]. The majority of the genes that we tested by whole mount in situ hybridization at 1, 2, and 3 dpf are detected in the brain regions, especially in the mid-hindbrain barrier. The pattern of PcG expression presented here shows high resemblance with the expression of the proliferation marker pcna [37]. PcG genes are described to enhance proliferation and the overlap in expression patterns found with the pattern of pcna could suggest that PcG genes are expressed in regions that are highly proliferative [38].
Some PcG genes are duplicated in the zebrafish genome, this includes the previously discussed pcgf5 gene [5]. Another example is the PRC1 component bmi1; the two paralogues were reported to have different expression patterns [4]. The phc2 gene is also duplicated. Additionally, there are two isoforms of phc2a described, which adds an extra layer of complexity to studying these type of genes [6]. The phc2a probe we have used for our analysis does not distinguish between these isoforms. The different paralogues of the PcG genes and the possibility of different isoforms should be taken into account when studying these genes in zebrafish.
The data presented in this study can contribute to our understanding of explaining the phenotypes of PcG gene mutants in zebrafish reported so far. For instance, we detect ezh2 expression in the pectoral fin buds, brain region, especially at the mid-hind brain barrier, and the intestinal tract. The MZezh2 mutants lack pectoral fins, and show malformation of the head, which could indicate a need for Ezh2 for proper outgrow of these tissues [11]. Normally during embryonic development, the gut is formed around 5 dpf. In this study, we could detect expression of ezh2 at 2 and 3 dpf in the presumptive gut tissue. Additionally, Dupret et al. reported intestinal defects in zygotic ezh2 mutant zebrafish [12]. PRC1 was also implicated to play a role in pectoral fin development, as zygotic rnf2 mutants show defects in fin bud outgrowth, due to incomplete activation of the Fgf signaling pathway. In these mutants, initial specification from presumptive pectoral fin precursors is correctly initiated. This observation confirms the view that PcG genes are involved in terminal differentiation of different tissue types [39]. Since eed and phc2a expression is detected in the pectoral fin buds at 2 dpf, we hypothesize that eed and phc2a zebrafish mutants would also lack pectoral fins.
The enzymatic subunit of PRC2 during zebrafish development
PRC2 contains one enzymatic subunit, Ezh1 or Ezh2, which are mutually exclusive [40,41]. The expression pattern of ezh1 is visually different from the other PRC2 components, since ezh1 seems to be the only PRC2 member which is not maternally loaded. Additionally, ezh1 cannot be detected at 2-cell stage and does not seem to be expressed up until 50% epiboly. This suggests that this gene is not activated at ZGA. A similar observation was made by Sun et al.; the expression of ezh2 was detected at 0.75 hpf (2-cell stage), 2 hpf, and 4 hpf, but no expression of ezh1 was observed at these stages [25]. At 1 dpf we observed ezh1 expression at low levels. Similar data about expression of ezh1 were also found in a high-resolution mRNA expression time course of embryonic development in zebrafish [24]. WISH at 2 and 3 dpf for ezh1 indicates an expression pattern that is enriched in the head region and the pectoral fins. At 5 dpf ezh1 expression is clearly detected in the RNA-sequencing dataset [24]. The expression levels in the study of White et al. show that at 1 dpf the levels are roughly 47 times higher for ezh2 compared to ezh1 [24]. The current view on Ezh1/Ezh2 is that Ezh2 is incorporated in PRC2 in cells that are pluripotent, such as embryonic stem cells. Ezh1 is believed to be present in adult tissue and non-proliferative cells [40,41]. Our observation of enrichment of ezh1 expression in muscle and eye tissue over gonad tissue is in line with this view. The cells of a 2-cell stage embryo have the potential to grow into all different cell types, which resembles a stem-cell like state, and this could explain why ezh2 is maternally loaded and ezh1 is not. Only at later stages of development ezh1 expression is detected. These observations support the hypothesis that Ezh2 and Ezh1 are part of PRC2 in less or more differentiated cells, respectively.
Summary and future perspectives
In this study we have shown the expression of a selection of PcG genes in the germ line and during early embryonic development. A multitude of the PcG genes that we tested showed enrichment in the germ line over somatic tissue. We also found that especially early stages of gametogenesis showed expression of the PcG genes that we tested. During zebrafish embryonic development the majority of PcG genes were also detected, this is a ubiquitous expression in the early stages and becomes more anteriorly enriched from 1 dpf onwards. The observations described here underline the likelihood for a role of PcG genes in specification of multiple lineages, including the germ line. Mutants are pivotal to study the function of a gene or multiple genes during development. Zebrafish are more frequently used to study epigenetics and some PcG mutants are already generated in the Sanger Institute and available via Zebrafish International Resourc Center (ZIRC). Nowadays, new zebrafish mutants are relatively easily generated using the CRISPR-Cas9 system [42]. This system allows for targeted mutations and provides opportunities to further study the role and function of PcG proteins in zebrafish embryonic and adult development. One could make mutants for the different paralogues of PcG genes and aim to mutate the different isoforms. Our results serve as an important resource of information on the expression patterns of a selection of PcG genes during embryonic and germ line development. This contributes to our understanding of the role of the PcG proteins, which were tested here, in embryogenesis and germ line development. Follow-up studies need to be performed in order to obtain detailed insights.
Supporting information
(XLSX)
Acknowledgments
The authors thank Bob Vlekke, Jeroen Korving, Huib Croes, Marco Betist, and the animal care takers for technical support. We thank Prof. dr. Pierre-Olivier Angrand for providing the pcgf1, pcgf5a, and pcgf5b plasmids for WISH probe generation. We thank Anna-Pavlina Haramis for providing the rnf2 plasmid for WISH probe generation. We would also like to express our gratitude to Marjo den Broeder from Brunel University, for extensive input about the project and critically reading the manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information file.
Funding Statement
The work was funded by the Innovative Research Scheme of the Netherlands Organisation for Scientific Research (www.nwo.nl, NWO-Veni 916.96.021, NWO-Vidi 864.12.009, NWO-Meervoud 836.13.003, L.M.K.), the Radboud University Nijmegen Medical Centre tenure track fellowship (www.radboudumc.nl, L.M.K.), and the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie Grant Agreement No. 705939 (K.A.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Li G, Reinberg D. Chromatin higher-order structures and gene regulation. Curr Opin Genet Dev. Elsevier Ltd; 2011;21: 175–186. doi: 10.1016/j.gde.2011.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lewis EB. A gene complex controlling segmentation in Drosophila. Nature. 1978;276: 565–70. [DOI] [PubMed] [Google Scholar]
- 3.Bracken AP, Helin K. Polycomb group proteins: navigators of lineage pathways led astray in cancer. Nat Rev Cancer. Nature Publishing Group; 2009;9: 773–84. doi: 10.1038/nrc2736 [DOI] [PubMed] [Google Scholar]
- 4.Dupret B, Völkel P, Bourhis X Le, Angrand PO. The Polycomb Group Protein Pcgf1 Is Dispensable in Zebrafish but Involved in Early Growth and Aging. PLoS One. 2016;11 doi: 10.1371/journal.pone.0158700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Le Faou P, Völkel P, Angrand P-O. The zebrafish genes encoding the Polycomb repressive complex (PRC) 1. Gene. 2011;475: 10–21. doi: 10.1016/j.gene.2010.12.012 [DOI] [PubMed] [Google Scholar]
- 6.Völkel P, Le Faou P, Vandamme J, Pira D, Angrand P-O. A human Polycomb isoform lacking the Pc box does not participate to PRC1 complexes but forms protein assemblies and represses transcription. Epigenetics. Taylor & Francis; 2012;7: 482–491. doi: 10.4161/epi.19741 [DOI] [PubMed] [Google Scholar]
- 7.Whitcomb SJ, Basu A, Allis CD, Bernstein E. Polycomb Group proteins: an evolutionary perspective. Trends Genet. 2007;23: 494–502. doi: 10.1016/j.tig.2007.08.006 [DOI] [PubMed] [Google Scholar]
- 8.Sowpati DT, Ramamoorthy S, Mishra RK. Expansion of the polycomb system and evolution of complexity. Mech Dev. Elsevier B.V; 2015;138: 97–112. doi: 10.1016/j.mod.2015.07.013 [DOI] [PubMed] [Google Scholar]
- 9.Voncken JW, Roelen BAJ, Roefs M, de Vries S, Verhoeven E, Marino S, et al. Rnf2 (Ring1b) deficiency causes gastrulation arrest and cell cycle inhibition. Proc Natl Acad Sci U S A. 2003;100: 2468–73. doi: 10.1073/pnas.0434312100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Carroll D, Erhardt S, Pagani M, Barton SC, Surani MA, Jenuwein T. The polycomb-group gene Ezh2 is required for early mouse development. Mol Cell Biol. 2001;21: 4330–6. doi: 10.1128/MCB.21.13.4330-4336.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.San B, Chrispijn ND, Wittkopp N, Van Heeringen SJ, Lagendijk AK, Aben M, et al. Normal formation of a vertebrate body plan and loss of tissue maintenance in the absence of ezh2. Sci Rep. 2016;6. doi: 10.1038/srep24658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dupret B, Völkel P, Vennin C, Toillon R-A, Le Bourhis X, Angrand P-O. The histone lysine methyltransferase Ezh2 is required for maintenance of the intestine integrity and for caudal fin regeneration in zebrafish. Biochim Biophys Acta—Gene Regul Mech. 2017;1860: 1079–1093. doi: 10.1016/j.bbagrm.2017.08.011 [DOI] [PubMed] [Google Scholar]
- 13.van der Velden YU, Wang L, van Lohuizen M, Haramis A-PG. The Polycomb group protein Ring1b is essential for pectoral fin development. Development. 2012;139: 2210–20. doi: 10.1242/dev.077156 [DOI] [PubMed] [Google Scholar]
- 14.van der Velden YU, Wang L, Querol Cano L, Haramis A-PG. The polycomb group protein ring1b/rnf2 is specifically required for craniofacial development. PLoS One. 2013;8: e73997 doi: 10.1371/journal.pone.0073997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holdeman R, Nehrt S, Strome S. MES-2, a maternal protein essential for viability of the germline in Caenorhabditis elegans, is homologous to a Drosophila Polycomb group protein. Development. 1998;125: 2457–67. [DOI] [PubMed] [Google Scholar]
- 16.Bender LB, Cao R, Zhang Y, Strome S. The MES-2/MES-3/MES-6 Complex and Regulation of Histone H3 Methylation in C. elegans. Curr Biol. 2004;14: 1639–1643. doi: 10.1016/j.cub.2004.08.062 [DOI] [PubMed] [Google Scholar]
- 17.Iovino N, Ciabrelli F, Cavalli G. PRC2 Controls Drosophila Oocyte Cell Fate by Repressing Cell Cycle Genes. Developmental Cell. 2013.. doi: 10.1016/j.devcel.2013.06.021 [DOI] [PubMed] [Google Scholar]
- 18.Prokopuk L, Stringer JM, Hogg K, Elgass KD, Western PS. PRC2 is required for extensive reorganization of H3K27me3 during epigenetic reprogramming in mouse fetal germ cells. Epigenetics Chromatin. 2017;10: 7 doi: 10.1186/s13072-017-0113-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Posfai E, Kunzmann R, Brochard V, Salvaing J, Cabuy E, Roloff TC, et al. Polycomb function during oogenesis is required for mouse embryonic development. Genes Dev. 2012;26: 920–32. doi: 10.1101/gad.188094.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Draper BW. Id entification of Germ-Line Stem Cells in Zebrafish Methods in molecular biology (Clifton, NJ: ). 2017. pp. 103–113. doi: 10.1007/978-1-4939-4017-2_8 [DOI] [PubMed] [Google Scholar]
- 21.Raz E. Primordial germ-cell development: the zebrafish perspective. Nat Rev Genet. 2003;4: 690–700. doi: 10.1038/nrg1154 [DOI] [PubMed] [Google Scholar]
- 22.Knaut H, Pelegri F, Bohmann K, Schwarz H, Nüsslein-Volhard C. Zebrafish vasa RNA but not its protein is a component of the germ plasm and segregates asymmetrically before germline specification. J Cell Biol. 2000;149: 875–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lindeman LC, Andersen IS, Reiner AH, Li N, Aanes H, Østrup O, et al. Prepatterning of Developmental Gene Expression by Modified Histones before Zygotic Genome Activation. Dev Cell. 2011;21: 993–1004. doi: 10.1016/j.devcel.2011.10.008 [DOI] [PubMed] [Google Scholar]
- 24.White RJ, Collins JE, Sealy IM, Wali N, Dooley CM, Digby Z, et al. A high-resolution mRNA expression time course of embryonic development in zebrafish. Elife. eLife Sciences Publications Limited; 2017;6: e30860 doi: 10.7554/eLife.30860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun X-J, Xu P-F, Zhou T, Hu M, Fu C-T, Zhang Y, et al. Genome-Wide Survey and Developmental Expression Mapping of Zebrafish SET Domain-Containing Genes. Hoheisel J, editor. PLoS One. Public Library of Science; 2008;3: e1499 doi: 10.1371/journal.pone.0001499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203: 253–310. doi: 10.1002/aja.1002030302 [DOI] [PubMed] [Google Scholar]
- 27.Gupta T, Mullins MC. Dissection of Organs from the Adult Zebrafish. J Vis Exp. 2010; doi: 10.3791/1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Houwing S, Kamminga LM, Berezikov E, Cronembold D, Girard A, van den Elst H, et al. A role for Piwi and piRNAs in germ cell maintenance and transposon silencing in Zebrafish. Cell. 2007;129: 69–82. doi: 10.1016/j.cell.2007.03.026 [DOI] [PubMed] [Google Scholar]
- 29.Thisse C, Thisse B. High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat Protoc. 2008;3: 59–69. doi: 10.1038/nprot.2007.514 [DOI] [PubMed] [Google Scholar]
- 30.Chambers EL, Gosden RG, Yap C, Picton HM. In situ identification of follicles in ovarian cortex as a tool for quantifying follicle density, viability and developmental potential in strategies to preserve female fertility. Hum Reprod. 2010;25: 2559–2568. doi: 10.1093/humrep/deq192 [DOI] [PubMed] [Google Scholar]
- 31.Allison AC, Young MR. Uptake of dyes and drugs by living cells in culture. Life Sci. 1964;3: 1407–14. [DOI] [PubMed] [Google Scholar]
- 32.Selman K, Wallace RA, Sarka A, Qi X. Stages of oocyte development in the zebrafish,Brachydanio rerio. J Morphol. Wiley Subscription Services, Inc., A Wiley Company; 1993;218: 203–224. doi: 10.1002/jmor.1052180209 [DOI] [PubMed] [Google Scholar]
- 33.Leal MC, Cardoso ER, Nóbrega RH, Batlouni SR, Bogerd J, França LR, et al. Histological and Stereological Evaluation of Zebrafish (Danio rerio) Spermatogenesis with an Emphasis on Spermatogonial Generations1. Biol Reprod. 2009;81: 177–187. doi: 10.1095/biolreprod.109.076299 [DOI] [PubMed] [Google Scholar]
- 34.Strome S, Updike D. Specifying and protecting germ cell fate. Nat Rev Mol Cell Biol. Nature Publishing Group; 2015;16: 406–416. doi: 10.1038/nrm4009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laugesen A, Helin K. Chromatin repressive complexes in stem cells, development, and cancer. Cell Stem Cell. Elsevier Inc.; 2014;14: 735–751. doi: 10.1016/j.stem.2014.05.006 [DOI] [PubMed] [Google Scholar]
- 36.Prokopuk L, Hogg K, Western PS. Pharmacological inhibition of EZH2 disrupts the female germline epigenome. Clin Epigenetics. BioMed Central; 2018;10: 33 doi: 10.1186/s13148-018-0465-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koudijs MJ, den Broeder MJ, Keijser A, Wienholds E, Houwing S, van Rooijen EMHC, et al. The Zebrafish Mutants dre, uki, and lep Encode Negative Regulators of the Hedgehog Signaling Pathway. PLoS Genet. Public Library of Science; 2005;1: e19 doi: 10.1371/journal.pgen.0010019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Orlando V. Polycomb, Epigenomes, and Control of Cell Identity. Cell. Cell Press; 2003;112: 599–606. doi: 10.1016/S0092-8674(03)00157-0 [DOI] [PubMed] [Google Scholar]
- 39.Juan AH, Wang S, Ko KD, Zare H, Tsai P- F, Feng X, et al. Roles of H3K27me2 and H3K27me3 Examined during Fate Specification of Embryonic Stem Cells. Cell Rep. ElsevierCompany.; 2016;17: 1369–1382. doi: 10.1016/j.celrep.2016.09.087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Margueron R, Li G, Sarma K, Blais A, Zavadil J, Woodcock CL, et al. Ezh1 and Ezh2 Maintain Repressive Chromatin through Different Mechanisms. Mol Cell. 2008;32: 503–518. doi: 10.1016/j.molcel.2008.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shen X, Liu Y, Hsu Y-J, Fujiwara Y, Kim J, Mao X, et al. EZH1 mediates methylation on histone H3 lysine 27 and complements EZH2 in maintaining stem cell identity and executing pluripotency. Mol Cell. 2008;32: 491–502. doi: 10.1016/j.molcel.2008.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ata H, Clark KJ, Ekker SC. The zebrafish genome editing toolkit. Biophysical Methods in Cell Biology. Elsevier Ltd; 2016. doi: 10.1016/bs.mcb.2016.04.023 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information file.