Abstract
Objective
To investigate the relationship between fractional anisotropy (FA), a suggested biomarker for tissue integrity, and motor recovery in stroke patients following post-acute rehabilitation.
Design
Retrospective study.
Setting
Acute rehabilitation hospital.
Participants
43 subjects, 28 diagnosed with ischemic stroke, and 15 diagnosed with hemorrhagic stroke. The average age for subjects was (68±14 years).
Interventions
MRI and Diffusion Tensor Imaging (DTI) were conducted on all patients.
Main Outcome Measure(s)
This is a retrospective study. The admission and discharge motor sub-scores of the Functional Independence Measure (FIM) were obtained from medical records, and relative gain was calculated using the Montebello Rehabilitation Factor Score (MRFS). K-means cluster analysis (K=3) using both MRFS and gain of FIM motor sub-score (ΔFIM) was performed. ANOVA test was used to determine the difference in FA among the clusters. Spearman analysis was conducted to examine the relationship between FA, ΔFIM, and MRFS in each cluster.
Results
FA was significantly higher in the clusters of good and moderate recovery in the corticospinal tract (CST), peduncle, and posterior limb of the internal capsule (PLIC) bilaterally (all P<0.05) compared to the poor recovery group. Significant positive correlations were observed in multiple regions along CST between FA, ΔFIM, and MRFS in the clusters of good and moderate recovery, but not in the poor recovery group.
Conclusion
Our results showed an association between FA values within the corticospinal tract and motor recovery in stroke patients undergoing post-acute rehabilitation. This finding may help to identify novel targets for new interventions to promote stroke recovery.
Keywords: stroke, motor, DTI, FIM, MRFS
Introduction
Stroke is the leading cause of long-term disability in adults.1 Despite advances in stroke treatment and rehabilitation, more than 50% of stroke patients still have motor dysfunction at the end of rehabilitation.2 Although the severity of initial motor deficit is a strong predictor of poor functional recovery following stroke, the clinical evaluation is often limited by floor/ceiling effects of the tests and compensatory strategies used by the subjects. 2, 3
Diffusion Tensor Imaging (DTI) is a non-invasive MRI-based neuroimaging technique that has been used to evaluate structural changes in white matter following stroke.4, 5 Fractional anisotropy (FA), a DTI metric, is widely used in the literature to evaluate the degree of anisotropy or directional diffusion within the white matter fibers in the brain tissue.6, 7 Many studies have evaluated the predictive value of FA in chronic stroke recovery.8, 9
However, little is known about the relationship between white matter abnormalities and the potential for functional gains and recovery of patients who underwent early inpatient rehabilitation (3–49 days of stroke onset). To the best of our knowledge this is one of the early studies10, 11 that examines the relationship between FA and the clinical scales of rehabilitation earlier in stroke patients.
Therefore, our objective in this study is to investigate the relationship between FA within CST and functional gain in the early stages of stroke following inpatient rehabilitation. We hypothesize that decreased FA within CST in the early stages of stroke is associated with poor functional recovery when compared to patients with higher FA values, and FA within CST correlates positively with the functional gain of motor function.
Methods
Study participants
This is a retrospective study. Medical records for 1510 stroke patients admitted to an acute rehabilitation hospital in Boston between January 2012 and January 2015 were screened with the following inclusion criteria: age >18 years, MRI including DTI performed in the same scanning session within 2 weeks of stroke onset, and standard inpatient rehabilitation was ≥ 6 days. Demographic data including age, gender, diagnosis, side of hemiplegia, discharge destination, and stroke risk factors were recorded. Forty-three patients with ischemic or hemorrhagic stroke who met these criteria were enrolled in the study. This study was approved by the Institutional Review Board.
Clinical assessments
Functional Independence Measure (FIM) scale
The Functional Independence Measure (FIM) scale12 is widely used to evaluate the amount of assistance required for a person with disabilities to perform basic daily activities after stroke. The scale includes 18 items graded on a 7-point ordinal scale, with a maximum total score of 126. The motor subscale includes 13 items: eating; grooming; bathing; dressing upper extremity; dressing lower extremity; toileting; bowel management; bladder management; transfers to bed, chair or wheelchair; transfer to tub; transfer to toilet and shower; walking or wheelchair propulsion; and stair climbing. The possible scores ranged from 1 to 7 where a higher score indicates higher independence.
The motor sub-scores of FIM at admission and discharge were obtained from the medical records of our patients. The gain of FIM motor sub-score (ΔFIM), which is commonly used to evaluate functional recovery after stroke13, 14, was determined for every patient by the difference between FIM motor sub-score at discharge and FIM sub-score at admission.
Montebello Rehabilitation Factor Score (MRFS)
A second method to evaluate the relative gain in patients is MRFS15, 16. This method depends on the validated FIM score. According to this method, the basis for calculating relative gain is a patient’s specific potential for improvement (maximal possible FIM − actual admission FIM). The actual score ranges from 0 to 1, and MRFS can overcome the misinterpretation of the “ceiling effect.”
MRFS was calculated using the following formula:
K-means cluster analysis
We used the k-means clustering algorithm implemented in JMP PRO 12.0.1. We fed ΔFIM and MRFS scores together into this analysis. The k-means algorithm demands a predefined number of clusters (K). We visually inspected the clusters formed by k-means for k=2,3,4, and 5 using the data of ΔFIM and MRFS scores. We subjectively decided to separate the data into three clusters because the resultant clusters of (K=3) matched in age, gender, stroke location, type of stroke, and risk factors (Table 1). As a result, the subjects were divided into three groups: Cluster A, good recovery, 11 subjects; Cluster B, moderate recovery, 21 subjects; Cluster C, poor recovery, 11 subjects.
Table 1.
characteristics of patients in study groups
| Characteristics | Cluster A (n=11) | Cluster B (n=21) | Cluster C (n=11) | P value |
|---|---|---|---|---|
| Demographics | ||||
| Age | 67.8±3.36 | 70.1±2.66 | 65.2±3.67 | 0.386 |
| Gender (male) | 3(27.27%) | 12(57.14%) | 6(54.55%) | 0.250 |
| Education | ||||
| College | 6(54.55%) | 12(57.14) | 4(36.36%) | 0.518 |
| High school | 5(45.45%) | 9(42.86%) | 7(63.64%) | |
| Handedness (right) | 11(100%) | 19(90.48%) | 10(90.91%) | 0.574 |
| Medical history | ||||
| Smoking | ||||
| Current | 1(9.09%) | 2(9.52%) | 2(18.18%) | 0.734 |
| Past | 5(45.45%) | 7(33.33%) | 2(18.18%) | 0.392 |
| Hypertension | 9(81.82%) | 18(85.71%) | 8(72.73%) | 0.668 |
| Diabetes | 3(27.27%) | 7(33.33%) | 1(9.09%) | 0.325 |
| Hyperlipidemia | 4(36.36%) | 9(42.86%) | 5(45.45%) | 0.903 |
| CVA history | 4(36.36%) | 7(33.33%) | 1(9.09%) | 0.268 |
| CAD history | 1(9.09%) | 4(36.36%) | 1(9.09%) | 0.642 |
| Atrial fibrillation | 2(18.18%) | 8(72.73%) | 1(9.09%) | 0.164 |
| Clinical assessment | ||||
| Days from onset to admission | 9±5 | 13±10 | 18±16 | 0.196 |
| Days from onset to MRI | 3±3 | 3±2 | 4±5 | 0.334 |
| Length of stay | 22±15 | 25±15 | 28±14 | 0.606 |
| Discharge Destination | ||||
| Home | 8(72.73%) | 11(52.38%) | 1(9.09%) | 0.010 |
| Skilled nursing facility | 1(9.09%) | 7(33.33%) | 9(81.82%) | |
| Acute unit | 2(18.18%) | 3(14.29%) | 1(9.09%) | |
| FIM motor | ||||
| Admission | 30.36±15.55 | 28.81±8.52 | 14.82±5.88 | 0.001 |
| Discharge | 53.45±22.20 | 51.19±6.90 | 23.09±7.41 | 0.001 |
| MRFS | 0.53±0.09 | 0.26±0.08 | 0.06±0.07 | 0.001 |
| ΔFIM | 31.3±6.06 | 16.6±6.99 | 4.36±7.1 | 0.001 |
| Stroke features | ||||
| Stroke type | ||||
| Ischemia | 9(81.82%) | 14(66.67%) | 5(45.45%) | 0.197 |
| Hemorrhage | 2(18.18%) | 7(33.33) | 6(54.55%) | |
| Stroke sites | ||||
| Supratentorial | 9(81.82%) | 18(85.71%) | 10(90.91%) | 0.800 |
| Infratentorial | 2(18.18%) | 2(9.52%) | 1(9.09%) | |
| Both | 0(0%) | 1(4.76%) | 0(0%) | |
| Side of hemiparesis | ||||
| Left side | 6(54.55%) | 6(28.57%) | 5(45.45%) | 0.061 |
| Right side | 2(18.18%) | 11(52.38%) | 4(36.36%) | |
| Both side | 0(0%) | 0(0%) | 2(18.18%) | |
| Within functional limits | 3(27.27%) | 4(19.05%) | 0(0%) | |
Values are shown as mean ± SD or number of patient’s n (%)
FIM=functional independence measure; CVA= cardio vascular disease; CVA= cerebrovascular accident; ΔFIM = gain of FIM motor subscore; MRFS = Montebello Rehabilitation Factor Score
MRI Data acquisition
MRI imaging data was retrospectively collected using the Research Patient Data Registry (RPDR),17 which is a centralized warehouse of clinical data within the Partners Network, including Massachusetts General Hospital (MGH) and Spaulding Rehabilitation Hospital (SRH). We accessed this data using the RPDR online Query Tool and mi2b2 Workbench software.
MRI imaging data within the RPDR vary in acquisition protocol and parameters. In order to reduce variation due to imaging method, we used the following inclusion criteria: DTI images were acquired using a single Siemens Skyra 3T (Siemens Medical Solutions, Erlangen, Germany) scanner and 20-channel head and neck coil. A single-shot echo planar imaging (EPI) sequence was used in DTI data, including 28 nonlinear diffusion directions with b=1000 s/mm2 and an additional three volumes with b=0 s/mm2. The acquisition parameters were as follows: Repetition Time (TR) = 5000 msec, Echo Time (TE) =96 msec, Inversion Time (TI) =-1 msec, flip angle=90 degrees, and field of view (FOV) = 220. The slice thickness was 5 mm, and the matrix dimension size was 160×160×28. As a result, only images from MGH were utilized for analysis.
Neuroimaging analysis
Details of DTI data processing, data motion evaluation, and region of interest (ROI) analysis were reported elsewhere.18–22 FA maps were derived from the diffusion data using the FMRIB software library (FSL V5.0.6, Oxford, UK). The mean FA value for each ROI was determined by averaging all values across all voxels included within each ROI. These ROIs were located on the white matter skeleton that was created by feeding all individuals’ FA maps into tract-based spatial statistics (TBSS) analysis. The JHU White-Matter Tractography atlas was used to determine the exact position for each ROI along the right and left CST in the posterior limb of the internal capsule, superior corona radiata, and the peduncle. We chose these areas based on their importance in preserving motor function.
Statistical analysis
All statistical analyses were carried out in JMP PRO 12.0.1. One-way ANOVA tests were used to statistically analyze the difference in the continuous variables among the clusters (A, B, C), and the Chi-square test was used to evaluate clinical categorical measures. ANOVA analysis was followed by a post-hoc t-test analysis to study the difference between clusters (A-C; B-C; A-B). Due to the small sample size, non-parametric spearman rho correlation coefficient analysis was computed to investigate the relationship between FA, ΔFIM, and MRFS within each cluster. The differences between the clusters as well as the correlations were considered significant in these analyses when P is <0.05 after correction with multiple comparisons (factor=12). Within each cluster, we ran 12 correlation analyses between 6 ROIs and 2 clinical scales.
Results
Clinical characteristic and demographics
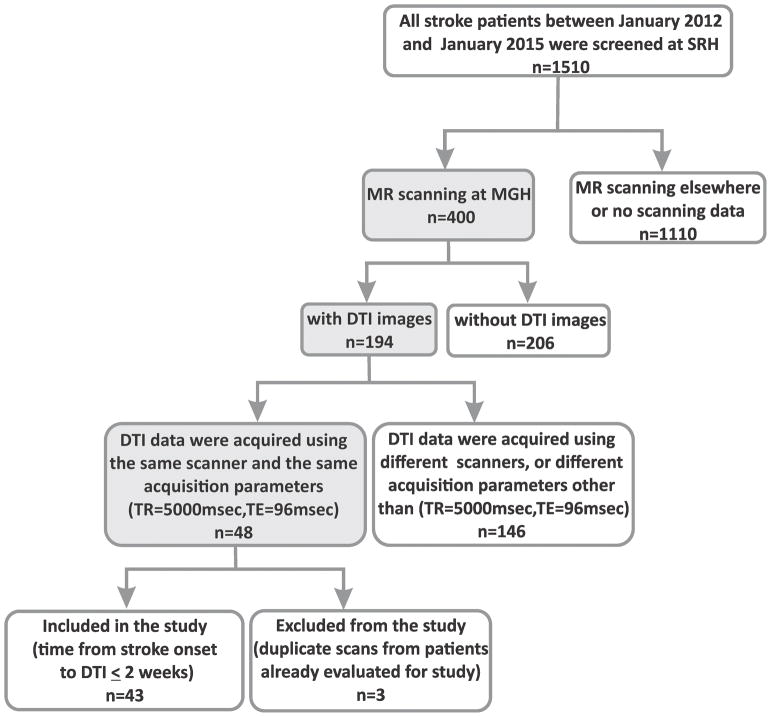
Forty-three subjects were included in the study. As shown in Figure 1, the limiting factor in enrollment was the availability of DTI images acquired using the same scanner, DTI protocol, and acquisition parameters. Among them, 21 (48.8%) were male. The average age was 68±14 years. 28 subjects were diagnosed with ischemic stroke and 15 with hemorrhagic stroke. The stroke location was supratentorial in 37 subjects, infratentorial in 5 subjects, and in one subject, the stroke was extending between the supra- and infratentorial areas. The hemiplegia involved the left side in 17 subjects, the right in 17 subjects, and in both sides in two subjects; the motor function was within the functional limits in 7 subjects. Number of days from onset to admission was 13±11 (3–49). Time from stroke onset to MRI scanning was 3±3 (1–13) days. Length of hospital stay was 25±15 (6–74) days. Twenty (46.5%) patients were discharged home, 17 (39.5%) to a skilled nursing facility, and 6 (14.0%) patients were transferred to an acute unit. The average FIM motor sub-score on admission was 25.63±11.91 (12–60) and on discharge was 44.58±17.79 (13–80).
Figure 1.
Flowchart of the study population.
SRH = Spaulding Rehabilitation Hospital; MR = Magnetic resonance; MGH = Massachusetts General Hospital; DTI = diffusion tensor imaging; TR = Repetition Time; TE = Echo Time
Five (11.6%) subjects were current smokers, 14 (32.6%) were former smokers, and 24 (56.8%) nonsmokers. Eleven (25.6%) subjects were diabetics, 18 (41.9%) were diagnosed with hyperlipidemia, 12 (27.9%) had a past history of cerebrovascular accident, six (14%) had a past history of cardiovascular disease, and 11 (25.6%) had an atrial fibrillation.
K-means cluster analysis (k=3) using both ΔFIM and MRFS generated three groups (cluster A-good recovery; cluster B-moderate recovery; cluster C-poor recovery). Cluster A had an average of ΔFIM motor subscore of 31.3; cluster B had an average of ΔFIM motor subscore of 16.6 and cluster C had an average of ΔFIM motor subscore of 4.36. According to the published minimally clinical important difference (MCID) for stroke recovery,13, 14 ΔFIM motor subscores from cluster A and cluster B but not cluster C reached the MCID which is 17 for FIM motor subscore.13, 14 There was no significant difference in the age among these clusters (cluster A 67.8 ± 3.36; cluster B 70.1 ± 2.66; cluster C 65.2 ± 3.67). ΔFIM and MRFS scores were significantly higher in cluster A (p<0.05; ANOVA) as a comparison with clusters B and C. There was significant difference in the discharge destination among the three groups (p=0.01). Seventy-three percent of patients in cluster A and 52% in cluster B were discharged home, whereas 9% of patients in cluster C were discharged home. There was no significant difference in the length of hospital stay among the clusters (cluster A 22±15 days; cluster B 25±15 days; cluster C 28±14 days) (see Table 1).
FA comparison among clusters
ANOVA analysis revealed significant difference in FA values among the clusters (A, B, C). Post-hoc t-test analysis revealed that FA values were significantly higher in cluster A (good recovery) as compared to FA values in cluster C (poor recovery). Also FA values were significantly higher in cluster B (moderate recovery) compared to cluster C (poor recovery).
The significant differences between the clusters (A-C) and (B-C) were observed in the right CST, left CST, right PLIC, left PLIC, right peduncle, and left peduncle (all P<0.05, corrected). We found a significant difference (p<0.05, corrected) in FA values between the clusters (A-B) in left-PLIC and left-peduncle (see Table 2 and Figure 2). We didn’t find any significant difference between the clusters in the left superior corona radiata (S-CR) or right S-CR.
Table 2.
FA value comparison between the clusters within each ROI
| ROI | Cluster | Mean | Std Dev | Std Err | Lower 95% | Upper 95% | P value |
|---|---|---|---|---|---|---|---|
| R-CST | A | 0.53 | 0.08 | 0.02 | 0.48 | 0.59 | <0.0001* |
| B | 0.53 | 0.07 | 0.01 | 0.50 | 0.57 | ||
| C | 0.19 | 0.06 | 0.02 | 0.15 | 0.23 | ||
|
| |||||||
| L-CST | A | 0.48 | 0.08 | 0.02 | 0.40 | 0.54 | <0.0001* |
| B | 0.45 | 0.05 | 0.01 | 0.43 | 0.48 | ||
| C | 0.17 | 0.05 | 0.01 | 0.14 | 0.20 | ||
|
| |||||||
| R-Peduncle | A | 0.45 | 0.12 | 0.04 | 0.37 | 0.54 | <0.0001* |
| B | 0.45 | 0.05 | 0.01 | 0.43 | 0.47 | ||
| C | 0.17 | 0.05 | 0.01 | 0.14 | 0.20 | ||
|
| |||||||
| L-Peduncle | A | 0.59 | 0.08 | 0.02 | 0.54 | 0.65 | 0.0056* |
| B | 0.52 | 0.09 | 0.02 | 0.48 | 0.57 | ||
| C | 0.45 | 0.13 | 0.04 | 0.36 | 0.54 | ||
|
| |||||||
| R-PLIC | A | 0.57 | 0.07 | 0.02 | 0.53 | 0.62 | <0.0001* |
| B | 0.56 | 0.06 | 0.01 | 0.53 | 0.59 | ||
| C | 0.16 | 0.04 | 0.01 | 0.13 | 0.19 | ||
|
| |||||||
| L-PLIC | A | 0.60 | 0.12 | 0.04 | 0.52 | 0.68 | <0.0001* |
| B | 0.58 | 0.08 | 0.02 | 0.54 | 0.61 | ||
| C | 0.28 | 0.12 | 0.04 | 0.19 | 0.36 | ||
C: Cluster, N: Number of subjects;
Significant difference between the clusters (A, B, C) (P<0.05; ANOVA)
L-CST=Left cortico spinal tract; R-CST= Right cortico spinal tract; L-PLIC= left posterior limb of the internal capsule; R-PLIC= Right posterior limb of the internal capsule; L-peduncle= Left peduncle; R-peduncle= Right peduncle
Figure 2.
Box plot graphic representation of Z-score fractional anisotropy (FA) values showing the median values (horizontal line inside the box), quartiles (box boundaries), and the largest and smallest FA values. *significant difference between the clusters (A–C; B–C) (P<0.05). ** significant difference between the clusters (A–B) (P<0.05)
L-CST: Left corticospinal tract; R-CST: Right cortico spinal tract; L-PLIC: left posterior limb of the internal capsule; R-PLIC: Right posterior limb of the internal capsule; L-peduncle: Left peduncle; R-peduncle: Right peduncle.
Correlations within the clusters between FA and ΔFIM
Cluster A-good recovery
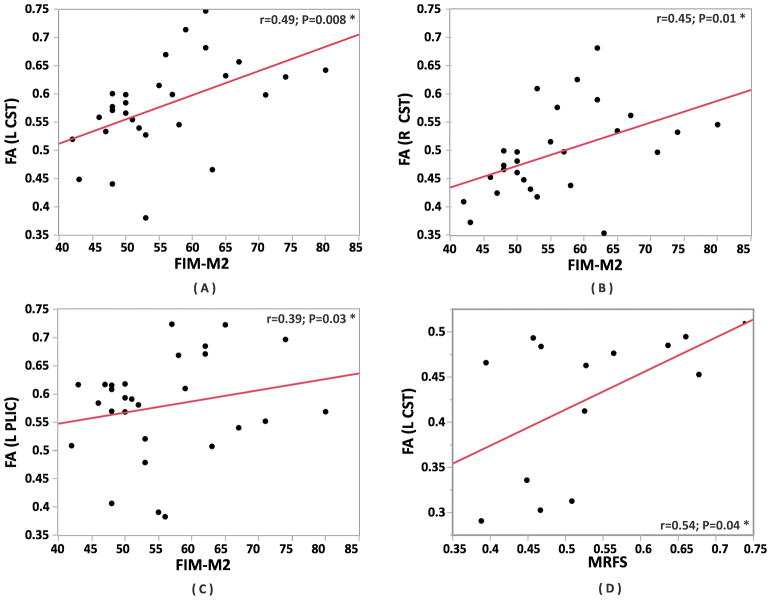
We found a significant positive correlation between FA and ΔFIM in the right peduncle (r=0.89; P=0.0002), right CST (r=0.80; P=0.0029), left CST (r=0.71; P=0.01), right PLIC (r=0.66; P=0.025), and left PLIC (r=0.63; P=0.037) (Figures 3A, 3C).
Figure 3.
Spearman rho correlation coefficient analyses within the clusters. The left panel showing the correlations. The correlations are sorted by the most significant on top. The right panel shows scatter plots between FA within the right peduncle and ΔFIM (A), FA within left CST and MRFS (B), FA within right CST and ΔFIM (C), FA within right CST and MRFS (D), FA within right PLIC and ΔFIM (E) and FA within right PLIC and MRFS (F). The subjects within each cluster are represented by (green=cluster A; red=cluster B; blue=cluster C).
*significant difference between the groups P<0.05 (corrected). ** significant difference between the groups P<0.004 (uncorrected).
L-CST = Left corticospinal tract; R-CST = Right corticospinal tract; L-PLIC = Left posterior limb of the internal capsule; R-PLIC = Right posterior limb of the internal capsule; ΔFIM = gain of FIM motor sub-score; FA = Fractional Anisotropy; MRFS = Montebello Rehabilitation Factor Score.
Cluster B-moderate recovery
We found a significant positive correlation between FA and ΔFIM in the right peduncle (r=0.91; P<0.0001), right CST (r=0.82; P<0.0001), and right PLIC (r=0.78; P<0.0001).(Figures 3A, 3C, 3E).
Cluster C-poor recovery
No significant correlations were detected between FA and ΔFIM.
Correlations within the clusters between FA and MRFS
Cluster A-good recovery
We found a significant positive correlation between FA and MRFS in the right CST (r=0.90; P=0.0002), left CST (r=0.73; P=0.0098), left PLIC (r=0.72; P=0.011), and right peduncle (r=0.66; P=0.026) (Figures 3B, 3D).
Cluster B-moderate recovery
We found a significant positive correlation between FA and MRFS in the right peduncle (r=0.92; P<0.0001), right CST (r=0.85; P<0.0001), right PLIC (r=0.76; P<0.0001), and left CST (r=0.45; P=0.38) (Figures 3D, 3F).
Cluster C-poor recovery
No significant correlations were detected between FA and MRFS.
Discussion
In this study, we show that FA values within bilateral CST are higher in acute/subacute stoke patients who have better functional recovery. We also found that FA values within CST correlated positively with motor functional recovery in patients with better recovery as measured by MRFS following post-acute rehabilitation.
Anisotropy is one of the most important metrics in DTI. It has been used widely as a general index of axonal integrity.23 It measures the degree to which water molecule diffusion is constrained in brain tissue. In other words, when the space between the axons is very small (e.g., the corpus callosum where the axons are tightly packed and highly organized), the diffusion of water molecules is more constrained, and as a result, the anisotropy is higher, reflecting normal tissue structure.
Until recently, the literature has either described the long-term changes in anisotropy following different etiologies that could lead to degenerative changes in brain tissue or used FA value to predict long-term consequences following an injury. Recently, shorter time frames are becoming relevant as studies have reported rapid changes in anisotropy following post-acute pathological changes in brain tissue or after treatments targeting specific conditions.23–26
The degree of anisotropy in a white or gray matter region reflects the degree of structural integrity of white or gray matter in that region. In the acute phase of stroke, the mean diffusivity (MD) decreases as the blood flow decreases,23, 27 and the space between the axons shrinks in association with increased FA; around 5–7 days after stroke, MD normalizes, and FA becomes normal. In sub-acute to chronic stroke (after 2 weeks), MD starts to increase as encephalomalacia ensues, and FA decreases below baseline levels.23, 24, 28 It appears that rather than being uniform across the brain, anisotropy is highly variable in different white and gray matter regions following not only chronic degenerative changes, but also after acute and sub-acute changes in brain pathophysiology. Thus, FA may potentially serve as an imaging biomarker during the recovery process.
Our results indicated that FA values within the CST, PLIC, and peduncle bilaterally were significantly higher in the good and moderate motor recovery groups compared to the poor motor recovery group. This suggests that the CST white matter structure in the good motor recovery group is tighter and has less space between the axons. Such a group difference may be related to fewer edematous changes in the acute stages following stroke and/or to greater regional restriction of axonal damage. On the other hand, in the poor motor recovery group, decreased FA values suggest that there is more space between the axons, with the CST white matter structure being looser due to primary tissue damage,29 remote anterograte Wallerian degeneration (WD) 30, 31, and/or retrograde axonal degeneration post stroke32.
Previous studies have indicated that anisotropy is mainly influenced by axonal membranes, whereas myelin, microtubules, and fast axonal transport have limited effects on diffusion anisotropy, and the degree of fiber organization in the white matter tracts mirrors the diffusion anisotropy33–38. While it has previously been shown that CST disruption is associated with degree of chronic functional impairment, our study provides more insight into this relationship due to the correlations we have identified between FA values and early functional recovery.
In this study, we found that FA values within CST correlate positively with degree of motor functional improvement in patients with better recovery. In line with our study, Fan et al39 showed that increased FA after training was coupled with greater improvement of motor function.
Our finding provides new insight into the mechanisms of stroke recovery and would potentially lead to developing novel targets for pharmacological or non-pharmacological inventions to promote stroke recovery.
Study limitations
This study has several limitations. First, as our data was collected using RPDR and mi2b2, we were restricted in our analysis to only the raw diffusion weighting images (DWI), so we were unable to do volumetric tractography because the number of gradient directions available to us was small. Second, small sample size (n = 43) and the heterogeneity of stroke patients were also a primary limitation of this study. Lesion type varied in these patients as 28 subjects were diagnosed with ischemic stroke and 15 with hemorrhagic stroke. Lesion location varied in these patients -- supratentorial in 37 subjects, infratentorial in 5 subjects, and in one subject extending between the supra- and infratentorial areas. Also, 21 patients had first-onset stroke, and 12 (27.9%) had a prior history of cerebrovascular accident. Third, we were specifically interested in the value of anisotropy in the CST, PLIC, and CR as a predictor for motor recovery only in one-time point. For better evaluation of the predictive value of FA, we suggest that future studies measure FA value in multiple time points. Fourth, we investigated only the corticospinal tract to evaluate motor function, and we did not investigate other tracts that could contribute to motor function or provide potential caveats. Fifth, our analysis did not control for demographics or other covariates. Nonetheless, we did not observe any significant group differences in univariate comparisons.
Conclusions
In summary, we have shown preliminary evidence that FA values within the corticospinal tracts correlate positively with degree of motor function improvement in acute stroke patients following early inpatient rehabilitation. Further studies are needed to identify the factors and approaches that lead to improved brain tissue functionality during rehabilitation as this may help to design more effective rehabilitation regimens.
Acknowledgments
This study was accepted for presentation at the annual assembly of American Academy of Physical Medicine and Rehabilitation October 1–4th, 2015.
This work was conducted with support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award UL1 TR001102) and financial contributions from Harvard University and its affiliated academic healthcare centers. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, or the National Institutes of Health.
Dr. Hongmei Wen was supported by the Fundamental Research Funds for the Central Universities (12ykpy39) and the National Natural Science Foundation of China (No.81101461; No.81472156).
List of abbreviations
- CST
Corticospinal tract
- DTI
Diffusion Tensor Imaging
- FA
Fractional Anisotropy
- FIM
Functional Independence Measure
- MD
Mean Diffusivity
- MRFS
Montebello Rehabilitation Factor Score
Footnotes
Conflict of Interest:
Dr. Ross Zafonte receives book royalty from Demos, personal fees for educational video from Oakstone and serves in the scientific advisory board at Oxeia Biopharma.
Conflict of interest
None.
References
- 1.Donnan GA, Fisher M, Macleod M, Macleod M. Stroke. Lancet. 2008;371:1612–23. doi: 10.1016/S0140-6736(08)60694-7. [DOI] [PubMed] [Google Scholar]
- 2.Hendricks HT, van Limbeek J, Geurts AC, Zwarts MJ. Motor recovery after stroke: a systematic review of the literature. Arch Phys Med Rehabil. 2002;83:1629–37. doi: 10.1053/apmr.2002.35473. [DOI] [PubMed] [Google Scholar]
- 3.Coupar F, Pollock A, Rowe P, et al. Predictors of upper limb recovery after stroke: a systematic review and meta-analysis. Clin Rehabil. 2012;26:291–313. doi: 10.1177/0269215511420305. [DOI] [PubMed] [Google Scholar]
- 4.Hagmann P, Jonasson L, Maeder P, et al. Understanding diffusion MR imaging techniques: from scalar diffusion-weighted imaging to diffusion tensor imaging and beyond. Radiographics. 2006;26(Suppl 1):S205–23. doi: 10.1148/rg.26si065510. [DOI] [PubMed] [Google Scholar]
- 5.Le Bihan D, Johansen-Berg H. Diffusion MRI at 25: exploring brain tissue structure and function. Neuroimage. 2012;61:324–41. doi: 10.1016/j.neuroimage.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Assaf Y, Pasternak O. Diffusion tensor imaging (DTI)-based white matter mapping in brain research: a review. J Mol Neurosci. 2008;34:51–61. doi: 10.1007/s12031-007-0029-0. [DOI] [PubMed] [Google Scholar]
- 7.Neil JJ. Diffusion imaging concepts for clinicians. J Magn Reson Imaging. 2008;27:1–7. doi: 10.1002/jmri.21087. [DOI] [PubMed] [Google Scholar]
- 8.Puig J, Blasco G, Daunis IEJ, et al. Decreased corticospinal tract fractional anisotropy predicts long-term motor outcome after stroke. Stroke. 2013;44:2016–8. doi: 10.1161/STROKEAHA.111.000382. [DOI] [PubMed] [Google Scholar]
- 9.Song J, Nair VA, Young BM, et al. DTI measures track and predict motor function outcomes in stroke rehabilitation utilizing BCI technology. Front Hum Neurosci. 2015;9:195. doi: 10.3389/fnhum.2015.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Groisser BN, Copen WA, Singhal AB, et al. Corticospinal tract diffusion abnormalities early after stroke predict motor outcome. Neurorehabil Neural Repair. 2014;28:751–60. doi: 10.1177/1545968314521896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koyama T, Tsuji M, Nishimura H, et al. Diffusion tensor imaging for intracerebral hemorrhage outcome prediction: comparison using data from the corona radiata/internal capsule and the cerebral peduncle. J Stroke Cerebrovasc Dis. 2013;22:72–9. doi: 10.1016/j.jstrokecerebrovasdis.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 12.Granger CV, Hamilton BB, Linacre JM, et al. Performance profiles of the functional independence measure. Am J Phys Med Rehabil. 1993;72:84–9. doi: 10.1097/00002060-199304000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Beninato M, Gill-Body KM, Salles S, et al. Determination of the minimal clinically important difference in the fim instrument in patients with stroke. Arch Phys Med Rehabil. 2006;87:32–9. doi: 10.1016/j.apmr.2005.08.130. [DOI] [PubMed] [Google Scholar]
- 14.Hayward KS, Kuys SS, Barker RN, et al. Clinically important improvements in motor function are achievable during inpatient rehabilitation by stroke patients with severe motor disability: A prospective observational study. NeuroRehabilitation. 2014;34:773–9. doi: 10.3233/NRE-141076. [DOI] [PubMed] [Google Scholar]
- 15.Mutai H, Furukawa T, Araki K, et al. Factors associated with functional recovery and home discharge in stroke patients admitted to a convalescent rehabilitation ward. Geriatrics & gerontology international. 2012;12:215–22. doi: 10.1111/j.1447-0594.2011.00747.x. [DOI] [PubMed] [Google Scholar]
- 16.Koh GC, Chen CH, Petrella R, Thind A. Rehabilitation impact indices and their independent predictors: a systematic review. BMJ open. 2013;3:e003483. doi: 10.1136/bmjopen-2013-003483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nalichowski R, Keogh D, Chueh HC, Murphy SN. Calculating the benefits of a Research Patient Data Repository. AMIA Annu Symp Proc. 2006:1044. [PMC free article] [PubMed] [Google Scholar]
- 18.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–55. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Behrens TE, Johansen-Berg H, Woolrich MW, et al. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci. 2003;6:750–7. doi: 10.1038/nn1075. [DOI] [PubMed] [Google Scholar]
- 20.Behrens TE, Woolrich MW, Jenkinson M, et al. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med. 2003;50:1077–88. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- 21.Johansen-Berg H, Behrens TE, Robson MD, et al. Changes in connectivity profiles define functionally distinct regions in human medial frontal cortex. Proc Natl Acad Sci U S A. 2004;101:13335–40. doi: 10.1073/pnas.0403743101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Behrens TE, Berg HJ, Jbabdi S, et al. Probabilistic diffusion tractography with multiple fibre orientations: what can we gain? Neuroimage. 2007;34:144–55. doi: 10.1016/j.neuroimage.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007;4:316–29. doi: 10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang Q, Tress BM, Barber PA, et al. Serial study of apparent diffusion coefficient and anisotropy in patients with acute stroke. Stroke. 1999;30:2382–90. doi: 10.1161/01.str.30.11.2382. [DOI] [PubMed] [Google Scholar]
- 25.Burke Quinlan E, Dodakian L, See J, et al. Neural function, injury, and stroke subtype predict treatment gains after stroke. Ann Neurol. 2015;77:132–45. doi: 10.1002/ana.24309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dervan L, Poliakov A, Friedman SD, et al. Change in fractional anisotropy during treatment of diabetic ketoacidosis in children. Pediatr Res. 2014;75:62–6. doi: 10.1038/pr.2013.168. [DOI] [PubMed] [Google Scholar]
- 27.van Gelderen P, de Vleeschouwer MH, DesPres D, et al. Water diffusion and acute stroke. Magn Reson Med. 1994;31:154–63. doi: 10.1002/mrm.1910310209. [DOI] [PubMed] [Google Scholar]
- 28.Liu Y, D’Arceuil HE, Westmoreland S, et al. Serial diffusion tensor MRI after transient and permanent cerebral ischemia in nonhuman primates. Stroke. 2007;38:138–45. doi: 10.1161/01.STR.0000252127.07428.9c. [DOI] [PubMed] [Google Scholar]
- 29.Radlinska B, Ghinani S, Leppert IR, et al. Diffusion tensor imaging, permanent pyramidal tract damage, and outcome in subcortical stroke. Neurology. 2010;75:1048–54. doi: 10.1212/WNL.0b013e3181f39aa0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomalla G, Glauche V, Koch MA, et al. Diffusion tensor imaging detects early Wallerian degeneration of the pyramidal tract after ischemic stroke. Neuroimage. 2004;22:1767–74. doi: 10.1016/j.neuroimage.2004.03.041. [DOI] [PubMed] [Google Scholar]
- 31.Lai C, Zhou HC, Ma XH, Zhang HX. Quantitative evaluation of the axonal degeneration of central motor neurons in chronic cerebral stroke with diffusion tensor imaging. Acta Radiol. 2014;55:114–20. doi: 10.1177/0284185113492456. [DOI] [PubMed] [Google Scholar]
- 32.Werring DJ, Toosy AT, Clark CA, et al. Diffusion tensor imaging can detect and quantify corticospinal tract degeneration after stroke. J Neurol Neurosurg Psychiatry. 2000;69:269–72. doi: 10.1136/jnnp.69.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15:435–55. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- 34.Beaulieu C, Allen PS. Water diffusion in the giant axon of the squid: implications for diffusion-weighted MRI of the nervous system. Magn Reson Med. 1994;32:579–83. doi: 10.1002/mrm.1910320506. [DOI] [PubMed] [Google Scholar]
- 35.Beaulieu C, Allen PS. Determinants of anisotropic water diffusion in nerves. Magn Reson Med. 1994;31:394–400. doi: 10.1002/mrm.1910310408. [DOI] [PubMed] [Google Scholar]
- 36.Gulani V, Webb AG, Duncan ID, Lauterbur PC. Apparent diffusion tensor measurements in myelin-deficient rat spinal cords. Magn Reson Med. 2001;45:191–5. doi: 10.1002/1522-2594(200102)45:2<191::aid-mrm1025>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 37.Ono J, Harada K, Takahashi M, et al. Differentiation between dysmyelination and demyelination using magnetic resonance diffusional anisotropy. Brain Res. 1995;671:141–8. doi: 10.1016/0006-8993(94)01335-f. [DOI] [PubMed] [Google Scholar]
- 38.Song SK, Sun SW, Ramsbottom MJ, et al. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17:1429–36. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- 39.Fan YT, Lin KC, Liu HL, et al. Changes in structural integrity are correlated with motor and functional recovery after post-stroke rehabilitation. Restor Neurol Neurosci. 2015;33:835–44. doi: 10.3233/RNN-150523. [DOI] [PubMed] [Google Scholar]